Abstract
The objective of this work is to examine the contribution of late Na+ current (INa,L) to the cardiac action potential (AP) and arrhythmogenic activities. In spite of the rapidly growing interest toward this current, there is no publication available on experimental recording of the dynamic INa,L current as it flows during AP with Ca2+ cycling. Also unknown is how the current profile changes when the Ca2+-calmodulin dependent protein kinase II (CaMKII) signaling is altered, and how the current contributes to the development of arrhythmias. In this study we use an innovative AP-clamp Sequential Dissection technique to directly record the INa,L current during the AP with Ca2+ cycling in the guinea pig ventricular myocytes. First, we found that the magnitude of INa,L measured under AP-clamp is substantially larger than earlier studies indicated. CaMKII inhibition using KN-93 significantly reduced the current. Second, we recorded INa,L together with IKs, IKr, and IK1 in the same cell to understand how these currents counterbalance to shape the AP morphology. We found that the amplitude and the total charge carried by INa,L exceed that of IKs. Third, facilitation of INa,L by Anemone toxin II prolonged APD and induced Ca2+ oscillations that led to early and delayed afterdepolarizations and triggered APs; these arrhythmogenic activities were eliminated by buffering Ca2+ with BAPTA. In conclusion, INa,L contributes a significantly large inward current that prolongs APD and unbalances the Ca2+ homeostasis to cause arrhythmogenic APs.
Keywords: Na+ channel, late Na+ current, action potential, calcium, myocyte, cardiac, arrhythmia
1. Introduction
The late Na+ current (INa,L) influences the cardiac action potential (AP) repolarization [1–5], participates in Na+-Ca2+ homeostasis of cardiac cells [6–13], and can promote arrhythmogenic activities [13–17]. This tiny, persistent Na+ current has received increasing attention since its selective inhibition eliminated arrhythmogenic APs [5, 15–17], but electrophysiological recording of the INa,L current has been technically challenging. Our current knowledge on INa,L is mostly based on the voltage-clamp data obtained under simplified conditions (rectangular voltage pulse, ion substitutions, exogenous Ca2+ buffer etc.). The data was then used in model simulations to predict the dynamic profile of the current during AP in order to understand its role in shaping the AP. Although this approach has contributed valuable knowledge, using standard voltage-clamp technique to study INa,L suffers a great limitation due to a unique feature of the Na+ channel called non-equilibrium gating. Clancy et al. [13] first discovered an enhancement of INa,L under a repolarizing voltage ramp (instead of a rectangular voltage step), and this can be explained by a fast recovery of the Na+ channel from inactivated state and reactivation during repolarization ramp (non-equilibrium condition). The non-equilibrium gating gives rise to a large portion of INa,L and, importantly, this portion of the current cannot be activated by rectangular pulse voltage-clamp protocol [13, 15]. The divergent INa,L profiles reported in previous literature highlight the complexity in Na+ channel gating and the necessity to directly record INa,L under AP-clamp, because dynamic changes of the membrane potential during AP would significantly influence INa. In this study we use a special version of AP-clamp technique called self AP-clamp (sAP-clamp) [18–20] to directly record the INa,L during AP in the guinea pig ventricular myocytes under a triad of conditions: use the cell’s own steady state AP as the voltage command; keep physiological ionic compositions in the internal and external solutions; and preserve the intracellular Ca2+ cycling. The goal is to visualize and analyze INa,L as it flows during cardiac AP under physiological conditions in order to understand its contribution to shaping cardiac APs.
Voltage gated Na+ channels are known to be regulated by Ca2+, calmodulin (CaM), and Ca2+-CaM dependent protein kinases II (CaMKII); each of these molecules also individually and collectively modulates INa,L [21–23]. Previous studies exploring the individual elements of this complex regulation reported conflicting results. For example, Wingo et al. proposed that Ca2+ modulates Na+ current by directly binding to the EF-hand motif in the C-terminus [24], but different studies provided evidence against this notion [25, 26]. Tan et al. showed that CaM enhances slow inactivation of INa [27], and Kim et al suggested that CaM binding minimizes the sustained channel activity [25]. The current consensus is that Ca2+-CaM-CaMKII signaling facilitates cardiac Na+ current [23]. However, the simplified conditions used in traditional voltage-clamp experiments make it difficult to determine the integrated effects of Ca2+-CaM-CaMKII signaling pathway on modulating the INa during AP. Furthermore, many previous studies used rectangular pulse voltage-clamp protocol and the non-equilibrium gating of Na+ channel was absent from those data [28–30]. In this study we use the sAP-clamp technique to directly record the profile of INa,L during the AP with Ca2+ cycling, after Ca2+ overloading or chelation, and following CaMKII inhibition. The results provide the first experimental measure of CaMKII modulation of the dynamic INa,L during cardiac AP. We also further investigated changes in the AP profile when INa,L is facilitated by Anemone toxin II (ATX-II) to explore the role of INa,L in arrhythmogenesis.
2. Methods
All animal handling and laboratory procedures conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and to our Institutional Animal Care and Use Committee approved protocols. Chemicals and reagents were purchased from Sigma-Aldrich if not specified otherwise. Tetrodotoxin (TTX) and ATX-II were from EMD Chemicals. E4031 and Chromanal-293B were from TOCRIS. All experiments were conducted at 36±0.2 °C.
2.1. Cell isolation
Hartley guinea pig (male, 3–5 months old, purchased from Charles River Laboratories USA) were first injected with heparin (800u, I.P.) and then anesthetized with nembutal (100 mg/kg, I.P.). After achieving deep anesthesia a standard enzymatic technique was used to isolate ventricular myocytes at 37 °C. Hearts were mounted on a Langendorff system, and retrogradely perfused for 3–5 minutes with oxygenated solution containing (in mmol/l): NaCl 135, KCl 5.4, CaCl2 1, MgCl2 1, NaH2PO4 0.3, HEPES 10, glucose 10; pH=7.2. Then a Ca2+-free solution containing (in mmol/l): NaCl 135, KCl 5.4, MgCl2 1, NaHPO4 0.3, HEPES 10, glucose 10, EGTA 0.05 (pH=7.2) was perfused for 3 minutes to stop the beating of the heart. Next, a solution containing (in mmol/l): NaCl 135, KCl 5.4, MgCl2 1, NaHPO4 0.3, HEPES 10, glucose 10), and supplemented with 0.6 mg/ml type II collagenase (298 U/mg; Worthington, USA) and 0.05 mg/ml protease type XIV (Sigma, USA) was perfused to enzymatically dissociate cells. After perfusion, the left ventricle was minced and further incubated in the above solution for 40, 60, 80 minutes at 37 °C. Cells were then harvested and stored in a modified Tyrode solution (BTY) containing (in mmol/l): NaCl 120, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, NaHCO3 25, Glucose 10, pH=7.3 (adjusted using NaOH) and osmolarity=295–300 mmol/kg.
2.2. Electrophysiology
Cells were placed in a temperature controlled plexiglass chamber (Cell Microsystems, USA) and superfused with BTY solution (see above). Electrodes were fabricated from borosilicate glass (World Precision Instruments, USA) with tip resistances of 2–2.5 MΩ when filled with internal solution. To preserve the normal Ca2+ cycling during AP, we used the internal solution containing (in mmol/l): K-Aspartate 100, KCl 45, Mg-ATP 3, HEPES 5, cAMP 0.1, phosphocreatine dipotassium salt 10, Fura-2 K+ salt 0.02 (pipette loading), with pH=7.3 (adjusted using KOH) and osmolarity = 284–288 mmol/kg. To buffer intracellular Ca2+ to 100 nM (MaxChalator calculation) we used the internal solution containing (in mmol/l): K-Aspartate 74, KCl 43, KOH 25, Mg-ATP 3, HEPES 5, cAMP 0.1, phosphocreatine dipotassium salt 10, BAPTA 10, CaCl2 2.48, Fura-2 K+ salt 0.02, with pH=7.3.
sAP-clamp Sequential Dissection experiments were conducted as described in our previous publication [19]. Basic steps include: (1) Record the cell’s steady state AP under I-clamp (I=0) at 1 Hz pacing frequency. (2) Apply this AP onto the same cell as the voltage command under V-clamp at 1 Hz. The net current output, IBG should be zero. (3) Isolate the current of interest by using its specific blocker to remove it from the net current output, Idrug. (4) The current of interest is then obtained by subtraction: I = IBG - Idrug. (5) Next, isolate the 2nd current of interest by applying the 2nd channel blocker, and then obtained the 2nd current by subtraction: I2 = Idrug1 - Idrug2. Repeat (5) to isolate the 3rd, the 4th, and more currents by sequentially adding the specific blocker for each channel. The currents were recorded after they had reached steady-state. The following blockers used for dissecting out the currents: 10 µM chromonal-293B for IKs, 1µM E4031 for IKr, and 50 µM Ba2+ for IK1.
Current-clamp experiments were conducted to record action potentials (APs). Cells were stimulated with supra-threshold depolarizing pulses (2 ms duration) delivered via the patch pipette. APs were recorded at various pacing frequencies. After reaching steady state at each frequency, at least 50 consecutive APs were recorded to examine the average behavior. The frequency series were first recorded under control condition (in BTY), and then repeated after treatment with 10, 30 and 100 nM of ATX-II.
2.3. Measurement of intracellular Ca2+ concentration, [Ca2+]i
Cells were loaded with 20 µM Fura-2 K+ salt (Invitrogen, USA) through the patch pipette for simultaneous recording of the electrical sigNa,L and the Ca2+ sigNa,L in the same cell. [Ca2+]i was measured using IonOptix system (Ionoptix, USA) with dual excitation at 340 nm and 380 nm, and emission at >510 nm (long pass filter). The background fluorescence was measured after tight seal formation. After subtracting the background signal, the fluorescence ratio (F340/F380) was then used to measure [Ca2+]i as described previously [31].
2.4. Ca2+ loading of ventricular myocytes
The myocytes were stimulated at 1 Hz under current-clamp mode during the entire Ca2+ loading and washing procedure below. To increase the cytosolic Ca2+ concentration the cells were superfused with a modified Tyrode’s solution with lower Na+ concentration (110 mM instead of 145 mM, osmolarity was compensated with NMDG) for 10 minutes (Ca2+ loading), and then returned to BTy solution for 5 minutes (washing). This protocol resulted in significant increases in the diastolic Ca2+ concentration as measured by Fura-2 at the end of the washing procedure. Then the sAP-clamp experiments were immediately carried out to measure the INa,L under high Ca2+ load condition.
2.5. Statistical Analysis
The numerical values are presented as Mean ± Standard Error of Mean (SEM) in figures if not noted otherwise. Paired or unpaired Student’s t-test with equal variance (after using F-test to confirm equal variance) and 2 tails is used to evaluate the difference in the mean values, and the difference between two groups is deemed significant if p<0.05.
3. Results
3.1. Profile of INa,L under sAP-clamp with Ca2+ cycling
In order to study the magnitude and time course of INa,L during AP in the ventricular myocyte, we used sAP-clamp technique to directly record INa,L under a triad of conditions that mimic in situ environment: during the cell’s steady state AP, in a physiological ionic milieu (no ion substitution), and with intracellular Ca2+ cycling (no exogenous Ca2+ buffer). Fig.1A shows a set of Na+ currents obtained as TTX-sensitive currents under sAP-clamp. Notice that while the increasing TTX concentrations (between 0.3–30 µM,) inhibit larger and larger fraction of the plateau current the profile of the Na+ current keeps the same shape, indicating that TTX is a highly specific blocker in this concentration range. The TTX-sensitive current displayed a fast component that declined within 20 ms after the AP upstroke and a slow component that turned around to increase during the AP plateau and persisted throughout the AP; these two components demonstrate that TTX blocked both the fast and the late Na+ current. The slow current seen from the turning point to the end of AP is late Na+ current, INa,L.
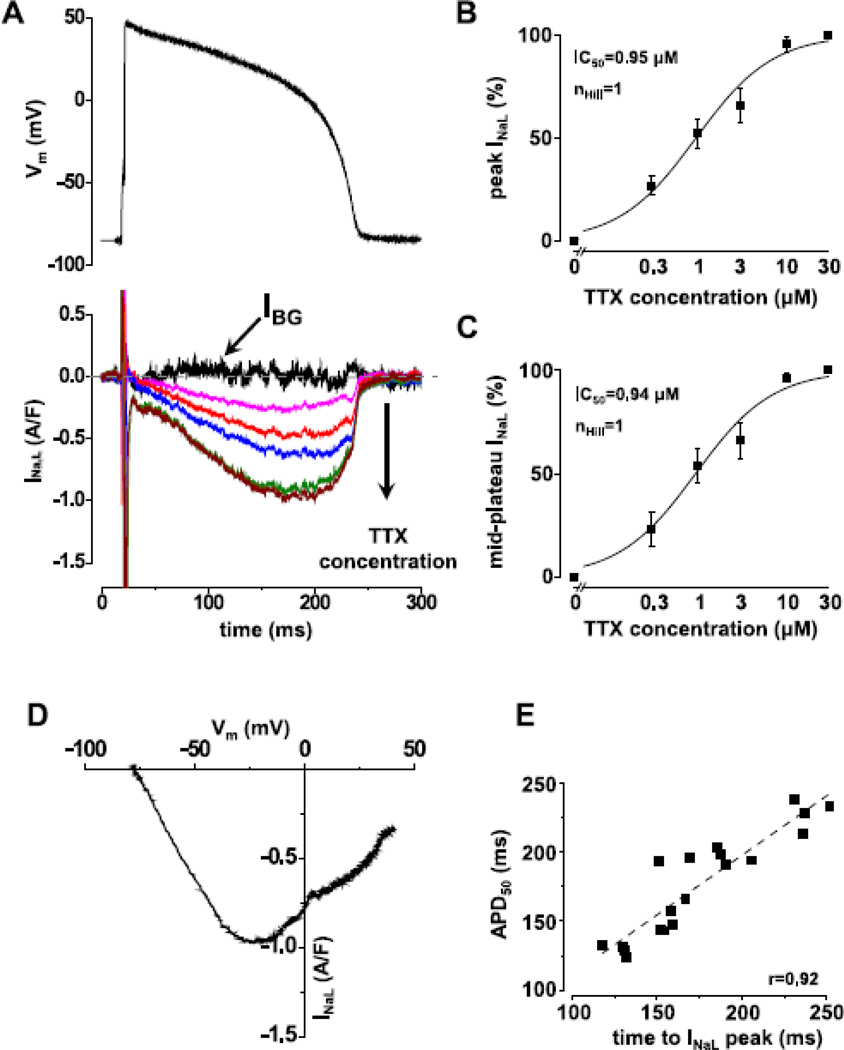
Fig.1. sAP-clamp recording of INa,L as TTX-sensitive current in guinea pig ventricular myocyte.
Panel A shows a representative set of INa,L current recorded under sAP-clamp by using TTX. As the TTX concentration increases (0, 0.3, 1, 3, 10, 30 nM), larger fraction of INa,L is visualized. B & C show the TTX concentration dependence of peak and mid-plateau current magnitudes (Mean±SEM, n=7cells/3animals). The Hill coefficient and IC50 values are the same at the peak and mid-plateau point, indicating the high specificity of TTX in blocking INa,L within the concentration range examined. Panel D shows the dynamic I-V relationship of the full amount of INa,L dissected out by 30 nM TTX plotted against the membrane potential. Panel E shows a tight correlation between APD50 and the time to peak INa,L (n=19cells/11animals). Similar correlations were observed for APD75 and APD90.
INa,L recorded under sAP-clamp displays a strikingly different profile from the small and monotonically decaying current seen under rectangular pulse voltage-clamp; the late Na+ current is now clearly separated from the fast Na+ current. As shown in Fig.1A, INa,L was a small current at AP phase-1, increased gradually during phase-2 and -3, reached a peak at APD50, and then declined rapidly. Fig.1B&C demonstrate TTX dose-response of the peak (Fig.1B) and the mid plateau (Fig.1C) values of INa,L; the data were fitted to Hill equation with Hill coefficient of 1 and IC50 of 0.94 µM and 0.95 µM, respectively. Hence, using 10–30 µM TTX was sufficient for dissecting out the full measure of INa,L. The peak current density of INa,L under sAP-clamp reached 0.78±0.07 A/F, demonstrating a surprisingly larger current. The instant I-V relationship of INa,L shows a bell-shaped curve with the peak current occurring at −15.3±4.6 mV (Fig.1D). A strong correlation also exists between the time to peak INa,L and APD50 (Fig.1E).
3.2. Modulation of INa,L during AP by Ca2+ and CaMKII
We further investigate how the INa,L profile during AP changes by increasing or decreasing the intracellular Ca2+, and by CaMKII inhibition. When the cytosolic Ca2+ concentration was clamped to 100 nM by using BAPTA, the AP was significantly lengthened and the INa,L recorded under sAP-clamp was significantly enlarged (Fig.2A). At the beginning, the INa,L in the Ca2+ clamped cell progressed at the same rate as in the control, but it continues to rise as the AP is lengthened and thus reached a higher peak. The instant I-V relationship for the control and the Ca2+ buffered cell showed difference in magnitude but no shift in the voltage dependency. The INa,L current density was significantly increased during AP (Fig.2C) and the total amount of Na inflow carried by INa,L was tripled in Ca2+ buffered cells (Fig.2D). Therefore, the lengthening of AP and elevation of plateau voltage under the low Ca2+ condition facilitates the INa,L during AP.
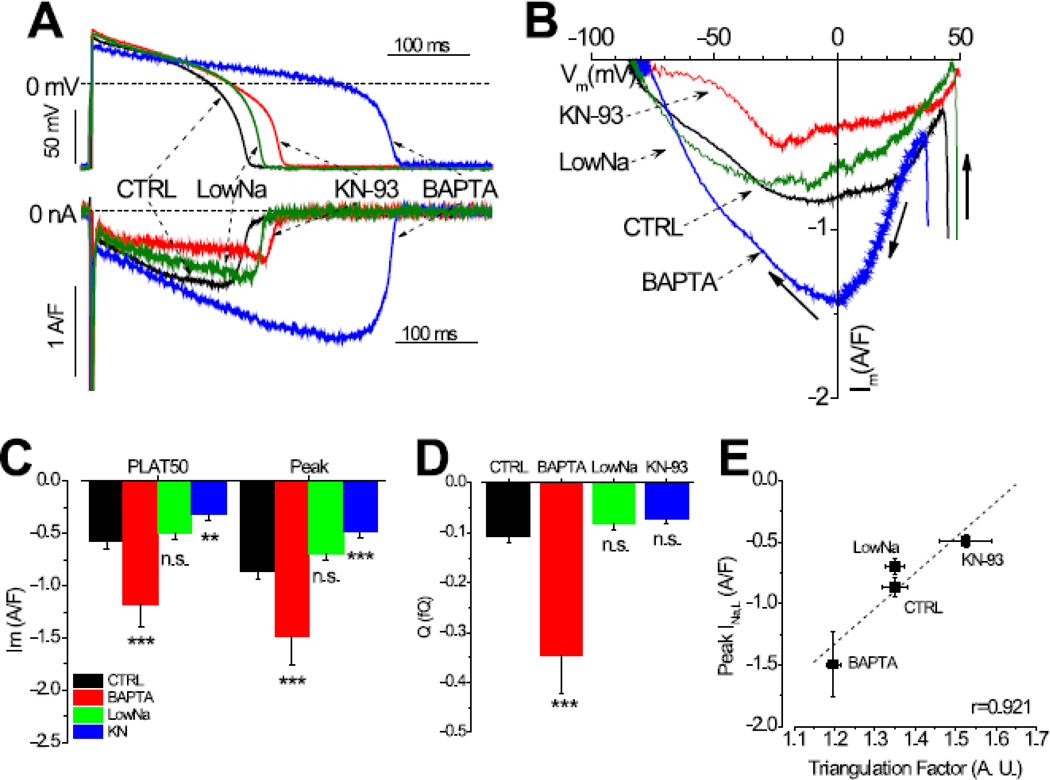
Fig.2. Modulation of INaL by Ca2+ and CaMKII.
Panel A shows representative traces of INa,L recorded in control cells (n=19cells/11animals), in BAPTA treated cells (n=11cells/5animals), in Low Na treated cells (n=7cells/3animals), and in 1 µM KN93 treated cells (n=10cells/5animals). Panel B shows the dynamic I-V relationship of INa,L when the Ca2+ and CaMKII signaling were normal and altered by the above different interventions. Panel C & D show the total charge carried by INa,L (QNa) and the peak current magnitude, respectively. Panel E shows a tight correlation between the amplitude of INa,L and the triangulation factor of the AP shape, demonstrating the presence of non-equilibrium gating.
Since INa,L was increased by chelating Ca2+, next we investigate whether increasing cytosolic Ca2+ would have the opposite effect. We used a Ca2+ loading procedure (see Method #2.4) to increase the amplitude and the baseline of the Ca2+ transient during AP, and recorded INa,L under sAP-clamp. Contrary to the expectation, the profile of INa,L during AP and the I-V relationship did not decrease but is similar to that of the control (Fig.2A & 2B); the current density and the total charge carried by the current are also similar to the control (Fig.2C & 2D). These data suggest two possibilities: one is that the INa,L sensitivity to Ca2+ might be already saturated at the control condition so there is no further change with Ca2+ overload; another is that the INa,L might not be sensitive to Ca2+ and the facilitation of INa,L in Ca2+ buffered cells solely results from the altered voltage profile of AP.
Furthermore, we investigated the effect of CaMKII inhibition on the INa,L profile during AP. The cells were treated with 1 µM KN-93 in bath while being paced at 1 Hz at current-clamp mode for 10 minutes. KN-93 treatment reduced the magnitude of INa,L during AP (Fig.2A) without significantly altering the I-V relationship (Fig.2B). The mid-plateau current magnitude and the peak amplitude were significantly decreased (Fig.2C). Nevertheless, the total amount of charge carried by the current during the AP was not significantly different from the control (Fig.2D), due to a lengthening of the APD90 by KN93 treatment (244.2±18.2ms vs. 204.7±9.2ms, p<0.05) that compensated for the decreased current amplitude.
To investigate the non-equilibrium gating, we use a Triangulation Factor (defined as TF=APD90/APD20) to characterized the membrane voltage change during AP. As shown in Fig.2E, BAPTA decreased TF as the AP lengthened and hence increased the peak INa,L amplitude. Low Na treated cells had similar TF and the peak INa,L as the control. KN93 treatment increased TF and reduced the peak INa,L. In fact, the peak INa,L current amplitude displays a tight positive correlation with TF (Pearson’s r value, r=0.921) under all experimental conditions tested, demonstrating a prominent influence of the non-equilibrium gating on the INa,L current amplitude during AP.
3.3. INa,L contributes significantly to the AP plateau
To understand the role of INa,L in shaping AP we investigated how the inward charge movement through INa,L is counterbalanced by the outward charge movement through K+ currents. We recorded the IKs, IKr IK1, and INa,L from the same cell using the sAP-clamp Sequential Dissection method (Fig.3A). Under control condition, the peak current density of INa,L is higher than that of IKs but lower than that of IKr or IK1. The INa,L peaks at the end of phase-2 of AP, about the same time when Ikr and Ik1 increase rapidly, but the magnitudes of the K+ currents become much larger and thus resulting in an acceleration of repolarization in the phase-3 of AP(Fig 3A). Surprisingly, the amount of inward charges carried by INa,L during the AP is comparable to the outward charges carried by each individual K+ current.
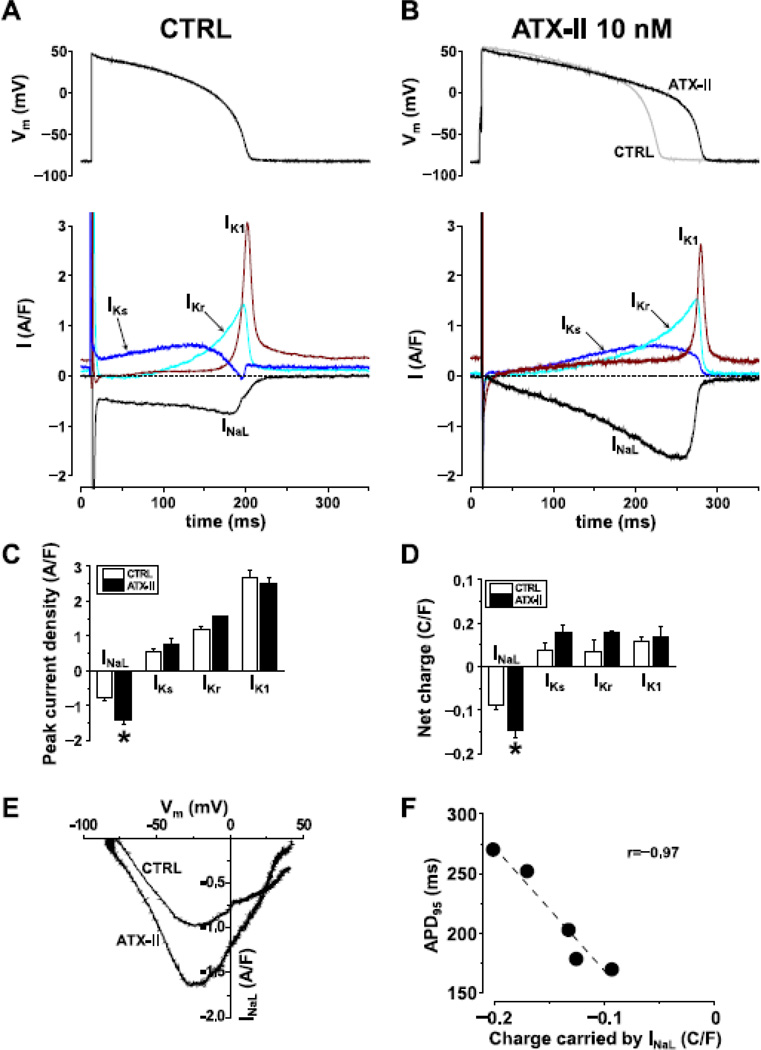
Fig.3. sAP-clamp Sequential Dissection of INaL and three major K+ currents from the same cell.
Representative traces of INaL, IKs, IKr and IK1 recorded from control cells (Panel A, n=6cells/5animals) and following facilitation by 10 nM ATX-II treatment (Panel B, n=5cells/3animals). ATX increased the peak current density (Panel C) and net charge (Panel D) without altering the K+ currents. Facilitation with ATX-II did not alter the dynamic I-V relationship (Panel E). When AP was lengthened the net charge carried by the current was increased as is shown by the good correlation with APD90 (Panel F).
Facilitation of INa,L with 10 nM ATX-II resulted in a robust increase in INa,L magnitude without altering the K+ currents (Fig.3B & 3C). Moreover, ATX-II treatment further increased the total charge carried by INa,L which significantly exceeds the outward charges carried by any individual K+ current (Fig.3D). The voltage dependence of INa,L shown in the instant I-V relationship was not altered (Fig.3E). Facilitation of INa,L by ATX-II resulted in significant lengthening of AP (APD90 = 212.0±19.9ms ATX-II vs. 192.5±15.6ms control; Fig.3B) without altering the voltage dependence of INa,L. A tight positive correlation is found between the net charge carried by INa,L and the AP duration (Fig.3F). These data reveals a significant contribution of the INa,L to shaping the AP morphology, and upregulation of INa,L can give rise to long APD.
3.4. Upregulation of INa,L leads to arrhythmogenic APs
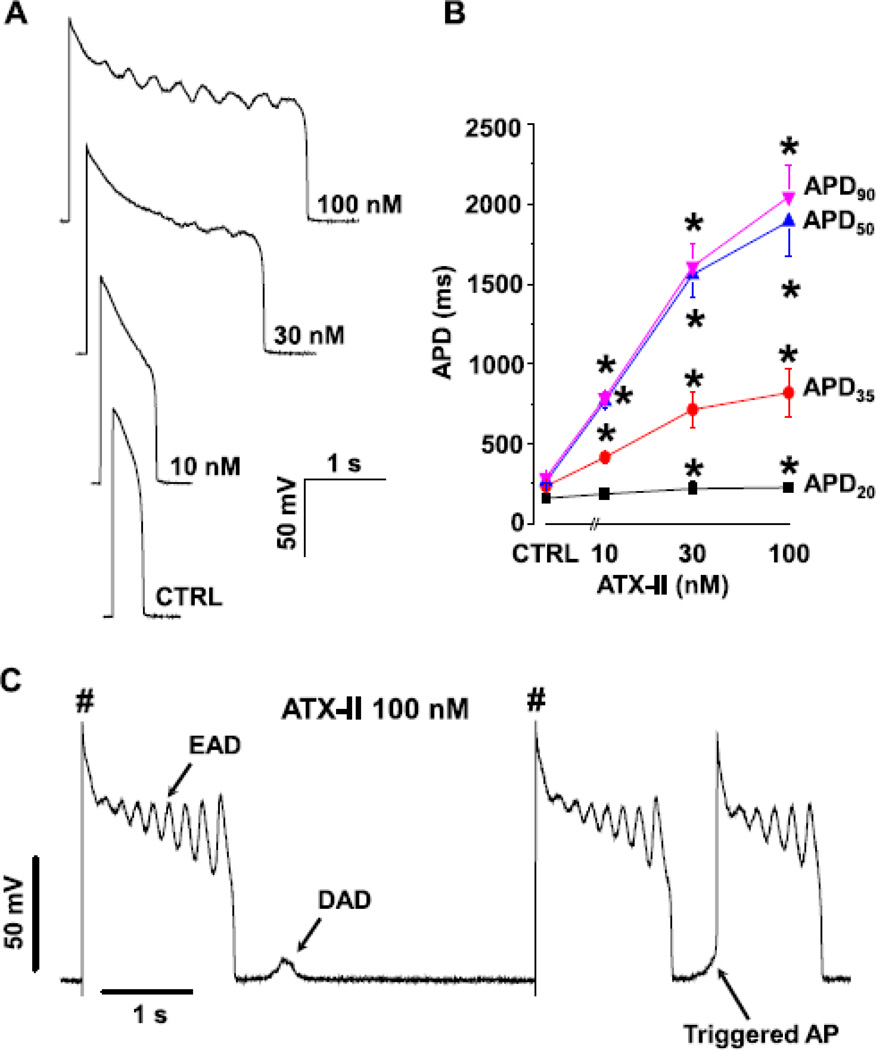
To further investigate the role of INa,L in arrhythmogenesis, we recorded the action potentials under current-clamp mode and studied the effect of upregulating INa,L using ATX-II. Our data show that ATX prolonged APD and induced arrhythmogenic APs in a dose-dependent manner (Fig.4A). While APD20 was not significantly altered by ATX-II in the range of 10–100 nM, APD35, APD50 and APD90 were greatly lengthen by ATX-II at 10 nM and above (Fig.4B). Early afterdepolarizations (EADs) emerged with 30–100 nM ATX-II treatment; EADs often occurred during early plateau phase, within 400–500 ms after the upstroke of AP. In addition to EADs, delayed afterdepolarizations (DADs) and triggered APs (TAPs) also emerged in 100 nM ATX-II in the same cell (Fig.4C), suggesting a common origin for these different forms of arrhythmogenic APs.
Fig.4. Effect of INa,L on AP morphology.
Panel A shows representative APs recorded in the presence of different ATX-II concentrations. Using ATX-II to upregulate INaL resulted in AP lengthening and generation of EAD. Panel B shows the concentration dependent AP lengthening effect of ATX-II at 0.2 Hz stimulation rate (n=6–11cells/6 animals). Panel C show the induction of various forms of arrhythmogenic APs (EAD, DAD, TAP) with ATX-II 100 nM in the same cell.
3.5. Spontaneous Ca2+ release from SR mediates INa,L induced arrhythmogenic APs
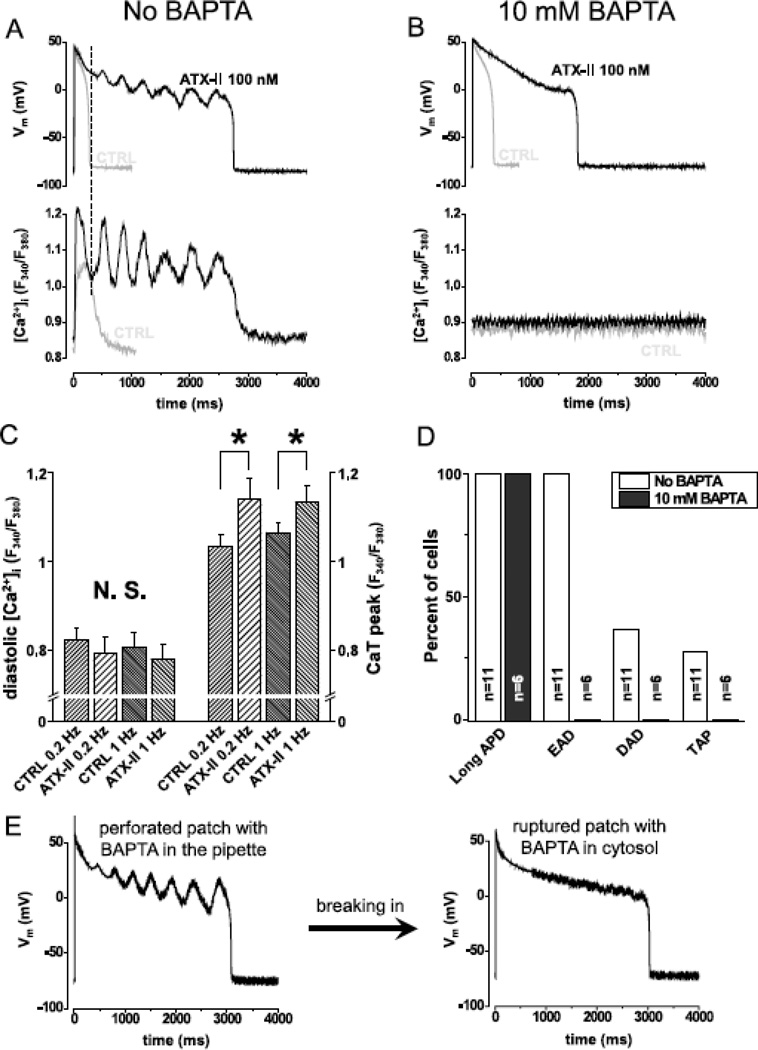
We hypothesized that ATX-II induced EADs are caused by Ca2+ release from SR (which also causes DADs and TAPs), rather than by reactivation of the L-type Ca2+ current during prolonged AP. To test this hypothesis we simultaneously recorded AP and the intracellular Ca2+ concentration ([Ca2+]i) using Fura-2 under the current-clamp mode. Fig.5A demonstrates that EADs occurred in concomitant with intracellular Ca2+ oscillations. As a tell-tale sign, the first spontaneous Ca2+ wave after the paced systolic Ca2+ transient always rises before the membrane voltage turns upward to form EAD. These data suggest that the Ca2+ wave drives the inward Na+/Ca2+ exchange current (forward mode) to give rise to EAD. In support of this notion, eliminating Ca2+ oscillations using BAPTA also abolished EADs (Fig.5B). Recall that BAPTA treatment also prolonged APD (Fig.2B) which should increase the reactivation of L-type Ca2+ current. Hence the cause of ATX-II induced EADs is not reactivation of L-type Ca2+ current but spontaneous Ca2+ release from SR. In further support of this notion, the systolic Ca2+ transient was higher in ATX-II treated cells than in the control (Fig.5C), demonstrating a higher SR load that would facilitate spontaneous Ca2+ release from SR. In relating to arrhythmogenic APs, INa,L upregulation by 100 nM ATX-II induced long APD and EADs in all cells examined, and also DADs and TAPs in some cells (Fig.5D). In contrast, Ca2+ buffering by BAPTA prolonged APD, but eliminated EAD, DAD and TAP. As a final confirmation for the role of Ca2+ oscillation in generating EAD, we conducted a self-controlled experiment in the same cell. As shown in Fig.5E, we first used perforated patch technique with the pipette solution containing 10 mM BAPTA and recorded EADs evoked by 100 nM ATX-II treatment; the Ca2+ oscillations were behind the EAD generation since the BAPTA could not get into the cell in the perforated patch configuration (Amphotericin was used to form small perforations in the cell membrane patch). Then we ruptured the membrane patch to form a ruptured whole-cell configuration to allow BAPTA diffusion into the cytosol; this eliminated Ca2+ oscillation and EAD but did not shorten APD. Therefore, INa,L directly contribute to lengthening APD, but the effect of INa,L on evoking EAD, DAD, and TAP is Ca2+ dependent through altered Na+ – Ca2+ homeostasis.
Fig.5. Spontaneous Ca2+ oscillation gives rise to EAD.
Panel A shows simultaneous recording of AP (upper trace) and Ca2+ concentration (lower trace) under sAP-clamp (n=9cells/5animals). Grey and black lines show traces recorded in the absence and the presence of 100 nM ATX-II, respectively. ATX-II lengthened APD but EADs were seen only when Ca2+ was not buffered. Dashed line in panel A shows that the rise in Ca2+ signal precedes the turning point of membrane potential indicating that Ca2+ drives EAD. Panel B shows that when Ca2+ was clamped to 100 nM using BAPTA (n=6cells/3animals), ATX-II lengthened AP but no EAD was observed (grey line control, black line ATX-II). Statistical analysis of the Ca2+ transients (Panel C,) reveal that systolic, but not diastolic Ca2+ concentration was increased by ATX-II at 0.2 Hz (n=9cells/5animals) and 1.0 Hz (n=8cells/5animals) pacing rate. Panel D shows the percent of cells developing various forms of arrhythmogenic APs in the presence of 100 nM ATX-II. When Ca2+ cycling was not buffered (n=11cells/6animals), all cells developed long APD and EADs and some developed DADs and TAPs. When Ca2+ was buffered with BAPTA (n=6cells/3animals), the cells developed long APD, but no EAD, DAD or TAP was seen.
4. DISCUSSION
In order to understand the contribution of INa,L to the normal and arrhythmogenic APs, we have recorded INa,L during the cell’s steady state AP in a physiological ionic milieu with Ca2+ cycling, while the cell was undergoing excitation-contraction at body temperature. To our best knowledge, this is the first time INa,L has been recorded under these conditions designed to closely mimic the physiological environment. Several pioneering studies used different variance of AP-clamp technique to measure INa,L [32–35]. However, all of them used AP waveforms recorded from other cells or reconstructed from model and therefore caused the measured current to deviate from the original current naturally flowing under the cell’s own AP. Moreover, the earlier AP-clamp experiments also used ion substitution (replacing Na+ with Cs+ or K+ with TEA) and exogenous Ca2+ buffer which, albeit useful for dissecting the biophysical properties of the channel, also deviate from physiological conditions and introduce some artifacts. The present sAP-clamp study was designed to directly record the INa,L following under the cell’s own AP during excitation-contraction coupling under physiological conditions. The data so obtained revealed novel findings on the dynamics of INa,L during AP and its role in arrhythmogenesis as discussed below.
4.1. Non-equilibrium gating is a chief factor determining the profile of INa,L during AP
Our data show that INa,L has a saddle-like profile during the AP in guinea pig ventricular myocyte (Fig.1A): after the fast Na+ current declines during phase-1, the late Na+ current (INa,L) takes a turn to increase during phase-2, reaches a peak at about APD50, and then rapidly declines during phase-3, and returns to baseline at phase-4. First of all, note that the profile of INa,L recorded with rectangular voltage-clamp protocol differs significantly from that INa,L current seen during AP. The former is invariably triangular in all species due to proliferating inactivation of sodium channels under a constant voltage. The latter, however, was traditionally visualized by computer simulation based on rectangular voltage-clamp data and such simulations often involve simplifications in the model hence still need to be verified experimentally. Our recordings consistently show saddle-like INa,L profile during AP under control condition and also under ATX-II treatment in guinea pig myocytes (Fig.3A&B). This does not preclude that INa,L profile could be somewhat different in different species. Saddle-like profile is consistent with the simulation results from some models (canine [36], human [37], and guinea pig ventricular myocyte [38], and recombinant Na+ channel expressed in cell line 293 [13]), but differ from some other model simulations predicting triangle-like profile during AP (canine [33]; guinea pig [32]). Thus, our experimental data confirm those models, where INa,L accumulates during the AP plateau giving a peak before terminal repolarization.
The profile of INa,L is shaped by several mechanisms including ‘window current’ and ‘non-equilibrium gating’ [16]. A ‘window current’ occurs when ion channels reactivate in a voltage range where the steady-state activation and inactivation curves overlap. The window current voltage range for the Na+ channel is quite negative (e.g. −80 mV to −50 mV) [39]. The AP plateau, however, falls in the positive voltage range before repolarization. Hence the window current should be negligible during plateau. On the other hand, repolarizing voltage ramp was found to facilitate INa,L [13], and this phenomenon was termed non-equilibrium gating. Therefore, a steep repolarization in AP is expected to boost INa,L. Moreover, non-equilibrium gating is not detectable by the rectangular pulse voltage-clamp experiments that measure steady-state currents [13]. Therefore, earlier models based on steady-state currents would miss the non-equilibrium gating and produce a triangle-like INa,L profile without the saddle-like profile obtained in our sAP-clamp experiment. Our data is consistent with non-equilibrium gating, and provide a realistic measure of the INa,L during dynamic changes of the membrane potential during AP for fine-tuning future models.
4.2. Ca2+ dependent modulation of INa,L during AP
Ca2+ was proposed to modulate INa,L by direct and indirect mechanisms. Direct Ca2+ binding to hH1 domain of sodium channel was shown to induce a rightward shift in the steady state inactivation increasing the availability of channels at more positive potentials [24], therefore buffering cytosolic Ca2+ should reduce Na+ current. Inhibition of CaM-CaMKII pathway showed divergent effects on INa,L in different previous reports. In our experiments when BAPTA was used to buffer cytosolic Ca2+, The APD and plateau height were significantly increased, which give rise to substantially increased INa,L during the AP (Fig 2). The positive correlation between the triangulation factor of AP and the peak amplitude of INa,L further supports the conclusion that INa,L is more strongly influenced by the voltage profile during the AP rather than by Ca2+.
CaMKII is known to facilitate Na+ current [23]. In agreement with this notion, in our experiments CaMKII inhibition using KN-93 reduced INa,L magnitude throughout the time course of AP and also in the I-V relationship. Meanwhile, increasing Ca2+ load did not further increase INa,L, indicating that the Ca2+-CaMKII modulation of INa,L is already saturated at the control condition. The voltage dependence of the INa,L recorded under sAP-clamp did not change under all the conditions tested, seen as no horizontal shift in the instant I-V relationship albeit changes in the amplitude of the current density (vertical shift in I-V),. Our data provide, for the first time, a visualization of the impact of Ca2+ chelation and CaMKII inhibition on the dynamic profile of INa,L during the AP.
4.3. The magnitude of INa,L during AP in comparing to K+ currents
We found that INa,L presents a substantial inward current with peak current density of 0.78±0.32 A/F (Mean±SD) and total charge movement of 88.3±43.2 mC/F during the AP in guinea pig ventricular myocytes (Fig.3C&D). For long time it was thought that INa,L was a tiny sustained current during AP, especially in comparing to the fast Na+ current with much larger amplitude. Now we found that the INa,L during the AP is surprisingly large, even in comparing to the major K+ currents. The inward charge movement through INa,L should depolarize the membrane potential, which is counterbalanced by the outward charge movement through K+ channels. To investigate the interplay of INa,L and K+ currents in shaping the AP morphology, we recorded INa,L, IKs, IKr and IK1 from the same cell. Previously, the relationship between INa,L and K+ channels was studied only in model simulations [36], and the ratio between INa,L and K+ channels was shown to affect arrhythmogenesis. Now our experimental data show, interestingly, the INa,L magnitude and time course of seems to mirror that of IKs (Fig.3A). The peak current density of INa,L is comparable to IKs, about 50% of IKr and 30% of IK1 (Fig.3C). The inward charge carried by INa,L is more than the outward charge carried by any individual K+ current during the AP cycle, although less than the total charge carried by all three K+ currents (Fig.3D). Therefore our data reveal that INa,L is a major determinant of the AP morphology, and significantly contributes to arrhythmogenic AP activities
4.4. Spontaneous Ca2+ release from SR mediates INa,L induced EAD, DAD and TAP
Upregulation of INa,L is linked to development of arrhythmias in heart failure and other acquired heart diseases [15, 40]. However, the exact role of INa,L in arrhythmogenesis remained unclear, especially in the genesis of EADs. At least two possible mechanisms have been proposed [16]: one involves reactivation of the L-type Ca2+ current; the other involves SR Ca2+ overload. In the first mechanism, upregulation of INa,L prolongs the AP plateau which allows recovery and reactivation of the L-type Ca2+ current; then the Ca2+ influx disrupts the membrane repolarization to cause EADs [14, 16]. This mechanism emphasizes the window current of the L-type Ca2+ channel as the depolarization drive for EADs. Nevertheless, our data show that the first EAD occurred at positive voltages (>10 mV, see Fig.3A) above the Ca2+ ‘window’ current voltage range (−30 mV to 0 mV) [41]; hence, the window current is not responsible for generating the EAD.
In the second mechanism, upregulation of INa,L increases intracellular Na+ concentration which causes SR Ca2+ overload; SR overload then causes spontaneous Ca2+ release from SR which drives forward mode Na+/Ca2+ exchange to depolarize the membrane, resulting in EADs [6, 42, 43]. Our data provide several evidences to support this mechanism. First, the ATX-II treated cells showed elevated systolic Ca2+ transient indicating elevated SR load (Fig.5C). Second, the spontaneous Ca2+ wave precedes the first EAD (Fig.5A, indicated by dash line); this timing is consistent with increased cytosolic Ca2+ drives inward (forward mode) Na+/Ca2+ exchanger to cause EAD. Third, using BAPTA to buffer Ca2+ abolished EADs (Fig.5B), although caused a prolonged phase-2; this long plateau feature is clearly different from the EADs driven by Ca2+ oscillations (Fig.5A). Therefore, spontaneous Ca2+ release from SR is a prerequisite for the generation of EADs. Taken together, our data suggest that spontaneous Ca2+ release from SR is a common mechanism underlying INa,L induced EADs, DADs and TAPs. The common mechanism of EAD and DAD was already proposed in Cs+ and isoproterenol induced early afterdepolarizations [44, 45] and Udrovinas et al. showed that augmented INa,L contributes to diastolic Ca2+ accumulation [6]. Our data provide experimental evidence to support the common mechanisms of afterdepolarizations proposed in these earlier works.
Highlights.
First recording of INa-L during action potential with Ca2+ cycling
Comparing INa-L with three major K+ currents in the same cell
Novel finding of large INa-L comparable to IKs and IKr
Reveal significant role of INa-L in action potential repolarization
Interplay between INa-L and Ca2+ dynamics causes EAD, DAD, TAP
Acknowledgement
We are grateful to Dr. Robert Hadley (University of Kentucky) for kindly providing the guinea pig cardiac cell isolation protocol. We thank Mr. Shaden Khabbaz for the excellent work in isolating cells.
Funding: This work was supported by the National Institute of Health R01 grant (HL90880) to LTI and YC, NIH R03 grant (AG031944) to YC, American Heart Association National Center Scientist Development Award (0335250N) to YC, European Society of Cardiology Visiting Scientist Award to BH, the Hungarian Research Found OTKA- K101196 and OTKA- K100151 to TB and PPN, and the funds from the University of California to LTI and YC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none.
Disclosures: None.
References
- 1.Yuill KH, Convery MK, Dooley PC, Doggrell SA, Hancox JC. Effects of BDF 9198 on action potentials and ionic currents from guinea-pig isolated ventricular myocytes. Br J Pharmacol. 2000;130:1753–1766. doi: 10.1038/sj.bjp.0703476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiyosue T, Arita M. Late Sodium Current And Its Contribution To Action-Potential Configuration In Guinea-Pig Ventricular Myocytes. Circ Res. 1989;64:389–397. doi: 10.1161/01.res.64.2.389. [DOI] [PubMed] [Google Scholar]
- 3.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cellular and Molecular Life Sciences. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92:6–14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. European Journal of Heart Failure. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. The journal of physiological sciences : JPS. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine Improves Abnormal Repolarization and Contraction in Left Ventricular Myocytes of Dogs with Heart Failure by Inhibiting Late Sodium Current. Journal of Cardiovascular Electrophysiology. 2006;17:S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, et al. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserstrom JA, Sharma R, O'Toole MJ, Zheng J, Kelly JE, Shryock J, et al. Ranolazine antagonizes the effects of increased late sodium current on intracellular calcium cycling in rat isolated intact heart. J Pharmacol Exp Ther. 2009;331:382–391. doi: 10.1124/jpet.109.156471. [DOI] [PubMed] [Google Scholar]
- 12.Hoyer K, Song YJ, Wang DS, Phan D, Balschi J, Ingwall JS, et al. Reducing the Late Sodium Current Improves Cardiac Function during Sodium Pump Inhibition by Ouabain. Journal of Pharmacology and Experimental Therapeutics. 2011;337:513–523. doi: 10.1124/jpet.110.176776. [DOI] [PubMed] [Google Scholar]
- 13.Clancy CE, Tateyama M, Liu H, Wehrens XH, Kass RS. Non-equilibrium gating in cardiac Na+ channels: an original mechanism of arrhythmia. Circulation. 2003;107:2233–2237. doi: 10.1161/01.CIR.0000069273.51375.BD. [DOI] [PubMed] [Google Scholar]
- 14.Trenor B, Cardona K, Gomez JF, Rajamani S, Ferrero JM, Jr, Belardinelli L, et al. Simulation and mechanistic investigation of the arrhythmogenic role of the late sodium current in human heart failure. PLoS One. 2012;7:e32659. doi: 10.1371/journal.pone.0032659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno JD, Clancy CE. Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol. 2012;52:608–619. doi: 10.1016/j.yjmcc.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac "late sodium current". Pharmacology & Therapeutics. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a Channels in Heart Reduces Late Sodium Current and Is Antiarrhythmic. Circ Res. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banyasz T, Horvath B, Jian Z, Izu LT, Chen-Izu Y. Profile of L-type Ca2+ current and Na+/Ca2+ exchange current during cardiac action potential in ventricular myocytes. Heart Rhythm. 2012;9:134–142. doi: 10.1016/j.hrthm.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banyasz T, Horvath B, Jian Z, Izu LT, Chen-Izu Y. Sequential dissection of multiple ionic currents in single cardiac myocytes under action potential-clamp. J Mol Cell Cardiol. 2011;50:578–581. doi: 10.1016/j.yjmcc.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banyasz T, Fulop L, Magyar J, Szentandrassy N, Varro A, Nanasi PP. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovascular Research. 2003;58:66–75. doi: 10.1016/s0008-6363(02)00853-2. [DOI] [PubMed] [Google Scholar]
- 21.Maier LS. CaMKII regulation of voltage-gated sodium channels and cell excitability. Heart Rhythm. 2011;8:474–477. doi: 10.1016/j.hrthm.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 22.Scheuer T. Regulation of sodium channel activity by phosphorylation. Seminars in Cell & Developmental Biology. 2011;22:160–165. doi: 10.1016/j.semcdb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bers DM, Grandi E. Calcium/Calmodulin-dependent Kinase II Regulation of Cardiac Ion Channels. J Cardiovasc Pharmacol. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nature Structural & Molecular Biology. 2004;11:219–225. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Ghosh S, Liu HJ, Tateyama M, Kass RS, Pitt GS. Calmodulin mediates Ca2+ sensitivity of sodium channels. Journal of Biological Chemistry. 2004;279:45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- 26.Cormier JW, Rivolta I, Tateyama M, Yang AS, Kass RS. Secondary structure of the human cardiac Na+ channel C terminus - Evidence for a role of helical structures in modulation of channel inactivation. Journal of Biological Chemistry. 2002;277:9233–9241. doi: 10.1074/jbc.M110204200. [DOI] [PubMed] [Google Scholar]
- 27.Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AAM, et al. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 28.Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Isoform-specific modulation of voltage-gated Na+ channels by calmodulin. Circ Res. 2002;90:E49–E57. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- 29.Young KA, Caldwell JH. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J Physiol-London. 2005;565:349–370. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy BJ, Rogers J, Perdichizzi AP, Colvin AA, Catterall WA. cAMP-dependent phosphorylation of two sites in the alpha subunit of the cardiac sodium channel. Journal of Biological Chemistry. 1996;271:28837–28843. doi: 10.1074/jbc.271.46.28837. [DOI] [PubMed] [Google Scholar]
- 31.Chen-Izu Y, Chen L, Banyasz T, McCulle SL, Norton B, Scharf SM, et al. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol. 2007;293:H3301–H3310. doi: 10.1152/ajpheart.00259.2007. [DOI] [PubMed] [Google Scholar]
- 32.Chorvatova A, Snowdon R, Hart G, Hussain M. Effects of pressure overload-induced hypertrophy on TTX-sensitive inward currents in guinea pig left ventricle. Mol Cell Biochem. 2004;261:217–226. doi: 10.1023/b:mcbi.0000028759.22274.cf. [DOI] [PubMed] [Google Scholar]
- 33.Murphy L, Renodin D, Antzelevitch C, Di Diego JM, Cordeiro JM. Extracellular proton depression of peak and late Na current in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2011;301:H936–H944. doi: 10.1152/ajpheart.00204.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marangoni S, Di Resta C, Rocchetti M, Barile L, Rizzetto R, Summa A, et al. A Brugada syndrome mutation (p.S216L) and its modulation by p.H558R polymorphism: standard and dynamic characterization. Cardiovasc Res. 2011;91:606–616. doi: 10.1093/cvr/cvr142. [DOI] [PubMed] [Google Scholar]
- 35.Magyar J, Kiper CE, Dumaine R, Burgess DE, Bányász T, Satin J. Divergent action potential morphologies reveal nonequilibrium properties of human cardiac Na channels. Cardiovasc Res. 2004;64:477–487. doi: 10.1016/j.cardiores.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Flaim SN, Giles WR, McCulloch AD. Contributions of sustained INa and IKv43 to transmural heterogeneity of early repolarization and arrhythmogenesis in canine left ventricular myocytes. American Journal of Physiology - Heart and Circulatory Physiology. 2006;291:H2617–H2629. doi: 10.1152/ajpheart.00350.2006. [DOI] [PubMed] [Google Scholar]
- 37.O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS computational biology. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakmann BFAS, Spindler AJ, Bryant SM, Linz KW, Noble D. Distribution of a Persistent Sodium Current Across the Ventricular Wall in Guinea Pigs. Circ Res. 2000;87:910–914. doi: 10.1161/01.res.87.10.910. [DOI] [PubMed] [Google Scholar]
- 39.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 40.Zaza A. Control of the cardiac action potential: The role of repolarization dynamics. J Mol Cell Cardiol. 2010;48:106–111. doi: 10.1016/j.yjmcc.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Madhvani RV, Xie Y, Pantazis A, Garfinkel A, Qu Z, Weiss JN, et al. Shaping a new Ca2+ conductance to suppress early afterdepolarizations in cardiac myocytes. J Physiol. 2011;589:6081–6092. doi: 10.1113/jphysiol.2011.219600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and Contractile Dysfunction in Heart Failure : Roles of Sodium-Calcium Exchange, Inward Rectifier Potassium Current, and Residual {beta}-Adrenergic Responsiveness. Circulation Research. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Z, Wen H, Fefelova N, Allen C, Baba A, Matsuda T, et al. Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: predominant by Ca waves or Ca currents? Am J Physiol Heart Circ Physiol. 2012;302:H1636–H1644. doi: 10.1152/ajpheart.00742.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo B, Sweidan R, Rajagopalan CV, Lazzara R. Role of Na+:Ca2+ exchange current in Cs(+)-induced early afterdepolarizations in Purkinje fibers. J Cardiovasc Electrophysiol. 1994;5:933–944. doi: 10.1111/j.1540-8167.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]