Resistance to antimicrobial drugs represents one of the greatest threats to the control of infectious diseases and is a particular problem in treating diseases caused by parasitic protists. These pathogens are of enormous medical importance, causing diseases such as malaria, toxoplasmosis, trypanosomiasis, and leishmaniasis. In the absence of effective vaccines, the control of these diseases is critically dependent on drug therapies that are often undermined by poor compliance or overuse in communities with struggling health care systems and endemic poverty. The development and spread of resistance to the antimalarial drug chloroquine from the 1950s onwards constitutes a spectacular example of drug failure caused by overuse. Similarly, the development of antimonial-resistance in Leishmania donovani in India in the 1970–1980s had all of the hallmarks of long-term misuse (1). L. donovani is the causative agent of visceral leishmaniasis (VL) or Kala-Azar, which kills many thousands of people in India each year. However, in PNAS, Perry et al. (2) provide compelling evidence that Leishmania-acquired antimonial resistance in India may be attributable, at least in part, to increased arsenic contamination of the drinking water. These findings highlight how environmental factors can contribute to the emergence of microbial drug resistance and have implications for drug development.
Leishmania are insect-transmitted protist parasites that cause a spectrum of diseases affecting more than 12 million people worldwide. Depending on the Leishmania species involved (there are 20 that infect humans) and the host immune status, symptoms can range from localized, self-resolving skin lesions to deadly visceral infections where parasites target immune cells in the liver and spleen (3). VL is a particular problem in the eastern Indian state of Bihar as a result of widespread poverty and hyperendemicity of L. donovani, which is transmitted from human to human by a sandfly vector. Pentavalent antimonials, such as pentostam, were first used to treat VL in the 1920s and deployed successfully for many decades. However, in the 1970s resistance was noted during epidemics caused by the cessation of vector-control programs in Bihar. Drug doses and length of treatments were progressively increased (from 6 d to 30 d) in the face of falling treatment success rates, and antimonials were finally replaced by lipid amphotericin B and miltefosine in 2002. Although the development of antimonial resistance in Bihar is often attributed to misuse, the fact that similar levels of resistance have not been observed in other parts of the world suggests that other factors unique to Bihar State were in play. Perry et al. noted that the development of antimonial resistance in Bihar State in the 1970s coincided with increased arsenic contamination of drinking water as a result of the widespread insertion of shallow tube wells for the provision of clean drinking water (4). Arsenic, like antimony, belongs to group 15 of the periodic table and the two metalloids share many chemical properties. In fact, organic arsenic formulations have previously been used to treat leishmaniasis and the arsenicals remain one of the mainstay therapies for treating human African trypanosomiasis (5).
Perry et al. hypothesized that long-term exposure and subsequent adaptation of L. donovani to sublethal levels of arsenic in their human hosts may have led to cross-resistance to antimonials (4). In support of this hypothesis they now show that antimonial-susceptible L. donovani strains repeatedly passaged in mice exposed to low levels of arsenic in their drinking water (comparable to those observed in Bihar) evolved strong resistance to pentostam. The acquisition of antimonial resistance was associated with elevated levels of arsenic in the liver and was stable after multiple passages through arsenic-free animals. Significantly, antimonial-resistant parasites seem to be more pathogenic than antimonial-susceptible lines, even in the absence of drug selection (2, 6), suggesting that antimonial/arsenic cross-resistant parasites might persist and be transferred to neighboring areas where arsenic contamination is not a problem. This finding contrasts with the more common situation where the development of drug resistance is associated with a loss of fitness in the mammalian host. For example, in some areas where chloroquine use for malaria treatment has been discontinued, chloroquine-sensitive strains have gradually replaced the resistant strains, allowing chloroquine to be effectively used again (7). A comparable scenario seems, unfortunately, less likely for antimonial-resistant Leishmania, particularly if environmental exposure to arsenic persists.
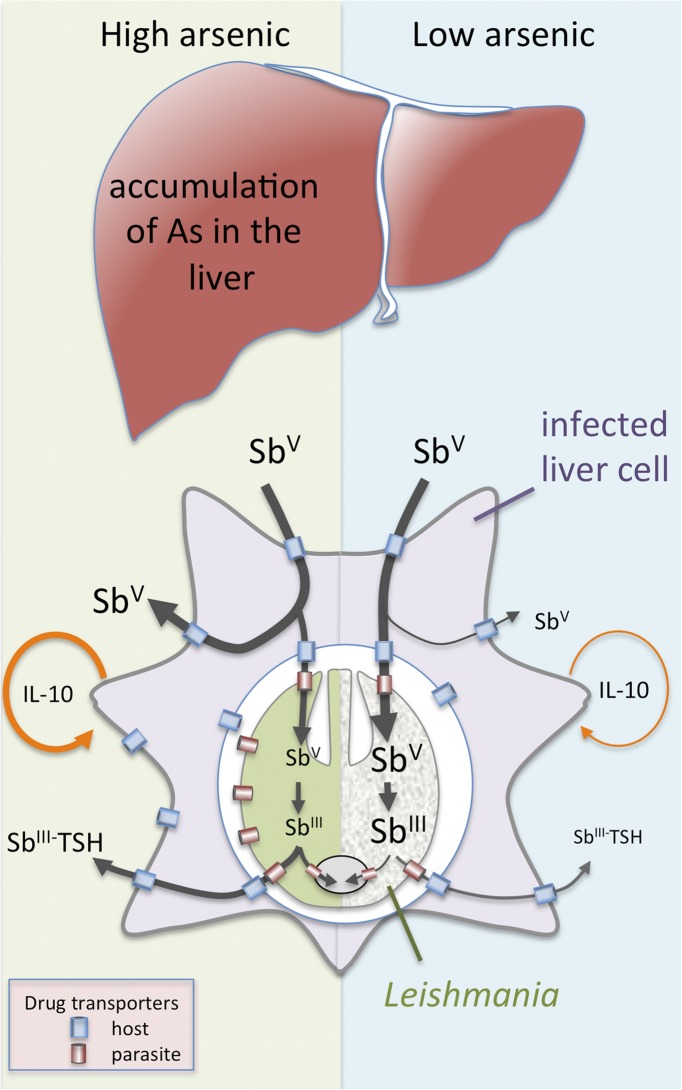
Pentavalent antimonials (Sbv) are transported across the host and parasite membranes and subsequently reduced to the bioactive SbIII form in either the host cell or intracellular parasite stages (8) (Fig. 1). SbIII is thought to primarily target parasite enzymes involved in maintaining reduced thiols, such as the unique bis-glutathione-spermidine conjugate, trypanothione (1). Resistance may arise as a result of increased expression of parasite transporter proteins that export trypanothione-SbIII conjugates out of the parasite or into intracellular organelles, reducing the intracellular accumulation of cytotoxic SbIII (8–10). Resistance may also be associated with global changes in parasite metabolism and a switch to a metabolically distinct state that facilitates survival in the mammalian macrophages (11), consistent with the data presented by Perry et al. indicating that chronic exposure to arsenic may lead to increased parasite loads in susceptible animals (2). Finally, there is evidence that antimonial-resistant L. donovani may induce changes in the rate of uptake or efflux of antimonials by infected host cells, as well as changes in the host immune response (12, 13). In particular, antimonial-resistant parasite lines can trigger host signaling pathways that lead to increased secretion of IL-10, a potent anti-inflammatory cytokine that promotes Leishmania growth in vivo (13). Thus, chronic exposure to arsenic may not only lead to activation of drug-resistance mechanisms that confer protection against antimonials, but also alter the balance of host–parasite interactions in favor of the parasite by increasing the fitness of the pathogen and polarizing the immune response to one that favors parasite proliferation (14).
Fig. 1.
Mechanisms of Leishmania resistance to antimonials that may be induced by chronic exposure to arsenic. Accumulation of arsenic (As) in the liver, one of the major organs targeted by L. donovani, leads to cross-resistance to related antimony (Sb). Resistance mechanisms include increased efflux or intracellular sequestration of SbV- and SbIII-trypanothione (TSH) conjugates, as well as activation of anti-inflammatory cytokines (such as IL-10) that promote parasite growth.
It is possible that chronic arsenic exposure underlies the development of antimonial resistance in other parts of the world. In particular, significant resistance to antimonials has been observed in Peru (although lower than in India), where these drugs are still used to treat cutaneous forms of the disease (15). Peru has several regions with greatly elevated arsenic in drinking and irrigation water (16), and this recent study raises the prospect that a similar association between arsenic contamination and antimonial resistance may be found. Another question raised by these studies is whether chronic exposure to arsenic leads to resistance to other antileishmanial drugs. Miltefosine, a chemically unrelated alkyl-lipid replaced antimonials as the major front-line treatment for VL in India and is now widely used to treat other forms of leishmaniasis worldwide (17). Resistance to miltefosine can be induced in the laboratory and a number of studies have shown that resistance is mediated by up-regulation of the same membrane transporters that are involved in efflux of antimonials (10). An increase in resistance to miltefosine in clinical isolates from the Bihar region has recently been reported, raising the possibility that it might also be linked to high levels of
Leishmania-acquired antimonial resistance in India may be attributable, at least in part, to increased arsenic contamination of the drinking water.
arsenic exposure (17). However, Perry et al. (2) found no evidence that chronic exposure to subclinical levels of arsenic or even higher “toxic” levels led to a decrease in the efficacy of miltefosine in vivo, although this was not tested on cultured parasite stages or in the macrophage assay.
This study highlights the potential of other environmental contaminants to contribute to the development of microbial drug resistance. As noted by Perry et al., it has previously been proposed that rapid spread of chloroquine resistance may have been facilitated by subtherapeutic exposure of communities in South America, Southeast Asia, and Africa to chloroquine containing medicated salts (4). Similarly, triclosan, another antimicrobial with broad activity against bacteria and many parasitic protists, is a common component of toothpaste, hand-washes, and other man-made products. The ubiquitous presence of triclosan in the environment, as well as long-term human exposure, could contribute to the development of triclosan-resistant pathogens (18) or microbial resistance to other drugs that share similar modes of action, such as the antituberculosis drug, isoniazid (19). Although a link between environmental triclosan levels and antibiotic resistance has yet to be established (20), the study by Perry et al. (2) provides a cautionary lesson. As phenotypic screens of large compound libraries are increasingly being used to identify new lead compounds for microbial chemotherapy (21), it will be important to be aware of compounds that share chemical similarity to common environmental “contaminants” to ensure that resistance is not already being induced before clinical trials.
Footnotes
The authors declare no conflict of interest.
See companion article on page 19932.
References
- 1.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry MR, Wyllie S, Raab A, Feldmann J, Fairlamb AH. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc Natl Acad Sci USA. 2013;110:19932–19937. doi: 10.1073/pnas.1311535110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Perry MR, et al. Visceral leishmaniasis and arsenic: An ancient poison contributing to antimonial treatment failure in the Indian subcontinent? PLoS Negl Trop Dis. 2011;5(9):e1227. doi: 10.1371/journal.pntd.0001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–196. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanaerschot M, et al. Antimonial resistance in Leishmania donovani is associated with increased in vivo parasite burden. PLoS ONE. 2011;6(8):e23120. doi: 10.1371/journal.pone.0023120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laufer MK, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355(19):1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 8.Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: Drugs in the clinic, reistance and new developments. Drug Resist Updat. 2004;7(4-5):257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Leprohon P, Légaré D, Ouellette M. Intracellular localization of the ABCC proteins of Leishmania and their role in resistance to antimonials. Antimicrob Agents Chemother. 2009;53(6):2646–2649. doi: 10.1128/AAC.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Victoria JM, et al. Sitamaquine overcomes ABC-mediated resistance to miltefosine and antimony in Leishmania. Antimicrob Agents Chemother. 2011;55(8):3838–3844. doi: 10.1128/AAC.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg M, et al. Metabolic adaptations of Leishmania donovani in relation to differentiation, drug resistance, and drug pressure. Mol Microbiol. 2013;90(2):428–442. doi: 10.1111/mmi.12374. [DOI] [PubMed] [Google Scholar]
- 12.Gómez MA, et al. Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: Impact on intracellular parasite survival. J Antimicrob Chemother. 2013;(Sep):12. doi: 10.1093/jac/dkt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee B, et al. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc Natl Acad Sci USA. 2013;110(7):E575–E582. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stauch A, et al. Treatment of visceral leishmaniasis: Model-based analyses on the spread of antimony-resistant L. donovani in Bihar, India. PLoS Negl Trop Dis. 2012;6(12):e1973. doi: 10.1371/journal.pntd.0001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llanos-Cuentas A, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46(2):223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 16.Bundschuh J, et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci Total Environ. 2012;429:2–35. doi: 10.1016/j.scitotenv.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Sundar S, et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55(4):543–550. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 18.Nietch CT, et al. Effects of a chronic lower range of triclosan exposure to a stream mesocosm community. Environ Toxicol Chem. 2013 doi: 10.1002/etc.2385. [DOI] [PubMed] [Google Scholar]
- 19.McMurry LM, McDermott PF, Levy SB. Genetic evidence that InhA of Mycobacterium smegmatis is a target for triclosan. Antimicrob Agents Chemother. 1999;43(3):711–713. doi: 10.1128/aac.43.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell AD. Whither triclosan? J Antimicrob Chemother. 2004;53(5):693–695. doi: 10.1093/jac/dkh171. [DOI] [PubMed] [Google Scholar]
- 21.Sykes ML, Avery VM. Approaches to protozoan drug discovery: Phenotypic screening. J Med Chem. 2013;56(20):7727–7740. doi: 10.1021/jm4004279. [DOI] [PubMed] [Google Scholar]