Significance
Currently, little is understood about how the transcriptional regulation of cartilage breakdown contributes to pathogenesis of osteoarthritis (OA). Here, we report that, within cartilage, the transcription factor Nuclear factor of activated T cells c1 (NFATc1) displays selective expression in superficial articular chondrocytes. Accordingly, mice lacking both NFATc1 and NFATc2 in cartilage were generated and found to develop a severe, spontaneous and early-onset OA. These findings establish NFATc1 as a key transcriptional repressor of cartilage breakdown and OA. Additionally, these findings provide a unique model of OA that is an attractive platform for the preclinical development of treatments to alter the course of OA.
Abstract
Osteoarthritis (OA) was once viewed originally as a mechanical disease of “wear and tear,” but advances made during the past two decades suggest that abnormal biomechanics contribute to active dysregulation of chondrocyte biology, leading to catabolism of the cartilage matrix. A number of signaling and transcriptional mechanisms have been studied in relation to the regulation of this catabolic program, but how they specifically regulate the initiation or progression of the disease is poorly understood. Here, we demonstrate that cartilage-specific ablation of Nuclear factor of activated T cells c1 (Nfatc1) in Nfatc2−/− mice leads to early onset, aggressive OA affecting multiple joints. This model recapitulates features of human OA, including loss of proteoglycans, collagen and aggrecan degradation, osteophyte formation, changes to subchondral bone architecture, and eventual progression to cartilage effacement and joint instability. Consistent with the notion that NFATC1 is an OA-suppressor gene, NFATC1 expression was significantly down-regulated in paired lesional vs. macroscopically normal cartilage samples from OA patients. The highly penetrant, early onset, and severe nature of this model make it an attractive platform for the preclinical development of treatments to alter the course of OA. Furthermore, these findings indicate that NFATs are key suppressors of OA, and regulating NFATs or their transcriptional targets in chondrocytes may lead to novel disease-modifying OA therapies.
Osteoarthritis (OA) is perhaps the condition with the greatest disparity between its wide prevalence and the absence of therapeutic options that alter the course of disease. More than 10% of the adult US population has clinically defined OA, and OA is the fourth leading cause of hospitalization and a leading cause of disability in the United States (1). The high prevalence of OA contrasts with the dearth of available therapeutic options. Although nonsteroidal anti-inflammatory drugs or intraarticular steroid and hyaluronic acid injections provide limited pain relief, the only intervention that definitively treats this condition is replacement of the affected joint with a prosthetic. The difficulty in advancing the management of this common disorder highlights the need for an improved basic understanding of the pathogenic mechanisms driving OA.
Advances made in the past two decades highlight the notion that OA is the product of an active dysregulation of the biology of cartilage and other joint tissues, as opposed to simple mechanical “wear and tear.” For instance, the complement cascade has been shown to play an active role in OA by promoting the expression of catabolic proteases (2). Similarly, a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) has been identified as a critical aggrecanase that breaks down cartilage matrix in OA, and further advances demonstrate that this protease is highly regulated, for example, via syndecan 4-mediated inhibition (3–5). Despite these advances, less is understood regarding the transcriptional regulation of OA gene expression programs.
The nuclear factor of activated T cells (NFAT) family of transcription factors play diverse roles in a wide range of tissues, ranging from the development of heart valves and brain vasculature to innate and adaptive immune responses (6–8). Specific cell lineages, such as osteoclasts and pancreatic beta cells, require particular NFAT family members for development (9–11). Additionally, NFATs cooperate with the transcription factor osterix in osteoblasts to promote bone formation (12). In other cell types, including T cells, NFATs manifest partially overlapping or redundant functions (13–15). Of the five NFATs, four are activated by dephosphorylation mediated by calcineurin in response to sustained increases in intracellular calcium (NFATc1–NFATc4). Mice with a germ-line deletion of the transcription factor Nfatc2 develop ectopic cartilage formation around synovial joints at 3 mo of age and older (16) along with loss of proteoglycan staining. Ultimately, between 12 and 24 mo of age, Nfatc2−/− mice develop OA (17). To further explore the role of NFATs in cartilage biology, the expression of NFATC1 was assessed in lesional tissue from patients with OA and was found to be reduced. To explore the significance of this finding, mice with a conditional deletion of Nfatc1 in cartilage mediated by the Collagen 2–Cre were bred onto the Nfatc2−/− strain. The resulting strain lacking expression of NFATc1 and NFATc2 in cartilage (hereafter Nfatc1col2Nfatc2−/− mice) develop severe, spontaneous OA. These data suggest that NFATC1 is an important repressor of OA and that Nfatc1col2Nfatc2−/− mice are a promising model of OA.
Results
NFATc1 Expression in Cartilage Is Restricted to Articular Chondrocytes in Vivo.
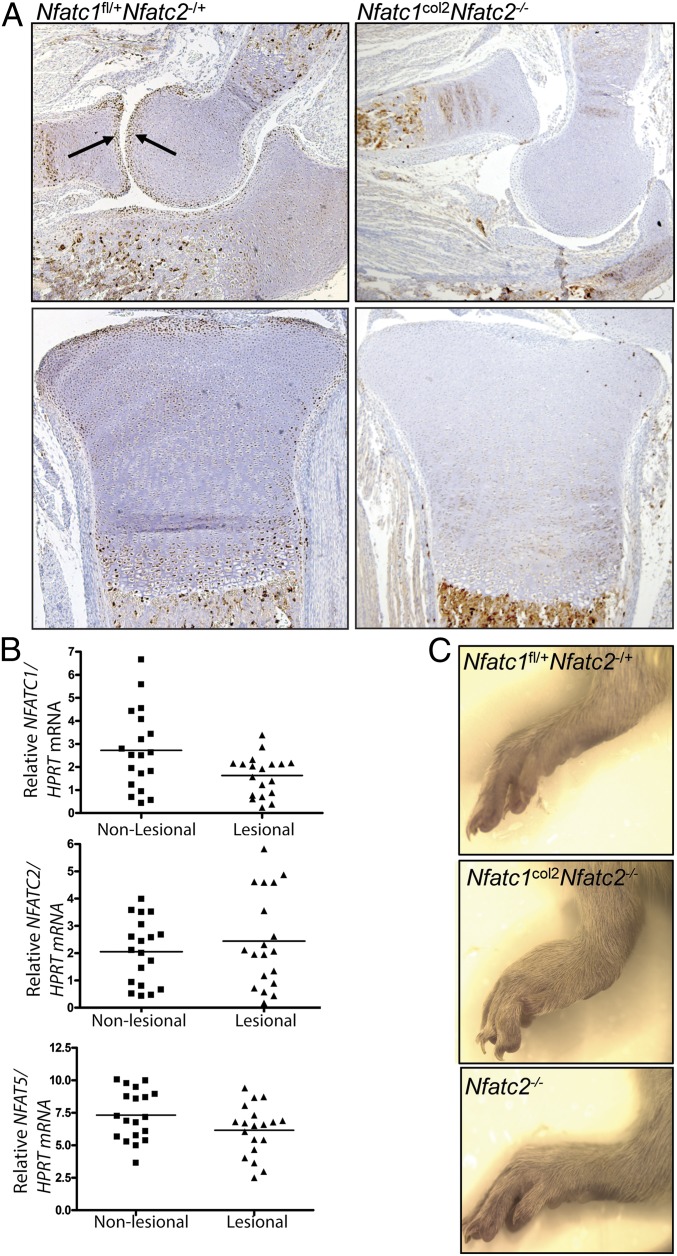
Previous studies of Nfatc2-deficient mice demonstrated that they develop ectopic cartilage surrounding synovial joints when >6 mo of age. Additionally, Nfatc2-deficient mice display an OA-like phenotype at advanced ages, including proteoglycan depletion in articular cartilage (16, 17). In one of these reports, Nfatc1, Nfatc3, and Nfatc4 mRNAs were found to be expressed in chondrocytes in vitro (16). To examine the expression of NFATs in vivo, immunohistochemistry was performed. Because of the limitations of the reagents available, only immunohistochemistry for NFATc1 was technically robust. This analysis demonstrated that NFATc1 is expressed at high levels in the most superficial layers of cartilage abutting the articular surface in all joints examined, including the elbow and knee (Fig. 1A). Additional expression in prehypertrophic and hypertrophic chondrocytes of the growth plate was also detected.
Fig. 1.
Expression of NFATc1 in murine and human OA cartilage and gross phenotype of Nfatc1col2Nfatc2−/− mice. (A) Immunohistochemistry with anti-NFATc1 on elbows from 1-wk-old mice. Shown are cross-sections through the elbow (Upper) and the proximal tibia (Lower). (B) Quantitative RT-PCR for NFATC1, NFATC2, and NFAT5 on mRNA isolated from paired lesional and grossly nonlesional cartilage from patients undergoing arthroplasty for end-stage knee OA. NFATC1, P = 0.0065; NFATC2, P = 0.214; NFAT5, P = 0.0134 (two-tailed, paired t test). (C) Gross images of the ankle of 3-wk-old mice showing subluxation of the tarsus in Nfatc1col2Nfatc2−/− mice.
NFATc1 Expression Is Reduced in Lesional Tissue from Human OA Patients.
To explore the relevance of these findings to human OA, surgical specimens were obtained from total-knee-replacement procedures performed for end-stage OA. RNA was isolated from paired biopsies of lesional and grossly nonlesional articular cartilage. Expression of NFATC1, but not NFATC2, was reduced in lesional tissue (Fig. 1B). NFAT5 levels were also lower in lesional tissue.
Mice Lacking NFATc1 in Cartilage Are Not More Susceptible to Posttraumatic OA.
To study the importance of decreased NFATC1 expression in lesional chondrocytes during OA, a mouse model with a conditional deletion of Nfatc1 in chondrocytes was generated. Because of the lethality of the homozygous germ-line Nfatc1-null allele, a floxed allele was bred to a strain that mediates deletion in chondrocytes via the collagen-2 promoter (9, 18). The resulting Nfatc1col2 strain was born at normal Mendelian ratios and displayed no gross or histologic evidence of cartilage pathology (Fig. S1 A and B). To test this strain in a provocative model of OA, we used the destabilization of the medial meniscus (DMM) model (2, 19). In this model, the medial meniscotibial ligament is transected by direct visualization. This transection destabilizes the joint, resulting in up-regulation of catabolic pathways culminating in OA over 8–16 wk (20, 21). Compared with WT littermate controls, no differences in DMM-induced OA was observed in Nfatc1col2 mice (Fig. S1C).
Generation of Mice Lacking both NFATc1 and NFATc2 in Cartilage.
Although no effect of NFATc1 deletion alone in chondrocytes was observed, in many organ systems the functions of NFATs are masked by redundancy among family members. Thus, mice bearing compound deletions of NFATs display substantially more severe or different phenotypes than mice lacking individual family members (13, 15, 22). Thus, to determine whether redundancy was masking an important function of NFATs in cartilage, Nfatc1col2 or Nfatc3col2 mice were intercrossed with the germ-line Nfatc2 null allele to generate mice lacking either NFATc1/c2 or NFATc2/c3 in cartilage. Similar to Nfatc1col2 mice, Nfatc3col2 mice displayed no histologic or clinical abnormalities (Fig. S1D). Unexpectedly, whereas mice lacking NFATc2/c3 in cartilage (Nfatc2−/−Nfatc3fl/fl col2–cre mice) displayed no phenotypic abnormalities at the histologic or clinical levels beyond those seen in Nfatc2-deficient mice (Fig. S1D), mice lacking NFATc1/c2 in cartilage (Nfatc1col2Nfatc2−/−) display severe, spontaneous OA with 100% penetrance (Figs. 1 A and C and 2). This phenomenon manifests grossly via subluxation of the elbow at ∼1 wk of age and the metatarsals at ∼3 wk of age (Figs. 1 A and C and 2A). Although joint subluxation is not a highly prevalent feature of human OA, it can occur either as an inciting factor or secondary to OA-induced joint instability (23, 24). Notably, another spontaneous model of murine OA, the STR/ORT strain, also displays similar joint subluxations, suggesting that subluxations are a feature of severe murine OA (25). Nfatc1col2Nfatc2−/− mice display a mild runting of the limbs, with an ∼10–15% reduction in the length of long bones. Additionally, mice walk with a wide-set, antalgic gait with eversion of the hindpaws relative to a normal murine gait.
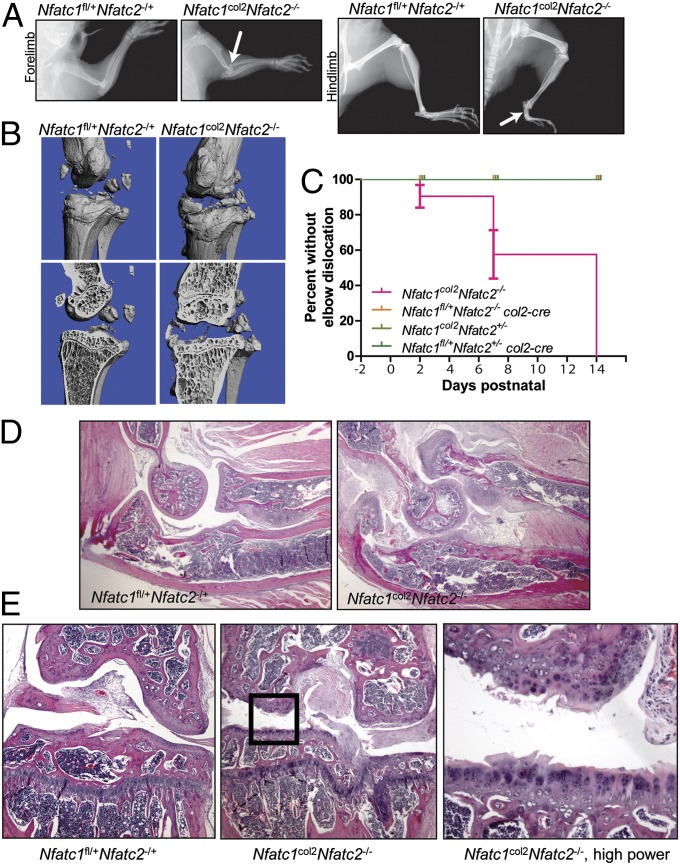
Fig. 2.
Radiographic and histologic characterization of Nfatc1col2Nfatc2−/− mice. (A) Plain radiographs of the forelimb (Left) and hindlimb (Right) of 3-wk-old mice. Dislocation of the elbow and subluxation of the tarsus are indicated with arrows. (B) Micro-CT analysis of the knee of 3-mo-old mice of the indicated genotypes, showing both the periosteal surface (Upper) and a coronal cut plane through the tibial plateau and femoral condyle (Lower). (C) Survival curves showing the incidence of elbow dislocation. Negative times indicate days prenatal. P < 0.0001 by Mantel–Cox test. (D) Micrographs of the elbow of 3-wk-old mice of the indicated genotypes. The elbow is oriented so that the humerus is in the upper left, the radius is in the upper right, and the ulna is in the lower half of the image. Images were taken at 100× magnification. (E) Micrographs of coronal sections through the knee of 3-mo-old mice. Left and Center images were taken at 100× magnification. Right displays a higher-power magnification of the boxed area in Center.
Bony Changes Characteristic of OA Are Present in Nfatc1col2Nfatc2−/− Mice.
Radiologic evaluation is the primary diagnostic modality used to support a clinical diagnosis of OA. In that respect, human OA is characterized by the formation of bony outcroppings termed osteophytes, sclerosis and cyst formation of the underlying bone, and narrowing of the joint space. At the microarchitectual level, advanced OA is associated with thickening of the underlying trabecular bone and flattening of the articular surface (26, 27). To evaluate whether Nfatc1col2Nfatc2−/− mice recapitulate these findings, the knee joints of affected animals and controls were evaluated by microcomputed tomography (μCT). As shown in Fig. 2B, Nfatc1col2Nfatc2−/− mice displayed a number of these radiographic hallmarks. First, osteophytes stud the tibia, fibula, and femur. The presence of osteophytes in the elbow and tarsus of Nfatc1col2Nfatc2−/− mice was confirmed in skeletal preparations (Fig. S1E). Second, the tibial plateau and condyles of the femur were flattened. Lastly, the underlying trabecular bone showed thickened, coarse trabeculae (Fig. 2B). Thus, Nfatc1col2Nfatc2−/− mice show typical radiographic features of human OA.
Histologic Manifestations of OA in Nfatc1col2Nfatc2−/− Mice.
Given that the gross and radiographic appearance of Nfatc1col2Nfatc2−/− mice is suggestive of OA, histologic sections of the affected joints were examined. Serial examination of Nfatc1col2Nfatc2−/− mice demonstrated that the onset of elbow dislocation is postnatal, largely occurring during the first 2 wk of life (Fig. 2C). In 3-wk-old mice, the articular cartilage surfaces of the elbow joints were fibrillated and thickened, with the underlying bone displaying a disorganized, woven appearance (Fig. 2D). The surrounding synovium was hypertrophied and hypervascular. By 6 mo of age, this OA phenotype progressed to near effacement of articular cartilage with extensive damage to the underlying bone (Fig. S2A). A similar appearance was observed in the knee joint and the femoral head, although the kinetics were slower than those observed in the elbow or tarsals, with the severity of disease at 3 mo being comparable with that of the elbow at 3 wk (Fig. 2E and Figs. S2 A and B and S3A). Dislocation was not observed at knee joints. Additionally, a similar pattern of disease to that seen in the elbow was observed in the tarsal joints, explaining the subluxation of the ankle (Fig. S3 B and C). In addition, OA characteristically induces inappropriate hypertrophy and proliferation of articular chondrocytes (28). Accordingly, high-power examination of the articular surface in of Nfatc1col2Nfatc2−/− mice demonstrated hypertrophy of chondrocytes, and a morphologic correlate of inappropriate proliferation, the presence of multiple chondrocytes filling a single lacunae in the cartilage matrix (Fig. 2E). Notably, no inflammatory infiltrates or pannus formation were observed, suggesting that disease in Nfatc1col2Nfatc2−/−mice is not attributable to an inflammatory or autoimmune arthritis. Lastly, Nfatc2−/−mice were previously observed to display cartilaginous neoplasms in mice >6 mo of age. In examination of Nfatc1col2Nfatc2−/−mice at ages up to 6 mo, no cartilaginous tumors were observed, suggesting that the absence of NFATc1 does not exacerbate the tumorigenic phenotype of Nfatc2−/− mice.
Molecular Characterization of Cartilage Matrix Degradation in Nfatc1col2Nfatc2−/− Mice.
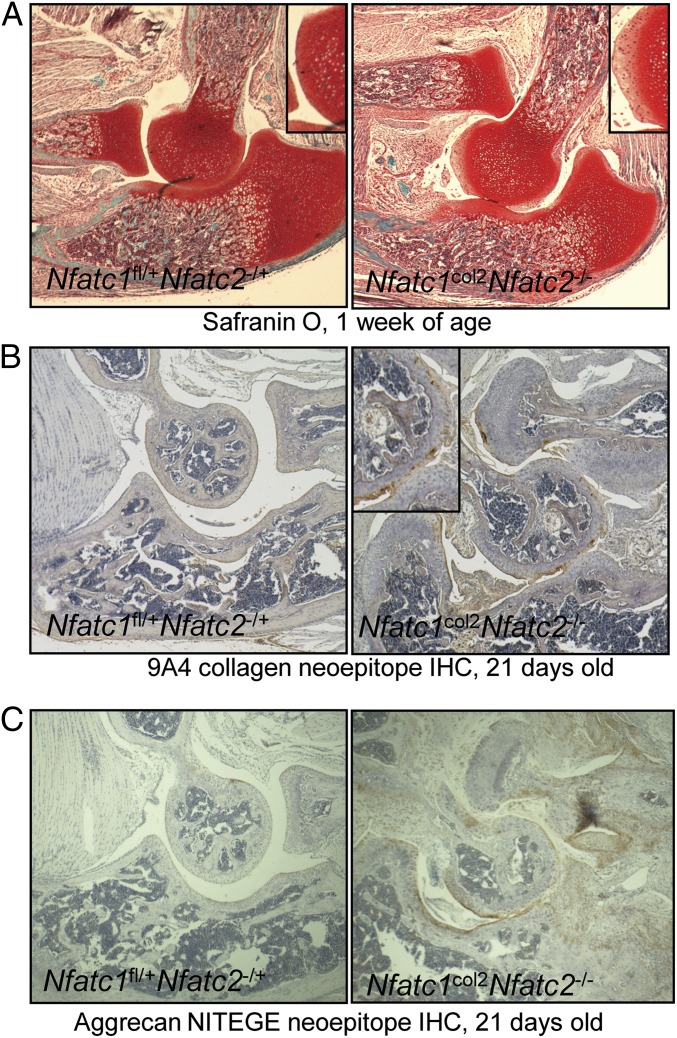
Markers of dysregulated cartilage matrix metabolism follow a stereotypic pattern of up-regulation in Nfatc1col2Nfatc2−/−mice. The first noticeable change was depletion of proteoglycan staining as assessed by safranin O staining. In the elbow, depletion of safranin O staining occurred by 1 wk of age and was observed concurrent with the onset of elbow dislocation (Fig. 3A). Similar loss of safranin O staining was observed in the knee at 3 mo of age (Fig. S3A). The tarsals likewise displayed fibrillation of the articular surfaces, proteoglycan depletion, and accompanying changes to the underlying bone by 3 mo of age (Fig. S3 B and C).
Fig. 3.
Characterization of cartilage matrix degradation in Nfatc1col2Nfatc2−/− mice. (A) Micrographs of elbows from 1-wk-old mice stained with safranin O to visualize cartilage proteoglycans. Images were taken at 100× magnification. (B) Micrographs of elbows from 21-d-old mice stained with the 9A4 antibody recognizing collagenase-generated neoepitope of collagen. Images were taken at 100× magnification. (C) Micrographs of elbows from 21-d-old mice stained with an antibody recognizing the NITEGE neoepitope generated by agggrecanase-mediated cleavage of aggrecan. Images were taken at 100× magnification.
Access to the epitope in either collagen type I or II recognized by the 9A4 antibody clone is blocked by steric hindrance in native triple-helical collagen. Upon cleavage of collagen by a collagenase, this epitope is exposed and staining can be observed by immunohistochemistry (29). Staining of this 9A4 neoepitope lagged behind loss of proteoglycan staining and was first observable by ∼21 d in the elbow (Fig. 3B and Fig. S4A). Similarly, the NITEGE neoepitope within the structural component aggrecan is only exposed upon cleavage with an aggrecanase (30). NITEGE staining was evident in the elbow by 21 d of age (Fig. 3C).
Loss of NFATc1/c2 Disrupts Gene Expression in Chondrocytes.
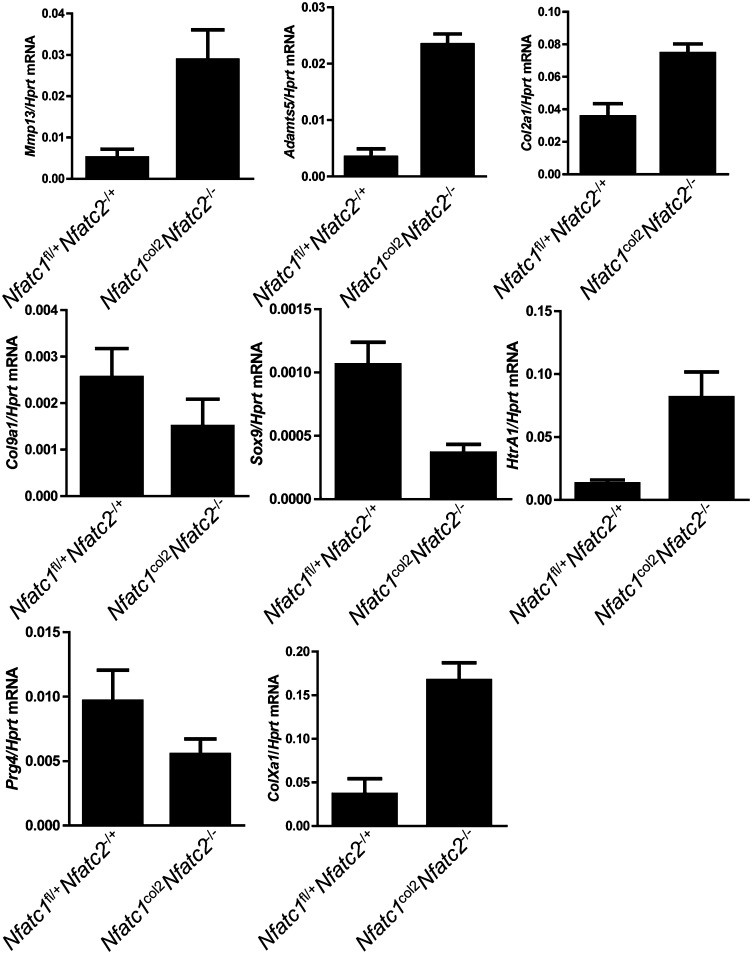
To further characterize how loss of NFATc1/c2 results in OA, articular cartilage was microdissected from the elbow of 3-wk-old Nfatc1col2Nfatc2−/− mice, and gene expression was examined by quantitative RT-PCR (Fig. 4). Corresponding to the acquisition of neoepitopes reflecting collagenase and aggrecanase activity, Mmp13 and Adamts5 expression was up-regulated in the absence of NFATc1/c2. Expression of Col2a1, a major organic component of cartilage matrix, was also up-regulated, suggesting that cartilage matrix turnover is hyperdynamic, perhaps in an effort to compensate for catabolism. Absence of type IX collagen in Col9a1−/− mice results in age-dependent OA-like changes in mice, and Col9a1 expression was moderately reduced in Nfatc1col2Nfatc2−/− mice (31). Inducible deletion of sex-determining region Y (SRY)–Box 9 (Sox9) in adult mice is associated with proteoglycan depletion at articular surfaces, and Sox9 expression was also reduced (32). Consistent with observing chondrocyte hypertrophy at affected articular surfaces, expression of type X collagen (ColXa1), a characteristic marker of hypertrophic chondrocytes, is increased in Nfatc1col2Nfatc2−/− mice. Additionally, high temperature requirement A1 (HTRA1) is a serine protease with aggrecanase activity that is up-regulated in human and murine OA, and Htra1 expression was increased in Nfatc1col2Nfatc2−/−mice (33–36). Expression of Htra3, which encodes a protein with similar proteolytic activity to HTRA1, was also up-regulated (Fig. S4B) (37). Lastly, humans and mice lacking expression of proteoglycan 4 (PRG4) develop chondrocyte dropout and proteoglycan depletion in the superficial layers of articular cartilage, and Prg4 expression was reduced in Nfatc1col2Nfatc2−/− mice (38). Thus, absence of NFATc1/c2 leads to invocation of several potential osteoarthritogenic pathways.
Fig. 4.
Characterization of gene expression in cartilage in mice lacking Nfatc1col2Nfatc2−/−. Quantitative RT-PCR analysis for the indicated genes in mRNA samples isolated from the articular elbow cartilage of 21-d-old mice. n = 4 mice per genotype. Values are mean + SD. P values calculated by an unpaired t test were as follows: Mmp13, P = 0.0008; Adamts5, P < 0.0001; Col2a1, P = 0.0487; Col9a1, P, not significant; Sox9, P = 0.0032; HtrA1 P = 0.0005; Prg4, P = 0.0217; ColXa1, P = 0.0003.
Discussion
A major factor contributing to the dearth of therapeutic options for OA is the limited number of small-animal models available for preclinical studies. Existing spontaneous mouse models of OA are commonly limited by the time to develop a full phenotype. For example, STR/ORT (39), collagen type IX (COL9A1)-deficient, and heterozygous Col11a1 chondrodysplasia mice each take >6 mo to develop robust disease, although subtle changes are present in the latter two models at 3 mo of age (31, 40). Mice lacking NFATc2 alone develop OA, although also with slow kinetics. By comparison, the articular changes at 9 mo of age in Nfatc2−/− mice are similar to those seen in 3-wk- to 3-mo-old Nfatc1col2Nfatc2−/− mice (17). From this perspective, a strength of the Nfatc1col2Nfatc2−/− model is that disease is highly penetrant and develops rapidly, which will facilitate further mechanistic and therapeutic studies.
Comparison with additional models of dysregulated cartilage metabolism offers insight into the Nfatc1col2Nfatc2−/− phenotype. Sox9-heterozygous mice also develop proteoglycan depletion without progression to a full OA phenotype (32). COL9A1-deficient mice and mice heterozygous for the Col11a1 chondrodysplasia mutation each develop an OA-like phenotype with articular cartilage fibrillation, proteoglycan depletion, and induction of the collagenase expression. Considering these phenotypes alongside the reduced expression of Sox9, Col9a1, and Prg4 in articular chondrocytes in Nfatc1col2Nfatc2−/− mice suggests a model in which multiple mediators act downstream of the loss of NFATc1/c2 expression to induce an OA phenotype. Our results also suggest that NFATs may play a role in the induction and maintenance of the identity of articular chondrocytes. In this respect, NFATs may mediate the maintenance of enduring epigenetic marks that sustain the transcriptional program needed to cope with the mechanical stresses present at the articular surface (41). This role for NFATc1 in the maintenance of chondrocyte identity correlates with the observation that NFATc1 displays restricted expression in superficial articular chondrocytes. In this respect, it is notable that human OA is associated with a selective reduction in NFATc1 in grossly lesional as opposed to nonlesional tissue. Loss of NFATc1 expression may then, at least partially, account for deregulation of catabolic and anabolic pathways that promote human OA. However, we cannot exclude the possibility that our finding simply reflects effacement of the superficial chondrocytes expressing NFATc1 in lesional tissue.
With these findings, NFATc1 joins a small group of other known transcriptional regulators of OA. One of these is SOX9, a transcription factor that determines chondrocyte identity. Loss of SOX9 expression results in proteoglycan depletion, indicating that this molecule contributes to matrix homeostasis (32). HIF-2α is also a critical regulator of OA, acting to promote expression of procatabolic proteases that degrade cartilage matrix, such as matrix metalloproteinases and aggrecanases (42). The transcription factors NF-κB, ELF3, and C/EBPβ all have also been shown to regulate expression of catabolic genes in chondrocytes and to contribute to the development of OA (43–45). It is unclear how NFATc1 may functionally interact with these other transcriptional regulators of OA in chondrocytes.
Mice lacking NFATc2 alone display cartilaginous neoplasms, largely in mice >6 mo of age (16). This phenotype was not observed in Nfatc1col2 mice and was not found to be exacerbated in Nfatc1col2Nfatc2−/− mice. Thus, the cartilaginous neoplasms occurring in Nfatc2−/− mice are not likely to be secondary to the OA phenotype of Nfatc2−/− mice, given that this aspect of the phenotype is much stronger in Nfatc1col2Nfatc2−/− mice. Additionally, the absence of a role for NFATc1 in promoting cartilaginous neoplasms may be consistent with our observation that NFATc1 displays relatively restricted expression in superficial zone articular chondrocytes. We speculate that the cell of origin for such tumors is not an NFATc1-expressing articular chondrocyte. Additionally, we speculate that the reason why ectopic cartilage growths and cartilaginous neoplasms are only observed in Nfatc2−/− as opposed to Nfatc1col2 mice is that NFATc2 may show a broader pattern of expression within chondrocytes than NFATc1. The dramatic difference in the kinetics of the onset of OA in NFATc2 singly deficient compared with Nfatc1col2Nfatc2−/− mice is also notable. Because NFATc2 is known to be a positive regulator of NFATc1 expression in other cell types (46), one explanation for this difference is that NFATc1 expression is lost over time in Nfatc2−/− mice. Thus, at older ages, Nfatc2−/− mice may functionally resemble Nfatc1col2Nfatc2−/− mice in terms of their NFATc1 and NFATc2 expression pattern.
It is unclear what signals act upstream of NFATs to either induce or mitigate their activation in chondrocytes in vivo. Cyclic mechanical stimulation is able to evoke calcium currents and is a possible activator of NFATs (47). Mechanical stimulation may provide a link between abnormal mechanical forces that may underlie the etiology of OA and NFATs. Another possibility is that stimulation with noncanonical WNT ligands activates NFATs in chondrocytes (48). Additionally, Nfatc1col2Nfatc2−/− mice raise the question of what factors may interrupt the activation of NFATs in chondrocytes. These factors could include signals that activate NFAT kinases, such as GSK3β, Protein Kinase A, or dual-specificity tyrosine-phosphorylation–regulated kinase 1A (DYRK1A), or factors that up-regulate endogenous cellular inhibitors of calcineurin, like Down syndrome critical region 1 (DSCR1) (49). Interestingly, patients with Down syndrome, which results in part from increased gene dosage of the NFAT inhibitors DYRK1A and DSCR1 (50), manifest joint instability, ligament laxity, and higher-than-expected rates of early degenerative arthritis of the hip and cervical spine (51, 52). Additionally, pharmacologic immunosuppression with cyclosporine A or tacrolimus disrupts activation of NFAT transcription factors, raising the possibility that these medications may predispose patients to OA. In contrast to this hypothesis, however, a recent publication found that tacrolimus increases anabolic (COL2, AGC1, and SOX9) and decreases catabolic (MMP1 and MMP13) gene expression in chondrocytes cultured under physiologic tonicity (53). Given that the phosphatase activity of calcineurin may also target non-NFAT proteins, it will of interest to monitor the incidence and progression of OA in patients taking cyclosporine A or tacrolimus.
In summary, we have identified that NFATc1 displays expression in articular chondrocytes and reduced expression in lesional cartilage from patients with OA. This corresponds to the ability of NFATc1 and NFATc2 to repress the development of spontaneous OA in mice. This is one of a few spontaneous OA models in a murine system, and further study may yield fundamental insight into the pathogenesis of OA.
Methods
Mouse Husbandry and Histologic Analysis.
The generation of NFATc2-deficient and NFATc1 floxed allele mice were described, with NFATc2-deficient mice being a gift from Anjana Rao (La Jolla Institute for Allergy and Immnology, La Jolla, CA) (54) (9, 55). The collagen 2 promoter cre deleter strain was described (18). All experiments were performed by using littermate controls. All mice were housed at the Harvard School of Public Health, and the Institutional Animal Care and Use Committee approved all experimental protocols. Mice bearing the Nfatc3 floxed allele were a kind gift of Gerald Crabtree (Stanford University School of Medicine, Stanford, CA).
Analysis of Expression by Real-Time PCR.
For analysis of mRNA expression, cartilage was dissected free from the surrounding tissues under a dissecting microscope. The resulting tissue fragments were homogenized by using a mortar and pestle in TRIzol (Quiagen), and subsequent RNA extraction was performed according to the manufacturer’s instructions. Sequences of the PCR primers used are provided in Table S1.
Micro-CT Analysis.
Micro-CT analysis was performed as described (56). Briefly, intact joints were scanned in a 7- or 12-mm sample tube on a Scanco µCT 35 on the medium-resolution default settings. The resulting images were then thresholded at 211 permille, and three-dimensional reconstructions were generated.
Immunohistochemistry.
Immunohistochemistry on decalcified, paraffin-embedded tissue was performed as described (57). Briefly, tissue sections were prewarmed at 55 °C, deparaffinized, and rehydrated. Endogenous peroxidase activity was quenched by 3% (vol/vol) hydrogen peroxide for 15 min. Antigen retrieval was performed by cooking in a pressure cooker in citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) for 15 min. Sections were blocked with 3% goat serum, 1% BSA, 0.1% Triton X-100 in PBS for 1 h at room temperature and then incubated with the specific primary antibody. The 9A4 neoepitope antibody was a gift from Olga Nemirovskiy and Anthony J. Milici (Pfizer, Groton, CT). Anti-NFATc1 was purchased from Santa Cruz, and anti-NITEGE antibody was purchased from MD Bioproducts. Sections were then treated with tyramide signal amplification (Perkin-Elmer) and streptavidin–HRP as per manufacturer's instructions, and HRP was visualized with 2,2′-diaminobenzidine tetrahydrochloride.
DMM Model.
The DMM model was performed as described (2, 19). Briefly, mice of the indicated genotype were anesthetized by using isofluorane, one knee was prepared for dissection, and the knee was exposed by incision through the skin, s.c. tissues, and synovium. The medial meniscal ligament was transected, and the incision was closed with sutures. Sterile technique and appropriate analgesia was used according to the guidelines of the Harvard Medical School Institutional Animal Care and Use Committee. DMM surgery was performed when the mice were 10–12 wk of age, and mice were euthanized at 8 wk postoperatively. Mice were euthanized at the indicated time points after the procedure. The scoring system used was a semiquantitative scale of 0–6, looking at cartilage features including loss of safranin O staining (indicating loss of proteoglycans without obvious cartilage loss) and fibrillations of cartilage and also evaluating frank cartilage loss down to the level of calcified cartilage (58). The area scored was the weight-bearing surface of the medial compartment.
Supplementary Material
Acknowledgments
This work was supported by an Arthritis Foundation grant (to L.H.G.), a Career Award for Medical Scientists from the Burroughs Wellcome Fund (to A.O.A.), and by National Institutes of Health Grants K08AR054859 (to A.O.A) and R01AR060363-01 (to A.O.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320036110/-/DCSupplemental.
References
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: A population-health perspective. Am J Nurs. 2012;112(3) Suppl 1:S13–S19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echtermeyer F, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15(9):1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 4.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Stanton H, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 6.Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med. 2010;207(5):923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranger AM, et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392(6672):186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 8.Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev. 2010;233(1):286–300. doi: 10.1111/j.0105-2896.2009.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliprantis AO, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118(11):3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heit JJ, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 11.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 12.Koga T, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11(8):880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 13.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14(1):13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 14.Rengarajan J, Tang B, Glimcher LH. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naïve T(H)cells. Nat Immunol. 2002;3(1):48–54. doi: 10.1038/ni744. [DOI] [PubMed] [Google Scholar]
- 15.Graef IA, et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113(5):657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 16.Ranger AM, et al. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191(1):9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009;219(2):163–172. doi: 10.1002/path.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26(2):145–146. [PubMed] [Google Scholar]
- 19.Kamekura S, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Loeser RF, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105(7):863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 23.Vollnberg B, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347–2356. doi: 10.1007/s00330-012-2493-3. [DOI] [PubMed] [Google Scholar]
- 24.Nomura E, Inoue M. Second-look arthroscopy of cartilage changes of the patellofemoral joint, especially the patella, following acute and recurrent patellar dislocation. Osteoarthritis Cartilage. 2005;13(11):1029–1036. doi: 10.1016/j.joca.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Walton M. A spontaneous ankle deformity in an inbred strain of mouse. J Pathol. 1978;124(4):189–194. doi: 10.1002/path.1711240403. [DOI] [PubMed] [Google Scholar]
- 26.Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12(Suppl A):S10–S19. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Walton M, Elves MW. Bone thickening in osteoarthrosis. Observations of an osteoarthrosis-prone strain of mouse. Acta Orthop Scand. 1979;50(5):501–506. doi: 10.3109/17453677908989795. [DOI] [PubMed] [Google Scholar]
- 28.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12(5):216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otterness IG, et al. Detection of collagenase-induced damage of collagen by 9A4, a monoclonal C-terminal neoepitope antibody. Matrix Biol. 1999;18(4):331–341. doi: 10.1016/s0945-053x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 30.Hughes CE, Caterson B, Fosang AJ, Roughley PJ, Mort JS. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: Application to catabolism in situ and in vitro. Biochem J. 1995;305(Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu K, et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54(9):2891–2900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 32.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Mineral Res. 2012;27(12):2511–2525. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchiya A, et al. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone. 2005;37(3):323–336. doi: 10.1016/j.bone.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, et al. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007;56(11):3675–3684. doi: 10.1002/art.22876. [DOI] [PubMed] [Google Scholar]
- 35.Grau S, et al. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281(10):6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 36.Chamberland A, et al. Identification of a novel HtrA1-susceptible cleavage site in human aggrecan: Evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J Biol Chem. 2009;284(40):27352–27359. doi: 10.1074/jbc.M109.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zurawa-Janicka D, Skorko-Glonek J, Lipinska B. HtrA proteins as targets in therapy of cancer and other diseases. Expert Opin Ther Targets. 2010;14(7):665–679. doi: 10.1517/14728222.2010.487867. [DOI] [PubMed] [Google Scholar]
- 38.Rhee DK, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton M. Degenerative joint disease in the mouse knee: Radiological and morphological observations. J Pathol. 1977;123(2):97–107. doi: 10.1002/path.1711230206. [DOI] [PubMed] [Google Scholar]
- 40.Xu L, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48(9):2509–2518. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- 41.Zaidi SK, et al. Architectural epigenetics: Mitotic retention of mammalian transcriptional regulatory information. Mol Cell Biol. 2010;30(20):4758–4766. doi: 10.1128/MCB.00646-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 43.Hirata M, et al. C/EBPβ and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2α as the inducer in chondrocytes. Hum Mol Genet. 2012;21(5):1111–1123. doi: 10.1093/hmg/ddr540. [DOI] [PubMed] [Google Scholar]
- 44.Otero M, et al. E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J Biol Chem. 2012;287(5):3559–3572. doi: 10.1074/jbc.M111.265744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: Multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asagiri M, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202(9):1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wann AK, et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26(4):1663–1671. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley EW, Drissi MH. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol Endocrinol. 2010;24(8):1581–1593. doi: 10.1210/me.2010-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller MR, Rao A. NFAT, immunity and cancer: A transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 50.Arron JR, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441(7093):595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 51.Fidone GS. Degenerative cervical arthritis and Down’s syndrome. N Engl J Med. 1986;314(5):320. doi: 10.1056/NEJM198601303140519. [DOI] [PubMed] [Google Scholar]
- 52.Kosashvili Y, et al. Total hip arthroplasty in patients with Down’s syndrome. Int Orthop. 2011;35(5):661–666. doi: 10.1007/s00264-010-1030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Windt AE, et al. Inhibiting calcineurin activity under physiologic tonicity elevates anabolic but suppresses catabolic chondrocyte markers. Arthritis Rheum. 2012;64(6):1929–1939. doi: 10.1002/art.34369. [DOI] [PubMed] [Google Scholar]
- 54.Canté-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179(1):103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- 55.Xanthoudakis S, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272(5263):892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 56.Greenblatt MB, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. 2010;120(7):2457–2473. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shim JH, et al. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28(14):2028–2041. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.