Significance
Reactivation is a key process that updates memory by strengthening existing memories and incorporating relevant new information, thus supporting the dynamic and flexible nature of memory. This adaptive function, however, can sometimes contribute to memory distortions. The current study examines how neural mechanisms that operate during reactivation of memories for a museum tour contribute to enhancement of existing memories, while also supporting integration of novel information that can contribute to false memories. Our results reveal similarities and differences in the neural mechanisms of reactivation associated with subsequent true and false memories for real-world events, thereby illuminating how memories change over time as a consequence of reactivation—a process that has important implications for understanding the unreliability of eyewitness memories.
Keywords: autobiographical memory, false memory, episodic memory
Abstract
We remember a considerable number of personal experiences because we are frequently reminded of them, a process known as memory reactivation. Although memory reactivation helps to stabilize and update memories, reactivation may also introduce distortions if novel information becomes incorporated with memory. Here we used functional magnetic resonance imaging (fMRI) to investigate the neural mechanisms mediating reactivation-induced updating in memory for events experienced during a museum tour. During scanning, participants were shown target photographs to reactivate memories from the museum tour followed by a novel lure photograph from an alternate tour. Later, participants were presented with target and lure photographs and asked to determine whether the photographs showed a stop they visited during the tour. We used a subsequent memory analysis to examine neural recruitment during reactivation that was associated with later true and false memories. We predicted that the quality of reactivation, as determined by online ratings of subjective recollection, would increase subsequent true memories but also facilitate incorporation of the lure photograph, thereby increasing subsequent false memories. The fMRI results revealed that the quality of reactivation modulated subsequent true and false memories via recruitment of left posterior parahippocampal, bilateral retrosplenial, and bilateral posterior inferior parietal cortices. However, the timing of neural recruitment and the way in which memories were reactivated contributed to differences in whether memory reactivation led to distortions or not. These data reveal the neural mechanisms recruited during memory reactivation that modify how memories will be subsequently retrieved, supporting the flexible and dynamic aspects of memory.
Research in psychology and neuroscience supports the idea that memory is not an exact reproduction of past experiences, but is instead a constructive process subject to a variety of errors and distortions (1–8). Both in the laboratory and everyday life, much evidence shows that people sometimes remember events differently from the way they actually unfolded and under some conditions remember events that never happened (9–12). Memory distortions can have serious consequences in everyday life, as illustrated by the frequent involvement of eyewitness memory errors (13) in wrongful convictions of individuals who were eventually exonerated on the basis of DNA evidence (14).
Memory distortions are often viewed as flaws in the memory system or as evidence of impairment, and there is evidence consistent with this view: increased susceptibility to memory distortions has been linked with such phenomena as low intelligence (15), frontal-lobe damage (16–18), and symptoms of posttraumatic stress disorder (19). An alternative approach characterizes memory distortions as byproducts of otherwise adaptive features of memory. An early example of this approach comes from Bartlett (7), who theorized that distortions he observed when people recalled folktales reflect the operation of structured prior knowledge—a schema—that contributes to comprehending and organizing the folktale. More recently, a growing number of researchers have advanced adaptive perspectives on memory distortion (3, 6, 20–22).
In a recent review (23) we delineated three kinds of evidence for adaptive-memory distortions. The first involves gist-based and associative-memory errors, which occur when individuals incorrectly recall or recognize novel information that is conceptually, perceptually, or associatively similar to material that they previously encoded (1, 24, 25). By an adaptive view, these kinds of memory distortions are consequences of beneficial cognitive processes that serve to structure and organize recall or support the retention of important themes that contribute to the ability to abstract and generalize. A second kind of evidence comes from studies showing that imagination can be easily confused with memory and that simply imagining that an experience might have occurred can lead to increased confidence that it actually occurred (10, 26–30). From an adaptive perspective, these findings may reflect, in part, the important role of memory in imagining future events (22, 31–35) and that memory and imagination depend, to a large extent, on similar underlying cognitive and neural processes (36).
A third kind of evidence for adaptive-memory distortion comes from the well-known postevent misinformation effect, where people incorporate erroneous information encountered after an original event into their memory of the original event (2). From an adaptive perspective, the misinformation effect may arise as a byproduct of memory-updating processes, which normally serve to strengthen existing memory representations and to incorporate relevant new information (37, 38) but can contribute to distortions or false memories if novel information is confused with old information (3, 23).
Updating relies on the process of memory reactivation, or the activation of a latent memory trace when we are reminded of a past experience, which is a key process that shapes long-term memory representations by reorganizing them over distributed brain networks (39–41). In this paper we examine the effects of memory reactivation on the neural mechanisms mediating subsequent true and false memories. In a previous behavioral study, we showed that the quality of memory reactivation, as indexed by an individual’s subjective sense of recollection, modulates the extent to which reactivation strengthens subsequent true memories for a recently presented target event or creates false memories of novel information encountered for the first time after the target event has already occurred (42). We found that subsequent true memories were improved when targets were highly reactivated (i.e., the retrieval cues during reactivation matched the encoding experience) compared with memories that were reactivated at lower levels (i.e., the retrieval cues during reactivation mismatched encoding); however, subsequent false memories were also greater for lures that followed targets that were highly reactivated. A primary challenge of such research is to understand how neural mechanisms that operate during memory reactivation contribute to enhancement of existing memory traces while also supporting integration of novel information that can contribute to false memories.
Memory retrieval recruits a typical pattern of brain regions (43, 44), including frontoparietal network regions associated with controlled processes and default network regions linked to recovery of memory details (45). In particular, hippocampus, parahippocampal cortex, retrosplenial cortex, and lateral posterior parietal cortex are associated with recollection processes during retrieval that contribute to the quality of memory reactivation (46). Recovery of contextual information associated with the encoding experience that supports these recollection processes, however, can sometimes contribute to memory distortions as predicted by some computational models of memory (47). Recruitment of parahippocampal, retrosplenial, and posterior inferior parietal cortices during encoding of items that are contextually related (e.g., bed, dresser, etc.) is associated with subsequent false recognition of novel related items (e.g., alarm clock) (48), and contextual reinstatement of scene-related activity in the posterior parahippocampal cortex underlies subsequent misattributions in memory (49). Further, manipulating contextual memory engrams in the hippocampus has been shown to implant false fear memories to reactivated contexts in mice (50), which is consistent with functional neuroimaging studies in humans, pointing to the contribution of the hippocampus in the formation of false memories (51–53).
A related line of research suggests that the hippocampus and ventromedial prefrontal cortex (vmPFC) support flexible memory processes that allow memories to be combined and used in novel ways, and that contribute to memory updating. The hippocampus and medial PFC are associated with memory reactivation in humans (54) and with replay in rodents (55). Moreover, hippocampal recruitment supports persistent changes due to social conformity in long-term memory (56) and hippocampal–vmPFC interactions support integrative encoding of overlapping memory representations (57, 58), in line with known anatomical connections between these regions (59). This research is supported by studies showing that the hippocampus and vmPFC enable rapid consolidation of novel information when it can be linked to existing memory representations (60).
Neural regions associated with recollection and memory-updating processes are typically recruited more during autobiographical memory compared with laboratory-based memory retrieval (43, 61, 62), suggesting that autobiographical memory may be ideal for testing whether these putative neural markers of reactivation-related updating support subsequent memory effects. In the current study, we used functional magnetic resonance imaging (fMRI) to investigate neural recruitment supporting reactivation-related updating, which leads to enhancement and distortion in autobiographical memories. We used a unique museum paradigm in which participants encoded events they experienced during a museum tour while wearing a sensor-based camera that automatically took photographs (42). The museum paradigm allowed us to exert control over the encoding of real-world events and to verify the accuracy of memories for subsequent memory analysis. During functional scanning, 48 h after the museum tour, photographs from the camera were presented to trigger reactivation of memories for stops experienced during the museum tour and then were immediately followed by a novel lure photograph from an alternate museum tour (Fig. 1A; Materials and Methods). A recognition-memory test occurred 48 h after scanning in which participants were presented with reactivated target and lure photographs they saw during scanning, as well as baseline target and lure photographs that were not previously presented. In our previous behavioral study (42) we showed that the quality of reactivation affected subsequent recognition-memory performance by improving memory for target photographs and increasing false memories for lure photographs in the reactivation condition compared with baseline photographs that were not shown during the reactivation condition. Thus, reactivating memories for stops from the museum improved later memory for the tour, but also contributed to false memories by facilitating encoding of the novel lure photographs from the alternate tour that followed target reactivation. Based on this work, we predicted that both subsequent true-memory and subsequent false-memory effects would be associated with recruitment of regions linked to recollection processes, such as the medial temporal lobe (MTL; hippocampus and posterior parahippocampus), retrosplenial, and lateral inferior parietal cortices. Additionally, we predicted that, compared with subsequent true memories, subsequent false memories would show greater involvement of vmPFC and hippocampus because, as reviewed above, these regions are associated with flexible memory processes that allow for updating of existing memory with novel information that may potentially support incorporation of the lure photograph into existing memory for the museum tour.
Fig. 1.
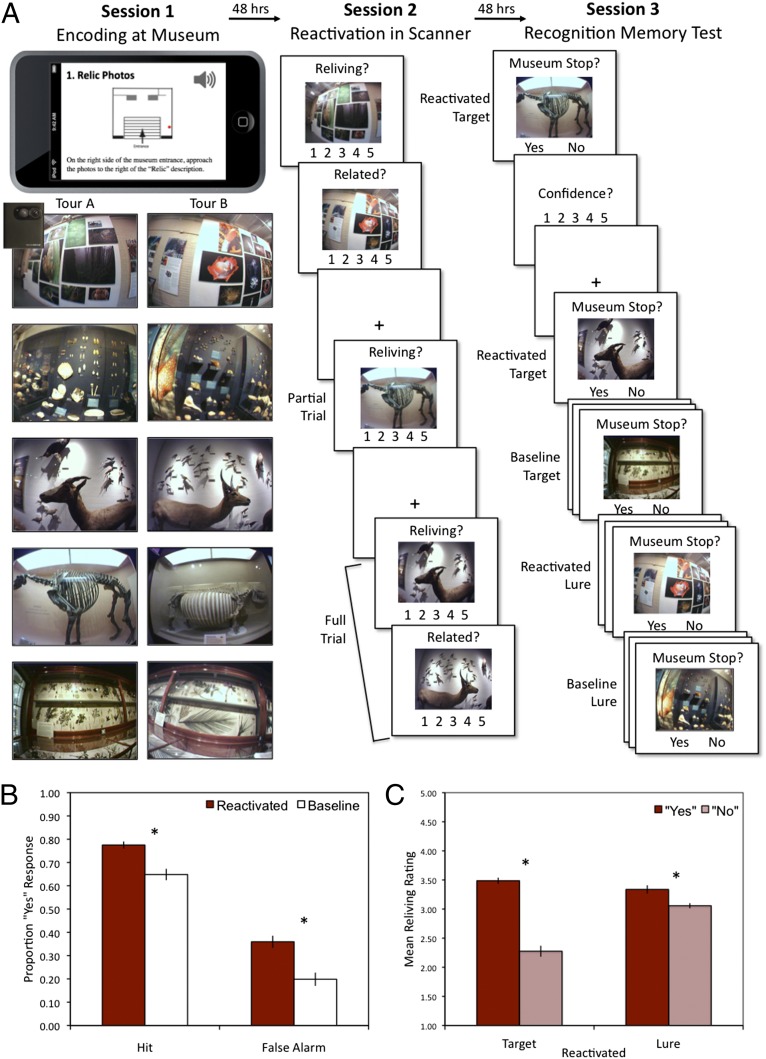
Experimental design and behavioral results. (A) In session 1, participants went on one of two audio-guided museum tours while wearing a camera that automatically took photographs of each stop using a timer. In session 2, during fMRI scanning, they were shown photographs from stops they visited (targets) and asked to make reliving ratings (partial trial). On some trials (full trial), this was immediately followed by a photograph taken from the alternate tour (lure) and they were asked to judge how related the two photographs were. Full and partial trials were used to separate the hemodynamic response associated with the target and lure presentation within the same trial (Materials and Methods). In session 3, participants were shown reactivated targets and lures (i.e., shown during scanning session) or baseline targets and lures (i.e., not shown during scanning session) and asked to indicate whether the photograph showed a stop they had visited during the museum tour. (B) Recognition memory performance revealed increased hit and false alarm rates in the reactivated condition versus baseline. (C) Linking the recognition memory performance to the quality of memory reactivation, mean reliving ratings were higher for hits (target: “yes”) than misses (target: “no”), and for false alarms (lure: “yes”) than correct rejections (lure: “no”). Error bars indicate ±SEM. *P < 0.001.
Results
The behavioral results indicated that reactivation improved memory for the targets, but also facilitated encoding of the lures that followed reactivated targets. The reactivation condition equally increased subsequent hits [t(32) = 6.05, P < 0.0001] and subsequent false alarms [t(32) = 6.67, P < 0.0001] compared with the baseline condition consisting of target and lure photographs that were not shown during session 2 (i.e., items that were not scanned; Fig. 1B). To link the reactivation-related increases in subsequent memory to the quality of reactivation, we examined reliving ratings, the subjective sense of reexperience or recollection, during session 2 according to the subsequent memory outcome in session 3. These results showed that stronger reliving of the target improved memory for the target, but also increased memory for the lure that followed (Fig. 1C). Participants made higher reliving ratings for photographs associated with subsequent hits versus subsequent misses [t(32) = 13.68, P < 0.0001] and with subsequent false alarms versus subsequent correct rejections [t(32) = 3.96, P = 0.0003]. The link between reliving and subsequent memory was also evident on a trial-by-trial level, such that there was a significant within-participant correlation between reliving ratings and subsequent true memories (r = 0.34, P < 0.0001) and between reliving ratings and subsequent false memories (r = 0.09, P = 0.0004). Additionally, the influence of reliving on subsequent memory remained after controlling for how quickly the memory was retrieved and the relatedness of novel information (SI Results). Thus, the quality of reactivation during session 2 uniquely influenced the outcome of both subsequent true and subsequent false memories, such that on a trial-by-trial basis, stronger reliving improved memory for the targets and increased memory for the lures that followed. In sum, we found an increase in subsequent true and false memories for reactivated items compared with baseline items, and that the quality of reactivation influenced the level of true and false memories. The current results support the findings of our previous behavioral study using a similar paradigm (42). For additional behavioral results, see SI Results.
Reactivation-related neural regions associated with subsequent memory.
The main goal of the fMRI analysis was to examine how neural mechanisms recruited during reactivation of memory for real-world events experienced during the museum tour influence updating processes that result in later enhancement or distortion in memory. Thus, we separated trials as a function of later true memories (i.e., hits minus misses) and later false memories (i.e., false alarms minus correct rejections; Materials and Methods). The subsequent memory analysis revealed that both subsequent true-memory and subsequent false-memory effects were associated with recruitment of left posterior parahippocampal, bilateral retrosplenial, and bilateral posterior inferior parietal cortices (Fig. 2A and Table S1).
Fig. 2.
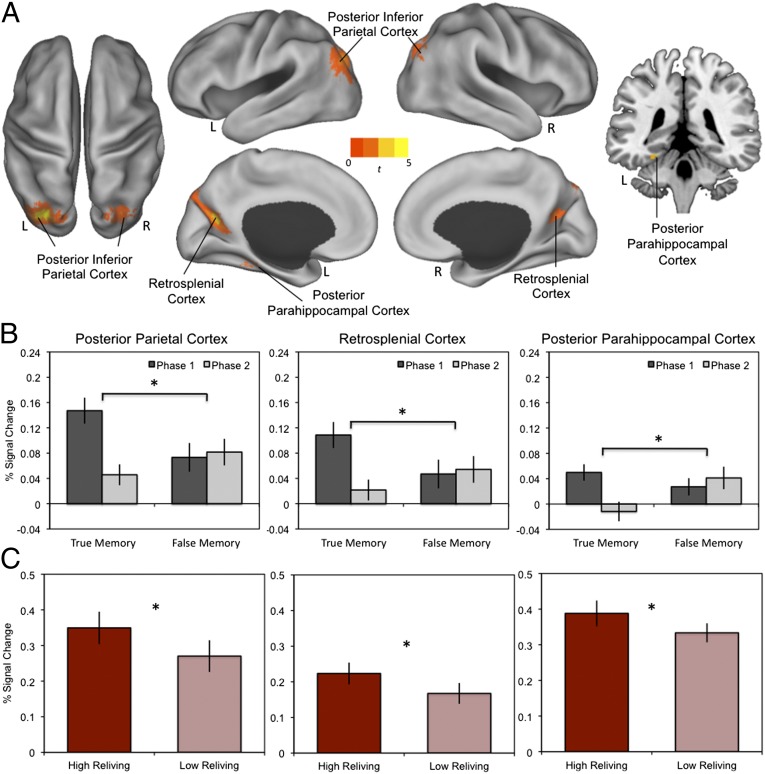
Common brain regions associated with subsequent true and false memories. (A) Subsequent true and false memories were associated with recruitment of the bilateral posterior inferior parietal cortex, bilateral retrosplenial cortex, and left posterior parahippocampal cortex (P < 0.001 uncorrected; for complete results, see Table S1). L, left; R, right. (B) Percent signal change showing a significant trial phase × memory condition interaction, reflected by a significant difference between the two phases for true memories, but not for false memories. Phase 1 = target presentation and phase 2 = lure presentation. (C) Percent signal change showing a significant difference in high reliving versus low reliving trials based on a median split. Error bars indicate ±SEM. *P < 0.001.
Although reactivation-related updating in later memory recruited similar neural mechanisms for subsequent true and false memories, we found that the timing of these effects differed by examining neural recruitment during target (phase 1) and lure (phase 2) presentation. There was a significant interaction between trial phase (target photograph, lure photograph) and subsequent memory (true, false) in the recruitment of the posterior parahippocampal, retrosplenial, and posterior inferior parietal cortices (Fig. 2B and Table S2). The interaction was reflected by greater recruitment during target than during lure phases for subsequent true memories but equal recruitment of these regions across both trial phases for subsequent false memories. Thus, true and false subsequent memories were distinguished by neural mechanisms recruited when processing the lure photograph. For false memories, sustained involvement of the posterior parahippocampal, retrosplenial, and posterior inferior cortices across memory reactivation and the presentation of novel information was associated with whether the lure photograph would be incorporated into later memories for the museum tour. In contrast, for true memories, reduced recruitment of these same brain regions during presentation of the lure was associated with accurate subsequent memories.
We conducted two additional follow-up analyses to determine whether the pattern of neural recruitment supporting memory updating was associated with memory reactivation. First, we examined how activation of these regions was related to trial-by-trial differences in the quality of memory reactivation by conducting a parametric modulation analysis using the reliving rating, our putative marker of the quality of reactivation. We found that recruitment of the posterior parahippocampal, retrosplenial, and posterior inferior parietal cortices increased on a trial-by-trial basis with increases in reliving, and thus were associated with the quality of reactivation (Fig. 2C). Importantly, recruitment of each of these regions was not associated with reaction time or the relatedness of novel information (Fig. S1).
Second, we examined the potential overlap in updating during memory reactivation with the formation of new memories during novel encoding (Materials and Methods). If the current findings reflect memory reactivation, there should be minimal overlap with memory-encoding processes related to the formation of a new memory. Consistent with this prediction, there was no overlap in neural recruitment for subsequent memory effects between the reactivation condition and the novel encoding condition (Table S3).
In sum, the recruitment of left posterior parahippocampal, bilateral retrosplenial, and bilateral posterior inferior parietal cortices during memory retrieval supports reactivation-related updating that impacts subsequent remembering. Although there were differences in neural recruitment associated with subsequent true and subsequent false memory effects (SI Results), these regions were unrelated to the quality of reactivation (Fig. S2).
Differential subsequent memory effects related to reactivation quality.
The behavioral results showed that reactivation quality was associated with both subsequent hits and false alarms. Both conditions are associated with a “yes” response during recognition memory; however, we reasoned that the way memory was reactivated could differentially support enhancement versus distortion effects in subsequent memory. To investigate this issue, we used a parametric modulation analysis on reliving ratings and examined subsequent memory effects in a 2 (trial phase: target photograph, lure photograph) × 2 (memory condition: hit, false alarm) ANOVA (Table S4; see Table S5 for a similar analysis on relatedness ratings). There was an overall main effect of trial phase reflecting greater neural recruitment of brain regions sensitive to reliving during the target versus lure trial phases. During the presentation of the target photograph compared with the lure photograph, reliving was associated with a number of regions typically recruited during autobiographical memory retrieval (43), including anterior and posterior hippocampus, left ventrolateral PFC, rostral medial and vmPFC, posterior cingulate, posterior inferior parietal cortex, and lateral temporal cortex (Fig. S3).
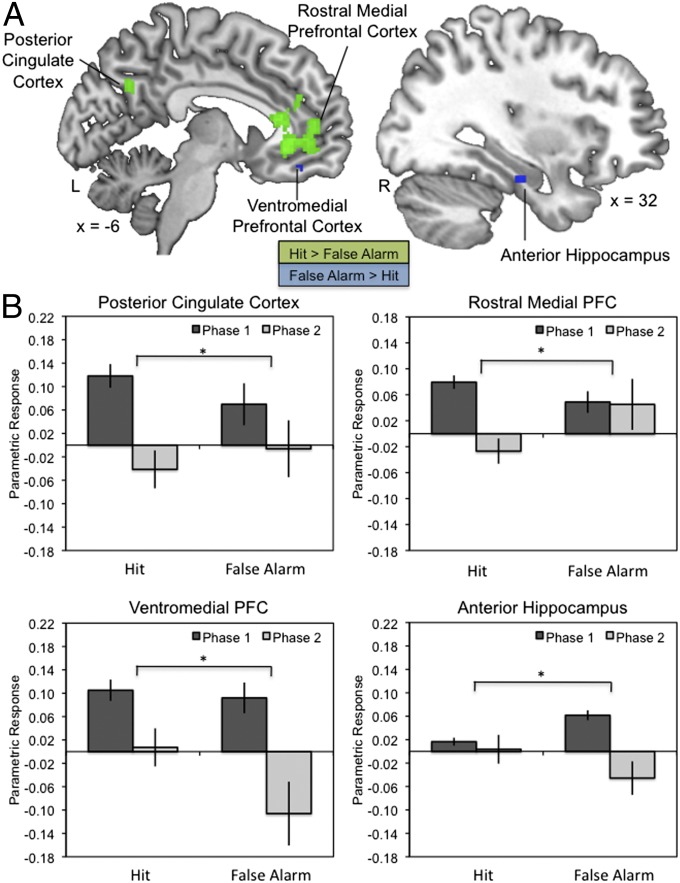
A significant trial phase × memory condition interaction on the neural mechanisms associated with the quality of reactivation, however, revealed that the way the memory was reactivated was associated with different outcomes in subsequent memory (Fig. 3). Subsequent hits were associated with greater recruitment of the rostral medial PFC and posterior cingulate during target compared with lure phases for highly relived memories. In contrast, subsequent false alarms were associated with greater recruitment of vmPFC, ventrolateral PFC, lateral temporal cortex, and right anterior hippocampus during target compared with lure phases for highly relived memories. Thus, the neural mechanisms of reactivation-related updating differed depending on whether memory was updated to include novel information presented later in the trial or whether it was protected from such distortions.
Fig. 3.
Differential subsequent memory effects related to reactivation quality. (A) Neural recruitment supporting reactivation quality differed for hits and false alarms across the two phases. For phase 1 versus phase 2, there was greater recruitment of posterior cingulate cortex and rostral medial PFC for hits, but greater recruitment of vmPFC and right hippocampus for false alarms (P < 0.001 uncorrected). (B) Parametric response, in beta values, showing the trial phase × memory condition interaction. Error bars indicate ±SEM. *P < 0.001.
Discussion
A challenge in memory research has been to understand the neurobiological mechanisms of memory updating and how updating supports enhancement of existing memory representations while also allowing for incorporation of novel information that sometimes leads to distortions in memory. Our results provide evidence for similarities and differences in the neural mechanisms of reactivation associated with updating of memory for real-world events. In particular, subsequent true and false memory effects were associated with common neural recruitment of posterior parahippocampus, retrosplenial cortex, and posterior inferior parietal cortex during memory reactivation. Equal neural recruitment in these regions during target and lure presentation supported the incorporation of novel information that contributed to false memories, whereas reduced recruitment during lure presentation was associated with true memories. Additionally, neural recruitment that increased according to the quality of reactivation differed depending on the outcome of memory. Subsequent hits were associated with greater activity in posterior cingulate cortex and rostral medial PFC; in contrast, false alarms were associated with greater activity in vmPFC and anterior hippocampus.
Memory reactivation is a central component of computational theories of memory (40), which hold that it supports the stabilization of memory over distributed brain networks. A number of studies have observed neural reactivation during offline periods of sleep that enhance later true memories, and sleep-related mechanisms have also been shown to contribute to restructuring and reorganization of experiences that may support the formation of false memories (63). However, there is conflicting evidence regarding the nature of awake reactivation, with some studies showing that reactivation enhances or even protects memory from new information (54, 64, 65) and other studies indicating that reactivation incorporates new information into memory (49, 65–67). The present study significantly extends this work by demonstrating that reactivation can both protect true memories and support the incorporation of new information leading to false memories, depending upon neural recruitment during reactivation.
Memory updating was associated with the recruitment of a subset of retrieval-related regions that were sensitive to the quality of reactivation. Here we defined reactivation quality according to subjective recollection, and thus, our findings could also reflect the contribution of memory strength during reactivation (68). There was little overlap in neural recruitment supporting subsequent memory effects during reactivation compared with novel encoding. Indeed, many of these retrieval-related brain regions are deactivated compared with baseline during novel encoding tasks (69), suggesting less contribution from the typical subsequent memory regions during memory updating. One way that reactivation may support memory updating is via contextual reinstatement, by linking novel information that occurs during retrieval with the reinstated context of the reactivated memory (47, 49). Contextual reinstatement could underlie the link between the quality of reactivation and subsequent memory effects (42). In the current study, contextual reinstatement would include reconstruction of the rich 3D spatial setting of the museum stop from the photographic retrieval cue. In line with this idea, subsequent memory effects were associated with recruitment of posterior parahippocampal, retrosplenial, and posterior inferior parietal cortices, which contribute to the recovery of spatial context and scene construction (70, 71). Retrosplenial cortex is thought to support integration and translation between egocentric spatial representations in posterior parietal cortex and allocentric spatial representations in the MTL (71), and these processes are recruited to a greater extent when spatial context needs to be updated and manipulated (72). Contextual reinstatement may strengthen existing memory and/or integrate existing memory with novel information provided by the retrieval cues, in much the same way that retrieval practice is thought to support memory via elaboration of the memory trace (73). This restructuring of memories could facilitate re-encoding and reconsolidation processes that contribute to updating of long-term memory representations.
Neural recruitment sensitive to reactivation quality differed depending upon the outcome of subsequent memory. Our finding that subsequent false alarms were associated with greater involvement of anterior hippocampus and vmPFC builds upon accumulating evidence linking these regions to flexible memory processes that enable memories to be combined and used in novel ways. Hippocampus and vmPFC are part of a MTL subsystem of the default network (74) that supports the formation of mental models based on mnemonic content during both memory retrieval and the simulation and encoding of future events (36, 75, 76). In particular, the anterior portion of the hippocampus is associated with relational processes (77–79) that contribute to the ability to integrate memory details across experiences (80). Additionally, the anterior hippocampus represents global context through its connections with vmPFC and related schematic processes that could support the incorporation of novel information (59). Our results converge with these findings, but extend this work by demonstrating that the quality of reactivation is a key mechanism and also by showing that these processes support the formation of false memories. We also found that anterior hippocampus was sensitive to relatedness ratings more for false alarms than hits during presentation of the target (Table S5). One explanation is that target reactivation and related pattern completion processes may linger during the processing of the lure and affect the degree of relatedness of the lures (81), thus contributing to false memories. In line with this idea, we found a significant association between reliving and relatedness ratings, and that this association contributed more to false than true memories. In contrast, posterior hippocampus contributed equally to subsequent hits and false alarms, consistent with its role in representing detailed contextual information that more generally supports recollection (59). Compared with subsequent false alarms, we found that subsequent hits were associated with posterior cingulate cortex and rostral medial PFC, which are core midline regions of the default network (74) that support self-referential processes (82, 83) and successful encoding of self-relevant information (84–86). These findings suggest that reactivation may enhance true memories by elaborating upon memory details related to oneself (87), but increase false memories via binding of novel information.
Our results add to the growing literature on the neural mechanisms supporting false memories (88). The memory distortion effects we observed here in some ways resemble those that occur in the postevent misinformation paradigm (2), in which erroneous information presented after encoding contributes to later false memories because of source-memory confusion. During encoding of misinformation, some studies have shown recruitment of anterior and posterior midline regions that protect true memories (51), and hippocampal recruitment that supports the formation of false memories (52). Our results converge with these findings, but further indicate that neural recruitment that differentiates subsequent true and false memories during presentation of novel information depends on the extent to which memory for the original experience is reactivated rather than source confusion between the presence or absence of a lure. Thus, here we show that the quality of reactivation modulates both the extent of subsequent memory effects, and the neural recruitment associated with these memory effects. Similarly, in our previous study using the museum paradigm (42), we manipulated the quality of reactivation and showed that subsequent false memories were greater when lures followed targets where memories were highly reactivated (i.e., the retrieval cues during reactivation matched encoding experience) than for memories that were reactivated at lower levels (i.e., the retrieval cues during reactivation mismatched encoding).
We frequently remember events from our personal pasts (89)—voluntarily, as we share a memory with another individual, or involuntarily, as we spontaneously bring to mind a past event—our findings build upon accumulating evidence that memory is shaped by such retrievals (90, 91). Our data show that the quality of reactivation is one mechanism by which retrieval influences memory, and we suggest a link between contextual reinstatement via neural recruitment of the posterior parahippocampal, retrosplenial, and posterior inferior parietal cortices in the enhancement and distortion in later memory. The current study reveals neural mechanisms that support the formation of false memories for naturalistic events experienced in a real-world setting, which has important implications for eyewitness memory and the law (92, 93). Indeed, Schacter and Loftus (92) argued that understanding the neural mechanisms of memory reactivation and reconsolidation could provide a foundation for understanding how memories change over time, which in turn could eventually help better understand why eyewitness memories sometimes change in response to repeated questioning. Our findings also fit with an adaptive perspective on memory distortion (6, 20–23), in which reactivation allows for the incorporation of relevant new information that is essential for the operation of a dynamic memory system, but which comes at the cost of memory distortions. It is an open question as to whether all varieties of memory distortion can be conceived as costs associated with adaptive features of memory, but our results are consistent with the view that reactivation-related false memories reflect one downside to the generally beneficial process of memory updating.
Materials and Methods
Participants.
There were 35 participants (18–30 y old, 19 women). All participants were right-handed and reported no history of neurological or psychiatric episodes or current use of medication known to affect cognitive function. Participants gave written consent for a protocol approved by the Harvard University Intuitional Review Board. Two participants were excluded due to computer issues. Seven additional participants were excluded from the fMRI analysis only because of quality control issues (SI Materials and Methods).
Procedure.
The study involved three sessions separated by 48 h: a museum tour, an fMRI scanning, and a recognition memory test. In session 1, participants were provided with an iTouch (Apple) outlining a self-guided audio tour of the adjoining Harvard Museum of Natural History and Peabody Museum and asked to wear a ViconRevue camera (Vicon, Oxford, UK), which automatically takes photographs every 15 s using a timer. The tour was composed of 208 museum stops (e.g., examining a display case, watching a video, etc.), and took ∼4–5 h to complete. There were two versions of the tour, which were counterbalanced between participants. Photographs of museum stops from the alternate tour were used as lures. Museum stops in the two tour versions were matched to be similar in content (e.g., one video versus another video in the same exhibit; Fig. 1A), but selected to minimize overlap in the route through the museum. However, to increase the number of trials available for subsequent memory fMRI analysis, the museum tour included 41 stops that overlapped in both tour versions (i.e., participants in each tour version visited the same museum stop). Overlapping museum stops included items that would be unavoidable during each route through the museum (i.e., a large display case in the middle of the exhibit) and/or items in which a lure from the same exhibit was unavailable (i.e., an odd numbered display case in an exhibit), and were included as partial trials in session 2 (i.e., trials in which the target photograph was not followed by a lure). Participants were instructed to complete only the museum stops described in the tour guide. Photographs from each participant’s camera were inspected to ensure that the participant adhered to the instructions, and if the camera captured a photograph of a unique museum stop from the alternate tour, it was excluded from further analysis. Photographs from each participant’s camera for each museum stop were selected to use in the later sessions. Lure photographs from the alternate tour were taken from a control set.
Session 2 took place in the MRI scanner. The scanning session included a 6-min run of quiet rest with eyes open, four runs of the reactivation condition, another 6-min resting state run, four runs of the novel encoding condition (SI Materials and Methods), and another 6-min resting state run. The order of reactivation and encoding conditions was counterbalanced between participants.
During the reactivation condition, participants were asked to retrieve memories for the museum stops they visited during the tour. On each trial, participants were shown a photograph of a museum stop taken from their camera and instructed to retrieve their memory for that museum stop and then to rate the sense of reliving, which refers to the subjective sense of recollection or reexperience, on a five-point scale from low to high. The photograph remained on the screen for 5 s. For partial trials (∼64 trials), fixation immediately followed. For full trials (∼112 trials), a second photograph of a museum stop from the alternate tour that was not seen during the participant’s tour (i.e., lure photograph) immediately followed. Full and partial trial types were included to separate neural recruitment during the target and lure presentation (fMRI analysis). Participants were instructed to indicate how related (i.e., “could it be taken from the same exhibit?”) the second photograph was to the preceding one, on a five-point scale from low to high. They were not told whether the lure photographs were from the tour or not. The photograph remained on the screen for 5 s. Trials were separated by a variable fixation (2.5–7.5 s) and distributed exponentially such that shorter intertrial intervals occurred more frequently than longer. Approximately 80% of the museum stops were shown during reactivation (i.e., 176 trials), and the remaining museum stops were used for the baseline condition (i.e., 32 trials).
Session 3 involved an old/new recognition task. Participants were shown target and lure photographs taken from the museums they visited during the tour and asked to make a yes/no decision whether the photograph was a stop from their museum tour. Photographs consisted of reactivated targets (both partial and full trials, ∼120 trials) and lures (i.e., photographs that were shown during scanning session, ∼56 trials) and baseline (i.e., photographs that were not shown during scanning session) targets (∼16 trials) and lures (∼16 trials). Participants were warned that the lure photographs would look very similar to stops that they had conducted during the tour and to look carefully at each before making their decision. Participants were allowed up to 6 s to make their decision, followed by a 6-s confidence rating on a five-point scale from low to high.
fMRI Methods.
Image acquisition.
Imaging was conducted on a 3T Siemens Magnetom TimTrio Scanner equipped with a 12-channel head coil at the Center for Brain Science (Harvard University). A laptop computer running Cogent 2000 (Wellcome Department of Imaging Neuroscience, University College London, London, UK) software implemented in MATLAB (MathWorks) controlled stimulus display via a liquid crystal display projector, which projected onto a screen placed at the head of the bore. Participants viewed the screen through a mirror fastened to the head coil. Cushions were used to minimize head movement and earplugs dampened scanner noise. Participants made responses using a five-button box placed in their right hand.
Anatomical images were acquired using a high-resolution 3D magnetization-prepared rapid gradient echo sequence (176 sagittal slices, echo time [TE] = 1.64 ms, repetition time [TR] = 2,530 ms, flip angle = 7°, voxel size = 1 × 1 × 1 mm). Functional images were collected using a T2* gradient echo, echo-planar imaging sequence sensitive to blood oxygen level-dependent (BOLD) contrast (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, 3 × 3 mm in-plane resolution). Whole-brain coverage was obtained with 39 contiguous slices, acquired parallel to the anterior–posterior commissure plane (3-mm slice thickness, 0.5-mm skip between slices).
Image processing.
Imaging data were preprocessed and statistically analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). First, data were preprocessed to remove sources of noise and artifact. Preprocessing included slice-time correction to correct for differences in acquisition time between slices for each whole brain volume; realignment within and across runs to correct for head movement; spatial normalization to the Montreal Neurological Institute template (resampled at 2 × 2 × 2 mm voxels) and spatial smoothing (8-mm full-width at half maximum) using a Gaussian kernel.
fMRI analysis.
Preprocessed data were analyzed using the general linear model (GLM). For each participant, trial onsets (i.e., time-locked to target or lure stimulus presentation) were modeled with a canonical hemodynamic response function and duration of 5 s.
We used a compound trial approach (94) to separate the hemodynamic response associated with target and lure presentation within the same trial in the reactivation condition. Because the stimulus onsets for phase 2 (lures) occurred at a fixed interval after the stimulus onset of phase 1 (targets), partial trials were used to separate the BOLD responses of the phases. Approximately 36% of the total trials were partial. A similar approach was used to separately analyze the pairs of novel photographs presented in the novel encoding condition (Fig. S4 and SI Materials and Methods).
To combine the compound trial approach and subsequent memory analysis on true and false memories, it was necessary to create two GLMs in the reactivation condition. The models differed in the ability to examine false alarm and correct rejection trial types in the two phases because the nature of the task design did not allow for partial trials within these categories. To address this issue, ∼50% of the partial trials were randomly assigned to a new partial trial type to effectively separate the BOLD response of phase 1 and phase 2 for false alarms and correct rejections. One GLM was created to examine phase-1 effects only; it included a combined phase-2 trial type (full hits, misses, false alarms, and correct rejections), phase-1 hits (full + partial trials), phase-1 misses (full + partial trials), phase-1 false alarms (full trials only), phase-1 correct rejections (full trials only), and phase-1 new partial trials. Another GLM was created to examine phase-2 effects only and included a combined phase-1 trial type, (full false alarms, full correct rejections, and new partial trials), phase-1 hits (full + partial trials), phase-1 misses (full + partial trials), phase-2 hits (full trials only), phase-2 misses (full trials only), phase-2 false alarms (full trials only), and phase-2 correct rejections (full trials only).
We then used a subsequent memory analysis. We examined neural activity during retrieval that was associated with subsequent true memories (i.e., subsequent hits minus subsequent misses) and subsequent false memories (i.e., subsequent false alarms minus subsequent correct rejections) separately for each stimulus onset. A minimum of eight trials per trial type was used as a cutoff for inclusion in the analysis. There were two primary analyses. First we conducted a 2 (trial phase: phase 1, phase 2) × 2 (memory condition: true, false) ANOVA. The main interest in the trial phase × memory condition interaction was to determine the presence of variation in the magnitude of trial phase effects by memory condition (i.e., quantitative or non–cross-over interactions), rather than completely reverse effects (i.e., qualitative or cross-over interaction). Thus, here we focused on quantitative interactions by weighting the interaction effects in ANOVA, as implemented in SPM8, to examine differences in magnitude across levels of trial phase for true and false subsequent memories.
Second, we examined the influence of trial-by-trial variation in the quality of reactivation on neural activity by using a parametric modulation analysis on the reliving ratings in two models: (i) irrespective of subsequent memory performance and condition or (ii) differentiating reliving sensitive neural activity for subsequent hits and subsequent false alarms. Additionally, we conducted ancillary parametric modulation analyses to examine neural recruitment sensitive to reaction time and relatedness ratings irrespective of memory performance, and a separate analysis that differentiated subsequent hits and subsequent false alarms for relatedness ratings.
A threshold of P < 0.001, uncorrected, with a cluster size of 10 voxels was used for all reported analyses. Region of interest analyses for reporting percent signal change were performed in MarsBaR (version 0.43) by extracting all significantly active voxels in an 8-mm sphere centered on the coordinate from the relevant contrast.
Supplementary Material
Acknowledgments
We thank Clifford A. Robbins, Annie Mitran, and Justin Kim for helpful assistance and the Harvard Museum of Natural History and Peabody Museum of Archaeology and Ethnology at Harvard University for cooperation. This work was supported by National Institute of Health Grant MH060941 (to D.L.S.) and by National Institute on Aging Grant National Research Service Award AG038079 and a L’Oreal USA for Women in Science Fellowship (both to P.L.S.J.).
Footnotes
The authors declare no conflict of interest.
See QnAs on page 19655.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319630110/-/DCSupplemental.
References
- 1.Brainerd CJ, Reyna VF. The Science of False Memory. New York: Oxford Univ Press; 2005. [Google Scholar]
- 2.Loftus EF. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learn Mem. 2005;12(4):361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- 3.Hardt O, Einarsson EO, Nader K. A bridge over troubled water: Reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu Rev Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. The restless engram: Consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 5.Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- 6.Schacter DL. The Seven Sins of Memory: How the Mind Forgets and Remembers. Boston: Houghton Mifflin; 2001. [Google Scholar]
- 7.Bartlett FC. Remembering. Cambridge: Cambridge Univ Press; 1932. [Google Scholar]
- 8.Squire LR. Biological foundations of accuracy and inaccuracy in memory. In: Schacter DL, Coyle JT, Fischbach GD, Mesulam MM, Sullivan LE, editors. How minds, brains, and societies reconstruct the past. Cambridge: Havard Univ Press; 1995. [Google Scholar]
- 9.Roediger HL, III, McDermott KB. Creating false memories: Remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21(4):803–814. [Google Scholar]
- 10.Loftus EF. Make-believe memories. Am Psychol. 2003;58(11):867–873. doi: 10.1037/0003-066X.58.11.867. [DOI] [PubMed] [Google Scholar]
- 11.Gallo DA. Associative Illusions of Memory: False Memory Research in DRM and Related Tasks. New York: Psychology Press; 2006. [Google Scholar]
- 12.Schacter DL. Searching for Memory: The Brain, the Mind, and the Past. New York: Basic Books; 1996. [Google Scholar]
- 13.Loftus E. Eyewitness Testimony. Cambridge: Harvard Univ Press; 1979. [Google Scholar]
- 14.Garrett BL. Convicting the Innocent. Cambridge: Harvard Univ Press; 2011. [Google Scholar]
- 15.Zhu B, et al. Individual differences in false memory from misinformation: Cognitive factors. Memory. 2010;18(5):543–555. doi: 10.1080/09658211.2010.487051. [DOI] [PubMed] [Google Scholar]
- 16.Moscovitch M. Confabulation and the frontal systems: Strategic versus associative retrieval in neuropsychological theories of memory. In: Roediger HL, Craik FIM, editors. Varieties of Memory and Consciousness: Essays in Honour of Endel Tulving. Hillsdale, NJ: Lawrence Erlbaum; 1989. pp. 133–160. [Google Scholar]
- 17.Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: A case study. Neuropsychologia. 1996;34(8):793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 18.Schnider A. The Confabulating Mind. Oxford: Oxford Univ Press; 2008. [Google Scholar]
- 19.Goodman GS, et al. False memory for trauma-related Deese-Roediger-McDermott lists in adolescents and adults with histories of child sexual abuse. Dev Psychopathol. 2011;23(2):423–438. doi: 10.1017/S0954579411000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howe ML. The adaptive nature of memory and its illusions. Curr Dir Psychol Sci. 2011;20(5):312–315. [Google Scholar]
- 21.Newman EJ, Lindsay DS. False memories: What the hell are they for? Appl Cogn Psychol. 2009;23(8):1105–1121. [Google Scholar]
- 22.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schacter DL, Guerin SA, St Jacques PL. Memory distortion: An adaptive perspective. Trends Cogn Sci. 2011;15(10):467–474. doi: 10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. J Mem Lang. 1997;37(4):555–583. [Google Scholar]
- 25.Gallo DA. False memories and fantastic beliefs: 15 years of the DRM illusion. Mem Cognit. 2010;38(7):833–848. doi: 10.3758/MC.38.7.833. [DOI] [PubMed] [Google Scholar]
- 26.Hyman IE, Jr, Pentland J. The role of mental imagery in the creation of false childhood memories. J Mem Lang. 1996;35(2):101–117. [Google Scholar]
- 27.Garry M, Manning CG, Loftus E, Sherman J. Imagination inflation: Imagining a childhood event inflates confidence that it occurred. Psychon Bull Rev. 1996;3(2):208–214. doi: 10.3758/BF03212420. [DOI] [PubMed] [Google Scholar]
- 28.Mazzoni G, Memon A. Imagination can create false autobiographical memories. Psychol Sci. 2003;14(2):186–188. doi: 10.1046/j.1432-1327.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MK, Raye CL. Reality monitoring. Psychol Rev. 1981;88(1):67–85. [Google Scholar]
- 30.Goff LM, Roediger HL., 3rd Imagination inflation for action events: Repeated imaginings lead to illusory recollections. Mem Cognit. 1998;26(1):20–33. doi: 10.3758/bf03211367. [DOI] [PubMed] [Google Scholar]
- 31.Schacter DL. Adaptive constructive processes and the future of memory. Am Psychol. 2012;67(8):603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suddendorf T, Corballis MC (2007) The evolution of foresight: What is mental time travel, and is it unique to humans? The Behavioral and Brain Sciences 30(3):299–313; discussion 313–351. [DOI] [PubMed]
- 33.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 35.Szpunar KK. Episodic future thought: An emerging concept. Perspect Psychol Sci. 2010;5(2):142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- 36.Schacter DL, et al. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JL. Reconsolidation: Maintaining memory relevance. Trends Neurosci. 2009;32(8):413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjork RA. The updating of human memory. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol 12. New York: Academic; 1978. [Google Scholar]
- 39.Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17(5):766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- 40.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci USA. 1994;91(15):7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St Jacques PL, Schacter DL. Modifying memory: Selectively enhancing and updating personal memories for a museum tour by reactivating them. Psychol Sci. 2013;24(4):537–543. doi: 10.1177/0956797612457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabeza R, St Jacques PL. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Spaniol J, et al. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8-9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 45.St Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011;57(2):608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 47.Sederberg PB, Gershman SJ, Polyn SM, Norman KA. Human memory reconsolidation can be explained using the temporal context model. Psychon Bull Rev. 2011;18(3):455–468. doi: 10.3758/s13423-011-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aminoff E, Schacter DL, Bar M. The cortical underpinnings of context-based memory distortion. J Cogn Neurosci. 2008;20(12):2226–2237. doi: 10.1162/jocn.2008.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershman SJ, Schapiro AC, Hupbach A, Norman KA. Neural context reinstatement predicts memory misattribution. J Neurosci. 2013;33(20):8590–8595. doi: 10.1523/JNEUROSCI.0096-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez S, et al. Creating a false memory in the hippocampus. Science. 2013;341(6144):387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 51.Baym CL, Gonsalves BD. Comparison of neural activity that leads to true memories, false memories, and forgetting: An fMRI study of the misinformation effect. Cogn Affect Behav Neurosci. 2010;10(3):339–348. doi: 10.3758/CABN.10.3.339. [DOI] [PubMed] [Google Scholar]
- 52.Okado Y, Stark CE. Neural activity during encoding predicts false memories created by misinformation. Learn Mem. 2005;12(1):3–11. doi: 10.1101/lm.87605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutchess AH, Schacter DL. The neural correlates of gist-based true and false recognition. Neuroimage. 2012;59(4):3418–3426. doi: 10.1016/j.neuroimage.2011.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13(4):501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 56.Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: Brain substrates of long-term memory conformity. Science. 2011;333(6038):108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Kesteren MTR, Fernández G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci USA. 2010;107(16):7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Tse D, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 61.McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47(11):2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Gilboa A. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Stickgold R. Parsing the role of sleep in memory processing. Curr Opin Neurobiol. 2013;23(5):847–853. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14(3):381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 66.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn Mem. 2007;14(1-2):47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwabe L, Wolf OT. New episodic learning interferes with the reconsolidation of autobiographical memories. PLoS ONE. 2009;4(10):e7519. doi: 10.1371/journal.pone.0007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15(5):210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daselaar SM, et al. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol Rev. 2007;114(2):340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10(11):792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 73.Roediger HL, 3rd, Butler AC. The critical role of retrieval practice in long-term retention. Trends Cogn Sci. 2011;15(1):20–27. doi: 10.1016/j.tics.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci USA. 2011;108(33):13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Addis DR, Schacter DL. The hippocampus and imagining the future: Where do we stand? Front Hum Neurosci. 2011;5:173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson O, 3rd, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21(1):456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 78.Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14(1):5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- 79.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9(1):7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 80.Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Front Hum Neurosci. 2012;6:70. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan K, Sadanand A, Davachi L. Memory’s penumbra: Episodic memory decisions induce lingering mnemonic biases. Science. 2012;337(6093):485–487. doi: 10.1126/science.1221936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.St Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J Cogn Neurosci. 2011;23(6):1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 84.Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 85.Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48(1):211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Craik F, et al. In search of self: A positron emission tomography study. Psychol Sci. 1999;1(1):26–34. [Google Scholar]
- 87.Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychol Bull. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- 88.Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44(1):149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Rubin DC, Berntsen D. The frequency of voluntary and involuntary autobiographical memories across the life span. Mem Cognit. 2009;37(5):679–688. doi: 10.3758/37.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsh EJ. Retelling Is not the same as recalling: Implications for memory. Curr Dir Psychol Sci. 2007;16(1):16–20. [Google Scholar]
- 91.Hirst W, Echterhoff G. Remembering in conversations: The social sharing and reshaping of memories. Annu Rev Psychol. 2012;63:55–79. doi: 10.1146/annurev-psych-120710-100340. [DOI] [PubMed] [Google Scholar]
- 92.Schacter DL, Loftus EF. Memory and law: What can cognitive neuroscience contribute? Nat Neurosci. 2013;16(2):119–123. doi: 10.1038/nn.3294. [DOI] [PubMed] [Google Scholar]
- 93.Lacy JW, Stark CE. The neuroscience of memory: Implications for the courtroom. Nat Rev Neurosci. 2013;14(9):649–658. doi: 10.1038/nrn3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.