Significance
Short and damaged DNA is ubiquitous in most environments and can survive more than half a million years. We show that naturally competent environmental bacteria can take up such degraded DNA and incorporate it into their genomes, including DNA from a 43,000-y-old woolly mammoth bone. The process occurs as part of cellular DNA replication and may resemble the earliest forms of horizontal gene transfer. Our findings suggest that natural genetic exchange of DNA from dead and even extinct organisms to contemporary bacteria can take place over hundreds of thousands of years. Hence damaged and degraded DNA may be a previous unrecognized driver of bacterial evolution with implications for evolutionary theory.
Keywords: microbial evolution, horizontal gene transfer, DNA degradation, early life, anachronistic evolution
Abstract
DNA molecules are continuously released through decomposition of organic matter and are ubiquitous in most environments. Such DNA becomes fragmented and damaged (often <100 bp) and may persist in the environment for more than half a million years. Fragmented DNA is recognized as nutrient source for microbes, but not as potential substrate for bacterial evolution. Here, we show that fragmented DNA molecules (≥20 bp) that additionally may contain abasic sites, cross-links, or miscoding lesions are acquired by the environmental bacterium Acinetobacter baylyi through natural transformation. With uptake of DNA from a 43,000-y-old woolly mammoth bone, we further demonstrate that such natural transformation events include ancient DNA molecules. We find that the DNA recombination is RecA recombinase independent and is directly linked to DNA replication. We show that the adjacent nucleotide variations generated by uptake of short DNA fragments escape mismatch repair. Moreover, double-nucleotide polymorphisms appear more common among genomes of transformable than nontransformable bacteria. Our findings reveal that short and damaged, including truly ancient, DNA molecules, which are present in large quantities in the environment, can be acquired by bacteria through natural transformation. Our findings open for the possibility that natural genetic exchange can occur with DNA up to several hundreds of thousands years old.
DNA molecules are continuously released into the surroundings through decomposition of organic matter and are ubiquitous in most environments (1). DNA degradation is initiated at cell death by coreleased cellular nucleases and continued by microbes feeding on organic matter (1). Because fragmentation proceeds quickly, larger (gene-length) fragments are not expected to persist in the environment (1). Extracellular DNA is further modified by spontaneous biochemical, chemical, or physical processes, of which the most important are hydrolysis and oxidation (2). These result in apurinic sites (depurination) and in loss of amino groups at the base-residues (deamination). Depurination affects the stability of the DNA backbone and leads to nicks and single-strand overhangs of the DNA fragments (2). Consequently, most free DNA fragments in the environment are <100 bp in size (3–8). Deamination particularly affects cytosine, creating uracil residues that can lead to cytosine-to-thymine exchanges, which result in DNA sequencing errors (9). Other chemical modifications of the DNA backbone and base residues also occur, but normally at lower rates than those of depurination and cytosine deamination (10–13). Despite continuous degradation of free DNA, short fragments often persist for thousands of years and may survive for more than a million years in the environment given optimal preservation conditions (4, 14–17). However, physical disturbance of preservation conditions may lead to release of such environmental ancient DNA. For instance, estimates suggest that 859–14,500 tons of sedimentary DNA are released per year from rivers alone (SI Text).
Fragmented and chemically damaged DNA is recognized as an important microbial nutrient source but has so far not been considered to contribute to bacterial genome evolution (1, 18–20). We decided to assess whether bacterial cells can acquire such degraded DNA, including genetic signatures of the deep past, by horizontal gene transfer—a driving force in prokaryotic evolution (21–24). Horizontal gene transfer by natural transformation is a process by which cells take up free (donor) DNA from the environment and integrate it into their own genomes (25, 26). The process likely occurs in most environments and is well described for high–molecular-weight DNA (chromosomal fragments typically ≥10,000 bp). Taken-up homologous DNA is efficiently incorporated into the genome by RecA-mediated homologous recombination. Successful natural transformation has not been described for DNA shorter than 250 bp (19, 20, 23). In this study we investigate (i) experimentally, to what extent is short (down to 20 bp) and damaged DNA taken up by natural transformation and integrated into the genome of the model organism Acinetobacter baylyi, a Gram-negative soil bacterium (27, 28); and (ii) in silico, the effect such recombination events have in genome evolution of transformable species.
Results
Short DNA Fragments Can Be Substrates for Natural Transformation of Bacteria.
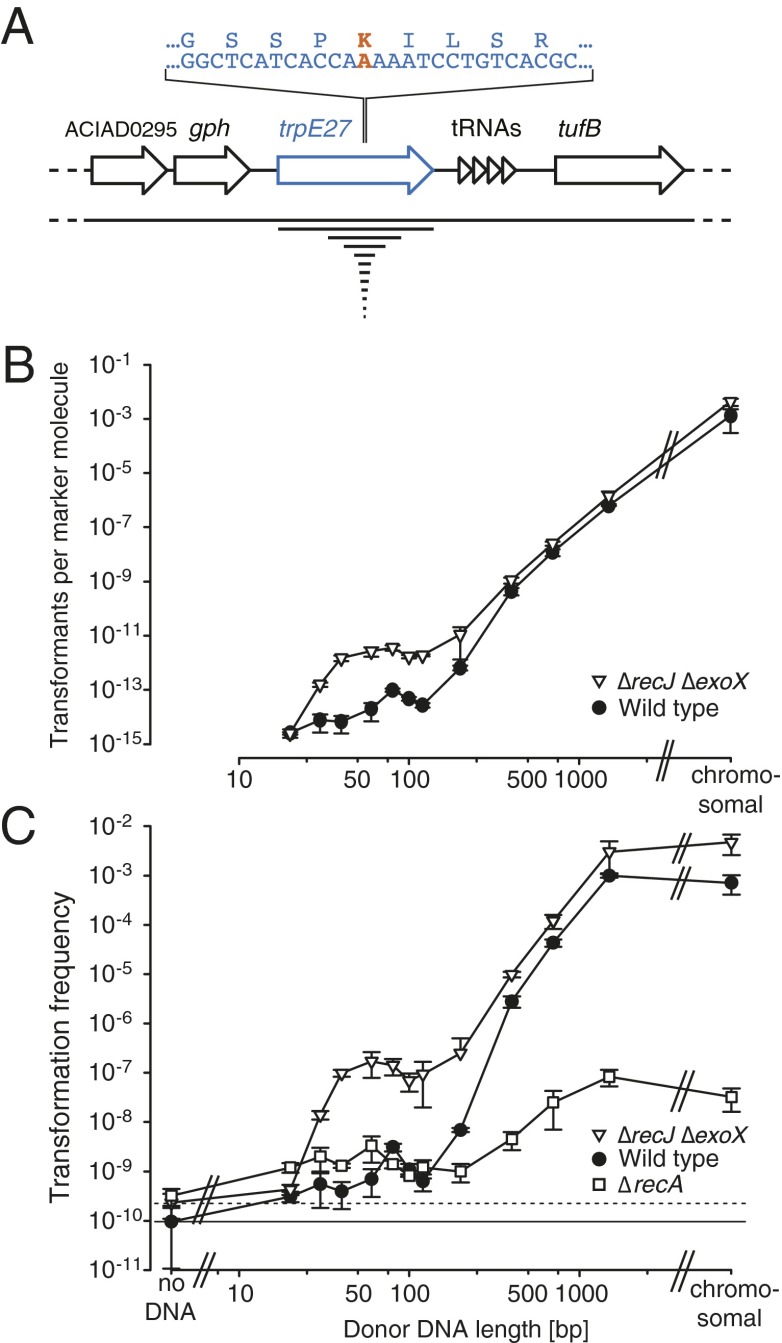
Naturally competent TrpE− A. baylyi cells were exposed to linear TrpE+ DNA ranging in size from 20 to ∼50,000 bp. TrpE encodes anthranilate synthase subunit I, which is part of the tryptophan biosynthesis pathway. Transformation was measured by the frequency of trpE+ cells that acquired the trpE+ single-nucleotide substitution (Fig. 1A). Transformation frequency decreased with fragment length (Fig. 1 B and C). DNA substrates ranging between 20 and 120 bp gave similar transformation frequencies, revealing that very short DNA molecules are capable of transforming bacteria. Using a RecA-deficient strain revealed that transformation by these short molecules occurred independently of the RecA recombinase (Fig. 1C). Experiments with a DNase knockout strain (∆recJ ∆exoX) (see SI Text, Table S1, and Fig. S1 for characterization of these A. baylyi mutants) revealed that single-strand DNA exonucleases are the main factors degrading short cytoplasmic DNA (Fig. 1C and SI Text), consistent with reports on limiting factors for artificial transformation by electroporation (18, 19). Experiments with different DNA concentrations showed that transformation by 60-bp substrates resulted in one-hit kinetics, as is the case with large molecules (SI Text and Fig. S2). Consequently, each DNA molecule has the same probability of transforming a cell independent of DNA concentration. In brief, transformation potential of DNA fragments varies with length, but not with concentration (Fig. 1B).
Fig. 1.
Natural transformation of A. baylyi by short DNA. (A) Chromosomal location of the single-nucleotide substitution marker (trpE27; SI Text) with sequence detail of the point mutation locus (bold) and proportional sizes of the donor DNA substrates (containing the wild-type residue G at the mutation locus). (B) Transformation efficiencies of wild type (circles; n = 3–7) and ∆recJ ∆exoX (triangles; n = 3–7; 26 for the 60-mer) calculated as transformants per marker-containing molecule with 100 ng/mL donor DNA of different lengths. (C) Transformation frequencies (mean with SD from three or more experiments) obtained with 100 ng/mL donor DNA of different lengths and calculated as transformants per recipient. Strains: wild type [circles; n = 3–7; frequencies were always higher than the mutational background (ANOVA: P = 0.033) determined in control experiments without DNA], ∆recA (squares; n = 3), and ∆recJ ∆exoX (triangles; n = 3–7; 26 for the 60-mer). The solid and dashed horizontal lines mark the background mutation frequency of the wild-type and ∆recJ ∆exoX strains, respectively. See also Table S1.
Natural Transformation Was Not Affected by the Presence of DNA Damages.
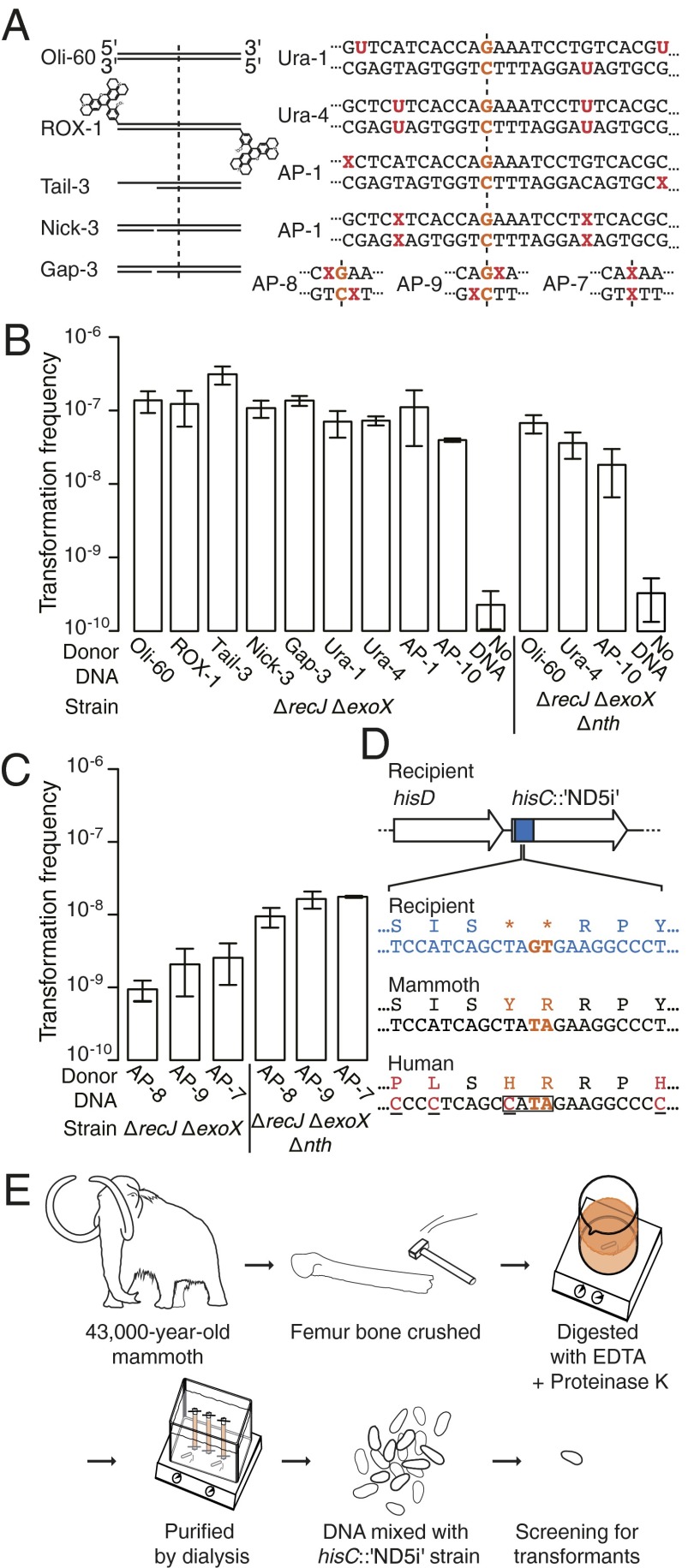
In a ∆recJ ∆exoX strain, damages such as nicks, gaps, or tails in DNA substrates of 40–60 bp had no substantial influence on transformation frequency (Fig. 2 A and B, and Fig. S3 A and B). Also, the presence of segments with no homology to the selective gene (terminal 20 bp of a 60-bp substrate) or chemical modifications of 5′-ends (carboxy-X-rhodamine and other adducts; nonphosphorylation) did not affect transformation frequency (Fig. 2 A and B, and Fig. S3 A and B), suggesting cellular processing of the 5′-end. Moreover, uracil residues or abasic (apurinic/apyrimidinic: AP) sites at trpE codon wobble positions did not substantially decrease transformation frequency even when located only 7 or 8 nt away from the marker (Fig. 2 A and B). AP sites directly neighboring or replacing the marker decreased but did not abolish transformation. The decrease was alleviated when a base excision repair (BER) endonuclease-deficient mutant (∆nth) was used (Fig. 2 A and C). This suggests that BER efficiently repaired these damages by nicking and gap repair (29). However, if not repaired by BER, the AP-7 DNA in the ∆nth strain had a fourfold decreased transformation frequency compared with the Oli-60 DNA (Fig. 2 B and C). This is consistent with insertion of a random nucleotide opposite the recombined DNA during the subsequent round of genome replication. Overall, the results indicate that the damages typically present in fragmented environmental DNA, including ancient DNA, have little influence on natural transformation in a ∆recJ ∆exoX background. Absence of RecJ and ExoX nucleases allows for elevated detection and robust quantification of rare transformation events while not affecting viability nor DNA repair functions of A. baylyi (Fig. 1 B and C; SI Text, Table S1, and Fig. S1).
Fig. 2.
Natural transformation by damage-containing DNA. (A) Sequence details of end modifications, internal lesions, uracil (U)- and AP site (X)-containing donor DNA substrates. Position of the marker nucleotide is indicated by the dashed line. (B and C) Transformation frequencies of the ∆recJ ∆exoX and ∆recJ ∆exoX ∆nth (BER endonuclease III-deficient) strains (n = 3–7; 26 for the Oli-60 with ∆recJ ∆exoX) with DNA substrates shown in A. Transformation frequencies were calculated as in Fig. 1C. (D) Chromosomal location and sequence detail of the detection construct for mammoth mtDNA (hisC::′ND5i′ with double stop codons and nucleotide variations in bold), and sequence details of mammoth and human mtDNA (additional nucleotide polymorphisms are underlined). In all transformants obtained in control experiments with human mtDNA substrates, the boxed sequence was present. See also SI Text. (E) Diagram of the ancient DNA experiment. Woolly mammoth DNA was used as donor DNA for natural transformation of the hisC::′ND5i′ strain.
Ancient DNA Can Be Taken Up and Integrated into the A. baylyi Genome by Natural Transformation.
To confirm that the results with modern fragmented and damaged DNA also apply to ancient DNA, we recovered 43,000-y-old DNA. Ancient bacterial DNA is extremely difficult to authenticate (30). Therefore, to exclude modern DNA contamination, we instead used DNA from an extinct animal—a woolly mammoth (Mammuthus primigenius) of which we had a large bone available. We used the mammoth DNA to naturally transform an A. baylyi ∆recJ ∆exoX strain carrying a DNA sequence similar to a defined stretch of mammoth mitochondrial DNA (Fig. 2 D and E). Using the 43,000-y-old DNA, resembling about 109 target molecules, we identified one isolate with a restored mammoth mtDNA sequence. In extensive control experiments without DNA, mutants resembling transformants were not encountered (for detailed descriptions, see SI Text). False-positive transformants (e.g., originating from contemporary human mtDNA; Fig. 2D) could be excluded by PCR screening and DNA sequencing (SI Text). This finding reveals that authentic ancient DNA can recombine with bacterial genomes through natural transformation. However, as ancient DNA molecules are fragmented to different sizes and may have undergone various types of damages, the experiment does not reveal the exact characteristics of the ancient donor DNA that recombined. To examine the general molecular process behind the recombination, we conducted a range of additional experiments.
Cellular Uptake of Short DNA Occurred by Natural Transformation, but Integration Occurred During DNA Replication of the Lagging Strand.
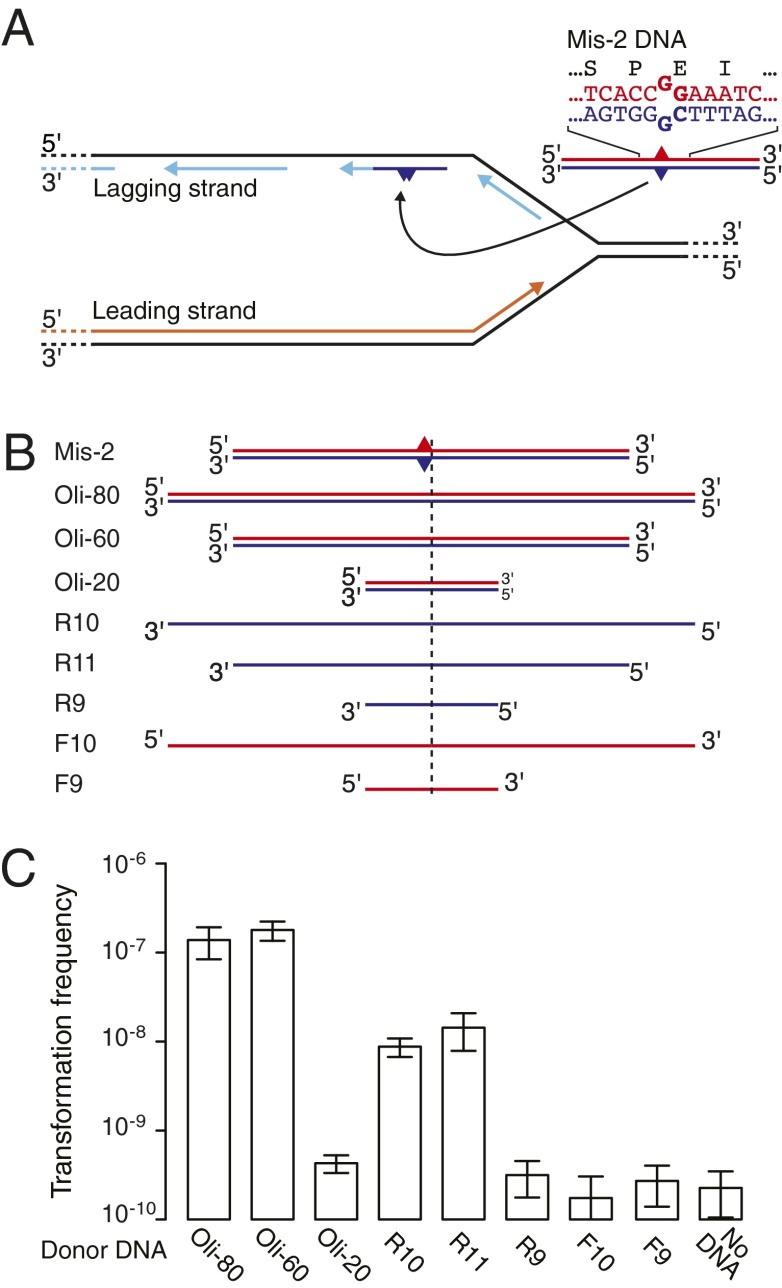
In type IV-pilus- and ComA-deficient mutants, natural transformation was abolished regardless of DNA size (SI Text), demonstrating that the same mechanism underlies the uptake of long and short DNA fragments into the bacterial cytoplasm (31, 32). We hypothesized that the RecA-independent recombination (Fig. 1C) with DNA fragments occurred by single-strand annealing of the incoming DNA strand with the discontinuously synthesized strand at replication forks (Fig. 3A), as previously described after artificial DNA exposure in Escherichia coli and Legionella pneumophila (18, 19). We confirmed this experimentally by using short mismatch-containing heteroduplex donor DNA and sequencing of transformants (Fig. 3A) and by using single-stranded donor DNA (Fig. 3 B and C). The data confirmed that transformation by short DNA was associated with DNA replication; in >97% of transformants, the DNA donor strand corresponded to the lagging strand of replication. The feasibility of short DNA molecules acting as primers in replication (Fig. 3A) is provided by trimming of the 5′-end of the incoming DNA during uptake/recombination (this study) and by cleavage of the 3′-end during uptake (33).
Fig. 3.
Recombination with very short DNA. (A and B) Donor DNA substrate illustrations: the top strand is depicted in red, and the bottom strand in blue. (A) Heteroduplex DNA with sequence detail and proposed integration mechanism. A bottom strand fragment can anneal with the discontinuously replicated strand and incorporate into the lagging strand at the replication fork. One hundred percent (n = 20) of wild-type transformants and >97% (n = 38) of ∆recJ ∆exoX transformants had the bottom strand integrated. (B) Schematic proportional sizes of double- and single-strand donor DNA substrates. Position of the marker nucleotide is indicated by the dashed line. (C) Transformation frequencies of the ∆recJ ∆exoX strain (n = 3–7; 26 for Oli-60) with various donor DNA substrates, and mutational background without DNA. Transformation frequencies were calculated as in Fig. 1C.
Double-Nucleotide Variations Escaped DNA Mismatch Repair.
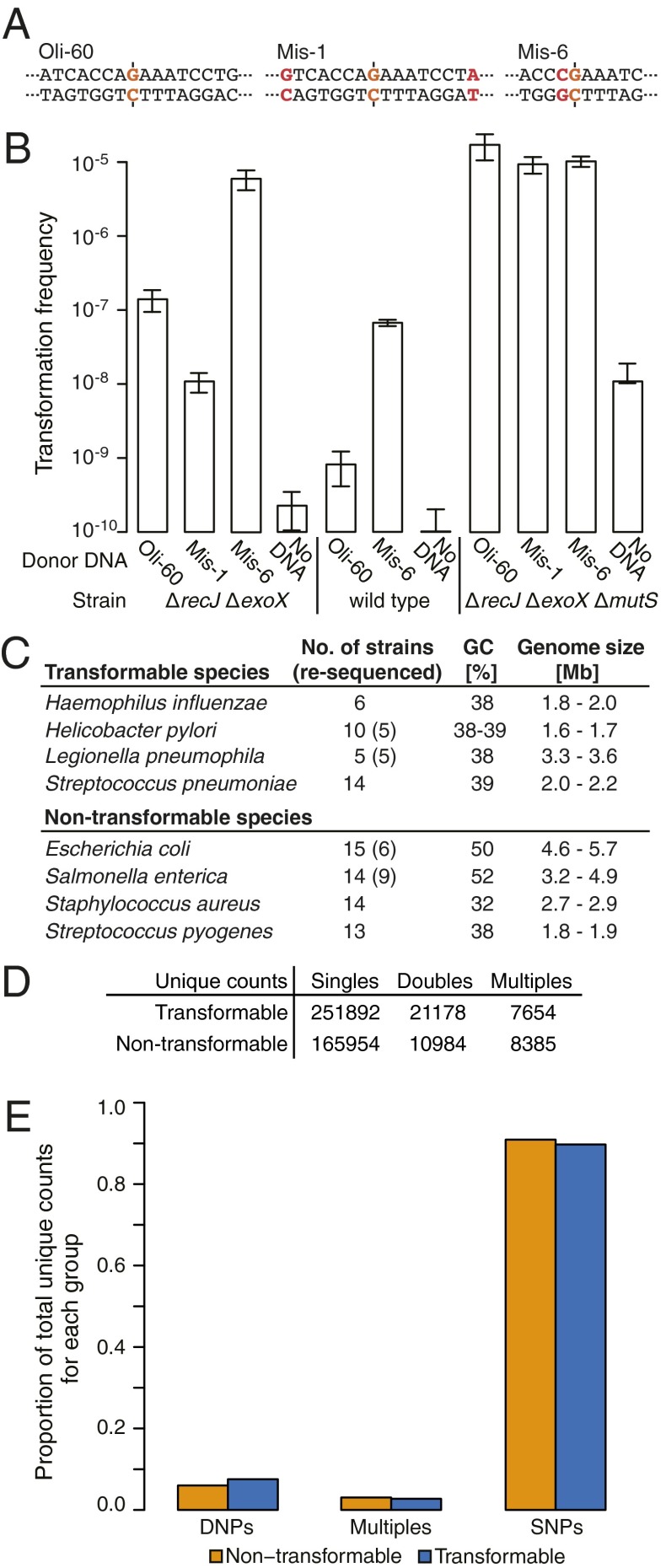
Because recombination with short DNA containing single-nucleotide variations (SNVs) results in mismatched base pairs, we investigated the influence of the DNA mismatch repair (MMR) on natural transformation. Transformation frequencies for short DNA molecules decreased when multiple nonadjacent SNVs were present, but were increased 50-fold for DNA molecules containing two neighboring SNVs, which we term double-nucleotide variations (DNVs) (Fig. 4 A and B). With an MMR-deficient (∆mutS) strain, transformation by all substrates was uniformly increased to the DNV level. These results demonstrate that MMR acts on mismatched recombination intermediates and that adjacent nucleotide mismatches escape this DNA repair mechanism. DNVs therefore have a higher likelihood of successful recombination, and if neutral or advantageous, they may accumulate as double-nucleotide polymorphisms (DNPs) in naturally transformable bacterial populations over time.
Fig. 4.
Double-nucleotide variants and polymorphism genome analysis. (A) Sequence details of nucleotide variation-containing donor DNA substrates (nucleotide exchanges in red). The dashed line indicates the position of the marker nucleotide. (B) Transformation frequencies of the ∆recJ ∆exoX, wild-type, and ∆recJ ∆exoX ∆mutS strains (n = 3–7; 26 for Oli-60 with ∆recJ ∆exoX) obtained with DNA substrates shown in A. Transformation frequencies were calculated as in Fig. 1C. (C–E) Distribution of DNPs, multiples, and SNPs in transformable and nontransformable bacterial species. (C) Bacterial genomes used for analysis. (D) Total polymorphism counts in transformable and nontransformable species. (E) Normalization of counts for direct visual comparison of the proportions of total polymorphisms of DNPs, multiples, and SNPs within nontransformable and transformable species, respectively.
DNPs Are More Frequent in Transformable than in Nontransformable Bacterial Species.
We investigated the prevalence of DNPs by collecting 91 GenBank genomes for transformable and nontransformable bacterial species (Fig. 4C, Table S2, and SI Text) and we resequenced 25 of the strains to test the genomes’ quality (SI Text and Table S3). Using a separate multiple alignment for each species, we counted the number of unique nucleotide polymorphisms for nontransformable and transformable species (Table S2). To ensure statistical independence of the counts, we define “unique” as a polymorphism that only occurs once in a multiple alignment column. We divided them into polymorphisms with no immediately adjacent nucleotide differences (SNPs), polymorphisms that occur as adjacent pairs (DNPs), and polymorphisms that occur in contiguous stretches of >2 nt (multiples: three to six adjacent polymorphisms; see SI Text for details). On the total counts of polymorphisms (Fig. 4D) we applied pairwise χ2 tests of homogeneity to statistically test whether the proportions of observed SNPs, DNPs, and multiples are the same in transformable and nontransformable bacteria (thereby accounting for difference in nucleotide diversity between the groups; see SI Text for further details). We find that the proportion of unique DNPs to SNPs is significantly higher in transformable than in nontransformable species (Fig. 4E; χ2 P value << 0.001). To investigate whether this pattern could be due to an overall higher rate of mutation in transformable species, we also assessed the proportion of DNPs relative to multiples. We find that nontransformable species have a higher proportion of multiples than DNPs (Fig. 4E; χ2 P value << 0.001). This shows that the excess of DNPs could not have been driven by a generally increased presence of multiples in transformable species. Consequently, these tests support the hypothesis of an increased proportion of DNPs in transformable bacterial species. It is pointed out that this analysis only concerns the proportions of observed polymorphisms and informs on neither the length of DNA recombination events, nor on the age of transforming DNA molecules. Despite using more nontransformable than transformable strains, we still count more polymorphisms in the latter group (Fig. 4D). To control for potential analysis artifacts arising from the greater number of nontransformable genomes, we created three reduced datasets that contained only five random strains of each species, thus equalizing the number of strains in each group of species. Multiple alignments, polymorphism counting, and statistical tests were conducted as for the complete dataset. The pattern of increased DNPs in transformable bacteria holds for all three reduced datasets with high statistical significance (all χ2 P values << 0.001). Furthermore, we always counted fewer total unique polymorphisms in the nontransformable species.
Discussion
Our findings reveal that short and damaged free DNA molecules, whether contemporary or ancient, remain available for natural genetic transformation of bacteria. The chemistry of damaged DNA does not in itself render the DNA biologically inactive. Importantly, and in contrast to transformation by longer DNA fragments, genomic incorporation of short and degraded DNA fragments is RecA independent, occurs at the replication fork, and does not require the DNA source and recipient cells to be present together in either space or time. Our analysis of DNPs in the genomes of naturally transformable bacteria match the nucleotide variation patterns we have detected and supports the experimental evidence provided here for a general genetic process in bacteria. Taken together, our observations suggest that natural transformation of short and degraded DNA takes place in nature. Consequently, our findings imply that highly fragmented DNA molecules may contribute to bacterial evolution. Further studies will reveal the broader impact of short fragment transformation in different species and environments.
The outcomes of natural transformation with short DNA fragments will differ substantially from those of transformation by longer DNA segments, such as those containing entire genes, operons, and mobile genetic elements (34). Natural transformation with very short DNA will, due to size constraints, lead to base pair substitutions resulting in modification or loss of resident gene functions, rather than acquisition and integration of entire genes. The outcome therefore resembles point mutational processes, and consequently short fragment length transformation may be a causal factor behind genetic polymorphisms so far attributed only to spontaneous mutation. Importantly, short DNA molecules do not encounter the same recombinational barriers to natural transformation as longer DNA fragments. The constraints on recombination due to conflicting gene order, function, and DNA similarity are minor. Although requirements for DNA similarity are still present, short similar DNA stretches are more likely to be conserved for a broader range of species. Also, the probability of random sequence similarity increases with shorter fragment size. Additionally, when DNA fragments containing abasic sites recombine through natural transformation, the outcome resembles a random mutagenic process with respect to the repair of the abasic nucleotide site. Consequently, the conditions for degradation of DNA in an environmental setting may influence the generation of genetic diversity in a bacterial community.
The recombination frequencies reported with short DNA are relatively low compared with transformation with high–molecular-weight DNA in laboratory models. However, the bacterial recombinational potential with DNA should be seen in the broader context of DNA exposure. Of the vast amounts of free DNA in the environment, the majority will exhibit various stages of degradation (3, 35, 36). Additionally, threshold levels for biologically significant gene transfer frequencies remains to be established for bacterial systems, and differences in transfer frequencies may have little or no impact on long-term evolutionary outcomes (37). The biological impact of DNA acquisitions will depend on a range of factors, including the fitness advantages they may provide (38, 39). It should be noted that in our study both short and long donor DNA molecules transform bacteria in compliance with a one-hit kinetic model (Fig. S2), as expected for DNA recombination during natural transformation. Consequently, the probability that a short DNA molecule transforms a bacterial cell depends on the absolute number of molecules available, and not the overall DNA concentration (Fig. 1B). For a DNV-containing 60-bp fragment about 8 × 1010 molecules (equivalent to 5 ng) yield one wild-type transformant within a short DNA exposure period (2 h). This efficiency is reproducible regardless of the DNA concentration applied or excess of nontransforming DNA present (Figs. S2 and S4).
The number of DNA molecules required is comparable to the number of cells needed (about 1 × 1010) for a specific point mutation to occur (Fig. 1A). For the probability of two adjacent point mutations to occur in one cell, the number of bacterial cells required is much higher, rendering the chance for a double mutation to spontaneously occur in an observable setting extremely low. In contrast, exposure to short DNA molecules can lead to dinucleotide exchanges at >8 orders of magnitude higher frequencies, because it occurs as a single recombination event and not two independent point mutations in the same cell. These considerations imply that natural transformation is a plausible way to acquire adjacent nucleotide polymorphisms such as DNPs, which is supported by the elevated ratio of DNPs over SNPs in naturally competent bacteria (Fig. 4 C–E). Taken together, the results suggest that natural transformation by fragmented and damaged DNA plays a role in shaping genomes of naturally competent bacteria.
Possible Implications and Perspectives
The ability of bacteria to recombine with fragmented and chemically damaged DNA may be of practical concern in some contexts. For example, two adjacent nucleotide changes can result in markedly increased antibiotic drug resistance in bacterial pathogens (40, 41). Biological waste disposal and decontamination practices, e.g., in hospitals where antibiotic-resistant infections are common, are focused on controlling organisms rather than free DNA molecules, and usually result in only partial DNA fragmentation (42). The possible impact of exposure to short DNA fragments on microbial evolution in hospital settings should be further studied.
Horizontal gene transfer is argued to have been a major evolutionary force in early life (43–45). In contrast to transformation by longer DNA sequences, which requires dedicated recombination functions, natural transformation by short and degraded DNA can occur during cellular replication. It is therefore tempting to speculate that this pathway of genetic recombination represents a plausible mechanistic model for the occurrence of passive genetic exchange in early single-celled populations, before the evolution of complex systems such as sexual reproduction or RecA-mediated homologous recombination. The genetic process described here suggests that early horizontal genetic transfer could have occurred in primitive cells after uptake of short DNA segments, which would have augmented evolutionary change. In addition to its main function as an important nutrient source, short DNA fragments may have contributed to exchange of beneficial mutations in early cells and continue to do so in extant microbial populations.
The potential for bacteria to take up degraded DNA, leading to single or a few nucleotide changes, adds another perspective to our understanding of the factors that drive microbial genome evolution. Models of population genetics and molecular evolution often rely on “memoryless” Markov processes, which predict the future genetic state of a reproducing population solely from its current state. Such models may not fully represent dynamical feedback between the diversity of environmental DNA and the replicating microbial gene pool. We propose that rates of molecular evolution in naturally transformable species may be influenced by the diversity of free environmental DNA. Furthermore, our findings suggest that bacterial recombination occurs with DNA fragments of considerable age, even from extinct microbial species. This suggests an additional, previously unrecognized contributor to molecular evolution. Recombination with DNA from temporally separated populations or species will bypass generations of cellular division and result in the transfer of genetic information over evolutionary time. We call this phenomenon “anachronistic evolution.”
Materials and Methods
Strains, Primers, and Plasmids.
The bacterial strains were constructed using standard procedures (see ref. 46 and SI Text). Primers are listed in Table S4. All strains are listed in Table S5. Details of strain construction and characterization are described in SI Text.
Natural Transformation Experiments.
Natural transformation experiments were performed as described in ref. 47 and SI Text, using as donor DNA chromosomal DNA, PCR products, or hybridized custom primers (including molecules containing 5′-adducts, uracil, or AP sites; Table S4). See SI Text for details on preparation of donor DNA. Unless stated otherwise, transformation frequencies are calculated as transformants per recipient and are given as means with SDs from three or more experiments obtained with 100 ng/mL donor DNA of different lengths. All experiments were done with ∼2.5 × 108 recipient cells per mL. Spontaneous (background) mutation frequencies were determined with “No DNA” natural transformation control experiments.
Genome Polymorphism Analysis.
The description in Results is supplemented with expanded explanations in SI Text.
DNA Sequencing.
Sanger sequencing followed standard protocols (SI Text). Illumina sequencing was performed at the Danish National High-Throughput DNA Sequencing Centre following regular protocols (SI Text).
Supplementary Material
Acknowledgments
Thanks to Christian Rohde and Nils Hülter for support with strain constructions. Thanks to all the people who kindly supplied DNA for resequencing of bacterial genomes (Table S3). We thank the people at the Danish National High-Throughput DNA Sequencing Centre for DNA sequencing support. Thanks to Christoph Tebbe for comments on the manuscript. We thank Anders Bjørk for information on sediment release estimates. Centre for GeoGenetics is funded by the Danish National Research Foundation (DNRF94) and the Faculty of Science, University of Copenhagen. The Microbiology, Molecular and Pharmaco-epidemiology research group at the Pharmacy Department, University of Tromsø, is funded by the Tromsø Research Foundation and the Research Council of Norway. The University of Oldenburg is funded by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequence data have been deposited with the European Nucleotide Archive, www.ebi.ac.uk/ena (accession no. PRJEB4698).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315278110/-/DCSupplemental.
References
- 1.Nielsen KM, Johnsen PJ, Bensasson D, Daffonchio D. Release and persistence of extracellular DNA in the environment. Environ Biosafety Res. 2007;6(1-2):37–53. doi: 10.1051/ebr:2007031. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 3.Pietramellara G, Ascher J, Borgogni F, Ceccherini GGMT, Nannipieri P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol Fertil Soils. 2009;45(3):219–235. [Google Scholar]
- 4.Willerslev E, et al. Long-term persistence of bacterial DNA. Curr Biol. 2004;14(1):R9–R10. doi: 10.1016/j.cub.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Willerslev E, Cooper A. Ancient DNA. Proc Biol Sci. 2005;272(1558):3–16. doi: 10.1098/rspb.2004.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poinar HN, et al. Metagenomics to paleogenomics: Large-scale sequencing of mammoth DNA. Science. 2006;311(5759):392–394. doi: 10.1126/science.1123360. [DOI] [PubMed] [Google Scholar]
- 7.Deagle BE, Eveson JP, Jarman SN. Quantification of damage in DNA recovered from highly degraded samples—a case study on DNA in faeces. Front Zool. 2006;3:11. doi: 10.1186/1742-9994-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allentoft ME, et al. The half-life of DNA in bone: Measuring decay kinetics in 158 dated fossils. Proc Biol Sci. 2012;279(1748):4724–4733. doi: 10.1098/rspb.2012.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brotherton P, et al. Novel high-resolution characterization of ancient DNA reveals C > U-type base modification events as the sole cause of post mortem miscoding lesions. Nucleic Acids Res. 2007;35(17):5717–5728. doi: 10.1093/nar/gkm588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Höss M, Jaruga P, Zastawny TH, Dizdaroglu M, Pääbo S. DNA damage and DNA sequence retrieval from ancient tissues. Nucleic Acids Res. 1996;24(7):1304–1307. doi: 10.1093/nar/24.7.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs AW, et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proc Natl Acad Sci USA. 2007;104(37):14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause J, et al. A complete mtDNA genome of an early modern human from Kostenki, Russia. Curr Biol. 2010;20(3):231–236. doi: 10.1016/j.cub.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 13.Orlando L, et al. True single-molecule DNA sequencing of a pleistocene horse bone. Genome Res. 2011;21(10):1705–1719. doi: 10.1101/gr.122747.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300(5620):791–795. doi: 10.1126/science.1084114. [DOI] [PubMed] [Google Scholar]
- 15.Willerslev E, Hansen AJ, Poinar HN. Isolation of nucleic acids and cultures from fossil ice and permafrost. Trends Ecol Evol. 2004;19(3):141–147. doi: 10.1016/j.tree.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Willerslev E, et al. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science. 2007;317(5834):111–114. doi: 10.1126/science.1141758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlando L, et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature. 2013;499(7456):74–78. doi: 10.1038/nature12323. [DOI] [PubMed] [Google Scholar]
- 18.Dutra BE, Sutera VA, Jr, Lovett ST. RecA-independent recombination is efficient but limited by exonucleases. Proc Natl Acad Sci USA. 2007;104(1):216–221. doi: 10.1073/pnas.0608293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan A, Swanson MS. Oligonucleotides stimulate genomic alterations of Legionella pneumophila. Mol Microbiol. 2011;80(1):231–247. doi: 10.1111/j.1365-2958.2011.07573.x. [DOI] [PubMed] [Google Scholar]
- 20.Palmen R, Vosman B, Buijsman P, Breek CK, Hellingwerf KJ. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993;139(2):295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- 21.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405(6784):299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 22.Gogarten JP, Doolittle WF, Lawrence JG. Prokaryotic evolution in light of gene transfer. Mol Biol Evol. 2002;19(12):2226–2238. doi: 10.1093/oxfordjournals.molbev.a004046. [DOI] [PubMed] [Google Scholar]
- 23.Fraser C, Hanage WP, Spratt BG. Recombination and the nature of bacterial speciation. Science. 2007;315(5811):476–480. doi: 10.1126/science.1127573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koonin EV, Wolf YI. Genomics of bacteria and archaea: The emerging dynamic view of the prokaryotic world. Nucleic Acids Res. 2008;36(21):6688–6719. doi: 10.1093/nar/gkn668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58(3):563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3(9):711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 27.Juni E, Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum) J Bacteriol. 1969;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbe V, et al. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 2004;32(19):5766–5779. doi: 10.1093/nar/gkh910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeberg E, Eide L, Bjørås M. The base excision repair pathway. Trends Biochem Sci. 1995;20(10):391–397. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 30.Hebsgaard MB, Phillips MJ, Willerslev E. Geologically ancient DNA: Fact or artefact? Trends Microbiol. 2005;13(5):212–220. doi: 10.1016/j.tim.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich A, Hartsch T, Averhoff B. Natural transformation in mesophilic and thermophilic bacteria: Identification and characterization of novel, closely related competence genes in Acinetobacter sp. strain BD413 and Thermus thermophilus HB27. Appl Environ Microbiol. 2001;67(7):3140–3148. doi: 10.1128/AEM.67.7.3140-3148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzgar D, et al. Acinetobacter sp. ADP1: An ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 2004;32(19):5780–5790. doi: 10.1093/nar/gkh881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries J, Wackernagel W. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc Natl Acad Sci USA. 2002;99(4):2094–2099. doi: 10.1073/pnas.042263399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domingues S, et al. Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog. 2012;8(8):e1002837. doi: 10.1371/journal.ppat.1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corinaldesi C, Beolchini F, Dell’Anno A. Damage and degradation rates of extracellular DNA in marine sediments: Implications for the preservation of gene sequences. Mol Ecol. 2008;17(17):3939–3951. doi: 10.1111/j.1365-294X.2008.03880.x. [DOI] [PubMed] [Google Scholar]
- 36.Dell’Anno A, Danovaro R. Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science. 2005;309(5744):2179. doi: 10.1126/science.1117475. [DOI] [PubMed] [Google Scholar]
- 37.Pettersen AK, et al. Modeling suggests frequency estimates are not informative for predicting the long-term effect of horizontal gene transfer in bacteria. Environ Biosafety Res. 2005;4(4):223–233. doi: 10.1051/ebr:2006008. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen KM, Townsend JP. Monitoring and modeling horizontal gene transfer. Nat Biotechnol. 2004;22(9):1110–1114. doi: 10.1038/nbt1006. [DOI] [PubMed] [Google Scholar]
- 39.Townsend JP, Bøhn T, Nielsen KM. Assessing the probability of detection of horizontal gene transfer events in bacterial populations. Front Microbiol. 2012;3:27. doi: 10.3389/fmicb.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreedharan S, Oram M, Jensen B, Peterson LR, Fisher LM. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: Close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casin I, Hanau-Berçot B, Podglajen I, Vahaboglu H, Collatz E. Salmonella enterica serovar Typhimurium bla(PER-1)-carrying plasmid pSTI1 encodes an extended-spectrum aminoglycoside 6′-N-acetyltransferase of type Ib. Antimicrob Agents Chemother. 2003;47(2):697–703. doi: 10.1128/AAC.47.2.697-703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gryson N. Effect of food processing on plant DNA degradation and PCR-based GMO analysis: A review. Anal Bioanal Chem. 2010;396(6):2003–2022. doi: 10.1007/s00216-009-3343-2. [DOI] [PubMed] [Google Scholar]
- 43.Woese CR. On the evolution of cells. Proc Natl Acad Sci USA. 2002;99(13):8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vetsigian K, Woese C, Goldenfeld N. Collective evolution and the genetic code. Proc Natl Acad Sci USA. 2006;103(28):10696–10701. doi: 10.1073/pnas.0603780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David LA, Alm EJ. Rapid evolutionary innovation during an Archaean genetic expansion. Nature. 2011;469(7328):93–96. doi: 10.1038/nature09649. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 47.Harms K, Schön V, Kickstein E, Wackernagel W. The RecJ DNase strongly suppresses genomic integration of short but not long foreign DNA fragments by homology-facilitated illegitimate recombination during transformation of Acinetobacter baylyi. Mol Microbiol. 2007;64(3):691–702. doi: 10.1111/j.1365-2958.2007.05692.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.