Significance
Bromo and extraterminal (BET) proteins have diverse roles in regulating tissue-specific transcriptional programs, raising safety concerns for their inhibition and suggesting that targeting of specific isoforms or even specific domains within this subfamily is important. We report the discovery and characterization of RVX-208 as a domain-selective inhibitor of BETs and provide a potential mechanism of action of a clinical compound that was identified based on phenotypic screens.
Keywords: small molecule inhibitor, epigenetics, microarray, ApoA1
Abstract
Bromodomains have emerged as attractive candidates for the development of inhibitors targeting gene transcription. Inhibitors of the bromo and extraterminal (BET) family recently showed promising activity in diverse disease models. However, the pleiotropic nature of BET proteins regulating tissue-specific transcription has raised safety concerns and suggested that attempts should be made for domain-specific targeting. Here, we report that RVX-208, a compound currently in phase II clinical trials, is a BET bromodomain inhibitor specific for second bromodomains (BD2s). Cocrystal structures revealed binding modes of RVX-208 and its synthetic precursor, and fluorescent recovery after photobleaching demonstrated that RVX-208 displaces BET proteins from chromatin. However, gene-expression data showed that BD2 inhibition only modestly affects BET-dependent gene transcription. Our data demonstrate the feasibility of specific targeting within the BET family resulting in different transcriptional outcomes and highlight the importance of BD1 in transcriptional regulation.
Bromodomains (BRDs) are protein-interaction modules that are selectively recruited to ε-N-acetylated lysine-containing sequences. BRDs are present in 46 diverse, mostly nuclear proteins functioning as effector domains of transcriptional regulators, chromatin modulators, and chromatin-modifying enzymes (1). BRD-containing proteins have been implicated in the development of many diverse diseases, and the architecture of their acetyl-lysine binding pocket makes them attractive targets for the development of potent and specific inhibitors (2, 3). All BRD modules share a conserved fold comprising a left-handed helical bundle creating a deep, largely hydrophobic and aromatic binding pocket for the specific recognition of peptide sequences containing one or more ε-N-acetylated lysine residues (1, 4–6).
In particular the bromo and extraterminal (BET) proteins, which comprise four members in human (BRD2, BRD3, BRD4, and the testis-specific BRDT), recently received a lot of attention after highly potent and cell-active pan-BET inhibitors were developed (7–10). BETs are transcriptional regulators that control expression of genes that play key regulatory roles in cellular proliferation, cell cycle progression, and apoptosis (11, 12). Dysfunction of BET proteins has been associated with the development of aggressive tumors, such as NUT midline carcinoma (NMC). In NMC, the N-terminal bromodomains of BRD3 or BRD4 are fused in frame with the testis-specific protein NUT (nuclear protein in testis), giving rise to an incurable fatal subtype of squamous carcinoma and in some cases tumors of other tissue origin (13). Importantly, BETs play a critical role in tumorigenesis also outside NMCs by driving the expression of genes that are essential for tumor growth and survival, such as c-Myc (14) and Aurora B (15). The potent pan-BET inhibitors (+)-JQ1 and GSK1210151A (I-BET151) have exhibited significant antitumor activity in murine models of NUT midline carcinoma (7), multiple myeloma (16), acute myeloid and mixed lineage leukemia (17), lung cancer (18), and glioblastoma (19).
BET family members play an essential role in diverse cellular processes, including general transcriptional elongation (20, 21), replication (22), hematopoiesis (23), adipogenesis (24, 25), and spermatogenesis (26), suggesting that drug discovery efforts should explore isoform, or even domain-specific targeting, to avoid adverse effects of prolonged pan-BET inhibition during treatment in different tissues.
The quinazolone RVX-208 (Fig. 1A) has been developed by Resverlogix Corporation for the treatment of cardiovascular diseases associated with atherosclerosis (27, 28) and has more recently entered clinical studies on Alzheimer’s disease (29). RVX-208 is a derivative of the plant polyphenol resveratrol (3,4',5-trihydroxy-transstilbene) that leads to an increase of plasma levels of the high-density lipid protein ApoA1. Increasing ApoA1 levels has emerged as a promising approach for the treatment of atherosclerosis (30), and recent phase IIb clinical trial data using RVX-208 as an ApoA1 modulator have been encouraging (28). ApoA1 expression is regulated by BET proteins, and chemical inhibition of BET bromodomains has been associated with ApoA1 up-regulation on transcriptional and protein levels (9, 31, 32). As a consequence, a similar mode of action has also been suggested for RVX-208, but no data characterizing the RVX-208/BET interaction have been published so far. The promising clinical outcome of RVX-208 trials and the presumed function of RVX-208 as a BRD inhibitor prompted us to study its role regulating BET-dependent transcription. Interestingly, we found that RVX-208 is specific for BET bromodomains and showed preferred binding to their second bromodomains (BD2s). Highest selectivity was observed for BD2 of BRD2 (23-fold) and BRD3 (21-fold). RVX-208 displaces BRD3 from chromatin at higher concentrations, and inhibition of BET bromodomains in the liver hepatocellular carcinoma cell line HepG2 resulted in weak regulation of only a subset of BET target genes. The study suggests that first and second BDs of BET family members can be selectively targeted despite high levels of sequence homology resulting in distinct transcriptional outcomes.
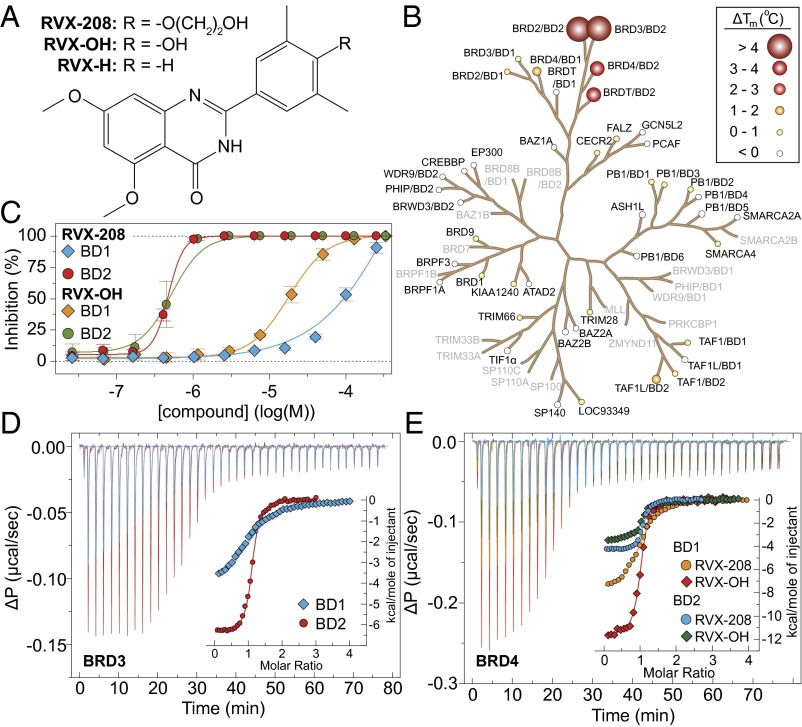
Fig. 1.
In vitro selectivity profile of RVX-208. (A) Structure of the inhibitor RVX-208 and its precursor RVX-OH. (B) Selectivity of RVX-208 within the human bromodomain family determined using a thermal shift assay. Temperature shifts (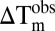 in °C, at 10 μM compound concentration) are shown as spheres as indicated in the Inset. Screened proteins are shown in bold. (C) Competitive displacement of a tetra-acetylated histone H4 peptide (H41–20K5ac/K8ac/K12ac/K16ac) from BD1 and BD2 of BRD3 using RVX-208 or RVX-OH (as indicated in the Inset) in a bead-based proximity assay (ALPHA assay). (D) Isothermal titration calorimetry (ITC) binding study. Data collected against the bromodomains of BRD3, showing raw injection heats for titrations of protein (BD1 or BD2) into compound. The Inset shows the normalized binding enthalpies corrected for the heat of protein dilution as a function of binding site saturation (symbols as indicated in the figure). Solid lines represent a nonlinear least squares fit using a single-site binding model. (E) Isothermal titration calorimetry (ITC) evaluation of RVX-208 and RVX-OH against the bromodomains of BRD4. Data have been corrected and displayed as described in D. All ITC titrations were carried out in 50 mM Hepes, pH 7.5 (at 25 °C), 150 mM NaCl and 15 °C while stirring at 1,000 rpm.
in °C, at 10 μM compound concentration) are shown as spheres as indicated in the Inset. Screened proteins are shown in bold. (C) Competitive displacement of a tetra-acetylated histone H4 peptide (H41–20K5ac/K8ac/K12ac/K16ac) from BD1 and BD2 of BRD3 using RVX-208 or RVX-OH (as indicated in the Inset) in a bead-based proximity assay (ALPHA assay). (D) Isothermal titration calorimetry (ITC) binding study. Data collected against the bromodomains of BRD3, showing raw injection heats for titrations of protein (BD1 or BD2) into compound. The Inset shows the normalized binding enthalpies corrected for the heat of protein dilution as a function of binding site saturation (symbols as indicated in the figure). Solid lines represent a nonlinear least squares fit using a single-site binding model. (E) Isothermal titration calorimetry (ITC) evaluation of RVX-208 and RVX-OH against the bromodomains of BRD4. Data have been corrected and displayed as described in D. All ITC titrations were carried out in 50 mM Hepes, pH 7.5 (at 25 °C), 150 mM NaCl and 15 °C while stirring at 1,000 rpm.
Results
Human BET proteins have a modular architecture comprising two N-terminal BRD modules, an extraterminal (ET) domain, and a C-terminal motif (BRD4 and BRDT only) (Fig. S1A). Their two highly conserved BRD modules share a high degree of sequence homology. Sequence homology is most pronounced comparing all four BD1s or BD2s, respectively, resulting in clustering of these interaction modules in sequence- and structure-based phylogenetic trees (1). Interestingly, sequence comparisons revealed three residue positions in close proximity to the acetyl-lysine peptide binding site that differ between BD1 and BD2 domains: with the exception of BRDT, the residue position corresponding to BRD4/BD1 Q85 is a lysine residue in BD2s; the position corresponding to the BRD4/BD1 residue D144 is a histidine residue in all BET BD2s; the position corresponding to the BRD4/BD1 residue I146 is a valine residue in BD2s. The location of these residues suggested that these sequence variations may be explored for the development of inhibitors that specifically recognize one of the two BET BRDs (Fig. S1B).
The established role of RVX-208 up-regulating the BET-regulated HDL protein ApoA1 without associated antiproliferative effects prompted us to synthesize this compound following the synthetic route disclosed by Resverlogix Corporation [previously established by Hansen—examples no. 4 and no 7 (33)] with minor changes (Fig. S1C) and to study its interaction with human BRD proteins.
RVX-208 Is a Potent Inhibitor of Second BET Bromodomains.
To establish a selectivity profile for RVX-208, we used temperature-shift assays (ΔTm) carried out on 44 of the 61 human bromodomains and found that its effect on BRD temperature stabilization is limited to the BET subfamily. Interestingly, we observed significant stabilization of only second bromodomains (BD2s) of BET proteins (Fig. 1B and Table S1), suggesting that RVX-208 selectively targets BD2s. In contrast, the synthetic precursor RVX-OH, which lacks the hydroxylether substitution (Fig. 1A), also specifically interacted with BET family members, although the differences in ΔTm between BD1s and BD2s were diminished. In addition, RVX-OH showed a number of weak ΔTm shifts on BRD9, CECR2, and some other BRDs (Table S1). AlphaScreen assays carried out using BRD3, for which a large difference in ΔTm values was observed, confirmed the interactions and demonstrated competitive displacement of histone H4 acetyl-lysine containing peptides by RVX-208. The IC50 values derived from the AlphaScreen data were 87 ± 10 μM and 0.510 ± 0.041 μM for BD1 and BD2, respectively, thus about 170-fold selectivity (Fig. 1C). In agreement with ΔTm data, RVX-OH exhibited a smaller window of selectivity between the two BRD3 bromodomains (IC50, 11.4 ± 5.9 μM and 0.379 ± 0.101 μM for BD1 and BD2, respectively) (Fig. 1C). Isothermal titration calorimetry (ITC) led to determination of accurate binding constants in solution for both RVX-OH and RVX-208 for all eight BET bromodomains (Fig. 1 D and E and Tables S2 and S3). The interaction of RVX-208 with BET bromodomains is driven by large negative binding enthalpy changes resulting in a KD of 4.06 ± 0.16 μM for BD1 and more than 20-fold stronger binding to BD2 (0.194 ± 0.013 μM) in the case of BRD3. ITC data measured on other BET bromodomains confirmed BD2 selectivity of RVX-208 but resulted in less-pronounced differences in binding affinities. In contrast, RVX-OH showed KD values between 0.15 and 1.4 μM, with no significant differences in ligand affinity for the two BRDs of BRD3. The more pronounced differences observed in AlphaScreen assays might be due to differences in peptide affinity for BD1 and BD2 domains.
RVX-208 Presents a Template for BD2 Rearrangement upon Binding.
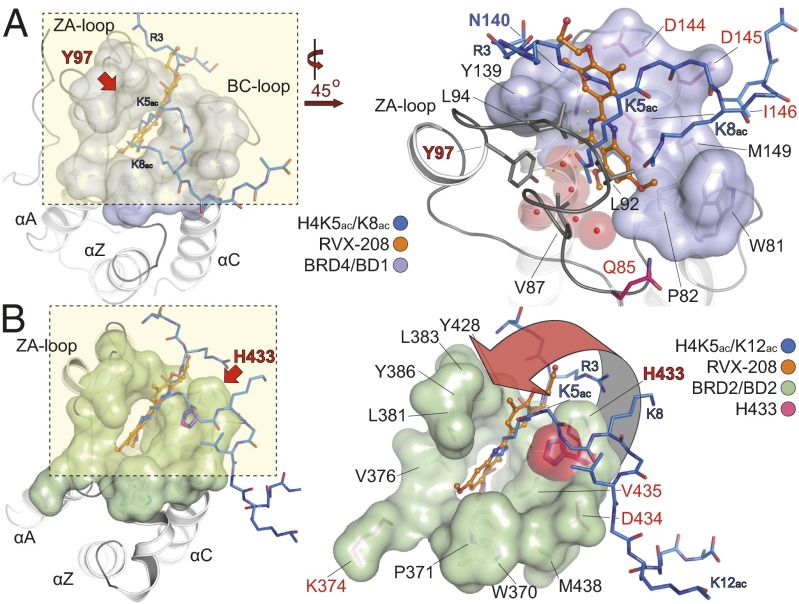
The observed selectivity profile for RVX-208 and the lack of selectivity of the closely related compound RVX-OH prompted us to determine the high-resolution cocrystal structures of RVX-208 and RVX-OH with representative BET bromodomains (Fig. 2 and Fig. S2). As predicted from our AlphaScreen data, RVX-208 bound to the acetyl-lysine binding pocket in a peptide-competitive manner. In the cocrystal structure of the first bromodomain of BRD4, the carbonyl oxygen and one of the nitrogen atoms of the quinazolinone ring system act as an acetyl-lysine mimetic moiety, forming a hydrogen bond with the conserved asparagine residue (N140), as well as a water-mediated hydrogen bond with Y97 (Fig. 2A and Fig. S2A). The hydroxy-ethylether moiety points out of the acetyl-lysine binding pocket and makes only a few contacts with the bromodomain surface. The WPF shelf, which contributes significantly to the affinity of phenyl-isoxazole and methyl-triazolo inhibitors (9, 34), is not occupied by the inhibitor but is occupied by an ethylene glycol solvent molecule. RVX-208 makes no direct interactions with residues unique to BD1 except to a water-mediated hydrogen bond with Q85. The binding mode of RVX-208 is largely conserved in BD2 domains, mimicking that of a histone substrate with the ligand occupying the entire channel used by a single acetyl-lysine. However, the BD2 unique residue H433 in BRD2 flips into the acetyl-lysine binding site packing against the phenyl ring of the inhibitor, providing a possible explanation for the tighter affinity for BD2 domains (Fig. 2B and Fig. S2B). Interestingly, the RVX-208 binding mode is not conserved in RVX-OH complexe with the BD1 of BRD4 (Fig. S2C). In this cocrystal structure, the ligand’s free hydroxyl group from the phenyl ring system acts as an acetyl-lysine mimetic moiety, forming a hydrogen bond with N140. Surprisingly, RVX-OH inverts its binding mode in the cocrystal complex with the BD2 of BRD2 assuming a similar interaction as observed in RVX-208 where the quinaxolinone function acts as the acetyl-lysine mimetic moiety (Fig. S2D and Fig. S3A). Both RVX-OH and RVX-208 are very well resolved in the high-resolution crystal structures (Fig. S3 B and C). The shift in binding mode of this inhibitor is also evident by the thermodynamic data of this interaction. The RVX-OH interaction with BD1s is characterized by a large negative binding enthalpy change for all BD1 interactions (∼ −7 to −10 kcal/mol) that is opposed by a negative entropy term (TΔS ∼ −2 kcal/mol). In contrast, interaction with BD2s gives rise to a modest negative enthalpy change (∼ −3.5 kcal/mol) associated with a favorable positive entropy change (TΔS ∼ +4 to +5 kcal/mol) (Table S3). However, the different binding modes of these two closely related inhibitors complicate the interpretation of the structural reasons for the observed selectivity of RVX-208. To further test the template's binding mode, we synthesized RVX-H, an analog lacking the Kac-mimetic free hydroxyl group and tested its ability to stabilize BET BRDs in thermal melt experiments. As expected, this scaffold significantly lost its affinity for BET BD1s (Table S1). We conclude that differences in binding affinity are due to small structural rearrangements and differences in binding mode that may include contributions of BD2 unique residues such as H433 in the case of the BD2 of BRD2, as well as possible differences in dynamic properties of BET bromodomains.
Fig. 2.
Cocrystal structures of RVX-208 and RVX-OH with the first and second bromdomains of human BET proteins. (A) Overview of RVX-208 binding onto BD1 of BRD4 (Left). A detail of the boxed area is shown on the Right (rotated 45° counterclockwise), highlighting the acetyl-lysine mimetic binding of the inhibitor, compared with a histone H4 di-acetyl peptide (H4K5ac/K8ac, PDB ID no. 3UVW, shown in stick representation, colored in blue), engaging the protein by directly interacting with the conserved asparagine (N140 in BRD4/BD1) in addition to a water-mediated hydrogen bond to Y97. Residues that differ between BD1 and BD2 are highlighted in red. (B) Comparison of the binding mode of RVX-208 and a histone H4 peptide onto BD2 of BRD2 (Left). The inhibitor engages the protein in the same orientation to that observed in the structure of a di-acetylated H4 peptide (H4K5ac/K12ac, PDB ID code 2E3K, colored as in A). A blow-up of the boxed area highlights the stabilization of the inhibitor complex, achieved by an inwards motion of H433 from the BC-loop region toward the front of the acetyl-lysine binding pocket, a unique feature of second site-BET bromodomains (Right). Residues that differ from the N-terminal BET BRDs are highlighted in red.
Selective Inhibition of BD2 Displaces BETs from Chromatin.
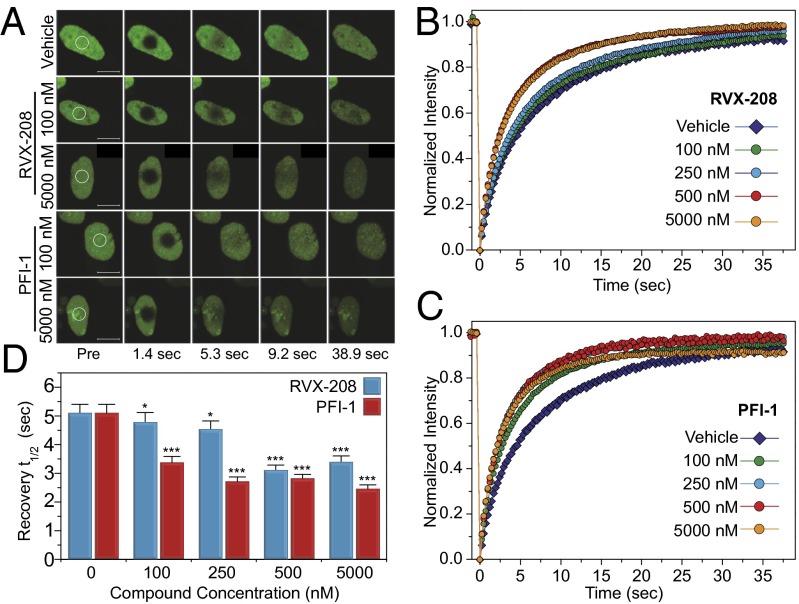
Given the weak interaction of RVX-208 with BET BD1s, we were interested in establishing whether this inhibitor can dissociate full-length BET proteins from acetylated chromatin. To this end, we established a FRAP (fluorescence recovery after photobleaching) assay using full-length human GFP-BRD3 transiently transfected into U2OS osteosarcoma cells. Exposure of these cells to the potent pan-BET inhibitor PFI-1 (10, 35) led to significant reduction of recovery times of the photobleached nuclear region, suggesting efficient dissociation of BRD3 from chromatin (Fig. 3), even at concentrations as low as 100 nM, given that the in vitro dissociation constant for this inhibitor is 80 nM for the BD1 of BRD3 and 76 nM for BD2 (35). At 250 nM, recovery times reached a plateau that did not decrease further at higher concentrations of the inhibitor. RVX-208 exhibited slightly weaker activity, displacing BRD3 at concentration of 500 nM and higher, thus demonstrating that both bromodomains are needed for efficient interactions with nucleosomes.
Fig. 3.
Fluorescence recovery after photobleaching (FRAP) evaluation of human BRD3 dissociation from chromatin. (A) Nuclei of DMSO treated (Top), RVX-208 treated (Middle), or PFI-1 treated (Bottom) cells. Target regions of photobleaching are indicated by a white circle. (Scale bars: 10 μm region.) (B and C) RVX-208 and PFI-1 accelerate fluorescence recovery in FRAP experiments performed in U2OS cells transfected with full-length GFP-BRD3 in a concentration-dependent manner. (B) RVX-208 can displace the protein from chromatin at concentrations above 500 nM whereas PFI-1 displaces the protein when used at 100 nM. (D) Quantitative comparison of time to half-maximal fluorescence recovery for FRAP studies using RVX-208 (blue bars) and PFI-1 (red bars) as a function of ligand concentration. Data represent the mean ± SEM (n = 15) and are annotated with P values as obtained from a two-tailed t test (*P < 0.05; ***P < 0.001).
BET Transcriptional Regulation Is Mainly Mediated by First Bromodomains in Liver.
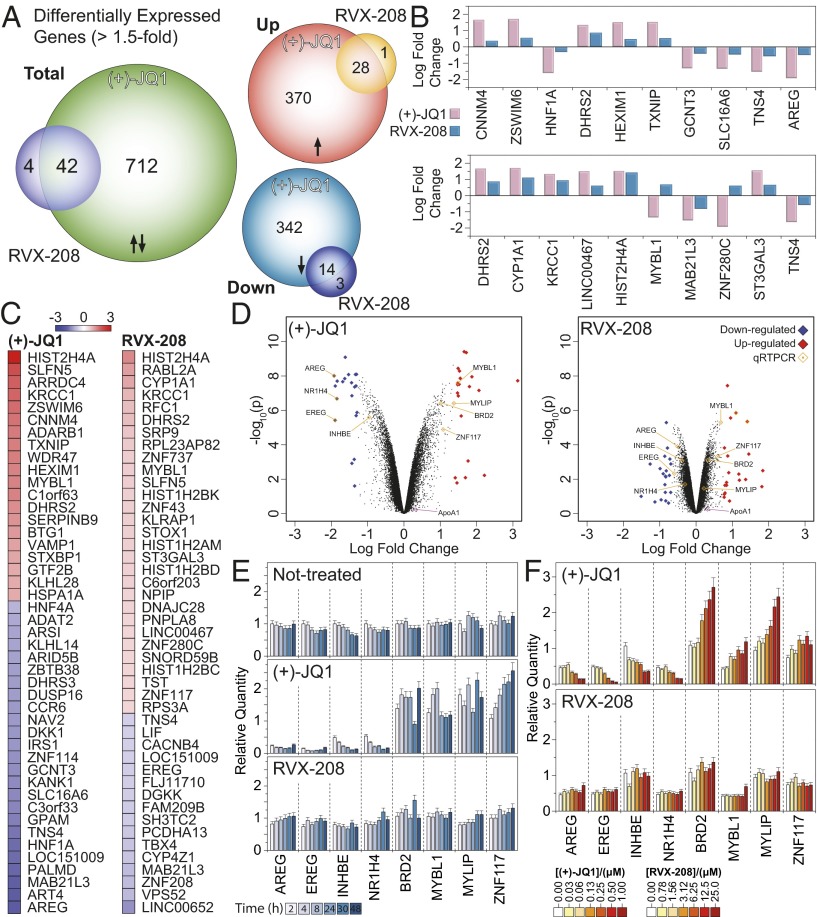
We next investigated the transcriptional regulation effect on gene expression in human liver carcinoma HepG2 cells by BET bromodomains, by either inhibiting both domains at the same time using the pan-BET inhibitor (+)-JQ1 (or JQ1 for simplicity) or RVX-208 seeking to inhibit mainly the second bromodomain (BD2). A microarray study of cells treated with either inhibitor for 4 h revealed large differences in gene expression, with the pan-BET inhibitor JQ1 strongly affecting transcription of genes with almost a 10-fold difference compared with the BD2-specific inhibitor RVX-208. Although inhibition of both BD1 and BD2 affected the gene expression of 754 genes within a 1.5-fold window, only 46 genes were affected by the inhibition of only BD2 using RVX-208 (Fig. 4A and Fig. S4A). Indeed, the top genes that seem to be affected by each inhibitor have small overlap, and the fold change in their expression is systematically higher when both domains are inhibited, using JQ1 (Fig. 4 B–D). This trend was also observed when we looked at the effect on the expression of the top 1,000 statistically significant genes (with an adjusted P value less than 0.05) for each inhibitor; genes that are strongly up-/down-regulated when both domains are inhibited by JQ1 are only weakly regulated when the second domain is inhibited by RVX-208 (Fig. S4B) whereas genes that are strongly up-/down-regulated when the second domain is inhibited by RVX-208 exhibit an even higher degree of regulation when both domains are inhibited by JQ1 (Fig. S4C). To further probe and verify the differences in gene expression initiated by BD1 or BD2, we selected a subset of four genes for qPCR studies that were strongly down-regulated (AREG, EREG, INHBE, and NR1H4) and four genes that were up-regulated (BRD2, MYBL1, MYLIP, and ZNF117). The effects of pan-BET versus BD2-selective inhibition were monitored in a time-dependent (Fig. 4E) and dose-dependent (Fig. 4F) manner. In the first instance, we observed a striking difference in gene expression when both BDs were inhibited by JQ1, with little or no effect when only BD2 was inhibited with RVX-208. This effect was verified by the dose experiment as well, despite the fact that we chose a 10-fold window between the two inhibitors in an effort to account for their differences in affinity for the two domains. Despite the lower concentrations used for JQ1 (0.3–1.0 μM), as opposed to higher doses of RVX-208 (0.78–25.0 μM), inhibition of the second domain was not sufficient to drive a strong transcriptional response. To establish that these effects are not inhibitor-dependent, we conducted a similar experiment and monitored gene expression by quantitative real-time PCR on a set of genes using either the pan-BET inhibitors (+)-JQ1 and PFI-1 in addition to the BD2 inhibitor RVX-208. We included also the precursor RVX-OH and used (−)-JQ1 as an inactive control compound. As expected, we observed strong up- or down-regulation when both BD1 and BD2 where inhibited by (+)-JQ1, PFI-1, or RVX-OH and a smaller effect when only BD2 was inhibited by RVX-208 (Fig. S4D) whereas the inactive variant, (−)-JQ1, had no significant effect on the expression of the selected genes. In agreement with the well-established role of JQ1 down-regulating c-MYC we found that pan-BET inhibition by JQ1, but not by RVX-208, led to transcriptional down-regulation of the c-MYC oncogene in liver cells (Fig. S4D). Interestingly ApoA1, a reported downstream target of BET proteins (9), was not affected by RVX-208 at the concentrations tested (27).
Fig. 4.
Microarray analysis of the transcriptional effect following 4-h treatment of HepG2 cells with 5 μM RVX-208 or 0.5 μM (+)-JQ1. (A) Venn diagram of statistically significant (P < 0.05) differential expression of genes that are up- or down-regulated with a 1.5-fold change (or greater) when cells are treated with compound. The transcriptional effect of (+)-JQ1 is 10x higher than that of RVX-208. (B) Log-fold change for the top 10 statistically significant (+)-JQ1 (Upper) or RVX-208 (Lower) regulated genes, highlighting the weaker effect of RVX-208 on transcription. (C) Top 45 up-/down-regulated genes in the case of (+)-JQ1 (Left) and RVX-208 (Right) treated cells. The color scale in the Inset represents log-fold change of expression compared with the untreated control. (D) Volcano plot of the top 1,000 genes that are up-/down-regulated in the case of (+)-JQ1 (Left) and RVX-208 (Right) after 4 h of treatment with 0.5 μM (+)-JQ1 or 5 μM RVX-208. Top genes are sorted by their fold-change and are highlighted and colored when they are up-regulated (red) or down-regulated (blue). Genes verified by qPCR are highlighted. (E) Quantitative real-time PCR on eight representative genes identified in A using gene-specific primers (Table S5). Cells were treated with 0.5 μM (+)-JQ1 or 5 μM RVX-208. Gene expression was monitored for 48 h, as indicated in the Inset. (F) Dose-dependent gene expression on the same set of eight genes tested in E, measured at 4 h. Even at the highest concentration, RVX-208 had only a modest transcriptional effect compared with (+)-JQ1. Error bars in E and F represent SD from triplicate experiments.
We found that ApoA1 RNA levels were not affected in a time- or dose-dependent manner whereas ApoA1 protein levels were only slightly affected by (+)-JQ1 treatment (Fig. S5 A–D). Using a luciferase reporter, we were also unable to observe a significant effect on ApoA1 transcription using a range of inhibitor concentrations (Fig. S5 E and F). Taken together, our data suggest that the transcriptional effect of BET proteins on their target genes is mainly dependent on both BDs, which seem to drive interactions with histones in the context of chromatin, suggesting that they are not functionally redundant. Indeed, deletion of only BD1 in BRDT is sufficient to impair spermatogenesis in mice (26), supporting the dominant role of BD1 in transcription control.
Discussion
BET proteins act as multidomain docking platforms that have a general role in transcription elongation by recruiting the positive transcription elongation factor b (P-TEFb) to acetylated chromatin (36, 37), as well as tissue-specific functions. The cell type-specific roles have been highlighted by recent reports on BRDT-dependent transcription programs that regulate spermatogenesis (37–39) and BRD3-dependent recruitment of GATA1 in hematopoietic cells regulating maturation of erythroid, megakaryocyte, and mast cell lineages (23, 40), as well as BRD2-dependent roles regulating differentiation of adipose tissue (25) and neurons (41). Application of BET inhibitors outside the area of oncology, given the pleiotropic nature of BET transcriptional regulation, strongly suggests a requirement for more selective targeting with respect to isoform and/or domains, to avoid adverse effects. Currently developed inhibitors are highly BET-specific but show no or little isoform or domain specificity (7–10).
In this work, we have established a first step toward isoform selective inhibition by characterizing interactions of RVX-208 with BET bromodomains, a template currently in phase I/II clinical trials for the treatment of cardiovascular diseases. Importantly, RVX-208 preferentially binds to the second bromodomain found on BET proteins, exhibiting selectivity over BD1 of up to 23-fold. With a KD of 195 nM against BD2 and 4 μM against BD1 of BRD3, we were able to show in vitro competitive displacement of a histone H4 tetra-acetylated peptide. Interestingly, cocrystal structures showed that RVX-208 induced a conformational switch upon binding to BD2, resulting in aromatic stacking accompanied by large entropic changes, as determined by isothermal titration calorimetry, which may explain in part its preference for BD2 over BD1 and suggests that dynamic properties of bromodomains need to be considered in the design of domain-specific inhibitors. BET BD1s and BD2s show a high degree of sequence similarity but differ in their recognition of acetylated target sequences (1). It is therefore likely that each BET bromodomain has a distinct function although the combined effect seems to be conferring a regulatory output in gene transcription. We observed in this study that targeting the BD2 had only a modest effect on transcription, an observation supported by previous studies of BRD3 that showed that its recruitment to acetylated sites on GATA1 is mediated by BD1 (40). Notably, deletion of the first bromodomain in BRDT in mice is sufficient to confer sterility by blocking BRDT-dependent sperm maturation (39). Unfortunately, no genetic knockout studies have been published that target only the BD2 in any of the BET family members, but biochemical studies and data presented here suggest a modulating role of BET function by BD2. In a recent study, Wu et al. showed that phosphorylation of flanking regions of BD2 in BRD4 triggers an intramolecular rearrangement that unmasks BD2 and directs it toward acetylated chromatin (42). This observation suggests that BET transcriptional regulators are controlled posttranslationally and that specific targeting, such as the one highlighted here toward BD2, may be able to perturb and modulate BET function in a context-dependent manner. However, further development of domain and isoform specific inhibitors will be necessary to unravel the exact role of BET bromodomains in gene transcription.
Materials and Methods
Cloning, Protein Expression, and Purification.
The BRD regions of human BRD3 and BRD4 were cloned/amplified as previously described (7). The full-length mouse BRD3 ortholog was used to reconstruct the human clone, by first mutating the C-terminal region and then cloning into pDONR223-hBRD3 according to Tillett and Neilan (43) and confirmed by sequencing. Protein expression and purification were carried out as previously described (7).
RVX-H, RVX-OH, and RVX-208 Synthesis.
The synthesis and characterization of these compounds were carried out following the synthetic route previously described (44) with minor changes, using the synthetic scheme highlighted in Fig. S1C.
Protein Stability Shift Assay (Tm Assay).
Thermal melting experiments were carried out using an Mx3005p Real-Time PCR machine as previously described (7). Temperature shifts (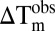 ) for three independent measurements per protein/compound are summarized in Table S1.
) for three independent measurements per protein/compound are summarized in Table S1.
Competitive Histone Displacement Assay (AlphaScreen Assay).
Experiments were run on a PHERAstar FS plate reader using an AlphaScreen 680 excitation/570 emission filter set. IC50 values were calculated in Prism 5 after normalization against corresponding DMSO controls. Assays were performed as previously described (7, 45), with minor modifications from the manufacturer’s protocol.
Isothermal Titration Calorimetry.
Experiments were carried out on an ITC200 microcalorimeter from MicroCal at 15 °C in 50 mM Hepes, pH 7.5 (at 25 °C), 150 mM NaCl by titrating protein into ligand solutions (reverse titrations), and data were corrected for protein heats of dilution and deconvoluted using the MicroCal Origin software as previously described (1, 7). Dissociation constants and thermodynamic parameters are listed in Tables S2 and S3.
Fluorescent Recovery After Photobleaching.
Fluorescent recovery after photobleaching (FRAP) studies were performed in U2OS cells transfected with mammalian overexpression constructs encoding GFP chimeras of BRD3, using a Zeiss LSM 710 scanhead coupled to an inverted Zeiss Axio Observer.Z1 microscope equipped with a high-numerical-aperture (N.A. 1.3) 40× oil immersion objective equipped with a heated chamber set to 37 °C, using a protocol modified from previous studies (7).
Crystallization, Data Collection, and Structure Refinement.
Cocrystallization and structure determination for complexes of BET BRDs with RVX-OH and RVX-208 were carried out following published procedures (7). Data collection and refinement statistics can be found in Table S4.
Cell Culture and RNA Extraction.
HepG2 cells were treated so that a final concentration of 0.1% DMSO was achieved. Cells were harvested, washed, and lysed in situ using standard protocols. Total RNA was extracted and prepared using RNeasy columns, and RNA was quantified and quality controlled using a Nanodrop spectrophotometer.
DNA Microarray Analysis.
An Affymetrix GeneChip WT Terminal Labeling and Controls Kit was used according to the manufacturer’s instructions and processed on an Affymetrix GeneChip Fluidics Station 450 and Scanner 3000. Data were processed in R using Bioconductor (46). Background correction and normalization were carried out using the Robust Multichip Array (RMA) (47). A linear model was applied (limma) followed by empirical Bayesian analysis, and genes were considered differentially expressed if the adjusted P value, calculated using the Benjamini–Hochberg method (48) to minimize false discovery rate, was less than 0.05 and the mean level of expression was greater than 1.5-fold.
Supplementary Material
Acknowledgments
We are grateful for support received by the Structural Genomics Consortium, a registered charity (number 1097737) that receives funds from the Canadian Institutes for Health Research, the Canada Foundation for Innovation, Genome Canada, GlaxoSmithKline, Pfizer, Eli Lilly, Takeda, AbbVie, the Novartis Research Foundation, the Ontario Ministry of Research and Innovation, and the Wellcome Trust (092809/Z/10/Z). P.F. and S.P. are supported by Wellcome Trust Career Development Fellowship (095751/Z/11/Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystal structures reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (4MR3–4MR6). Microarray data have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE51143).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310658110/-/DCSupplemental.
References
- 1.Filippakopoulos P, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidler LR, Brown N, Knapp S, Hoelder S. Druggability analysis and structural classification of bromodomain acetyl-lysine binding sites. J Med Chem. 2012;55(17):7346–7359. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhalluin C, et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399(6735):491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 5.Owen DJ, et al. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000;19(22):6141–6149. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morinière J, et al. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature. 2009;461(7264):664–668. doi: 10.1038/nature08397. [DOI] [PubMed] [Google Scholar]
- 7.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson MA, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicodeme E, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fish PV, et al. Identification of a chemical probe for bromo and extra C-terminal bromodomain inhibition through optimization of a fragment-derived hit. J Med Chem. 2012;55(22):9831–9837. doi: 10.1021/jm3010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama T, et al. A mammalian bromodomain protein, Brd4, interacts with replication factor C and inhibits progression to S phase. Mol Cell Biol. 2002;22(18):6509–6520. doi: 10.1128/MCB.22.18.6509-6520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20(23):4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 14.Rahl PB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141(3):432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You J, et al. Regulation of aurora B expression by the bromodomain protein Brd4. Mol Cell Biol. 2009;29(18):5094–5103. doi: 10.1128/MCB.00299-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuber J, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci USA. 2012;109(47):19408–19413. doi: 10.1073/pnas.1216363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Z, et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res. 2013;19(7):1748–1759. doi: 10.1158/1078-0432.CCR-12-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem. 2012;287(43):36609–36616. doi: 10.1074/jbc.M112.410746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, et al. Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J Biol Chem. 2012;287(51):43137–43155. doi: 10.1074/jbc.M112.413047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R, Liu M, Zhang K, Zhou Q. Isolation and functional characterization of P-TEFb-associated factors that control general and HIV-1 transcriptional elongation. Methods. 2011;53(1):85–90. doi: 10.1016/j.ymeth.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamsjaeger R, et al. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein Brd3. Mol Cell Biol. 2011;31(13):2632–2640. doi: 10.1128/MCB.05413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denis GV. Bromodomain coactivators in cancer, obesity, type 2 diabetes, and inflammation. Discov Med. 2010;10(55):489–499. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, et al. Brd2 disruption in mice causes severe obesity without Type 2 diabetes. Biochem J. 2010;425(1):71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development. 2007;134(19):3507–3515. doi: 10.1242/dev.004481. [DOI] [PubMed] [Google Scholar]
- 27.Bailey D, et al. RVX-208: A small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55(23):2580–2589. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls SJ, et al. ApoA-I induction as a potential cardioprotective strategy: Rationale for the SUSTAIN and ASSURE studies. Cardiovasc Drugs Ther. 2012;26(2):181–187. doi: 10.1007/s10557-012-6373-5. [DOI] [PubMed] [Google Scholar]
- 29.McNeill E. RVX-208, a stimulator of apolipoprotein AI gene expression for the treatment of cardiovascular diseases. Curr Opin Investig Drugs. 2010;11(3):357–364. [PubMed] [Google Scholar]
- 30.Joy TR. Novel HDL-based therapeutic agents. Pharmacol Ther. 2012;135(1):18–30. doi: 10.1016/j.pharmthera.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Chung CW, et al. Discovery and characterization of small molecule inhibitors of the BET family bromodomains. J Med Chem. 2011;54(11):3827–3838. doi: 10.1021/jm200108t. [DOI] [PubMed] [Google Scholar]
- 32.Mirguet O, et al. From ApoA1 upregulation to BET family bromodomain inhibition: Discovery of I-BET151. Bioorg Med Chem Lett. 2012;22(8):2963–2967. doi: 10.1016/j.bmcl.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 33. Hansen H (2008) Compounds for the prevention and treatment of cardiovascular diseases. International Patent WO 2008/092231 A1.
- 34.Bamborough P, et al. Fragment-based discovery of bromodomain inhibitors part 2: optimization of phenylisoxazole sulfonamides. J Med Chem. 2012;55(2):587–596. doi: 10.1021/jm201283q. [DOI] [PubMed] [Google Scholar]
- 35.Picaud S, et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET Bromodomains. Cancer Res. 2013;73(11):3336–3346. doi: 10.1158/0008-5472.CAN-12-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19(4):535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Gaucher J, et al. Bromodomain-dependent stage-specific male genome programming by Brdt. EMBO J. 2012;31(19):3809–3820. doi: 10.1038/emboj.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matzuk MM, et al. Small-molecule inhibition of BRDT for male contraception. Cell. 2012;150(4):673–684. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkovits BD, Wolgemuth DJ. The first bromodomain of the testis-specific double bromodomain protein Brdt is required for chromocenter organization that is modulated by genetic background. Dev Biol. 2011;360(2):358–368. doi: 10.1016/j.ydbio.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamonica JM, et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc Natl Acad Sci USA. 2011;108(22):E159–E168. doi: 10.1073/pnas.1102140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsume M, et al. Brd2 is required for cell cycle exit and neuronal differentiation through the E2F1 pathway in mouse neuroepithelial cells. Biochem Biophys Res Commun. 2012;425(4):762–768. doi: 10.1016/j.bbrc.2012.07.149. [DOI] [PubMed] [Google Scholar]
- 42.Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell. 2013;49(5):843–857. doi: 10.1016/j.molcel.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillett D, Neilan BA. Enzyme-free cloning: A rapid method to clone PCR products independent of vector restriction enzyme sites. Nucleic Acids Res. 1999;27(19):e26. doi: 10.1093/nar/27.19.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansen HC, Wagner GS, Attwell SC, McLure KG, Kulikowski EB (2010) Novel antiinflammatory agents. International Patent WO 2010/123975 A1.
- 45.Wigle TJ, et al. Screening for inhibitors of low-affinity epigenetic peptide-protein interactions: An AlphaScreen-based assay for antagonists of methyl-lysine binding proteins. J Biomol Screen. 2010;15(1):62–71. doi: 10.1177/1087057109352902. [DOI] [PubMed] [Google Scholar]
- 46.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.