Abstract
A vaccine adjuvant that can effectively promote cell-mediated immunity is currently not available. Because of the ability of a Candida skin test reagent injection to induce common wart regression, our group is using it as a novel adjuvant in a clinical trial of a peptide-based human papillomavirus therapeutic vaccine. The goal of this current study was to investigate the mechanisms of how Candida enhances the vaccine immune responses. Maturation effects on Langerhans cells, capacity to proliferate T-cells, expression of cytokines and pattern recognition receptors by Langerhans cells, and ability to induce Th1, Th2, and Th17 responses were investigated in healthy subjects. The vaccine, human papillomavirus peptides with Candida, demonstrated partial maturation effects on Langerhans cells indicated by significantly up-regulated CD40 (p=0.00007) and CD80 (p<0.00001) levels, and showed T-cell proliferative capacity (p<0.00001) when presented by Langerhans cells in vitro. Interestingly, the maturation effects were due to the peptides while Candida was responsible for the T-cell proliferation. The cytokine profile (IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-23Ap19, IFN-γ, and TNF-α) of Langerhans cells treated with the vaccine or Candida alone showed that IL-12p40 mRNA was most frequently induced, and IL-12p70 protein was detected in the supernatants. The presence of pattern recognition receptors known to associate with Candida albicans (DC-SIGN, dectin-1, dectin-2, galectin-3, mincle, mannose receptor, Toll-like receptors-1, 2, 4, 6, and 9) were demonstrated in all subjects. On the other hand, the induction of Th1 response demonstrated by IFN-γ secretion by CD4 cells stimulated with the vaccine or Candida pulsed Langerhans cells was demonstrated only in one subject. In summary, the Langerhans cell maturation effects of the vaccine were due to the peptides while the T-cell proliferative capacity was derived from Candida, and the most frequently induced cytokine was IL-12.
Keywords: Candida, human papillomavirus, adjuvant
1. Introduction
The most widely used adjuvant in approved human vaccines is an alum-based adjuvant that has been shown to elicit a predominantly Th2 immune response [1]. Therefore, the alum-based adjuvant would be useful in a vaccine designed to boost antibody responses, but not for a vaccine designed to stimulate cellular immune responses. Since successful clearance of human papillomavirus (HPV) infection is believed to be induced by cell-mediated immunity [2, 3], an adjuvant that would promote such an immunity is necessary, but not available.
Our group and others have shown that serial intra-lesional injections of common warts with skin testing reagents such as Candida, mumps, and/or Trichophyton can induce regression not only of treated warts but also of distant untreated warts [4-9]. In a Phase I clinical trial (NCT00569231), our group used Candin® (Allermed, San Diego, CA), a colorless extract of Candida albicans, to treat common warts. Resolution of treated warts occurred in 82% of the subjects, and anti-HPV T-cell responses were demonstrated [8]. Given that Candin is derived from C. albicans, it should contain numerous pathogen-associated molecular patterns (PAMPs). We hypothesized that Candin would be an effective vaccine adjuvant which would stimulate multiple pattern recognition receptors (PRRs) and induce innate as well as adaptive immunity.
Cervical cancer is almost always caused by high-risk HPV infection, and is the 2nd most common cancer among women in the world. Two very effective prophylactic HPV vaccines, Gardasil® (Merck, NJ, USA) and Cervarix® (GlaxoSmithKline, Middlesex, UK), are available, and they work by inducing high titers of neutralizing antibody [10-12]. However, they are not effective for women with pre-existing HPV infection [10, 12, 13]. Therefore, a therapeutic HPV vaccine that can be used for those already infected with HPV and/or have developed HPV-associated neoplasia is not available. Our group studied naturally induced immunity in women with HPV infection and/or cervical lesions, and have found that the ability to induce T-cell responses against E6, one of the oncoproteins of high-risk HPVs, is associated with HPV clearance and regression of cervical lesions [3, 14, 15]. Therefore, we designed an HPV therapeutic vaccine which consists of four HPV type 16 E6 peptides and Candin, and are conducting a Phase I clinical trial (NCT01653249).
In the current study, we examined the immune enhancing effects of Candin as a vaccine adjuvant. Surprisingly, the E6 peptides were responsible for the partial maturation of Langerhans cells (LCs) while Candin was responsible for the T-cell proliferative effects. The most commonly induced cytokine by the LCs was IL-12.
2. Materials and Methods
2.1 Generation of monocytes-derived LCs
Mononuclear cells were collected from healthy blood donors (n=10) by apheresis (Key Biologics, LLC, Memphis, TN). The subjects were numbered in a chronological order. Peripheral blood mononuclear cells (PBMCs) were purified using the ficoll gradient centrifugation method. Monocytes were negatively isolated from PBMC using Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA), and were converted to LCs using granulocyte-macrophage colony-stimulating factor, IL-4, and transforming growth factor β-1 as described by Fahey et al. [17]. The effectiveness of conversion to LCs was demonstrated by detecting CD1a (eBioscience, San Diego, CA), Langerin (Beckman-Coulter, Brea, CA), and E-cadherin (eBioscience) using FACS Fortessa (University of Arkansas for Medical Sciences Microbiology and Immunology Flow Cytometry Core Laboratory) and CellQuest Pro software (BD Biosciences, San Jose, CA) in selected experiments (Fig. 1). Sufficient number of cells were available from all subjects except for subject 1 in whom the LC maturation experiment could not be performed.
Fig. 1.
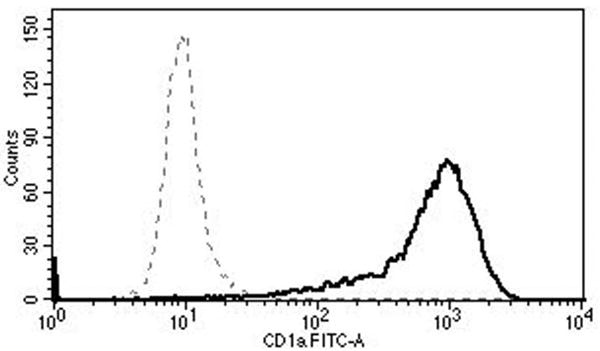
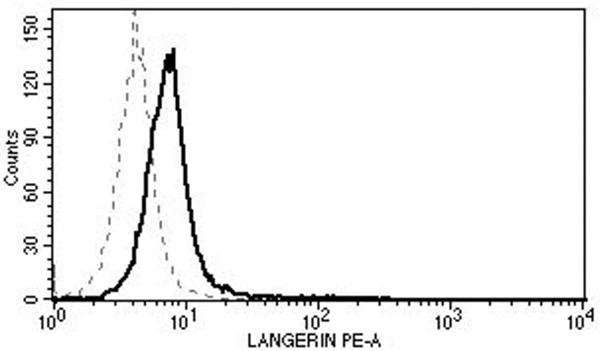
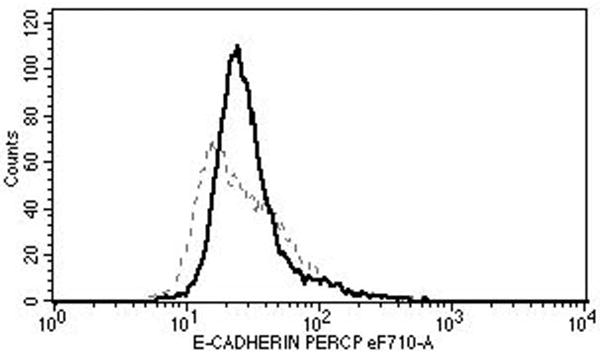
Surface expressions of CD1a (top), Langerin (middle), and E-cadherin (bottom) show successful conversion to LCs (solid lines). The dotted lines represent the relevant isotype controls.
2.2 Maturation analysis of LCs treated with Candin and/or HPV peptides
Candin was dialyzed before use to remove a small amount of solvent (0.4% phenol) using Slide-A-Lyzer G2 Dialysis Cassette (Thermo Scientific, Rockford, IL). LCs were prepared as described above, and one million LCs each were treated with Candin (150 μl/ml), four current good manufacturing practice-grade HPV16 E6 peptides [E6 1-45, E6 46-80, E6 81-115, and E6 116-158 (referred to as “peptides” hereafter); 10μg/ml/peptide; made by CPC Scientific, Sunnyvale, CA and vialed by Integrity Bio, Camarillo, CA], or Candin/“peptides”. Zymosan (10μg/ml, InvivoGen, San Diego, CA), a yeast cell wall particle containing many polysaccharides including β-glucan and mannan [18], was used as a positive control. After 48 hour incubation, cells were stained with anti-human CD40 phycoerythrin (PE)-Cy5.5, CD80 fluorescein isothiocyanate, CD86 PE-Cy5 and HLA-DR PE (eBioscience, San Diego, CA). Ten thousand events were acquired, and the data were analyzed using Flowjo software (BD Biosciences).
2.3 Analysis of T cell Proliferation induced by LCs treated with Candin and/or “peptides”
On day 7 of LCs conversion, CD3 T cells from the same subjects were negatively isolated from PBMCs using Pan T-Cell Isolation Kit II (Miltenyi Biotec). To remove CD25 regulatory T cells, human CD25 Antibody-Biotin (Miltenyi Biotec) was added. T cell proliferation assay was performed in 6 replicate wells by co-culturing T cells (1.5×106cells/ml) with autologous LCs (3×104cells/ml) in 100 μl of complete Yssel's media (Gemini Bioproducts Inc, Woodland, CA) containing 1% human serum in each well of a 96-well plate. Wells containing cells only (T-cells and LCs), cells and Candin (150μl/ml), cells and Candin/“peptides”, and cells and tetanus toxoid (500ng/ml, EMD Milipore, Billerica, MA) were set up. After 7 days of incubation, 10μl of alamarBlue (Life Technologies, Grand Island, NY) was used to replace the corresponding volume of media in each well, then the plate was incubated at 37°C for 6 hours. Fluorescence was measured (530nm excitation wavelength and 590nm emission wavelength) in media using BioTek Synergy-2 Multi Plate Reader (US BioTek, Seattle, WA).
2.4 Cytokine and PRR analyses by quantitative real-time PCR (qRT-PCR)
One million LCs each were treated with Candin (50 μl/ml, 100 μl/ml, and 150 μl/ml) with or without “peptides” (10μg/ml/peptide) at each Candin concentration. Zymosan was used as a positive control at 10ug/ml and media only as a negative control. Cells were harvested for RNA after 8 and 24 hours. RNA was extracted using RNeasy kit (Qiagen, Valencia, CA), and treated with DNase I (Promega, Madison, WI). cDNA synthesis was carried out using SuperScript III first-strand synthesis system (Life Technologies).
Quantitative PCR analysis was performed in duplicate for cytokines including IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-23Ap19, IFN-γ and TNF-α using an iQ-SYBR mix (Bio-Rad, Hercules, CA). In addition, expressions of PRRs (DC-SIGN, dectin-1, dectin-2, galectin-3, mincle, mannose receptor, TLR-1, TLR-2, TLR-4, TLR-6, and TLR-9) known to associate with C. albicans[19-28] were examined. The primers used to detect IL-12 were previously reported by Vernal et al. [29]. All other primers were designed using Beacon Design software (Bio-Rad, Table 1). The threshold cycles were normalized to a human housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase, and were calculated as fold change over untreated LCs at 8 hours. mRNA was considered to be detected when amplification of cDNA was demonstrated.
Table 1. Primers used for qRT-PCR.
| Description | Gene name | Accession no. | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|---|
| Interleukin 1 beta | hIL-1β | NM_000576.2 | CAG GGA CAG GAT ATG GAG CAA C | CAC GCA GGA CAG GTA CAG ATT C |
| Interleukin 6 (interferon, beta 2) | hIL-6 | NM_000600.3 | GTA GTG AGG AAC AAG CCA GAG C | GGC ATT TGT GGT TGG GTC AGG |
| Interleukin 8 | hIL-8 | NM_000584.3 | GAC CAC ACT GCG CCA ACA C | AAA CTT CTC CAC AAC CCT CTG C |
| Interleukin 10 | hIL-10 | NM_000572.2 | GGG TTG CCA AGC CTT GTC TG | CGC CGT AGC CTC AGC CTG |
| Interleukin 12B | hIL-12p40 | NM_002187.2 | CCC TGA CAT TCT GCG TTC A | AGG TCT TGT CCG TGA AGA CTC TA |
| Interleukin 23 alpha subunit p19 (IL23A) | hIL23A p19 | NM_016584.2 | AGT GTG GAG ATG GCT GTG ACC | GGG CTA TCA GGG AGC AGA GAA G |
| interferon, gamma | hIFN-γ | NM_000619.2 | TGT GGA GAC CAT CAA GGA AGA C | TGC TTT GCG TTG GAC ATT CAA G |
| Tumor Necrosis Factor alpha | hTNF-α | NM_000594.3 | GGG GTG GAG CTG AGA GAT AAC C | ACG GCG ATG CGG CTG ATG |
| DC-SIGN, CD 209 | hDCSIGN | NM_001144899.1 | TGC AGT CTT CCA GAA GTA ACC GCT | TGT TGG GCT CTC CTC TGT TCC AAT |
| C-type lectin domain family 7, member A (CLEC7A) | hDectin1 | NM_197947.2 | TGC TTG GTA ATA CTG GTG ATA G | GGT TGA CTG TGG TTC TCT T |
| C-type lectin domain family 6, member A (CLEC6A) | hDectin2 | NM_001007033 | AAC ACA GAA GCA GAG CAG AAT | TCC AGA AGA CTA TTG AAG CAC ATT |
| Lectin, galactoside-binding, soluble, 3 (LGAL3) | hGalectin3 | NM_001177388.1 | TGT GCC TTA TAA CCT GCC TTT GCC | TTC TGT TTG CAT TGG GCT TCA CCG |
| C-type lectin domain family 4, member E (CLEC4E) | hMincle | NM_014358.2 | TCA GAA TAC CGG TGT GGC CTT TCT | TGG TTA CAG CCT GTT TGG AGC TGA |
| Mannose receptor, C type2 | hMRC2 | NM_006039.4 | AGC AAC GTC ACC AAA GAA ACG CAG | AGA ACT GTG CCT CTG ACC ACT TCA |
| Toll-Like Receptor 1/6* | hTLR1 or TLR6 | NM_003263.3 or NM_006068.4 | ATG TGG CAG CTT TAG CAG CCT TTC | TCT GGA AGA AAT CAG CCG ATG GGT |
| Toll-Like Receptor 2 | hTLR2 | NM_003264 | TGC TGC CAT TCT CAT TCT | CAC TCC AGG TAG GTC TTG |
| Toll-Like Receptor 4 | hTLR4 | NM_138557 | CGT GCT GGT ATC ATC TTC AT | GGT AAG TGT TCC TGC TGA G |
| Toll-Like Receptor 9 | hTLR9 | NM_017442.3 | ATC TGC ACT TCT TCC AAG GCC TGA | AAG GCC AGG TAA TTG TCA CGG AGA |
| Glyceraldehyde-3-phosphate dehydrogenase | hGAPDH | NM_002046.4 | GGA CCT GAC CTG CCG TCT AG | GTA GCC CAG GAT GCC CTT GA |
The same primers were used to analyze TLR 1 and 6 amplifying a 100% homologous region between the two genes.
2.5 IL-12p70 protein analysis by ELISA
Supernants from LCs treated with Candin (50μl/ml, 100μl/ml and 150μl/ml) with or without “peptides” (10μg/ml/peptide) from the qRT-PCR experiments at 24 hours were collected and tested using the IL-12p70 High Sensitivity ELISA kit (eBioscience). Values from media only wells were subtracted from experimental wells.
2.6 Intracellular Cytokine Staining
The methods were adapted according to those described by Zielinski et al. [30]. CD4 T-cells were negatively isolated from PBMCs using CD4 T Cell Isolation Kit II (Miltenyi Biotec) and were co-cultured with autologous LCs at a ratio of 50:1 (CD4 T-cells : LCs). Candin (150μ1/ml) with or without “peptides” (10μg/ml/peptide) were added to stimulate cells. Media alone was used as a negative control. After 6 days of co-culture, the cells were stimulated with phorbol 12- myristate 13-acetate (200nM, Sigma, St. Louis, MO), and ionomycin (1μg/ml, Sigma) for 2 hours. Then, Brefeldin A (10μg/ml, eBioscience) was added for additional 2 hours. After being stained using fixable viability dye eFluor 450® (eBioscience), the cells were permeabilized/fixed and stained with anti-human IFN-γ PE, IL-17A peridinin chlorophyll protein-Cy5.5, IL-4 allophycocyanin, or relevant isotype controls (eBioscience). Ten thousand events were acquired using FACS Fortessa. Live lymphocytes were gated, and the percentages of IFN-γ, IL-17A and IL-4 positive CD4 T-cells were analyzed using FACS Diva (BD Biosciences) and Flowjo softwares.
2.7 Statistical analysis
A mixed effects ANOVA was used to compare the groups while accounting for the dependence between groups. Tukey's multiple comparison procedure was used to perform all pairwise comparisons for maturation markers (Fig. 2B) while Dunnet's test was used to compare the media control values to the remaining groups for T-cell proliferation (Fig. 3).
Fig. 2.
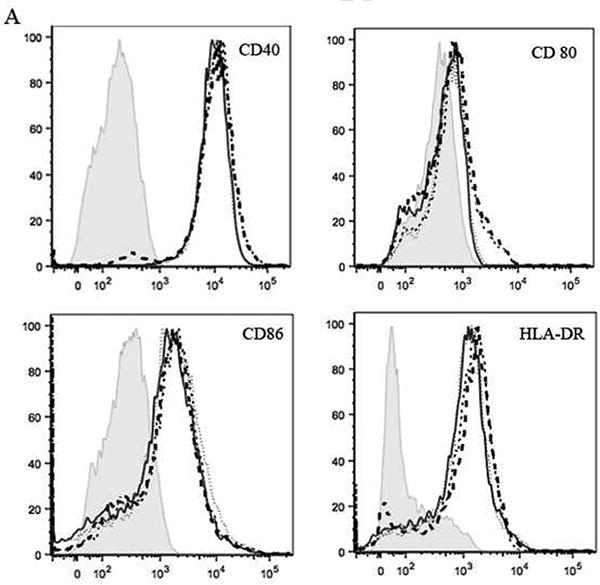
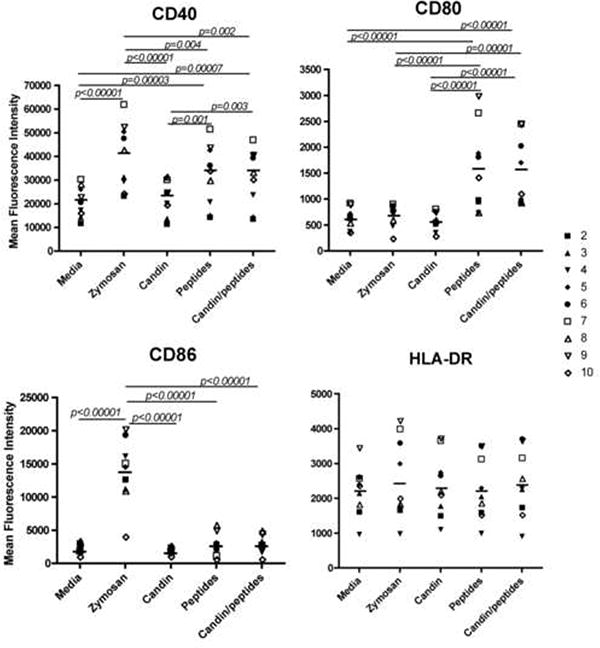
Maturation effects on LCs examined by surface expression of CD40, CD80, CD86, and HLA-DR. (A) Representative FACS histograms from subject 2. The shaded gray area, the black dotted line, the black solid line, the short dashed line and the long dashed line represent the isotype control, media, Candin, “peptides” and Candin/“peptides” respectively. (B) Summary of results from all subjects examined.
Fig. 3.
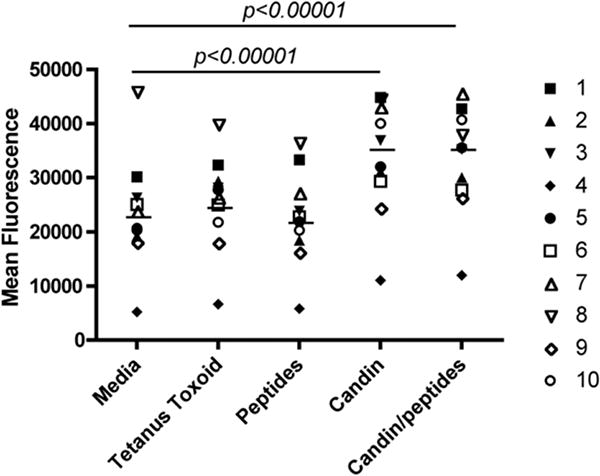
T-cell proliferation measured using alamarBlue. Candin and Candin/“peptides” pulsed LCs induce significantly increased T-cell proliferation compared to media. All wells contained CD3 T-cells (1.5 × 105 cells) and autologous LCs (3 × 103 cells).
3. Results
3.1 Phenotypic maturation of LCs
We evaluated the maturation effects of Candin, and/or “peptides” on LCs (Figs. 1-2). For CD40, statistically significant increases in mean fluorescence intensity (MFI) were observed with LCs treated with zymosan (p<0.00001), “peptides” (p=0.00003) and Candin/“peptides” (p=0.00007) compared to untreated LCs. In addition, MFIs of LCs treated with “peptides” and Candin/“peptides” were significantly higher than the MFI of LCs treated with Candin alone (p=0.001 and 0.003 respectively). For CD80, significant increases in MFIs were observed with LCs treated with “peptides” (p<0.00001) and Candin/“peptides” (p<0.00001) over media. Compared to Candin treated LCs, CD80 expression was significantly higher in “peptide” and Candin/“peptide” treated LCs (p<0.00001 for both). Only zymosan increased the MFI for CD86 significantly (p<0.00001). No significant increases were observed for HLA-DR. In summary, the “peptides” exerted partial LC maturation effects while Candin did not. Endotoxin levels for the “peptides” tested individually were all undetectable (< 1.0 EU/mg).
3.2 T-cell proliferation measured with alamarBlue
Proliferation was significantly increased with Candin (p<0.00001) and Candin/“peptides” (p<0.00001) over media (Fig. 3). “Peptides” did not induce measureable proliferation. Measurable proliferation with tetanus toxoid (increased fluorescence of ≥5000) was demonstrated in subjects 2 and 5, but overall no significant increase over media was observed (Fig. 3). Though unlikely, a possibility that LCs may have proliferated in addition to T-cells cannot be ruled out.
3.3 Expression of cytokines by LCs pulsed with Candin or Candin/“peptides”
LCs from ten subjects were treated with Candin or Candin/“peptides”, and mRNA expression of 8 cytokines (Table 1) were examined by qRT-PCR (Fig. 4, Table 2). The amplifications of the intended products were confirmed by DNA sequencing after gel-purification from selected experiments. Overall, the cytokine expression profiles of LCs treated with Candin and Candin/“peptide” were similar. IL-12p40 was the most commonly enhanced cytokine (≥ 5 fold over untreated), and expression was detected in 5 subjects with Candin and in 7 subjects with Candin/“peptides”. IFN- γ was the 2nd most commonly induced cytokine (6 subjects), and was detected in 5 subjects with Candin and in 4 subjects with Candin/“peptides”. IL-1β was also induced in 6 subjects: 4 subjects with Candin and 6 subjects with Candin/“peptide”. IL-6 and IL-23p19 were induced only with Candin (2 subjects for IL-6 and 1 subject for IL-23p19.) TNF-α was expressed only with Candin/“peptide” in 1 subject. IL-8 and IL-10 were not expressed in any subjects.
Fig. 4.
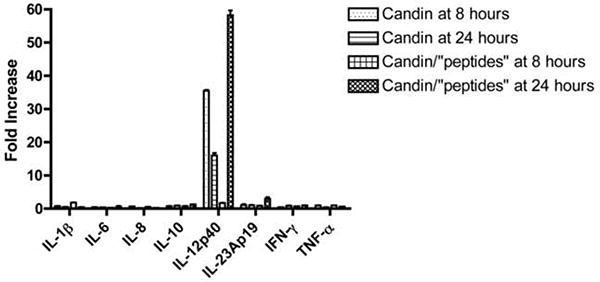
Representative results of cytokine expression by LCs treated with Candin (150μl/ml) or Candin/“peptides” from subject 4 are shown. The bars represent SD of the replicates.
Table 2. A summary of qRT-PCR results for the three most commonly increased cytokines.
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Candin±peptides, time | ||||||||||
| IL-12p40 | 50μl/ml, 8h | 64 | |||||||||
| 100μl/ml, 8h | 31 | 20 | 18 | ||||||||
| 150μl/ml, 8h | 10 | 36 | 9 | ||||||||
| 50μl/ml+peptides, 8h | 76 | ||||||||||
| 100μl/ml+peptides, 8h | 22 | 11 | 5 | ||||||||
| 150μl/ml+peptides, 8h | 39 | 5 | |||||||||
| 50μl/ml, 24h | 16 | 21 | 19 | 7 | |||||||
| 100μl/ml, 24h | 43 | 20 | 14 | 37 | |||||||
| 150μl/ml, 24h | 44 | 16 | 15 | 12 | 5 | ||||||
| 50μl/ml+peptides, 24h | 86 | 17 | 11 | ||||||||
| 100μl/ml+peptides, 24h | 5 | 40 | 11 | 6 | |||||||
| 150μl/ml+peptides, 24h | 32 | 58 | 34 | 11 | |||||||
|
| |||||||||||
| IFN-γ | 50μl/ml, 8h | 29 | |||||||||
| 100μl/ml, 8h | 9 | 13 | 64 | ||||||||
| 150μl/ml, 8h | 12 | 44 | |||||||||
| 50μl/ml+peptides, 8h | 15 | 5 | |||||||||
| 100μl/ml+peptides, 8h | 11 | 18 | 37 | ||||||||
| 150μl/ml+peptides, 8h | 42 | 25 | |||||||||
| 50μl/ml, 24h | 29 | 5 | |||||||||
| 100μl/ml, 24h | 92 | 13 | 17 | 49 | |||||||
| 150μl/ml, 24h | 10 | 23 | |||||||||
| 50μl/ml+peptides, 24h | 13 | 19 | |||||||||
| 100μl/ml+peptides, 24h | 12 | 23 | |||||||||
| 150μl/ml+peptides, 24h | 17 | ||||||||||
|
| |||||||||||
| IL-1β | 50μl/ml, 8h | ||||||||||
| 100μl/ml, 8h | 5 | 20 | |||||||||
| 150μl/ml, 8h | 5 | ||||||||||
| 50μl/ml+peptides, 8h | 23 | 8 | 14 | 7 | |||||||
| 100μl/ml+peptides, 8h | 7 | 19 | 8 | 7 | 7 | ||||||
| 150μl/ml+peptides, 8h | 10 | 10 | 8 | ||||||||
| 50μl/ml, 24h | 91 | ||||||||||
| 100μl/ml, 24h | 5 | 5 | |||||||||
| 150μl/ml, 24h | |||||||||||
| 50μ1/ml+peptides, 24h | 8 | ||||||||||
| 100μ1/ml+peptides, 24h | 7 | 7 | |||||||||
| 150μ1/ml+peptides, 24h | 6 | ||||||||||
Fold increases of ≥5 are shown.
Supernatants from LCs treated with Candin or Candin/“peptides” for 24 hours were analyzed for the presence of IL12p70 protein. IL12p70 was detected in 27 of 30 samples treated with Candin (range 38 to 177 ng/ml) and in 27 of 30 samples treated with Candin/“peptides” (range 38 to 299 ng/ml).
3.4 Expression of PRRs on LCs
All 11 PRRs examined were detectable in untreated LCs of all subjects (data not shown). Upon stimulation with Candin or Candin/“peptides”, few PRRs showed increased expression (≥ 5 fold over untreated). No obvious differences were observed in PRRs expressed between Candin and Candin/“peptide” treated LCs. The expression of TLR-9 was increased in 3 subjects (5 to 18 fold with Candin and 9 to 16 fold with Candin/“peptides”), mincle in 2 subjects (5 fold with Candin and Candin/“peptides”), mannose receptor in 2 subjects (5 to 9 fold with Candin and 5 to 11 fold with Candin/“peptides”), dectin-2 in 2 subjects (5 to 54 fold with Candin and 5 to 8 fold with Candin/“peptides”), and DC-SIGN in 1 subject (5 to 22 fold with Candin). In 5 subjects with increased expression of PRRs, 3 of them showed the increased expressions of two or more PRRs in LCs.
3.5 Intracellular cytokine expression of CD4 T-cells stimulated with Candin-pulsed LCs or Candin/“peptides”-pulsed LCs
CD4 T-cells stimulated with Candin or Candin/“peptides”-treated LCs from ten subjects were stained for intracellular secretion of IFN-γ (Th1), IL-4 (Th2) and IL-17A (Th17) (Fig. 5). Increased IFN- γ secretions (>5%) were observed in CD4 T-cells exposed to Candin or Candin/“peptides”-treated LCs over media in subject 4 (9.5% and 6.9% respectively). Overall, no differences were seen in the secretion of IFN-γ, IL-4 and IL-17A between CD4 T-cells treated with LCs alone and LCs treated with Candin as well as between LCs alone and LCs treated with Candin/“peptides”.
Fig. 5.
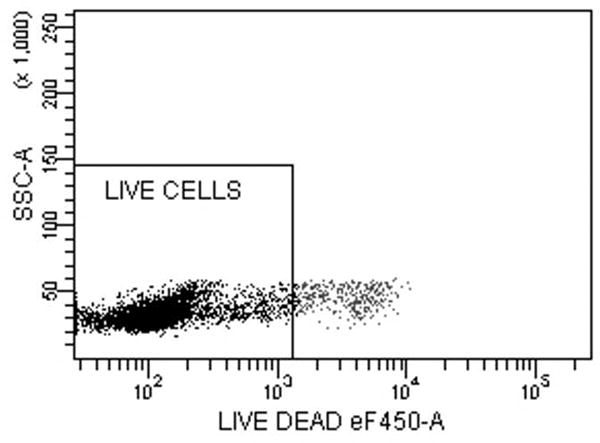
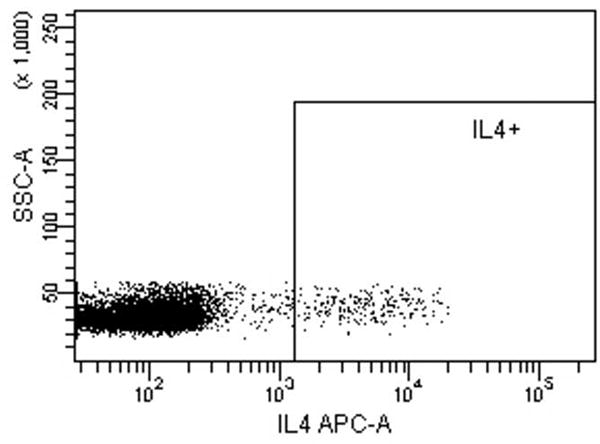
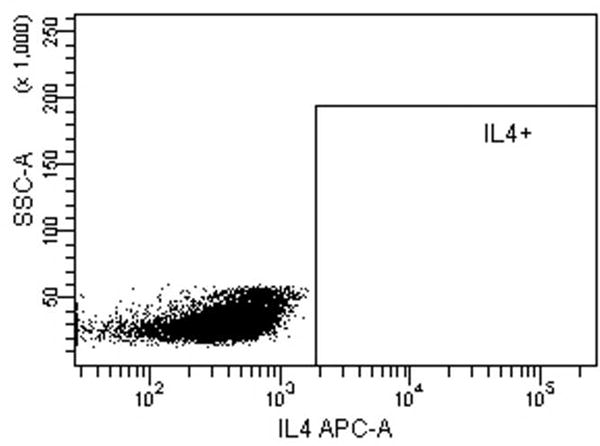
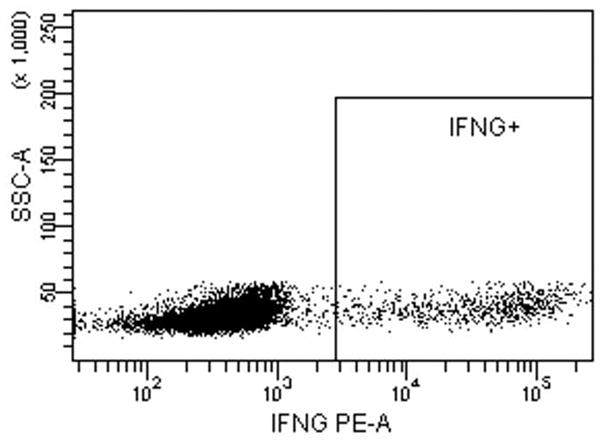
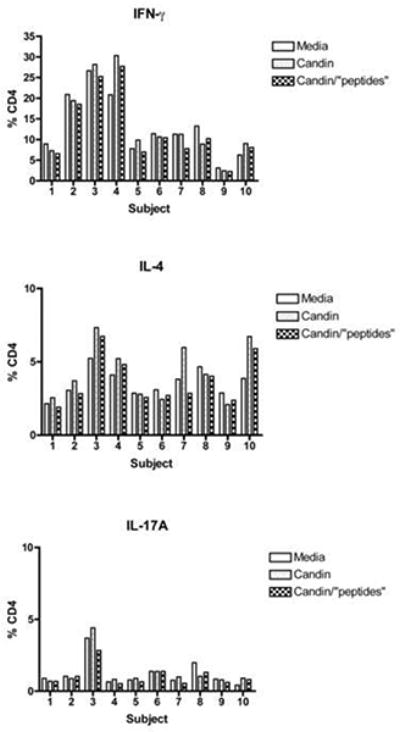
Intracellular cytokine staining for IFN-γ, IL-4 and IL-17A of CD4 T-cells stimulated with LCs pulsed with Candin or Candin/“peptides”. (A) A representative dot plot for subject 1 showing the gating on lymphocytes. (B) A representative dot plot for subject 1 showing gating on live cells discriminated using eFluor 450. (C) A representative dot plot for subject 1 showing IL-4 secreting CD4 cells that were exposed to LCs pulsed with Candin/ “peptides”. (D) Corresponding isotype control for IL-4. (E) A representative dot plot for subject 1 showing IFN-γ secreting CD4 cells that were exposed to LCs pulsed with Candin/ “peptides”. (F) Corresponding isotype control for IFN- γ. (G) A representative dot plot showing IL-17A secreting CD4 cells that were exposed to LCs pulsed with Candin/“peptides”. (H) Corresponding isotype control for IL-17A. (I) Diagrams summarizing the results from all subjects.
4. Discussion
“Adjuvant” is derived from a Latin word, adjuvare, and means to help or to enhance. An effective vaccine adjuvant should be able to promote a strong immune response against the vaccine antigen in terms of size and durability. Antigen presenting cells (APCs) play a critical role in the initiation of immune responses. One of the desired features of an adjuvant is the ability to enhance maturation of APCs and the consequent priming of effective T-cell responses. CD40 and CD80 have been demonstrated to be critical for the activation of antigen-specific T-helper cells [31] and cytotoxic T-cells [32]. Our results have shown that the “peptides” can induce significantly higher expression of CD40 and CD80. This HPV therapeutic vaccine may be a rare vaccine in that the peptide antigens rather than the adjuvant are more able to mature APCs. These results are different from those reported by Romagnoli et al. who showed up-regulation of CD40, CD80, CD86 and HLA-DR on dendritic cells by C. albicans [33]. Since endotoxin was undetectable in “peptides”, it is unlikely that contamination may have contributed to the unexpected partial maturation effects on the LCs. We focused on examining maturation effects of LCs because our vaccine was formulated for intradermal route in order to take advantage of abundant LCs in epidermis. Studying maturation effects on other APCs such as dendritic cells and monocytes would be important in the future.
C. albicans as a component of the normal flora often colonizes the skin and the mucosal surfaces of healthy individuals. Underlying acquired immunity to C. albicans is usually present in immunocompetent individuals [34]. In this study, Candin and Candin/“peptides”, but not “peptides”, induced significant T-cell proliferation. The results are consistent with the observations showing that C. albicans is capable of triggering an expansion of specific or naïve T-cells [33]. Similar to our results, Gordon et al. demonstrated skin test positive reactions to C. albicans in 92% of healthy subjects [35], and Bauerle et al. demonstrated Candida-specific T-cell responses in 71% of healthy subjects [36]. It is expected for Candin, which is an extract derived from C. albicans, to have a T-cell proliferative effect. After all Candin is being used clinically to assess the intactness of cell-mediated immunity. Unfortunately, the maturation effects of C. albicans [33] are lost in the extract. On the other hand, the “peptides” exert some maturation effects. In creating this vaccine, an obstacle was encountered in being able to develop a formulation in which the “peptides” were soluble as the E6 protein is known to be hydrophobic. While they remain soluble in acidic pH of the formulation, they are insoluble and form microparticles at a neutral pH (unpublished data). This unusual property may be contributing to the maturation effects by stimulating LCs to phagocytose these microparticles.
PRR signaling can induce APCs to express co-stimulatory molecules and cytokines necessary for activation and differentiation of T lymphocytes [37]. The cooperation of different PRRs in APCs by stimulating multiple PRRs leads to synergistic Th1 [20, 38] and cytotoxic T-lymphocyte responses [39]. C. albicans has been shown to activate many PRRs including DC-SIGN [19], dectin-1 [20], dectin-2 [21], galectin-3 [22], mannose receptor [19], mincle [40], and some TLRs [25-27, 41, 42]. Since some PRRs are increased during activation [43, 44], we investigated the presence and amplified expression of these PRRs. In this study, all PRRs examined were expressed by Candin and Candin/“peptide” pulsed LCs, and increased expressions of certain PRRs (DC-SIGN, dectin-2, mincle, monocyte receptor and TLR-9) were demonstrated in 5 of 10 subjects. Further investigations are necessary to determine which PRRs may have a role in transducing the signals from this HPV therapeutic vaccine. Dectin-1 in conjunction with TLR-2 can activate NF-κB [20], and dectin-1 can also independently mediate NFAT activation in dendritic cells leading to expression of inflammatory mediators such as IL-12p70 [45]. Therefore, it would be interesting to investigate whether Candin or Candin/”peptide” has any role in NF-κB and NFAT activation in the future.
Cytokines secreted by APCs play important roles in the process of differentiation of T-helper cells into Th1, Th2, or Th17 cells. IL-12p70 directs Th1 response while IL-1β and IL-6 direct the Th17 response [37, 46]. The cytokine profile in treated LCs showed IL-12p40 was the most commonly enhanced cytokine and IL-12p70 was also detected at a protein level. Published studies showed that C. albicans can induce the differentiation of specific Th1 and Th17 cells [30, 33], and Candida-specific Th1 immune responses can be detected in healthy subjects [47, 48]. These data lead us to anticipate the extract of C. albicans, Candin, to induce a Th1 and Th17 skewing effect. Though an increased Th1 response (IFN-γ secretion >5%) was observed in one subject, the overall results from ten subjects showed no skewing towards Th1 and Th17 responses. It may be that Candida exerts Th1 and Th17 effects through multiple mechanisms. There exist other subsets of APCs in dermis, like dermal DCs [49], which may play roles in the process of antigen presentation and T-cell activation. Furthermore, it would be important to assess the ability of this HPV therapeutic vaccine to induce HPV-specific T-cell responses. This is being investigated in the context of the ongoing clinical trial.
In summary, “peptides” (antigens) are responsible for the LC maturation effects while Candin (adjuvant) induces significant T-cell proliferation for this HPV therapeutic vaccine. Therefore, the antigens and the adjuvant have complementary immune enhancing effects. With time, the ongoing clinical trial will reveal whether these complementing effects will translate into effective clinical responses.
Highlights.
The vaccine antigens are 4 human papillomavirus type 16 E6 peptides.
Candida skin test reagent is the vaccine adjuvant.
The partial Langerhans cell maturation effects are due to the peptides.
Candida has the T-cell proliferative effect.
IL-12 is the most commonly expressed cytokine by the Langerhans cells.
Acknowledgments
This study was supported by grants from NIH (R01 CA143130, UL1TR000039, and P20GM103625), and the National Natural Science Foundation of China (No. 81101989).
Abbreviations
- APCs
antigen presenting cells
- HPV
human papillomavirus
- LCs
Langerhans cells
- MFI
mean fluorescence intensity
- PAMPs
pathogen-associated molecular patterns
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- qRT-PCR
quantitative real-time PCR
- PRRs
pattern recognition receptors
Footnotes
Author contributions: X.W.: Designed the study; acquired, analyzed and interpreted data; wrote and approved the manuscript.
H.N.C: Trained X.W. in experimental methods; acquired, and analyzed data; edited and approved the manuscript.
U.N.: Conceived and designed the study; analyzed and interpreted data; edited and approved the manuscript.
H.J.S.: Performed statistical analyses; edited and approved the manuscript.
M.N.: Conceived and designed the study; analyzed and interpreted data; wrote and approved the manuscript.
Conflict of Interest Statement: Mayumi Nakagawa is an inventor named in the patent application for the peptide-based HPV therapeutic vaccine (U. S. Serial No. 12/286,822).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–72. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 2.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa M, Gupta SK, Coleman HN, Sellers MA, Banken JA, Greenfield WW. A favorable clinical trend is associated with CD8 T-cell immune responses to the human papillomavirus type 16 e6 antigens in women being studied for abnormal pap smear results. J Low Genit Tract Dis. 2010;14:124–9. doi: 10.1097/LGT.0b013e3181c6f01e. [DOI] [PubMed] [Google Scholar]
- 4.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- 5.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: a safe and effective therapy. J Drugs Dermatol. 2004;3:263–5. [PubMed] [Google Scholar]
- 7.Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001;137:451–5. [PubMed] [Google Scholar]
- 8.Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O'Bryan K, et al. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol. 2010;146:1431–3. doi: 10.1001/archdermatol.2010.350. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RC, Ruhl TS, Pfenninger JL, Garber MR. Treatment of warts with Candida antigen injection. Arch Dermatol. 2000;136:1274–5. doi: 10.1001/archderm.136.10.1274-a. [DOI] [PubMed] [Google Scholar]
- 10.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 11.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8:1663–79. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 12.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Greenfield WW, Cannon MJ, Coleman HN, Spencer HJ, Nakagawa M. CD4+ T-cell response against human papillomavirus type 16 E6 protein is associated with a favorable clinical trend. Cancer Immunol Immunother. 2012;61:63–70. doi: 10.1007/s00262-011-1092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, et al. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–8. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 16.Igyarto BZ, Kaplan DH. Antigen presentation by Langerhans cells. Curr Opin Immunol. 2013;25:115–9. doi: 10.1016/j.coi.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahey LM, Raff AB, Da Silva DM, Kast WM. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J Immunol. 2009;182:2919–28. doi: 10.4049/jimmunol.0803645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, et al. Direct binding of Tolllike receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–25. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 19.Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–9. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 22.Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–87. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 23.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–85. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–50. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, et al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–43. doi: 10.1093/infdis/jir867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villamon E, Gozalbo D, Roig P, O'Connor JE, Fradelizi D, Gil ML. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–9. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 28.Salvenmoser S, Seidler MJ, Dalpke A, Muller FM. Effects of caspofungin, Candida albicans and Aspergillus fumigatus on toll-like receptor 9 of GM-CSF-stimulated PMNs. FEMS Immunol Med Microbiol. 2010;60:74–7. doi: 10.1111/j.1574-695X.2010.00720.x. [DOI] [PubMed] [Google Scholar]
- 29.Vernal R, Leon R, Silva A, van Winkelhoff AJ, Garcia-Sanz JA, Sanz M. Differential cytokine expression by human dendritic cells in response to different Porphyromonas gingivalis capsular serotypes. J Clin Periodontol. 2009;36:823–9. doi: 10.1111/j.1600-051X.2009.01462.x. [DOI] [PubMed] [Google Scholar]
- 30.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 31.Schweitzer AN, Borriello F, Wong RC, Abbas AK, Sharpe AH. Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–22. [PubMed] [Google Scholar]
- 32.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 33.Romagnoli G, Nisini R, Chiani P, Mariotti S, Teloni R, Cassone A, et al. The interaction of human dendritic cells with yeast and germ-tube forms of Candida albicans leads to efficient fungal processing, dendritic cell maturation, and acquisition of a Th1 response-promoting function. J Leukoc Biol. 2004;75:117–26. doi: 10.1189/jlb.0503226. [DOI] [PubMed] [Google Scholar]
- 34.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J Leukoc Biol. 2000;68:175–9. [PubMed] [Google Scholar]
- 35.Gordon EH, Krouse HA, Kinney JL, Stiehm ER, Klaustermeyer WB. Delayed cutaneous hypersensitivity in normals: choice of antigens and comparison to in vitro assays of cell-mediated immunity. J Allergy Clin Immunol. 1983;72:487–94. doi: 10.1016/0091-6749(83)90586-9. [DOI] [PubMed] [Google Scholar]
- 36.Bauerle M, Schroppel K, Taylor B, Bergmann S, Schmitt-Haendle M, Harrer T. Analysis of the Candida albicans - specific T-cell response and oropharyngeal Candida colonization in a cohort of HIV-1-infected patients. Eur J Med Res. 2006;11:479–84. [PubMed] [Google Scholar]
- 37.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, et al. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 40.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–13. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 41.Miyazato A, Nakamura K, Yamamoto N, Mora-Montes HM, Tanaka M, Abe Y, et al. Tolllike receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–64. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–23. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 43.Biswas I, Garg I, Singh B, Khan GA. A key role of toll-like receptor 3 in tissue factor activation through extracellular signal regulated kinase 1/2 pathway in a murine hypoxia model. Blood Cells Mol Dis. 2012;49:92–101. doi: 10.1016/j.bcmd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Sinha S, Guo Y, Thet S, Yuan D. IFN type I and type II independent enhancement of B cell TLR7 expression by natural killer cells. J Leukoc Biol. 2012;92:713–22. doi: 10.1189/jlb.0212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 47.La Sala A, Urbani F, Torosantucci A, Cassone A, Ausiello CM. Mannoproteins from Candida albicans elicit a Th-type-1 cytokine profile in human Candida specific long-term T cell cultures. J Biol Regul Homeost Agents. 1996;10:8–12. [PubMed] [Google Scholar]
- 48.Nisini R, Romagnoli G, Gomez MJ, La Valle R, Torosantucci A, Mariotti S, et al. Antigenic properties and processing requirements of 65-kilodalton mannoprotein, a major antigen target of anti-Candida human T-cell response, as disclosed by specific human T-cell clones. Infect Immun. 2001;69:3728–36. doi: 10.1128/IAI.69.6.3728-3736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–83. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]


