Abstract
Isoform selective inhibitors of the sirtuins (NAD+-dependent histone deacetylases) should enable an in depth study of the molecular biology underpinning these targets and how they are deregulated in diseases such as cancer and neurodegeneration. Herein, we present the discovery of structurally novel SIRT2 inhibitors. Hit molecule 8 was discovered through the chemical synthesis and biological characterization of a small-molecule compound library based around the 10,11-dihydro-5H-dibenz[b,f]azepine scaffold. In vitro screening assays revealed compound 8 to have an IC50 of 18 μM against SIRT2 and to exhibit more than 30-fold selectivity compared to SIRT1. Cellular assays, performed on MCF-7 cells, confirmed the in vitro selectivity and showed hit 8 to have antiproliferative activity at a concentration of 30 μM. Computational studies were performed to predict the SIRT2 binding mode and to rationalise the observed selectivity.
Introduction
Acetylation is a key post-translational modification of proteins that regulates function.1 Enzymatic removal of such modifications, most notably by the histone deacetylases,2 is therefore gaining increasing importance in translational science. Indeed deacetylation of histone proteins is one of the most studied epigenetic phenomena,3 regulating gene expression through chromatin remodeling. As such, epigenetic therapeutic4 strategies that target histone acetylation have the potential to make important therapeutic interventions against human diseases driven by aberrant epigenetic pathways, such as cancer5 and neurological disorders.6 Sirtuins7,8 or class III histone deacetylases are the mammalian homologues of yeast Sir2 (silent information regulator-2) and deacetylate various histone and non-histone proteins, playing a fundamental role in several physiological and pathological pathways. Moreover, while the other classes of HDACs are metalloproteins, sirtuins use NAD+ as a cofactor to affect deacetylation. To date, seven human sirtuin isoforms (SIRTs 1-7) have been discovered, with SIRT19 and SIRT210 being the most studied.
In this paper we present our discovery of selective SIRT2 inhibitors. SIRT2 is predominantly a cytoplasmic protein but has also been shown to deacetylate histone H4-K1611 and a variety of non-histone proteins, modulating various biological processes: it has recently been found to inhibit adipocyte differentiation by regulating FoxO1 acetylation status,12 to deacetylate FoxO3a in response to oxidative stress and caloric restriction,13 to deacetylate α-tubulin,14 and as we previously showed through pharmacological disruption, it has a role in p53 deacetylation.15 Moreover SIRT2 is overexpressed during mitosis, affecting mitotic exit,16 and its activity has been found to be deregulated in several types of cancer,17,18 metabolic12 and neurological disorders.19 Potent and selective SIRT2 modulators could be used as valuable pharmacological tools to gain insights into the specific cellular functions of their effector proteins, and potentially as therapeutic agents. While a number of moderately potent SIRT2 inhibitors have been reported to date: salermide,20 AGK2,19 β-phenyl-splitomicins,21 AK-7,22 the recently discovered coumarin and NAD derivatives;23,24 isoform selectivity still remains a major challenge for such compounds.
Results and Discussion
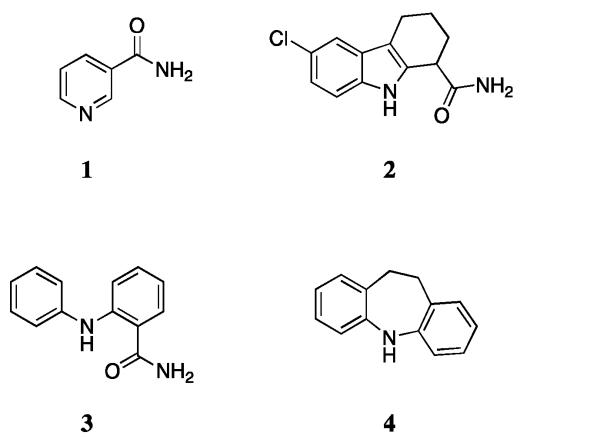
Previously, nicotinamide25 (1), EX-52726 (2) and 2-anilino-benzamide27 (3) have been reported as SIRT inhibitors, corresponding to the physiological inhibitor, a selective nanomolar SIRT1 inhibitor and a SIRT1/2 inhibitor respectively (Fig. 1). In an effort to discover alternative selective nicotinamide pocket (C-pocket)25 SIRT inhibitors, we considered the 10,11-dihydro-5H-dibenz[b,f]azepine framework (4). This tricyclic heterocycle has been used as the core structure in a variety of drugs, mainly for the treatment of several central nervous system (CNS) disorders:28 the antidepressants Imipramine and Clomipramine are two examples. In light of its structural similarity to the scaffolds present in EX-527 (2) and 2-anilino-benzamide (3), we decided to embark on the synthesis of a small-molecule compound library based around this core and assess for SIRT activity.
Fig. 1.
Molecular structures of 1, 2, 3 and 4.
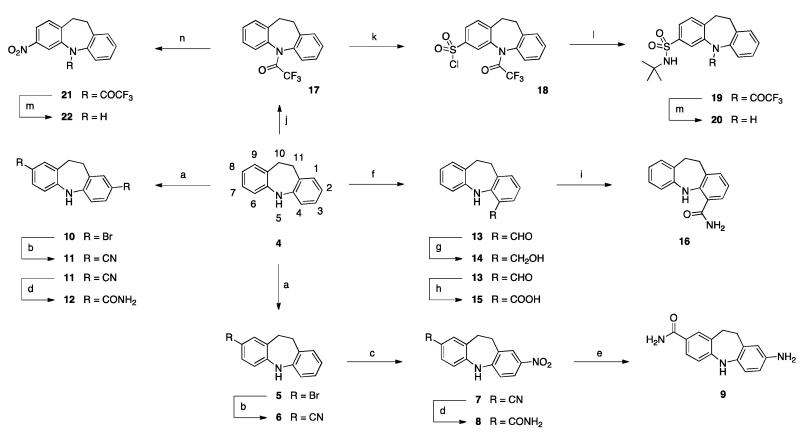
The synthetic studies commenced using the commercially available 10,11-dihydro-5H-dibenz[b,f]azepine heterocycle (4) (Scheme 1). Extensive use of electrophilic aromatic substitution was found to be advantageous, giving access to a variety of analogues bearing small, polar groups in various positions of the tricyclic template. In order to access derivatives bearing an amido group in the 2-position, bromination of 4 with NBS and silica gel, followed by palladium-catalysed cyanation, was performed to afford nitrile 6.
Scheme 1.
Reagents and conditions: (a) NBS, SiO2, DCM, rt, 1 h. (b) Zn(CN)2, Pd(PPh3)4, DMF, 90 °C, 2 h. (c) AgNO3, BzCl, MeCN, rt, 16 h. (d) H2O2, KOH, EtOH, 60 °C, 2 h. (e) NH4HCO2, Pd/C, MeOH, 50 °C, 1 h. (f) i) n-BuLi, Et2O, rt, 4 days; ii) DMF, rt, 24 h. (g) NaBH4, MeOH, rt, 1 h. (h) NaClO2, NaH2PO4, H2O, t-BuOH, 2-methyl-2-butene, rt, 16 h. (i) NH3/1,4-dioxane, HOBt, EDC•HCl, rt, 16 h. (j) TFAA, DIPEA, DMAP, DCM, rt, 16 h. (k) ClSO3H, 0 °C, 3 h. (l) t-BuNH2, DCM, rt, 16 h. (m) K2CO3, THF, MeOH, H2O, rt, 16 h. (n) Ac2O, HNO3, rt, 16 h.
Nitration of compound 6 was successfully carried out employing AgNO3 and BzCl, to yield nitrile 7 in moderate yield but high regioselectivity. Several conditions were explored in order to partially hydrolyse compound 7 into its corresponding primary amide 8. H2O2 in basic ethanolic solution was found to be the most effective, giving the desired product in excellent yield (95%). Reduction of the nitro group with NH4HCO2 afforded amide 9. Double bromination of 4, using 2 equivalents of NBS, afforded regioselectively compound 10, which was converted to 11 and 12, under the same conditions used for the synthesis of the mono derivatives.
Alternatively, in order to access derivatives bearing an amido group in the 4-position, compound 4 was formylated in a highly regioselective fashion via two-step-one-pot reaction, using n-BuLi as base and DMF as an electrophile. Curiously, optimized conditions required incubation for several days with n-BuLi in order to afford 13 in high yield (85%). Reduction of 13 with NaBH4 gave the corresponding primary alcohol 14, while oxidation yielded carboxylic acid 15, which was then converted to the desired amide 16. Finally, we found that compounds bearing polar groups in the 3-position could be obtained following protection of the secondary amine functionality of 4 with the acid stable and meta-directing trifluoroacetyl group. Following this protection strategy, chlorosulfonation or nitration reactions were performed on compound 17 to give 18 and 21 respectively. Nucleophilic displacement of the chloride ion in 18 by tert-butylamine afforded 19 in quantitative yield. Facile removal of the trifluoroacetyl orienting group, under mild basic conditions, afforded sulphonamide 20 and nitro compound 22.
The in vitro enzymatic screening assays for SIRT1 and SIRT2 inhibitory activity were performed using a fluorimetric assay29 (Table 1). IC50 values were determined for all compounds which showed over 25% inhibition at 50 μM. This led to the identification of two low-micromolar SIRT2 inhibitors with selectivity over SIRT1.
Table 1.
SIRT1 and SIRT2 enzymatic inhibitory assay data of reference compounds and key compounds in the generated small-molecule compound library
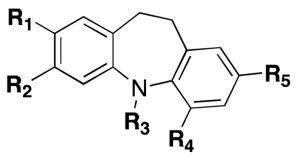
|
|||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| COMPD | R1 | R2 | R3 | R4 | R5 | SIRT1a | SIRT2b |
| AK-722 | - | - | - | - | - | -c | 15.5 |
| Salermide15,20 | - | - | - | - | - | 76 (IC50-μM) | 45.0 |
| Sirtinol15,30 | - | - | - | - | - | 38 (IC50-μM) | 103.4 |
| EX-52726 | - | - | - | - | - | 98 (IC50-nM) | 19.6 |
| 4 | H | H | H | H | H | - | - |
| 6 | CN | H | H | H | H | - | - |
| 7 | CN | H | H | H | NO2 | - | - |
| 8 | CONH2 | H | H | H | NO2 | 1% | 17.6 ± 3.4 |
| 9 | CONH2 | H | H | H | NH2 | - | - |
| 11 | CN | H | H | H | CN | - | - |
| 12 | CONH2 | H | H | H | CONH2 | - | - |
| 16 | H | H | H | CONH2 | H | - | - |
| 20 | H | SO2NHtBu | H | H | H | 12% | 29.8 ± 4.9 |
| 22 | H | NO2 | H | H | H | - | - |
| 23 | CONH2 | H | H | H | H | - | - |
| 24 | CONHtBu | H | H | H | H | - | 100.0 ± 6.8 |
| 25 | NO2 | H | H | H | H | - | - |
| 26 | H | H | CONH2 | H | H | - | - |
| 27 | CONH2 | H | H | H | Cl | - | - |
| 28 | CONH2 | H | H | H | CN | - | - |
| 29 | CONH2 | H | CH3 | H | NO2 | 23% | 77.5 ± 12.0 |
| 30 | CONH2 | H | H | NO2 | H | - | - |
| 31 | CONH2 | H | H | CH2OH | H | - | - |
| 32 | CH2OH | H | H | CH2OH | H | - | - |
| 33 | CH2OH | H | H | H | CH2OH | - | - |
Inhibition at 100 μM;
IC50 (μM) ± SD;
Inhibition <25% at 50 μM
The most potent SIRT2 inhibitor in this series was found to be compound 8 (SIRT2 IC50 = 18 μM), which showed more than 30-fold selectivity over its isoform SIRT1 (11% inhibition at 500 μM). Compound 20 showed a comparable SIRT2 inhibition (SIRT2 IC50 = 30 μM), and selectivity over SIRT1 (SIRT1 = 12% inhibition at 100 μM). Interestingly, none of the synthesised compounds were found to have any significant SIRT1 inhibitory activity in vitro.
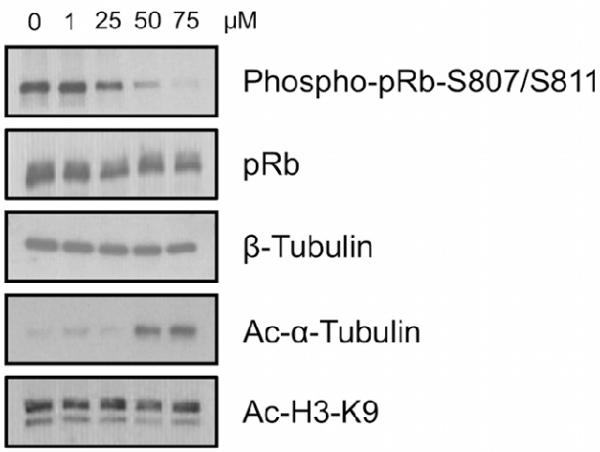
In light of the reported issues surrounding certain in vitro SIRT assays,31 we felt it was crucial to validate the in vitro results in cells (Fig. 2). Each compound, at increasing concentrations (from 0 to 100 μM), was incubated with MCF-7 cells for 24 hours.
Fig. 2.
Western blot analysis performed on MCF-7 cells upon 24 h treatment with compound 8 at increasing concentrations.
The acetylation status of H3-K932 and α-tubulin,14 which are direct cellular biomarkers of SIRT1 and SIRT2 respectively, was monitored. At concentrations up to 50 μM, 8 was the sole compound able to induce hyperacetylation of α-tubulin without increasing the acetylation levels of H3-K9, confirming its in vitro SIRT2 inhibitory effect and isoform selectivity. Furthermore, at 50 μM, compound 8 was able to impede pRb phosphorylation at Ser807/Ser811. pRB is phosphorylated by cyclin D, E and A-CDK complexes in the G1 and S phases of the cell cycle, but becomes unphosphorylated in G2/M.33,34,35 The low levels of pRB phosphorylation observed upon compound treatment is consistent with cell-cycle arrest at G2/M.
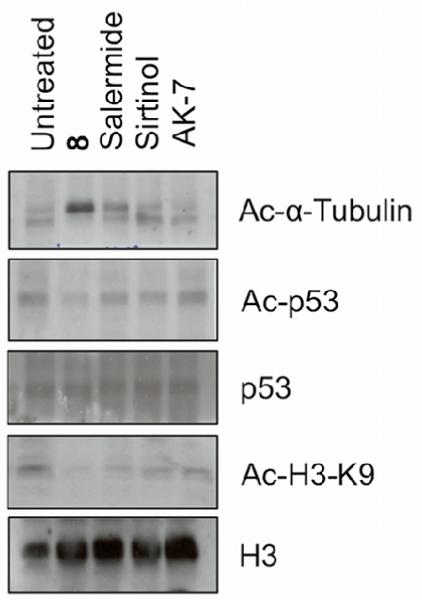
To allow for a more in depth comparison, we monitored the acetylation status of α-tubulin, H3-K9 and p53 in MCF-7 cells upon treatment with compound 8, and other key SIRT inhibitors: sirtinol,30 salermide and AK-7. While sirtinol shows moderate SIRT1 selectivity vs SIRT2, salermide and AK-7 have both been reported to have some SIRT2 selectivity. Western blot analysis showed that 8 induced a substantial accumulation of acetyl-α-tubulin (Fig. 3). Salermide also induced a slight increase in acetyl-α-tubulin, whereas no significant effect was observed for sirtinol- and AK-7-treated cells. Interestingly, sirtinol, AK-7 and salermide, but not compound 8, could moderately induce p53 acetylation at lysine 382, suggesting their ability to inhibit SIRT1. None of the compounds had a significant effect on H3-K9 acetylation. Clearly these data show compound 8 to have a much more significant effect on acetyl-α-tubulin, a specific SIRT2 target, than established SIRT2 inhibitors. Since our previous work has suggested both SIRT1 and SIRT2 need to be inhibited to significantly effect p53 acetylation in MCF-7 cells,15 our data suggest additional SIRT1 activity of AK-7 and salermide, but not compound 8. This is therefore supportive of a greater degree of SIRT2 selectivity for compound 8 than salermide and AK-7, despite a lack of an effect on Ac-H3-K9.
Fig. 3.
Western blot analysis performed on MCF-7 cells upon 24 h treatment with SIRT inhibitors. MCF-7 cells, treated with 50 μM of 8, salermide, sirtinol and AK-7.
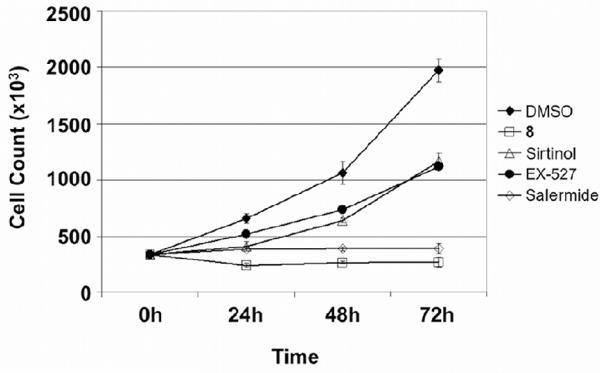
The antiproliferative effect of various known SIRT inhibitors has been previously demonstrated in the literature.15,20,36 Since compound 8 was able to retard cell cycle progression, we next compared the cytostatic effects of compound 8 with three other established SIRT inhibitors. To this end, we performed a proliferative analysis on MCF-7 cells treated with 30 μM of compound 8, EX-527 (SIRT1 inhibitor), sirtinol (SIRT1/2 inhibitor) and salermide (SIRT2 inhibitor) for 0, 24, 48 and 72 hours (Fig. 4). While MCF-7 cells treated with vehicle (DMSO) were able to proliferate normally with a cell doubling time of approximately 24 hours, a significant reduction in proliferation rate was observed with all inhibitors tested. EX-527 treatment reduced the cell proliferation by approximately 50% of the DMSO vehicle control, whereas both compound 8 and salermide abolished cell proliferation completely.
Fig. 4.
Cell viability assay on MCF-7 cells treated with compound 8, sirtinol, salermide and EX-527 at 30 μM.
Finally, the effects of compound 8 on the cell cycle progression, of the MCF-7 breast carcinoma cell line, were investigated (Fig. 5). The established SIRT2 inhibitors salermide and AK-7, as well as the SIRT1 inhibitors sirtinol and EX-527 were also included in the study as reference controls.
Fig. 5.
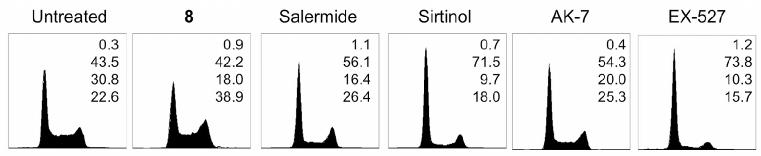
Effects of SIRT inhibitors on the MCF-7 cell cycle. MCF-7 cells were treated with 50 μM of compound 8, salermide, sirtinol, AK-7 and EX-527 for 24 h. Flow cytometric analysis of DNA content was performed after propidium iodide staining. Percentage of cells in each phase of the cell cycle (sub-G1, G1, S and G2-M) is indicated. Representative data from three independent experiments are shown.
Consistent with our previous data,15 cell cycle analysis showed that treatment with 50 μM of salermide and AK-7 resulted in an increase in cells primarily in G2/M phase of the cell cycle, whereas sirtinol and EX-527 caused cells to accumulate predominantly in G1. The results also demonstrated that 8 caused a high number of cells to accumulate at G2/M phase of the cell cycle, suggesting that 8 has a phenotypic response akin to other reported selective SIRT2 inhibitors. Such cell-cycle arrest is consistent with the reported role of SIRT2 in mitosis.14,37
In an attempt to rationalise the high selectivity and predict the binding mode of this novel chemical series, compound 8 was docked into the active site of human SIRT1 homology model (predicted by the PHYRE server)38 and human SIRT2 X-ray crystal structure (PDB entry code: 1J8F).39 Docking studies were initially performed on SIRT2 (Fig. 6).
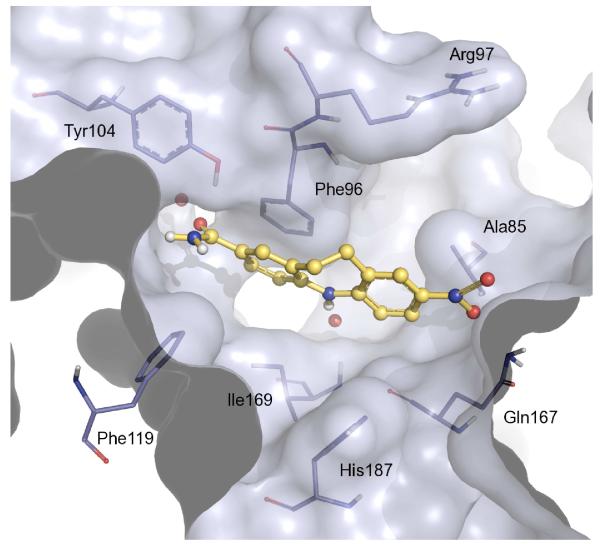
Fig. 6.
Predicted binding mode of compound 8 (ball and stick representation) at the active site of SIRT2 (PDB ID: 1J8F). Water molecules are displayed as red balls and key active site residues (stick representation) are indicated.
Analysis of the top-ranked pose of compound 8 within the SIRT2 cofactor binding site, demonstrated several plausible intermolecular interactions. Firstly, the free heterocyclic N-H functionality is hydrogen bonded to a tightly bound water molecule. One of the aromatic rings is involved in a π-stacking interaction with Phe96, and the amide carbonyl group is hydrogen bonded to Tyr104. Finally interactions are observable between the nitro group and the backbone N-H moiety of Ala85 and the side chain amino group of Gln167.
We therefore propose that the secondary N-H group in the heterocyclic scaffold is essential for the SIRT2 inhibitory activity, as it seems to form a key interaction, mediated through a water molecule. Indeed, as described the N-methyl derivative (29, Table 1) is 4-fold less potent. While one of the two aromatic rings present within 8 is clearly involved in a π-π interaction, both rings are necessary for the suitable positioning of the nitro and amido substituents within the catalytic pocket. To rationalise the SIRT1 vs SIRT2 selectivity of compound 8, we compared our SIRT1 model to SIRT2, and indeed, we also performed independent docking studies on SIRT1 (Fig. 7). The RMSD between the SIRT1 and SIRT2 structures, after rigid superimposition, was calculated to be 3.89 Å. A clear observable difference between the two structures is the orientation of the flexible loop in the large groove.
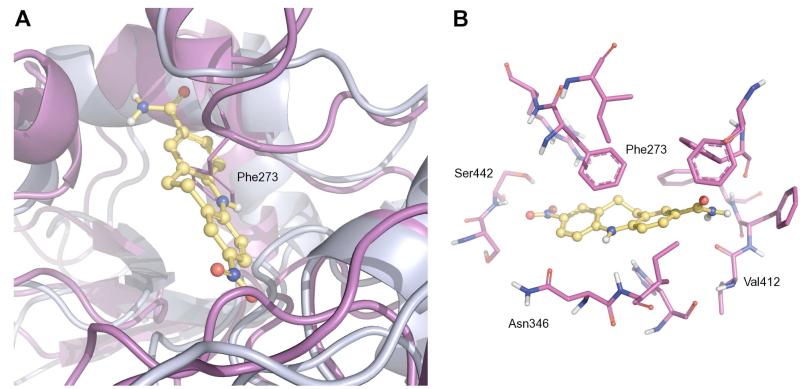
Fig. 7.
A) Rigid superposition of the SIRT1 homology model (magenta) and the SIRT2 crystal structure (light blue, PDB ID: 1J8F). 8 (ball and stick representation), docked to the SIRT2 catalytic pocket. B) Predicted binding mode of compound 8 at the active site of the homology-modeled SIRT1 structure. Key active site residues (stick representation) are indicated.
In SIRT1 the loop is oriented closer inside the NAD+ binding pocket, which creates an unfavourable clash between the top-ranked SIRT2 pose of 8, and Phe273 of SIRT1. This suggests that compound 8 can no longer occupy this pose in SIRT1.
Analysis of the top-scoring pose of compound 8 in the active site of SIRT1 model shows that the nitro moiety seems to be the only group able to form significant intermolecular interactions within the catalytic pocket. Hydrogen bonds are observable between this group and the backbone N-H and side chain hydroxyl group of Ser442.
No other relevant interactions appear to be present. Additionally, the polar amide group is placed in a highly hydrophobic cleft, most likely resulting in a high desolvation cost. According to the proposed binding poses we can speculate that while compound 8 can fit into a suitable pocket within the SIRT2 active site, it cannot within the SIRT1 pocket, giving rise to the observed selectivity.
Conclusions
Structurally novel and selective SIRT2 inhibitors were discovered through the diverse functionalisation of the 10,11-dihydro-5H-dibenz[b,f]azepine framework. Hit compounds 8 and 20 showed low micromolar SIRT2 inhibitory activity in vitro and more than 30-fold selectivity over SIRT1 in vitro. Cellular studies in MCF-7 cells confirmed compound 8 as a SIRT2 selective compound. Docking studies were performed on compound 8 to gain insights into its high SIRT2 selectivity and to propose its binding mode within the SIRT2 catalytic domain. Although it is premature to comment on the translational potential of this class of SIRT2 inhibitors, their resemblance to the established tricyclic antidepressants and related CNS drugs26 such as Imipramine and Clomipramine is striking. The fact that SIRT2 has been proposed as a target in the treatment of neurological diseases,19 coupled with the clinical validation of structurally similar CNS drugs, certainly warrants further study. Structure- and ligand-based approaches are currently being employed to further optimise these novel hit compounds.
Supplementary Material
Acknowledgments
We gratefully acknowledge Cancer Research UK for their financial support. K. S. and M. J. acknowledge funding by the EU (Project SEtTReND, Nr. 241865 – FP7 Health).
References
- 1.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 2.Yang XJ, Seto E. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg AD, Allis CD, Bernstein E. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Egger G, Liang G, Aparicio A, Jones PA. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 5.Taby R, Issa JP. CA Cancer J. Clin. 2010;60:376–392. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 6.Urdinguio RG, Sanchez-Mut JV, Esteller M. Lancet Neurol. 2009;8:1056–1072. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- 7.Schemies J, Uciechowska U, Sippl W, Jung M. Med. Res. Rev. 2009;30:861–889. doi: 10.1002/med.20178. [DOI] [PubMed] [Google Scholar]
- 8.Finkel T, Deng CX, Mostoslavsky R. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milner J. Curr. Pharm. Des. 2009;15:39–44. doi: 10.2174/138161209787185841. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Hiratsuka M, Osaki M, Oshimura M. Cell Cycle. 2007;6:1011–1018. doi: 10.4161/cc.6.9.4219. [DOI] [PubMed] [Google Scholar]
- 11.Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. Genes Dev. 2006;20:1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing E, Gesta S, Kahn CR. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Nguyen M, Qin FX, Tong Q. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 14.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. Mol. Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 15.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD, Lam EW. Mol. Cancer Ther. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 16.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Mol. Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, Depinho RA, Gu Y, Simon JA, Bedalov A. Cancer Res. 2006;66:4368–4377. doi: 10.1158/0008-5472.CAN-05-3617. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, Ji J, Wang XW, Park SH, Cha YI, Gius D, Deng CX. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 20.Lara E, Mai A, Calvanese V, Altucci L, Lopez-Nieva P, Martinez-Chantar ML, Varela-Rey M, Rotili D, Nebbioso A, Ropero S, Montoya G, Oyarzabal J, Velasco S, Serrano M, Witt M, Villar-Garea A, Imhof A, Mato JM, Esteller M, Fraga MF. Oncogene. 2009;28:781–791. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer RC, Uchiechowska U, Meier R, Hruby H, Valkov V, Verdin E, Sippl W, Jung M. J. Med. Chem. 2008;51:1203–1213. doi: 10.1021/jm700972e. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PA, Kazantsev AG. ACS Chem. Biol. 2011;6:540–546. doi: 10.1021/cb100376q. [DOI] [PubMed] [Google Scholar]
- 23.Rotili D, Carafa V, Tarantino D, Botta G, Nebbioso A, Altucci L, Mai A. Bioorg Med Chem. 2011;19:3659–3668. doi: 10.1016/j.bmc.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Pesnot T, Kempter J, Schemies J, Pergolizzi G, Uciechowska U, Rumpf T, Sippl W, Jung M, Wagner GK. J Med Chem. 2011;54:3492–3499. doi: 10.1021/jm1013852. [DOI] [PubMed] [Google Scholar]
- 25.Avalos JL, Bever KM, Wolberger C. Mol. Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Napper AD, Hixon J, McDonagh T, Keavey K, Pons JF, Barker J, Yau WT, Amouzegh P, Flegg A, Hamelin E, Thomas RJ, Kates M, Jones S, Navia MA, Saunders JO, DiStefano PS, Curtis R. J. Med. Chem. 2005;48:8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Imai K, Nakagawa H, Miyata N. ChemMedChem. 2006;1:1059–1062. doi: 10.1002/cmdc.200600162. [DOI] [PubMed] [Google Scholar]
- 28.Scuvee-Moreau JJ, Dresse AE. Eur. J. Pharmacol. 1979;57:219–225. doi: 10.1016/0014-2999(79)90368-6. [DOI] [PubMed] [Google Scholar]
- 29.Heltweg B, Trapp J, Jung M. Methods. 2005;36:332–337. doi: 10.1016/j.ymeth.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. J. Biol. Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- 31.Smith BC, Hallows WC, Denu JM. Anal. Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Mol. Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen ES, Knudsen KE. Nat Rev Cancer. 2008;9:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludlow JW, Glendening CL, Livingston DM, DeCarprio JA. Mol Cell Biol. 1993;1:367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschi A, Cecchini M, Steinhardt RC, Schamber MR, Dick FA, Rubin SM. Nat Struct Mol Biol. 2010;9:1051–1057. doi: 10.1038/nsmb.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Nakayama Y, Yamada H, Li YC, Yamaguchi S, Osaki M, Kurimasa A, Hiratsuka M, Katoh M, Oshimura M. Cell Cycle. 2009;8:1279–1291. doi: 10.4161/cc.8.8.8245. [DOI] [PubMed] [Google Scholar]
- 38.Kelley LA, Sternberg MJ. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 39.Finnin MS, Donigian JR, Pavletich NP. Nat. Struct. Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.