Summary
Rationale
Cystic fibrosis (CF) lung disease is characterized by structural changes and remodeling in airway architecture and lung parenchyma. Neutrophilic inflammation and infection lead to injury and breakdown of airway matrix constituents, including elastin. The non-invasive measurement of urinary desmosine (UDes), a breakdown product of elastin, may be reflective of ongoing lung injury and may serve as a biomarker of active short-term damage during pulmonary exacerbation. Our objectives were to measure desmosine in the urine of CF patients hospitalized for treatment of a pulmonary exacerbation and to explore the correlation between desmosine concentration and other markers of clinical improvement, including lung function and inflammatory mediators.
Methods
Urine and blood samples plus lung function measurements were collected at up to three points during hospitalization for treatment of a CF pulmonary exacerbation. We used a repeated measures model, adjusted for age and time between measurements, to compare log transformed urine desmosine concentrations across multiple time points and to correlate those concentrations with related clinical variables. Change in UDes concentration was investigated using a statistical model that incorporated normalization factors to account for variations in urinary concentration.
Results
Desmosine was measured by radioimmunoassay in 155 spot urine samples from 53 CF patients hospitalized for 63 pulmonary exacerbations (range of results: 0–235 pmol Des/mL). Specific gravity adjusted UDes concentration decreased significantly during admission for CF pulmonary exacerbation, p<0.01 (average length of stay = 11 days). No correlation was observed between UDes concentration and lung function or inflammatory markers.
Conclusions
Urinary desmosine decreased significantly following treatment for an acute pulmonary exacerbation and may be a useful biomarker of short-term injury to the CF lung. Further investigation is needed to evaluate the utility of UDes concentration in the long-term progression of CF lung disease.
Keywords: elastin breakdown, matrix, airway, inflammation
Introduction
Progressive, obstructive lung disease remains the leading cause of morbidity and mortality in cystic fibrosis (CF) patients (1). Impaired cAMP-regulated chloride transport in CF respiratory epithelial cells results in a vicious cycle of airway obstruction, chronic endobronchial infection and neutrophilic inflammation (2). Neutrophils and neutrophil products, including neutrophil elastase (NE), are abundant in the CF airway, resulting in the proteolytic destruction of lung connective tissue and eventual permanent fibrotic change (3).
Elastin is a major component of the extracellular matrix of the lung, largely responsible for its architectural foundation and elastic recoil properties (4). Mature elastin is cross-linked by the small molecule desmosine, forming a delicate latticework throughout the respiratory system (4). Lung injury that involves extracellular matrix catabolism is associated with elastin destruction and the release of quantifiable desmosine cross-links. Desmosine has characteristics that render it attractive for use as a biomarker of elastin catabolism: 1) There is limited absorption of dietary desmosine, 2) Desmosine is not metabolized by the body after its release from elastin, 3) Desmosine is unique to mature elastin, and 4) Desmosine is excreted by the kidney in measurable quantities (5,6). Given that CF lung disease is characterized by the proteolytic degradation of elastin, urinary desmosine (UDes) concentration has long been identified as a potential surrogate marker of active lung injury and remains a target of continued investigation (7).
The natural course of CF lung disease is a progressive decline of lung function compounded by intermittent pulmonary exacerbations. Pulmonary exacerbations are characterized by an increase in pulmonary symptoms, a decrease in lung function, loss of energy, weight loss and changes in chest physical findings; although a standardized definition of a CF pulmonary exacerbation is currently unavailable (8,9). Previous studies have shown that aggressive treatment of a pulmonary exacerbation decreases sputum bacterial burden, improves pulmonary function and decreases acute airway markers of inflammation and infection (10–12). However, up to 25% of CF patients may not return to baseline forced expiratory volume in one second (FEV1) after treatment for an exacerbation, increasing the risk for permanent loss of lung function (13,14). Short-term evaluation of disease progression remains difficult in this population and the relationship between an exacerbation and lung injury is currently not known.
UDes concentration may reflect ongoing lung injury and may serve as a biomarker of active short-term damage during pulmonary exacerbation. Desmosine is known to be excreted in the urine and has been shown to be elevated in patients with CF (3,15–17). Sputum desmosine concentration is also known to decrease during the first week of hospitalization for CF pulmonary exacerbation (18). We hypothesized that pulmonary exacerbation in patients with CF would result in active lung injury and that inpatient treatment would cause a significant decrease in UDes concentration. We sought to explore the use of desmosine as an objective biomarker in CF through determination of its concentration in urine and by its correlation with known markers of improvement during exacerbation.
Materials and Methods
Study Population
Fifty-three patients with a confirmed diagnosis of CF were identified on admission to The Children’s Hospital, Denver for treatment of a pulmonary exacerbation and recruited into our study (19). A pulmonary exacerbation was defined as an increase in pulmonary symptoms (i.e. cough or sputum production), a >10% decrease in FEV1 compared to baseline and/or attending physician clinical judgment. Patients were required to have two or more intravenous (IV) antibiotics administered for treatment of a pulmonary exacerbation to enroll in the study. All study protocols were approved by the Colorado Multiple Institutional Review Board (COMIRB#05–0690) and informed consent and/or assent were obtained from each of the subjects and/or their parents or guardians.
Study Design
This was a prospective cohort study of patients with CF who were hospitalized for treatment of a pulmonary exacerbation. All patients received standard-of-care therapies including airway clearance, nutritional support and IV antibiotics. Standard doses of IV antibiotics were used and blood levels were monitored where appropriate. Each subject provided a spot urine sample (16,20) and blood sample, and pulmonary function tests (PFT’s) within 72 hours of the initiation of IV antibiotics, on day 3–8 of therapy and on day 8–12. Sputum samples were collected at similar time-points on a subset of patients who could spontaneously expectorate, the results of which have been reported previously (18). Every attempt was made to obtain three sample sets from each patient. Urine samples were centrifuged and frozen immediately after collection at −80°C prior to shipment for desmosine analysis. Sputum was processed as previously described and frozen immediately after collection at −80°C prior to shipment for desmosine analysis (21).
Laboratory Assays
Urine Sample Analysis
Desmosine concentration was measured using a radioimmunoassay (RIA) (16,22–24). Urinary creatinine (UCr) and specific gravity (SG) were both measured for sample normalization purposes (25). Results are expressed as picomoles of desmosine/milliliter of urine (pmol Des/mL) or picomoles of desmosine normalized to either UCr or SG. The lower limit of detection for the desmosine RIA was 1 pmol.
Sputum Sample Analysis
Sputum desmosine, interleukin-8 (IL-8) and free neutrophil elastase (NE) were measured on each specimen in duplicate as previously described (18). Bacterial cultures were performed once during each admission.
Blood Sample Analysis
Circulating C-reactive protein (CRP) and interleukin-8 (IL-8) were measured on each sample. CRP was measured using nephelometry (Dade Behring BN11 instrument) and IL-8 was measured using Luminex Multiplex Beads (R&D Systems; Abingham, Oxon, UK). The lower limit of detection for these assays was: CRP, 0.1 mg/L and IL-8, 0.5 pg/mL.
Pulmonary Function Testing
Pulmonary function testing was attempted on each child ≥ 4 years of age, and performed according to American Thoracic Society (ATS) Guidelines (26). Given that a number of young subjects were unable to meet ATS Guidelines, we looked for reproducibility in the flow-volume loops to allow for analysis. The functional indices measured included forced expiratory volume in one second (FEV1) and forced vital capacity (FVC).
Power Analysis
This study was powered based on the primary objective which was to detect a decrease in urinary desmosine concentration during hospitalization for a pulmonary exacerbation. Fifty-four patients provided 88% power to detect 56% reduction in desmosine using an alpha level of 0.05.
Statistical Analysis
Descriptive statistics were calculated using medians, ranges, and interquartile ranges (IQR) where specified, for continuous variables and percentages for categorical variables. The marker variables were log transformed (base 10) and anchored at 1 to satisfy normality assumptions of the statistical tests. The UDes measurements were investigated using both unadjusted values and values adjusted for either SG or Cr given our previous finding that urinary Cr decreases significantly during treatment for pulmonary exacerbation and the findings of others (25,27–29). It was determined the SG-adjusted UDes was the optimal way to express UDes concentration, so further analysis and correlations were performed with this measure. To estimate the means in the outcome variables for multiple time points, a repeated measures model was fit with time between measurements included as a covariate. This model included a compound symmetric covariance structure for the repeated measures and contrasts testing the least square (LS) means between two time points. To assess the correlation between UDes and other outcome variables, a bivariate version of the repeated measures model was fit. This modeling approach is achieved by simultaneously fitting two univariate repeated measures models, one for each outcome, and specifying a joint multivariate distribution on the random effects and calculating their correlation (30). Differences between a patient’s first and last log transformed measurements were used to investigate the correlation of changes in the outcome variables, the correlation of the differences were calculated using Spearman’s rank correlation coefficient. Additionally, a sensitivity analysis was performed in which a nested random effect was included in the mixed effects models to account for any correlation due to repeated admissions. All models were fit using SAS PROC MIXED version 9.2 software (SAS Institute Inc.: Cary, NC, 2008).
Results
Patient Demographics
Clinical characteristics from the 53 CF patients hospitalized for treatment of a CF pulmonary exacerbation are presented in Table 1.
Table 1.
CF patient clinical characteristics.
| Median (range) or No. (%) | Hospitalized CF patients |
|---|---|
| Number of subjects | 53 |
| Female | 33 (62%) |
| Caucasian | 52 (98%) |
| ≥ 1 ΔF508 mutation | 49 (92%) |
| Number of admissions | 63 |
| Admit FEV1 (% predicted) | 65 (26 – 123) |
| Length of stay (days) | 11 (4 – 53) |
| Duration of Antibiotic treatment (days) | 10 (3 – 53) |
| Antibiotic Use | |
| Aminoglycoside | 56 (82%) |
| Fluoroquinolone | 14 (21%) |
| Anti-fungal | 25 (36%) |
| Carbapenem | 18 (26%) |
| Macrolide | 43 (63%) |
| Time between IV antibiotics and first urine sample collection (days) | 1 (0 – 3) |
| Sputum Culture Results | |
| > 1 pathogen | 46 (68%) |
| Pseudomonas Aeruginosa | 33 (49%) |
| Staphylococcus Aureus | 21 (31%) |
| Methcillin-resistant Staphylococcus Aureus | 11 (16%) |
| Atypical Mycobacteria | 10 (15%) |
| Fungus | 10 (15%) |
| Other | 21 (31%) |
Sputum Desmosine Concentration
We collected 71 expectorated sputum samples from 19 CF patients during 26 hospitalizations for exacerbation. Demographic data, desmosine concentration, and infectious and inflammatory marker levels for this cohort have been reported previously (18).
Urinary Desmosine Concentration
We collected 155 spot urine samples from 53 patients during 63 separate hospitalizations for treatment of a pulmonary exacerbation. The untransformed UDes concentration ranged from 0–235 pmol Des/mL. The initial median UDes concentration was 61.0 pmol Des/mL (IQR: 29.0–95.0 pmol Des/mL, n=57), the interim concentration was 45.0 pmol Des/mL (IQR: 27.5–79.7 pmol Des/mL, n=56) and the final concentration was 37.5 pmol Des/mL (IQR: 19.0–69.0 pmol Des/mL, n=42). Unadjusted UDes concentration decreased significantly during inpatient treatment for a CF pulmonary exacerbation (p=0.01).
Change in SG-adjusted UDes, Lung Function, Blood and Sputum Inflammatory Markers during Hospitalization
Figure 1 illustrates the LS means of log transformed, SG-adjusted UDes measurements at each time point. Table 2 summarizes the differences between LS means at each of three time points in the primary outcome variables during inpatient treatment. Our patient population improved clinically as demonstrated by a significant increase in absolute FEV1 and a significant decrease in sputum NE, sputum IL-8 and plasma CRP. Both unadjusted and SG-adjusted UDes values decreased significantly between the first and third measurements (p=0.01 and p<0.01, respectively). The decrease in SG-adjusted UDes leveled off between the second and third measurements, as did sputum NE, sputum IL-8, and plasma CRP. SG-adjusted UDes values also decreased significantly in those patients with CF who were unable to spontaneously expectorate sputum. These results were robust to the inclusion of the nested random effect and sensitivity analysis performed to account for repeated admissions.
Figure 1.
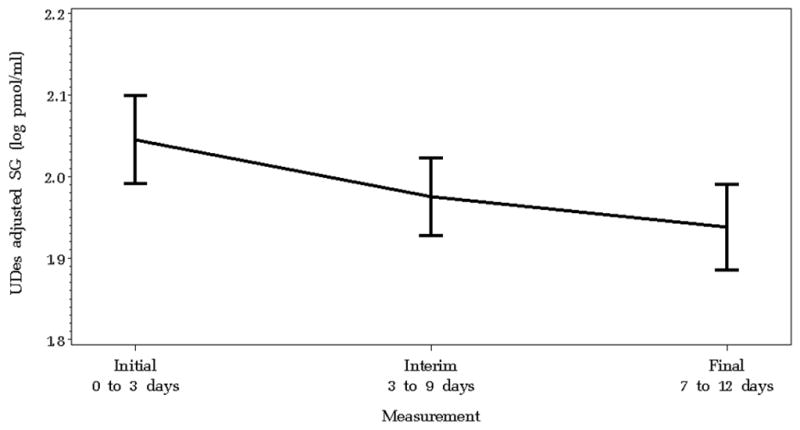
Least square mean values of log-transformed urinary desmosine concentration adjusted for specific gravity for each patient hospitalized for pulmonary exacerbation at each of three collection points. The bold lines connect the least square mean values and the bars represent the corresponding 95% confidence intervals. Specific gravity-adjusted urine desmosine concentration decreased significantly during treatment for pulmonary exacerbation (p<0.01).
Table 2.
Summary of differences in primary outcome variables at each of three time points during inpatient treatment for pulmonary exacerbation. Values are given as the estimate ± SE and p values for differences between LS means. Results of a repeated measures analysis of covariance model, adjusted for differences in time between measurements, are given.
| Interim vs. Initial | Final vs. Interim | Final vs. Initial | ||||
|---|---|---|---|---|---|---|
| Difference ± SE | p-value | Difference ± SE | p-value | Difference ± SE | p-value | |
| Urinary Desmosine (log pmol/mL) | −0.11 ± 0.08 | 0.18 | −0.11 ± 0.09 | 0.20 | −0.22 ± 0.09 | 0.01 |
| UDes adjusted SG (log pmol/mL) | −0.06 ± 0.02 | <.01 | −0.01 ± 0.02 | 0.62 | −0.07 ± 0.02 | <.01 |
| UDes adjusted UCr (log pmol/mg UCr) | −0.04 ± 0.03 | 0.25 | 0.01 ± 0.03 | 0.75 | −0.03 ± 0.04 | 0.47 |
| Sputum Des (log pmol/mL) | −0.21 ± 0.11 | 0.06 | 0.09 ± 0.11 | 0.43 | −0.12 ±0.11 | 0.29 |
| FEV1 absolute (L) | 0.30 ± 0.04 | <.01 | 0.11 ± 0.05 | 0.02 | 0.40 ± 0.05 | <.01 |
| Sputum NE (log μg/mL) | −0.25 ± 0.11 | 0.02 | −0.17 ± 0.11 | 0.14 | −0.42 ± 0.12 | <.01 |
| Sputum IL-8 (log pg/mL) | −0.32 ± 0.09 | <.01 | 0.01 ± 0.09 | 0.96 | −0.31 ± 0.10 | <.01 |
| Plasma IL-8 (log pg/mL) | 0.02 ± 0.05 | 0.75 | −0. 01 ± 0.05 | 0.80 | 0.003 ± 0.05 | 0.96 |
| Plasma CRP (log mg/L) | −0.21 ± 0.07 | <.01 | −0.03 ± 0.07 | 0.66 | −0.24 ± 0.07 | <.01 |
Correlations of SG-adjusted UDes with Lung Function, Blood and Sputum Inflammatory Markers, and Sputum Desmosine
There were no significant associations observed between SG-adjusted UDes concentration and sputum desmosine, lung function or blood/sputum inflammatory markers. The correlations ranged from absolute values of 0.11 to 0.56, with the largest correlation coefficient describing the association between SG-adjusted UDes and sputum desmosine, although this association was negative.
Correlations of Changes in SG-adjusted UDes with Changes in Lung Function, Blood and Sputum Inflammatory Markers
There was a non-significant, negative association observed between change in SG-adjusted UDes and change in absolute FEV1 [ρ=−0.22, p=0.16] (Figure 2). A positive, non-significant association was observed between change in SG-adjusted urinary desmosine and plasma CRP [ρ=0.40, p=0.10, respectively].
Figure 2.
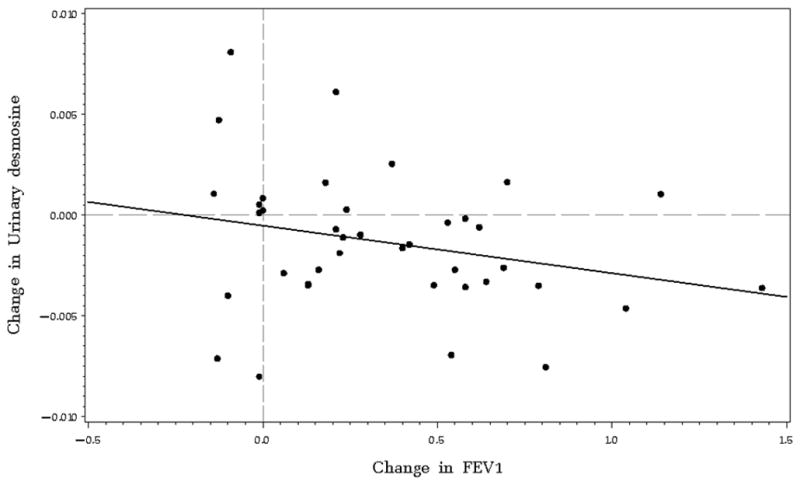
Change in urinary desmosine concentration plotted versus change in absolute FEV1. Reference lines divide the plot into quadrants at the zero values. Change in urinary desmosine concentration is not statistically significantly correlated with change in absolute FEV1, although a negative association is observed [ρ= −0.46, 95% CI (−2.10, 1.19)]. The majority of the points lie in the lower right quadrant which corresponds to an increase FEV1 absolute and a decrease in urinary desmosine concentration over time.
Discussion
Our aim was to explore UDes concentration as a potential biochemical marker of acute structural lung injury during hospitalization for a CF pulmonary exacerbation. Pulmonary exacerbation is associated with an increase in free neutrophil elastase activity in the CF airway (11). Therefore, we hypothesized that increased elastin breakdown would result in an elevation in UDes concentration with a subsequent decrease as the pulmonary exacerbation resolves. We chose an acute pulmonary exacerbation because subjects treated with IV antibiotics and enhanced mucus clearance generally display clinical improvement within 2 weeks. Our study demonstrates a significant decrease in UDes concentration across three time-points during inpatient treatment for a CF pulmonary exacerbation.
UDes concentration has long been a target of biomarker research in destructive lung diseases. The utility of UDes as a marker of lung destruction was first suggested by animal models of lung injury and emphysema (5,31–33). Early cross-sectional studies investigating UDes in a heterogeneous CF population revealed increased concentrations of desmosine compared to healthy controls, with positive correlations between concentration and proteolytic lung destruction as indicated by chest roentgenogram score (3,15). Subsequent longitudinal studies confirmed an elevated UDes concentration in CF compared to age-matched healthy controls, although these studies did not involve young children (16,17,34). UDes has also been evaluated in chronic obstructive pulmonary disease (COPD), smokers and non-smokers with similar results (35–40). During COPD exacerbation, UDes concentration increased significantly and decreased 2 months after discharge (41). Our study of a large number of well-characterized pediatric and adult CF patients hospitalized for a pulmonary exacerbation aimed to build on the foundation of previous studies and to critically evaluate UDes concentration in an acute, pulmonary exacerbation setting.
Our study is the largest to evaluate UDes concentration in a CF population during pulmonary exacerbation. As expected, absolute FEV1 increased significantly, and sputum NE, sputum IL-8 and plasma CRP concentrations decreased significantly during hospitalization. UDes concentration was positively correlated with plasma IL-8, but no significant association was observed with inflammatory markers or absolute FEV1. There was no correlation observed between urinary and sputum desmosine concentration. The lack of relationship between a local (sputum) and systemic (urine) biomarker in CF is not surprising, given that previous studies have failed to find such a correlation (42). The lack of correlation between UDes concentration and FEV1 is also not surprising, given the lack of such a correlation in previous studies (38,43). It is possible that FEV1 may not adequately reflect acute changes in lung elastin composition. A physiologic parameter such as FEV1 is likely the result of resolving mucus plugging and decreased airway inflammation, whereas UDes concentration may better reflect a more acute, injurious process (7). In a cohort of patients with acute respiratory distress syndrome/acute lung injury, no correlations were observed between UDes concentration and markers of clinical disease severity; however, higher UDes concentration was still associated with a higher risk of death (44). UDes concentration was also associated with a higher risk of a poor outcome in a cohort of stable patients with CF, even though no correlations were observed with any inflammatory variable (34). In COPD, UDes concentration was found to be inversely correlated with the extent of emphysema, suggesting that there may be a decrease in elastin catabolism secondary to decreased lung mass in severe disease (40). This evidence suggests that UDes as a measure of structural lung injury may be potentially valuable in determining the severity of clinical disease. The lack of correlation with other currently collected measures indicates its potential as an orthogonal marker measuring a different disease process (45).
Urinary concentration of any biomarker is dependent on both the biomarker excretion rate and on the urinary flow rate. Previous studies have utilized a normalization ratio incorporating UCr concentration to report UDes concentration (UDes/UCr) (12,15–17). The underlying assumption to this approach is that the UCr excretion rate is constant within an individual over time and also across individuals, which may not be the case, especially in a sick CF population receiving nephrotoxic intravenous medications (25,27,29), We chose to report both unadjusted and a novel, SG-adjusted UDes concentration given our finding that UCr concentration significantly decreased during hospitalization, making the use of a UCr normalization ratio inappropriate in this population (27). Knowing that a method of normalization is imperative to urinary biomarker measurements, we chose to normalize UDes concentration using a regression model incorporating specific gravity as a covariate, as opposed to using a simple ratio with urinary creatinine (27,28). Our finding of a significant decrease in UDes held true in the analysis of both unadjusted UDes concentration and UDes concentration incorporating specific gravity as a normalization factor into a statistical model (27). SG has been previously utilized as a normalization factor for urinary biomarker measurement and may be a more appropriate measure of urinary concentration in the CF population during exacerbation (46).
Our study is also not without limitation. We selected an RIA to measure UDes concentration, given this is a validated assay that has been utilized in similar published clinical studies (24,34,44). However, recent methodological advances in liquid chromatography and mass spectrometry may allow for more sensitive and specific measurements of UDes concentration (7). We also chose to normalize our urinary desmosine measurements to specific gravity, which differs from what has been used previously (UCr), making cross-comparison amongst studies challenging. Finally, the specificity of UDes for lung elastin catabolism remains a question. Since the lung is a major source of elastin in the body and lung disease was the major disease process in these patients, it appears that higher UDes concentration represents increased structural lung damage (44).
Desmosine measured non-invasively in urine may represent a biomarker of structural lung injury during pulmonary exacerbation in CF. It may prove to be most useful in those patients with CF unable to expectorate sputum, making the measurement of a non-invasive biomarker of lung injury attractive. We have demonstrated its utility during an acute setting; however, further investigation should be performed to compare its concentration in clinically stable times with its concentration during pulmonary exacerbation. It would also be useful to evaluate the association between UDes concentration and length of hospitalization for pulmonary exacerbation or decline in FEV1 over time, to help elucidate its usefulness as a potential long-term biomarker of lung disease progression in the CF population.
Acknowledgments
This work was supported by grants from the Cystic Fibrosis Foundation [CFF LAGUNA06A0]; and the National Institutes of Health [1U01HL081335-01 and M01RR00069].
The authors did not receive any financial assistance to conduct this study or write this manuscript.
Abbreviation List
- ATS
American Thoracic Society
- cAMP
Adenosine 3′,5′- Cyclic Monophosphate
- CF
Cystic Fibrosis
- COPD
Chronic Obstructive Pulmonary Disease
- CRP
C-Reactive Protein
- FEV1
Forced Expiratory Volume in One Second
- FVC
Forced Vital Capacity
- IL-8
Interleukin-8
- IQR
Interquartile Range
- IRB
Institutional Review Board
- IV
Intravenous
- LS
Least Square
- NE
Neutrophil Elastase
- PFTs
Pulmonary Function Tests
- RIA
Radioimmunoassay
- SG
Specific Gravity
- UCr
Urinary Creatinine
- UDes
Urinary Desmosine
- WBC
White Blood Cell
Footnotes
Conflict of Interest Statement: The authors have no potential, perceived or real conflicts of interest. The authors did not receive any financial assistance to conduct this study or write this manuscript.
Results of this work have been previously reported in abstract form at the American Thoracic Society International Conference, 2008.
The authors have no potential, perceived or real conflicts of interest.
References
- 1.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic Fibrosis. Cystic Fibrosis in the Metabolic and Molecular Basis of Inherited Disease. 7. New York: McGraw-Hill; 2001. pp. 521–588. [Google Scholar]
- 2.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23(1):5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 3.Bruce MC, Poncz L, Klinger JD, Stern RC, Tomashefski JF, Jr, Dearborn DG. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985;132(3):529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- 4.Starcher BC. Lung elastin and matrix. Chest. 2000;117(5 Suppl 1):229S–34S. doi: 10.1378/chest.117.5_suppl_1.229s-a. [DOI] [PubMed] [Google Scholar]
- 5.Starcher BC, Goldstein RA. Studies on the absorption of desmosine and isodesmosine. J Lab Clin Med. 1979;94(6):848–852. [PubMed] [Google Scholar]
- 6.Stone PJ, Lucey EC, Snider GL, Franzblau C. Effect of diet on urinary excretion of desmosine and hydroxylysyl pyridinoline. Am J Respir Crit Care Med. 1994;149(1):174–177. doi: 10.1164/ajrccm.149.1.8111578. [DOI] [PubMed] [Google Scholar]
- 7.Luisetti M, Ma S, Iadarola P, Stone PJ, Viglio S, Casado B, Lin YY, Snider GL, Turino GM. Desmosine as a biomarker of elastin degradation in COPD: Current status and future directions. Eur Respir J. 2008;32(5):1146–1157. doi: 10.1183/09031936.00174807. [DOI] [PubMed] [Google Scholar]
- 8.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. Pediatr. 2006;148(2):259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 10.Smith AL, Redding G, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, Moss R, Ramsey B, Rubio T. Sputum changes associated with therapy for endobronchial exacerbation in cystic fibrosis. J Pediatr. 1988;112(4):547–554. doi: 10.1016/s0022-3476(88)80165-3. [DOI] [PubMed] [Google Scholar]
- 11.Ordonez CL, Henig NR, Mayer-Hamblett N, Accurso FJ, Burns JL, Chmiel JF, Daines CL, Gibson RL, McNamara S, Retsch-Bogart GZ, Zeitlin PL, Aitken ML. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(12):1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 12.Downey DG, Brockbank S, Martin SL, Ennis M, Elborn JS. The effect of treatment of cystic fibrosis pulmonary exacerbations on airways and systemic inflammation. Pediatr Pulmonol. 2007;42(8):729–735. doi: 10.1002/ppul.20646. [DOI] [PubMed] [Google Scholar]
- 13.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, Goss CH. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010;45(2):127–134. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 15.Stone PJ, Konstan MW, Berger M, Dorkin HL, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995;152(1):157–162. doi: 10.1164/ajrccm.152.1.7599816. [DOI] [PubMed] [Google Scholar]
- 16.Starcher B, Green M, Scott M. Measurement of urinary desmosine as an indicator of acute pulmonary disease. Respiration. 1995;62(5):252–257. doi: 10.1159/000196458. [DOI] [PubMed] [Google Scholar]
- 17.Bode DC, Pagani ED, Cumiskey WR, von Roemeling R, Hamel L, Silver PJ. Comparison of urinary desmosine excretion in patients with chronic obstructive pulmonary disease or cystic fibrosis. Pulm Pharmacol Ther. 2000;13(4):175–180. doi: 10.1006/pupt.2000.0245. [DOI] [PubMed] [Google Scholar]
- 18.Laguna TA, Wagner BD, Luckey HK, Mann SA, Sagel SD, Regelmann W, Accurso FJ. Sputum desmosine during hospital admission for pulmonary exacerbation in cystic fibrosis. Chest. 2009;136(6):1561–1568. doi: 10.1378/chest.09-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW, 3rd Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic fibrosis foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winfield KR, Gard S, Kent GN, Sly PD, Brennan S. Assay for urinary desmosines in a healthy pre-pubertal population using an improved extraction technique. Ann Clin Biochem. 2006;43(Pt 2):146–152. doi: 10.1258/000456306776021571. [DOI] [PubMed] [Google Scholar]
- 21.Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 22.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292(1):125–129. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 23.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31(2):133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- 24.King GS, Mohan VS, Starcher BC. Radioimmunoassay for desmosine. Connect Tissue Res. 1980;7(4):263–267. doi: 10.3109/03008208009152362. [DOI] [PubMed] [Google Scholar]
- 25.Gaines LG, Fent KW, Flack SL, Thomasen JM, Ball LM, Zhou H, Whittaker SG, Nylander-French LA. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6-hexamethylene diamine. J Environ Monit. 2010;12(3):591–599. doi: 10.1039/b921073c. [DOI] [PubMed] [Google Scholar]
- 26.Lung function testing: Selection of reference values and interpretative strategies. american thoracic society. Am Rev Respir Dis. 1991;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 27.Wagner BD, Accurso FJ, Laguna TA. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J Cyst Fibros. 2010;9(3):212–216. doi: 10.1016/j.jcf.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Schutz RW. Statistical validity of using ratio variables in human kinetics research. Res Q Exerc Sport. 2003;74(3):226–235. doi: 10.1080/02701367.2003.10609087. [DOI] [PubMed] [Google Scholar]
- 29.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78(5):486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiebaut R, Jacqmin-Gadda H, Chene G, Leport C, Commenges D. Bivariate linear mixed models using SAS proc MIXED. Comput Methods Programs Biomed. 2002;69(3):249–256. doi: 10.1016/s0169-2607(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein RA, Starcher BC. Urinary excretion of elastin peptides containing desmosin after intratracheal injection of elastase in hamsters. J Clin Invest. 1978;61(5):1286–1290. doi: 10.1172/JCI109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janoff A, Chanana AD, Joel DD, Susskind H, Laurent P, Yu SY, Dearing R. Evaluation of the urinary desmosine radioimmunoassay as a monitor of lung injury after endobronchial elastase instillation in sheep. Am Rev Respir Dis. 1983;128(3):545–551. doi: 10.1164/arrd.1983.128.3.545. [DOI] [PubMed] [Google Scholar]
- 33.Stone PJ, Bryan-Rhadfi J, Lucey EC, Ciccolella DE, Crombie G, Faris B, Snider GL, Franzblau C. Measurement of urinary desmosine by isotope dilution and high performance liquid chromatography. correlation between elastase-induced air-space enlargement in the hamster and elevation of urinary desmosine. Am Rev Respir Dis. 1991;144(2):284–290. doi: 10.1164/ajrccm/144.2.284. [DOI] [PubMed] [Google Scholar]
- 34.Downey DG, Martin SL, Dempster M, Moore JE, Keogan MT, Starcher B, Edgar J, Bilton D, Elborn JS. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(3):216–220. doi: 10.1002/ppul.20553. [DOI] [PubMed] [Google Scholar]
- 35.Harel S, Yu SY, Janoff A, Hurewitz A, Bergofsky EH. Measurement of elastin degradation in vivo by desmosine radioimmunoassay. Bull Eur Physiopathol Respir. 1980;16 (Suppl):75–82. doi: 10.1016/b978-0-08-027379-2.50008-9. [DOI] [PubMed] [Google Scholar]
- 36.Stone PJ, Gottlieb DJ, O’Connor GT, Ciccolella DE, Breuer R, Bryan-Rhadfi J, Shaw HA, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151(4):952–959. doi: 10.1164/ajrccm.151.4.7697272. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb DJ, Stone PJ, Sparrow D, Gale ME, Weiss ST, Snider GL, O’Connor GT. Urinary desmosine excretion in smokers with and without rapid decline of lung function: The normative aging study. Am J Respir Crit Care Med. 1996;154(5):1290–1295. doi: 10.1164/ajrccm.154.5.8912738. [DOI] [PubMed] [Google Scholar]
- 38.Viglio S, Iadarola P, Lupi A, Trisolini R, Tinelli C, Balbi B, Grassi V, Worlitzsch D, Doring G, Meloni F, Meyer KC, Dowson L, Hill SL, Stockley RA, Luisetti M. MEKC of desmosine and isodesmosine in urine of chronic destructive lung disease patients. Eur Respir J. 2000;15(6):1039–1045. doi: 10.1034/j.1399-3003.2000.01511.x. [DOI] [PubMed] [Google Scholar]
- 39.Ma S, Lin YY, Turino GM. Measurements of desmosine and isodesmosine by mass spectrometry in COPD. Chest. 2007;131(5):1363–1371. doi: 10.1378/chest.06-2251. [DOI] [PubMed] [Google Scholar]
- 40.Cocci F, Miniati M, Monti S, Cavarra E, Gambelli F, Battolla L, Lucattelli M, Lungarella G. Urinary desmosine excretion is inversely correlated with the extent of emphysema in patients with chronic obstructive pulmonary disease. Int J Biochem Cell Biol. 2002;34(6):594–604. doi: 10.1016/s1357-2725(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 41.Fiorenza D, Viglio S, Lupi A, Baccheschi J, Tinelli C, Trisolini R, Iadarola R, Luisetti M, Snider GL. Urinary desmosine excretion in acute exacerbations of COPD: A preliminary report. Respir Med. 2002;96(2):110–114. doi: 10.1053/rmed.2001.1224. [DOI] [PubMed] [Google Scholar]
- 42.Sagel SD. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Curr Opin Pulm Med. 2003;9(6):516–521. doi: 10.1097/00063198-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Davies SF, Offord KP, Brown MG, Campe H, Niewoehner D. Urine desmosine is unrelated to cigarette smoking or to spirometric function. Am Rev Respir Dis. 1983;128(3):473–475. doi: 10.1164/arrd.1983.128.3.473. [DOI] [PubMed] [Google Scholar]
- 44.McClintock DE, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L566–71. doi: 10.1152/ajplung.00457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerszten RE, Accurso F, Bernard GR, Caprioli RM, Klee EW, Klee GG, Kullo I, Laguna TA, Roth FP, Sabatine M, Srinivas P, Wang TJ, Ware LB. Challenges in translating plasma proteomics from bench to bedside: Update from the NHLBI clinical proteomics programs. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L16–22. doi: 10.1152/ajplung.00044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot and 24-h urines. J Pharm Biomed Anal. 2006;40(4):928–942. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]


