Abstract
Fatty acid metabolism has received significant attention as a route for producing high-energy density, liquid transportation fuels and high-value oleochemicals from renewable feedstocks. If microbes can be engineered to produce these compounds at yields that approach the theoretical limits of 0.3–0.4 g/g glucose, then processes can be developed to replace current petrochemical technologies. Here, we review recent metabolic engineering efforts to maximize production of free fatty acids (FFA) in Escherichia coli, the first step towards production of downstream products. To date, metabolic engineers have succeeded in achieving higher yields of FFA than any downstream products. Regulation of fatty acid metabolism and the physiological effects of fatty acid production will also be reviewed from the perspective of identifying future engineering targets.
Keywords: fatty acid, metabolic engineering, Escherichia coli, thioesterase, biofuel, β-oxidation
Motivations for engineering fatty acid metabolism
Fatty acids are an integral component of living systems and have therefore been studied from many perspectives. Physiological studies focus on fatty acids as a structural component of cell membranes that provide barrier properties and are home to a wide range of metabolic, regulatory, and transport functions [1]. In medicine, fatty acids are a component of lipid signaling that controls cell processes in both healthy and diseased cells [2]. From a biotechnology perspective, fatty acids are energy rich and are therefore incorporated into intracellular lipid bodies (e.g., oils, fats) as a form of storage [3]. Recently, fatty acid metabolism has received significant attention as a route for producing high-energy density, liquid transportation fuels. In addition, microbially produced fatty acids are attractive chemical feedstocks that could replace plant oils, especially in cases where specific chain lengths or modifications (e.g., branches, desaturations) are desired. In the oleochemical industry, FFAs (see Glossary) are precursors to compounds used as agrochemicals, biocidal agents, textile processing agents, soaps, surfactants, and polymer additives [4–6]. Pathways for producing many of these fatty acid derivatives intracellularly have recently been elucidated and catalogued in reviews [7–10]. Briefly, fatty acids or fatty acyl thioesters can be esterified to make triacylglycerides (TAG) and acetyl triacylglycerides [11,12], and fatty acid methyl/ethyl esters (FAMEs/FAEEs) [13–15]. Acyl thioesters can also be reduced to alcohols [14,16–18], decarboxylated to yield α-olefins [19,20], reduced and deformylated to yield alkanes [17], decarboxylated (from β-ketoacids) to form methyl ketones [21], oxidized and polymerized to form polyesters [22], and condensed to form internal ketones and olefins [23,24] (see Figure Id in Box 1). Chemical catalysis has also been used to convert microbially synthesized FFAs or TAGs to many of these compounds [25–27]. The challenge in utilizing any of these pathways for industrial chemical production is optimizing their function in a living cell, such that the yield of product approaches its theoretical limit. Here, we review recent metabolic engineering efforts in the versatile bacterium E. coli to maximize microbial production of FFA, which has advanced further than in vivo production of fatty acid derivatives.
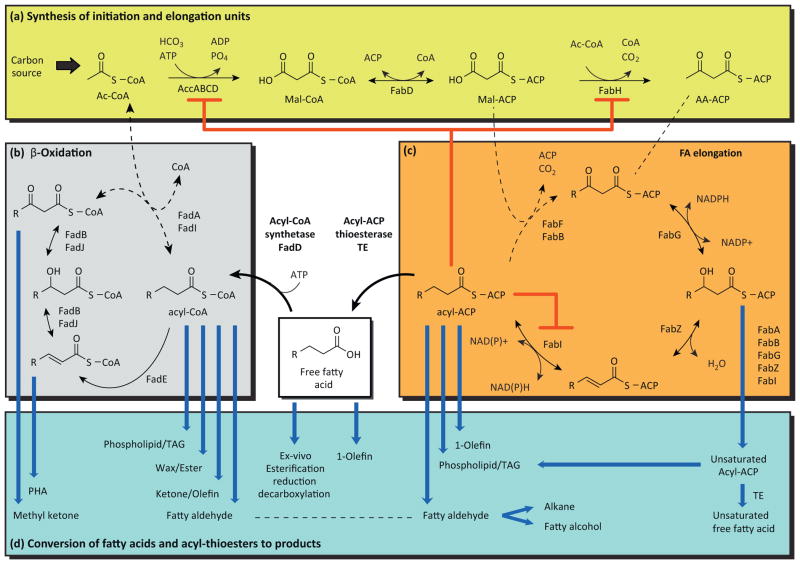
Box 1. Fatty acid metabolism in Escherichia coli.
Synthesis of starter and extender units (Figure Ia)
Acetyl-CoA (Ac-CoA) is a central metabolite made from numerous carbon sources and used in many biosynthetic pathways. In reaction (1), Ac-CoA is a substrate of acetyl-CoA carboxylase (ACC) in the production of malonyl-CoA (Mal-CoA) which, in E. coli, is exclusively consumed for FAB. ACC consists of four subunits, biotin carboxyl carrier protein (AccB), biotin carboxylase (AccC), and acetyl CoA carboxytransferase (heterodimer of AccA and AccD). Mal-CoA is converted to malonyl-ACP (Mal-ACP) in reaction (2) by malonyl-CoA:ACP transacylase (FabD), and to the first β-ketoacyl-ACP in the fatty acid elongation cycle (acetoacetyl-ACP–AA-ACP) in reaction (3) catalyzed by βketoacyl-ACP synthase III (FabH).
Synthesis of long-chain acyl-ACPs (Figure Ib)
E. coli utilizes a type II fatty acid biosynthesis pathway wherein discrete enzymes synthesize and reduce ketoacyl-ACPs. Acetoacetyl-ACP and subsequent ketoacyl-ACPs are reduced by β-ketoacyl-ACP reductase (4, catalyzed by FabG) to yield 3-hydroxyacyl-ACP, which is dehydrated by βhydroxyacyl-ACP dehydratase (5, FabZ) to yield enoyl-ACPs that are reduced by enoyl-ACP reductase (6, FabI). The resulting saturated acyl-ACP is condensed with Mal-ACP by β-ketoacyl-ACP synthase I (FabB) or II (FabF) in reaction (7) to elongate the chain by two carbons. Cis-unsaturated fatty acids are formed at the C10 chain length by 3-hydroxydecanoyl-ACP dehydrase (FabA), which produces both cis and trans species. FabB is essential for elongating cis-2-decenoyl-ACP, the committed step in unsaturated FAB (8). The cycle continues until long-chain acyl-ACPs are incorporated into phospholipids by acyltransferases (9, PlsB, PlsC, PlsX, PlsY). Long-chain acyl-ACPs are also a key regulatory signal that reduces the activity of reactions 1, 3, and 6 (red arrows).
Degradation of fatty acids via β-oxidation (Figure Ic)
FFAs are activated for β-oxidation by acyl-CoA synthetase (11, FadD, FadK). A catabolic oxidation cycle consisting of dehydrogenation by FadE (12), hydration and dehydrogenation by FadB or FadJ (13/14), and thiolation with CoA by FadA or FadI (15) completes one turn of the cycle and generates one molecule of acetyl-CoA.
Conversion of acyl-thioesters and FFAs to final products (Figure Id)
Acyl-ACP thioesterases (Box 2) hydrolyze thioester bonds to produce FFAs. Routes for converting FFAs to esters and alkanes ex vivo and αolefins in vivo have been demonstrated. In addition to phospholipids and FFAs, acyl-ACPs are converted to α-olefins, fatty aldehydes, fatty alcohols, and alkanes via distinct metabolic pathways. Acyl-CoAs made from FFAs are the starting point for synthesis of phospholipids, oils, waxes, esters, ketones, olefins, aldehydes, alcohols, and alkanes. Intermediates in β-oxidation provide the substrates for making bioplastics [e.g., enoyl-CoAs to polyhydroxyalkanoates (PHA) via 3- hydroxyacyl-CoA] and methyl ketones (via ketoacyl-CoAs).
Figure I.
Metabolic pathways for biosynthesis of fatty acids (a,b), β-oxidation (c), and production of high-value chemicals from intermediates in fatty acid metabolism (d).
Brief review of fatty acid metabolism
Fatty acid biosynthesis (FAB) and degradation (β-oxidation) are best understood in E. coli where decades of research have been well summarized in reviews [28–30]. The pathways for FAB and β-oxidation are detailed in Box 1 and Figure Ia–c. Briefly, fatty acids are made via an iterative reduction cycle that operates on acyl carrier protein (ACP) thioesters. In each iteration, two carbons (acetate) are added from malonyl-ACP to a growing acyl-chain and the resulting β-keto group is reduced to a saturated methylene. The process continues until long-chain acyl-ACPs (primarily C16:0, C16:1, C18:1) are incorporated into phospholipids by acyltransferases or converted to other metabolites (e.g., FFAs, fatty aldehydes, olefins; see Figure Id in Box 1). E. coli can also scavenge FFAs and use them as a sole carbon source by generating acetyl-CoA via the βoxidation pathway [30]. This cycle operates similar to FAB, but in reverse, removing one acetyl-CoA per turn. In contrast to fatty acid synthesis, β-oxidation operates on acyl-CoA thioester intermediates instead of acyl-ACP thioesters. The first step of β-oxidation is activation of FFAs to acyl-CoA by acyl-CoA synthetases (e.g., FadD). Acyl-CoAs are also the starting point for synthesizing many attractive oleochemical products including FAEEs, bioplastics, oils, fatty alcohols, alkanes, ketones, and olefins (see Figure Id in Box 1).
Regulation of fatty acid biosynthesis and degradation
In E. coli, the key regulatory signal for controlling FAB is long chain acyl-ACP. The initial connection came from an observation that cultures starved of glycerol (to limit phospholipid biosynthesis) exhibited a decreased rate of acyl-ACP synthesis [31]. FAB was also shut down when the stationary phase alarmone guanosine tetraphosphate (ppGpp) accumulated via ppGpp inhibition of sn-glycerol-3-phosphate acyltransferase (PlsB) [32]. These observations suggested a product-regulated control point for FAB. Concomitantly, flux through FAB was found to be increased by overexpression of a cytosolic form of E. coli thioesterase I (TesA′) [31], which hydrolyzes both acyl-CoAs and acyl-ACPs to generate FFAs and consequently depletes long chain acyl-ACPs [33]. When heterologously expressed, many other acyl-ACP thioesterases generate the same effect (Box 2). Therefore, it was postulated that acyl-ACPs feedback inhibited enzymes in FAB. In vitro studies later confirmed that acyl-ACPs directly inhibit acetyl-CoA carboxylase (ACC) [34] and to lesser degrees β-ketoacyl-ACP synthase III (FabH) and enoyl-ACP reductase (FabI) [35].
Box 2. Acyl-ACP thioesterases.
Expression of an acyl-ACP thioesterase is critical for producing FFAs in E. coli for several reasons. First, it provides the catalytic link between FAB and a product sink. Second, it depletes long-chain acyl-ACPs, the key regulatory signal in E. coli, thereby increasing flux through FAB. Last, the substrate specificity of a thioesterase determines the distribution of product chain lengths similar to the manner in which the composition of plant seed oils is determined. In plants, fatty acids are synthesized in the chloroplast, whereas lipid synthesis occurs in the cytoplasm [68]. Acyl-thioesters cannot cross the plastid membrane, so thioesterases cleave acyl-ACPs of a desired length to enable transport. Plant thioesterases fall into two classes based on homology to enzymes from Arabidopsis thaliana. FatA isoforms target unsaturated acyl chains, particularly oleoyl- ACP (18:1Δ9). Conversely FatB isoforms target saturated acyl chains [69]. As expected, most thioesterases exhibit the highest specificity towards C16–C18 acyl-chains, corresponding to the most abundant acyl-ACPs. Other seed oils are enriched in medium-length acyl chains and thioesterases have been isolated with the corresponding specificity.
Bacteria also possess hundreds of annotated acyl-ACP thioesterases that were uncharacterized prior to a recent study in which activity was studied by heterologous expression in E. coli [70]. Although many appeared to express poorly, functional thioesterases hydrolyzed a wider range of acyl groups. Most commonly, 8:0, 12:0, 12:1, 14:0, and 14:1 FFAs were detected, with some thioesterases producing significant amounts of butyric and hexanoic acids as well. E. coli possesses two native thioesterases, thioesterase I (TesA) and II (TesB), which predominantly cleave acyl- CoAs but also acyl-ACPs with much lower activity in vitro [71]. TesA is a periplasm-directed enzyme that probably does not have access to acyl-CoA or acyl-ACP species. Deletion of a 5′ leader peptide region traps TesA in the cytosol (TesA′), where it hydrolyzes predominantly C14 and C16, and a smaller amount of C12 saturated and unsaturated acyl-ACPs in β-oxidation deficient strains [47]. The physiological roles of both TesA and TesB remain unknown.
Thioesterases have evolved to produce both narrow (e.g., UcTE produces 80% C12, 20% C14 FFA) and broad ranges of FFA products. Methods for modulating chain length specificity have been reported in the patent literature and elsewhere [48,72]. Structural determination and comparative analysis of acyl-ACP thioesterase structures are likely to contribute to protein engineering efforts targeted at controlling product chain length, particularly for medium chain length products.
Identifying the rate-limiting step in FAB would be highly valuable to engineering strains for FFA production. Unfortunately, few kinetic parameters have been determined due to the dozens of acyl-ACP intermediates (saturated-, cis- and trans-enoyl-, β-keto-, and β-hydroxy-containing 4–18 carbons) and the difficulty in quantifying the protein-bound substrates and products. Furthermore, the full spectrum of acyl-ACP-mediated inhibition of all enzymes in FAB has yet to be determined. Despite the lack of kinetic data, candidate rate-limiting steps have been identified by both in vivo and in vitro experiments. Co-overexpression of TesA′ and ACC resulted in higher levels of FFA production than overexpressing TesA′ alone; suggesting that malonyl-CoA synthesis was a rate-limiting step under these conditions [36]. In vitro reconstitution of a full cycle of FAB from acetyl-CoA and malonyl-CoA to butyryl-ACP (using purified FabD, FabH, FabG, FabZ, and FabI) concluded that FabI catalyzed the rate-limiting step [37]. A more detailed in vitro reconstitution that also included FabB, FabF, FabA, and TesA′ to allow consecutive elongation cycles and a product sink for acyl-ACPs revealed that only FabI and FabZ enhanced the rate of fatty acid production in a dose-dependent manner [38].
Transcriptional and translational regulation of FAB is not completely understood. Two transcriptional regulators, FabR and FadR, are involved in controlling unsaturated FAB and β-oxidation [39], but a transcription factor has not been associated with controlling expression of genes encoding all FAB enzymes. This is in contrast to other bacteria (e.g., Bacillus subtilis and Streptococcus pneumoniae) where transcription factors have more universal control over expression of FAB genes [40]. In E. coli, the genes encoding ACC are located in three distal operons (accA, accBC, and accD). Levels of all four ACC subunit transcripts have been found to correlate with growth rate [41], similar to the overall rate of FAB. This finding further supports the role of ACC as a primary gatekeeper of FAB. It is also known that AccB autoregulates transcription of accBC, most likely by DNA binding within its promoter region [42]. Furthermore, translation of accD and accA is autoregulated by RNA binding to the AccAD complex, thereby providing feedback control of translation [43]. However, beyond these connections, the regulation of ACC is poorly understood. Given the key role of malonyl-CoA synthesis, understanding the regulation of ACC, and how it coordinates with the remainder of FAB, warrants further investigation.
Current state of metabolic engineering for FFA production
In order to meet low price targets, the key metrics for judging FFA production are yield (g product per g substrate) and productivity (g product per volume per time). Feedstock costs are predicted to contribute a large percentage of total costs for producing chemicals from biomass [44]. Yield and product price sets the maximum price that can be paid for feedstock. This relationship helps to explain why only processes for producing high-value products such as omega-3 polyunsaturated fatty acids have been successfully commercialized to date. The maximum theoretical yield (TY) of C12, C14, and C16 FFAs, as determined by constraint-based modeling using the iAF1260 metabolic reconstruction of E. coli, [45] range from 0.3 to 0.4 g FFA/g carbon source (Table 1). Although no study has achieved 90% TY, recent studies have shown significant advances and identified novel targets for further engineering. Productivity is also an important characteristic because many oleaginous, high yielding species such as algae and yeasts often require long periods of time to accumulate lipid. These long timescales would require capital expenditure for either larger reactor volumes or a larger number of reactors to meet demand. Rapid rates of growth and fatty acid synthesis are advantages that support E. coli as a host for metabolic engineering. However, significant work will be required to achieve yields comparable to native lipid-accumulating organisms such as Yarrowia lipolytica [12]. The remainder of this review will highlight recent progress and speculate on future directions.
Table 1.
| TY (g FFA per g carbon source) | ||||
|---|---|---|---|---|
| FFA | Glycerol | D-Glucose | D-Xylose | L-Arabinose |
| Lauric (12:0) | 0.39 | 0.35 | 0.29 | 0.29 |
| Myristic (14:0) | 0.38 | 0.34 | 0.29 | 0.28 |
| Palmitic (16:0) | 0.37 | 0.34 | 0.28 | 0.28 |
| Lauroleic (12:1Δ5) | 0.40 | 0.37 | 0.31 | 0.30 |
| Myristoleic (14:1Δ7) | 0.39 | 0.35 | 0.30 | 0.29 |
| Palmitoleic (16:1Δ9) | 0.38 | 0.35 | 0.29 | 0.28 |
Values were calculated by constraint-based modeling using the iAF1260 metabolic network reconstruction of E. coli with reactions added to enable extracellular transport of all listed FFA species and hydrolysis of acyl-ACP species. Maximization of extracellular FFA flux was performed, with reactions catalyzed by PlsB constrained to zero flux to force FFA formation by hydrolysis of acyl-ACPs.
It should be noted that the theoretical mass yields presented here do not reflect the yield of chemical energy, which is significantly higher. For example, theoretical conversion of 2.83 mol of glucose to 1 mol of lauric acid, 5 mol of CO2, and 5 mol of H2O has an enthalpy of reaction of –558 kcal/mol based on standard heats of formation. This represents retention of 84% of the enthalpy in the glucose. This compares favorably with the case of ethanol production in which 94% of the enthalpy is retained.
Early studies of thioesterase expression in β-oxidation-deficient E. coli achieved low titers of FFA (less than 0.3 g/l), where reported. These studies were designed to characterize the substrate specificity of plant acyl-ACP thioesterases or to explore regulation of FAB, and did not report yields or pursue strain optimization. In the first report of a concerted metabolic engineering effort to maximize production of FFA [46], three modifications were made based on knowledge of fatty acid regulation in E. coli: elimination of β-oxidation (ΔfadD); overexpression of two thioesterases (TesA′ and FatB1 from Cinnamomum camphorum, CcTE); and overexpression of E. coli ACC. An optimized (induction, temperature) combination of these modifications yielded a 19-fold increase in total fatty acids compared to the original strain. The fatty acid profile consisted predominantly of saturated and unsaturated C14–C16 species on account of the substrate specificities of TesA′ and CcTE [47,48]. Increased yields were achieved by altering expression levels of TesA′ and CcTE in an ACC-overexpressing ΔfadD strain (from 0.38 to 0.94 g/l) in a rich batch culture [49]. A fed-batch culture in a defined glycerol-supplemented medium, achieved a titer of 2.5 g/l and a yield of 0.048 g FA/g glycerol, representing 12.8% TY. Many subsequent studies utilized similar sets of genetic modifications (Table 2). In general, most strains overproduced FFAs in the C12–C16 range. Some report total fatty acid yields including bound lipids, and others report strictly FFA titers, depending on the analytical work-up procedure employed. TYs in Table 1 were used to normalize each study and enable a straightforward comparison.
Table 2.
Literature summary of FFA titers, yields, and percent of maximum TYa
| Base strain | Modifications | Thioesterase | Titer (g/l) | Mediac | Yieldc (% w/w) | % TY | Time (h) | Culture type | Refs |
|---|---|---|---|---|---|---|---|---|---|
| BL21(DE3) | ΔfadD, ACC+ | TesA′ + CcTE | 0.38 | LB/none | N/A | N/A | > 18 | Batch | [46] |
| BL21(DE3) | ΔfadD, ACC+ | TesA′ + CcTE | 2.5 | M9/gly | 4.8 | 12.8 | 22 | Fed-batch | [46] |
| BL21(DE3) | ΔfadD, ACC+ | TesA′ + CcTE | 0.94 | LB/None | N/A | N/A | > 20 | Batch | [49] |
| DH1 | ΔfadD | TesA′ | 0.7b | M9/2% glu | 3.5 | 10.3 | N/A | Batch | [14] |
| DH1 | ΔfadE | TesA′ | 1.2b | M9/2% glu | 6 | 17.6 | N/A | Batch | [14] |
| K-12 MG1655 | ΔfadD, ACC+ | BTE | 0.81 | LB/0.4% gly | < 16.1 | < 42 | 29 | Batch | [26] |
| K-12 MG1655 | ΔfadD | BTE | 0.77 | LB/0.4% gly | < 15.3 | < 40 | 24 | Batch | [55] |
| K-12 MG1655 | ΔfadD ΔfadE ΔfadAB | BTE | 0.22 | MOPSc/0.4% glu | 5.5 | 15.8 | 24 | Batch | [52] |
| K-12 MG1655 | ACC+, FabD+ | SpTE | 0.16 | LB/none | N/A | N/A | 24 | Batch | [76] |
| K-12 MG1655 | ΔfadD | RcTE | 2.1 | LB/1.5% glu | < 14 | < 41 | 36 | Batch | [62,77] |
| K-12 MG1655 | ΔfadD | JcTE | 1.5 | LB/1.5% glu | < 10 | < 29 | 36 | Batch | [78] |
| BL21(DE3) | ΔfadE | TesA′ + CcTE | 0.45 | LB? | N/A | N/A | > 18 | Batch | [38] |
| BL21(DE3) | ΔfadE, FabZ+, FabG+, FabI+ | TesA′ + CcTE | 0.65 | LB? | N/A | N/A | > 18 | Batch | [38] |
| K-12 MG1655 | ΔfadD, SaFabD+ | RcTE | 1.4 | LB/1.5% glu | < 9.3 | < 27 | 24 | Batch | [77] |
| BL21(DE3) | TesA′ | 5.1b | M9/glu | 4.1 | 12.9 | 38 | Fed-batch | [79] | |
| BL21(DE3) | ΔfadL | TesA′ | 4.8b | M9/glu | 4.4 | 12.1 | 38 | Fed-batch | [79] |
| K-12 MG1655 | See text, FadAB+ | TesA′ | ~0.45b | Minimal/2% glu | 2.3/7.4d | 6.6/22d | 48 | Batch | [63] |
| K-12 MG1655 | See text, FadAB+ | TesB | ~0.70b | Minimal/2% glu | 3.5/13.3d | 10/39d | 48 | Batch | [63] |
| K-12 MG1655 | See text, FadAB+ | FadM | ~0.87b | Minimal/2% glu | 4.4/28d | 13/85d | 48 | Batch | [63] |
| K-12 MG1655 | See text, FadAB+ | FadM | ~7 | Minimal/3% glu | 23/28d | 70/85d | 60 | Batch bioreactor | [63] |
| DH1 | ΔfadE | TesA′ | 3.8 | Minimal/2% glu | 19 | 56% | 72 | Batch | [54] |
Abbreviations: TesA′, cytosolic form of E. coli thioesterase I; CcTE, acyl-ACP TE from Cinnamomum camphorum; BTE, acyl-ACP TE from Umbellularia californica; SpTE, oleoyl-ACP TE from Streptococcus pyogenes; RcTE, acyl-ACP TE from Ricinus communis; JcTE, acyl-ACP TE from Jatropha curcus; SaFabD, FabD from Streptomyces avermitilis; FadM, E. coli acyl-CoA thioesterase; TesB, E. coli thioesterase II (acyl-CoA thioesterase); glu, glucose; gly, glycerol; LB, Luria Bertani Broth; N/A, either not applicable because it cannot be calculated from information given, or not available because information was not provided. See references for further details.
FFA or extracellular fatty acids only.
Several groups performed experiments in rich media such as LB. Because LB contains other usable carbon sources (e.g., amino acids), yields from supplemented carbon (glucose, glycerol) are probably lower than the maxima reported (indicated with <).
Authors’ calculation per g carbon source consumed (other values are per g carbon source supplied).
One of the advantages of using E. coli as a platform for producing fatty acids is the ability to target different chain length products. E. coli uses a Type II FAB system in which intermediates are accessible. By contrast, fungi, animals, and some bacteria use a Type I system where all catalytic activities are located in a single megasynthase complex that prevents access to intermediates and generates a narrow range of fatty acid products [50]. Although abundant in many organisms, long chain (C16–C18) fatty acids and corresponding downstream products have properties that are incompatible with cold temperature applications. For this reason, several groups have targeted production of shorter products. For example, medium-chain length FFA were produced by expressing the FatB acyl-ACP thioesterase from Umbellularia californica (UcTE), which acts on predominantly saturated C12 acyl-ACPs, and to a lesser degree unsaturated C12 and saturated and unsaturated C14 acyl-ACPs [51]. Titers of ~0.8 g/l in a rich undefined medium were reported (maximum 42% TY) when UcTE and ACC were overexpressed in a ΔfadD derivative of MG1655 [26]. An improved production strain harboring three chromosomal integrations of UcTE under control of the IPTG-inducible trc promoter in the fadD, fadE, and fadAB loci [52] achieved 16% TY in a batch culture and 20% TY in a chemostat operating with a dilution rate of 0.05/h [52].
The aforementioned studies demonstrate that thioesterase expression is a key step in producing FFA in E. coli. In general, higher titers were achieved in strains that maintained lower levels of thioesterase expression when using plasmid copy number [26] or promoter strength [49,53] to vary expression levels. When cell-free lysates or an in vitro reconstituted FAB system were supplemented with high concentrations of TesA′, rates of FFA production were inhibited [38,49]. Surprisingly, titration of CcTE to cell-free lysates resulted in no inhibition [49] indicating that this effect is thioesterase-specific. Interestingly, titration of the non-functional TesA′-S10A active site mutant enhanced FAB activity in the in vitro system when functional TesA′ was also present, suggesting a possible activity in sequestration of certain acyl-ACP intermediates [38]. Supporting this hypothesis, matrix-assisted laser desorption/ionization- time of flight (MALDI-TOF) analysis of acyl-ACP species present in an in vitro FAB reaction mixture containing TesA′ revealed a higher abundance of C6, C10, C16, and C18 chain lengths. As mentioned above, depletion of long-chain acyl-ACP pools via thioesterase activity derepresses enzymes leading to elevated flux through FAB. The decreased titers observed in strains with high levels of thioesterase expression could be due to depleting acyl-ACP pools to the point where lipid biosynthesis and cell growth are impaired or to physiological defects caused by elevated rates of FFA biosynthesis. Optimizing thioesterase expression and addressing the unintended consequences of modulating acyl-ACP pools will be critical steps towards developing commercial production strains. For example, a recent study reported a 3.8 g/l titer of FFAs over the course of 72 h in which the only modification to a prior study [14] was the use of a low copy vector for the thioesterase [54]. Although maximizing FFA production was not the objective of this study, it represents 56% TY, the highest yet achieved via FAB.
Blocking FFA consumption and optimizing thioesterase expression are two critical steps required for FFA production in E. coli but result in yields that remain less than 50% TY in most cases. Many studies have co-overexpressed additional FAB enzymes in attempt to identify and circumvent rate-limiting steps. Many of the steps in FAB are reversible and an effort to overexpress enzymes (FabG, FabZ, and FabI) that catalyze these reactions resulted in a modest improvement in titer (from 0.45 to 0.65 g/l) [38] from batch LB cultures using a ΔfadE strain expressing TesA′ and CcTE. In many organisms, the irreversible synthesis of malonyl-CoA by ACC is a key regulatory point. Overexpression of E. coli ACC in UcTE-expressing strains resulted in little to no increase in FFA titers at most sampling times [26,55]. Supporting this result, malonyl- ACP, an intermediate one step downstream of the ACC reaction, was found to accumulate in another E. coli strain expressing UcTE during the exponential phase [56]. By contrast, a 24% increase in molar titer was reported from additional overexpression of ACC in a strain harboring CcTE [46], and a sixfold increase in FFA production was demonstrated when ACC was overexpressed in a TesA′ expressing strain [36], suggesting that ACC catalyzes the rate-limiting step in these systems. The latter experiment was performed using radiolabeled substrates over a short period of time and final FFA titers were not reported. Expression of UcTE in E. coli leads to increased levels of at least one key ACC subunit, biotin carboxyl carrier protein (AccB) [55,56], which could partially explain this thioesterase-specific effect. Variations in thioesterase expression and activity, as well as the specific profiles of accumulating inhibitory acyl-ACP intermediates (as inferred by [38]) could also be responsible for these disparate observations. Further investigation of the dynamic interplay of thioesterase expression, accumulating acyl-ACP pools, and regulation of ACC would be valuable toward discovering rate-limiting steps in FFA production in vivo.
In an effort to determine whether cofactor or enzyme levels constituted bottlenecks to FFA production, FAB substrates were titrated into cell-free lysates of an E. coli ΔfadD strain [49]. Titration of NADPH resulted in an apparent Km of 35 μM, well below typical intracellular levels, indicating that NADPH levels are not limiting. Titration of both malonyl-CoA and ACC from Mycobacterium tuberculosis increased fatty acid productivity (it was unclear if a thioesterase was supplied to provide a product sink). The apparent Km for malonyl-CoA (15.7 μM) exceeded measured intracellular concentrations, thus it was concluded that malonyl-CoA could be limiting. It should be noted that in the absence of a product sink, the apparent Km may be increased by allosteric inhibition of ACC with acyl-ACPs. In a subsequent in vitro FAB reconstitution (with supplied TesA′) study [38], the same initial rates of FFA biosynthesis were observed with the addition of 0.3 mM and 1.5 mM malonyl-CoA, suggesting that malonyl-CoA was not limiting.
Recent efforts to improve malonyl-CoA availability for metabolic engineering of flavonoid and polyketide biosynthesis have targeted heterologous expression of ACC and biotin ligase [57,58], deletion of acetate kinase (ackA) and phosphotransacetylase (pta) involved in acetate formation, deletion of alcohol dehydrogenase (adhE), and overexpression of acetyl-CoA synthetase (Acs) [59], and other targets determined by metabolic network modeling [60,61]. Although these strategies were successful in increasing malonyl-CoA levels and flavonone titers, each were lower on a carbon-basis than FFA titers achieved by thioesterase expression. Many of these strategies have also proven ineffective for improving FFA yields both in our laboratory and others [49,62], indicating that acetyl-CoA consumption is not a limiting factor. This is consistent with the observation that acetate accumulation is greatly decreased in E. coli expressing acyl-ACP thioesterase from Ricinus communis (RcTE) [62].
A promising alternative route for producing FFAs and chemical derivatives involves reversing β-oxidation, such that it operates anabolically rather than in the usual catabolic direction [63]. The pathway is possible because of the reversibility of FadA and the two reactions catalyzed by FadB. This pathway is advantageous because it eliminates the need for ATP in synthesizing malonyl-CoA, thereby increasing TYs under both aerobic (~7%) and anaerobic (~38%) conditions. Recently, a highly engineered strain was designed to overexpress β-oxidation enzymes, prevent alternative fermentative pathways, and block re-uptake of FFA (ΔfadD). When FadA, FadB, and various acyl-CoA thioesterases were expressed, high titers of C16–C18 FFAs were observed (Table 2). Interestingly, fadE was not deleted and YdiO, which was suggested experimentally to catalyze reduction of enoyl-CoAs, was not overexpressed. In shake flask cultures, up to 85% TY (for traditional FAB) was attained on the basis of the glucose consumed by a thioesterase-expressing strain approaching levels required for commercialization. Of note was the addition to the bioreactor medium of 1 mM betaine, an osmolyte that has previously been shown to greatly improve microbial production of lactate [64].
Effects of FFA production on physiology of E. coli
Given that hydrophobic solvents can have a severe, negative impact on microbial physiology [65,66], additional effort may be needed to engineer stable FFA producers. An extensive literature exists on the antibacterial properties of FFAs [4]. Many attempts have been made to correlate FFA antibacterial activity with chain length and degree/position of unsaturation, however a lack of standardized testing methodology renders the interpretation difficult. In general, unsaturated FFAs appear more potent against a range of microorganisms than saturated species, and amongst saturated species, C10 to C14 FFAs exhibit the most commonly cited activities against bacteria. The modes of action are largely membrane-associated and include: disruption of the electron transport chain due to displacement or impaired function of electron carriers, uncoupling of oxidative phosphorylation via dissipation of proton motive force due to FFAs flipping across the membrane and acting as proton carriers, or from direct inhibition of ATP synthase, and cell lysis due to membrane intercalation resulting in the formation of pores.
Although many effects of exogenous FFA addition are known, the effects of endogenous production are less characterized and expected to be more severe given the higher local concentration. In a direct comparison, a lower degree of cell lysis and lower loss of viability was observed from exogenous addition of lauric acid than from endogenous production using UcTE [55]. Transcript and protein expression profiling revealed activation of the phage shock regulon and increased expression of NADH dehydrogenase I and cytochrome bo3 oxidase, indicative of uncoupling of oxidative phosphorylation and impairment of aerobic respiration [55]. Induction of toxic small molecule and oxidative stresses (MarA/Rob/SoxS regulons) was also observed. Identifying transporters capable of increasing transport of FFA (and reduced derivatives) across the cell membrane may be key to reducing or eliminating these stresses (see Figure I in Box 3).
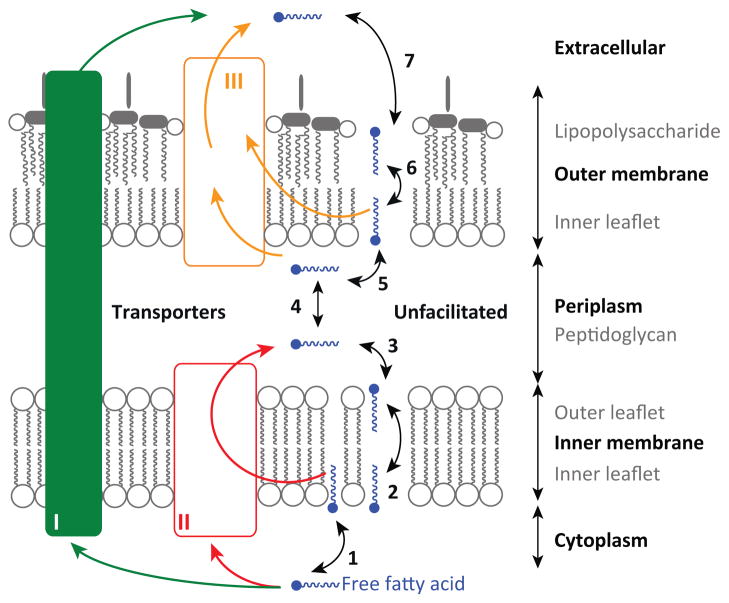
Box 3. Future directions for improving fatty acid production.
Given the current understanding of FAB in E. coli, four areas warrant pursuit.
Increase export rates. Despite the fact that FFA are predominantly found extracellularly, FFA export is poorly understood. The boundary of E. coli consists of an inner membrane, periplasm, and outer membrane (Figure I). For FFA to exit without the help of transporters, a molecule must (1) insert into the inner membrane, (2) flip across to the outer leaflet, (3) leave the inner membrane, (4) diffuse (bypassing the peptidoglycan layer) to, and (5) insert into the outer membrane, (6) flip to the outer leaflet, and (7) exit the lipopolysaccharide layer into the environment. Although a FFA exporter has not yet been identified in E. coli, transport proteins that span all three layers (I, green), the inner membrane (II, red), or the outer membrane (III, orange) may facilitate this process. Import of FFA across the outer membrane (III) is facilitated by FadL [30]. Lipopolysaccharide precursor, lipid A is exported to the periplasm (II) by MsbA [73]. Hydrophobic drugs and toxins are exported (I) by transporter complexes such as AcrAB/TolC [74]. Once exporters are identified, strains could be engineered to increase efflux rates and avoid toxic effects of desired products [75].
Regulate membrane saturation. E. coli uses a unique pathway that branches at C10-acyl-ACP for synthesizing unsaturated fatty acids (Figure II). (a) Flux through the unsaturated branch is controlled by the relative amount of unsaturated long-chain acyl- ACP via FabR. When a thioesterase is expressed, the pools of long-chain acyl-ACP are modified. (b) If the thioesterase primarily acts on saturated acyl-ACPs, the ratio of saturated to unsaturated long-chain acyl-ACPs available for incorporation into phospholipids can be significantly altered despite the presence of FabR [51,55]. (c) When the substrate specificity is relaxed, the effect is dampened. Given the impact of membrane unsaturation, efforts to control membrane content could have a strong impact on cell physiology.
-
Address metabolic and regulatory bottlenecks. Despite significant effort, the barriers that prevent FFA production from approaching TYs remain unknown. Once found, incorporation of genetic manipulations that circumvent these barriers should improve FFA yields. In addition, studies that facilitate anaerobic production of FFA or reduced products could reduce operating costs associated with aeration. Future directions include:
Overexpression of limiting enzymes from metabolic modeling and biochemistry studies
Engineer a stable stationary phase metabolism wherein carbon is directed solely to FFA production and not biomass
Downregulate competing pathways that consume ATP, NAD(P)H, and acetyl-CoA
Alter cofactor utilization and supply
Design strains to make fatty acids as a fermentation (anaerobic) product
Balancing fatty acid synthesis and reductive pathways for producing fatty acid derivatives
Identify regulatory connections between growth rate, acyl-ACP pools, and FAB enzyme levels
-
Structural, biochemistry, and genetic studies. Additional fundamental studies are needed to support metabolic engineering efforts targeted at altering product properties (controlling chain length), increasing yields (additional regulatory studies), and utilizing other species. Additional directions for future study include:
Structures of thioesterases and downstream enzymes
Understanding the features that control substrate specificity of thioesterases
Understanding the features that control substrate specificity of acyl-ACP reductases
Kinetic and inhibition studies of FAB enzymes to facilitate kinetic models
Cofactor utilization
Modes of allosteric and transcriptional/translational regulation of ACC in E. coli
Figure I.
FFA export from Gram-negative bacteria.
Figure II.
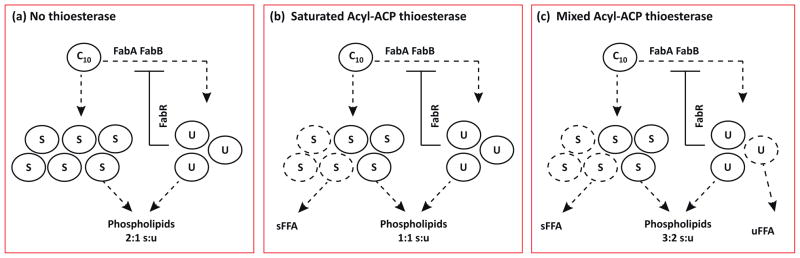
Impact of thioesterase specificity on membrane saturation.
In the same study, thioesterase expression also resulted in elevated (~60%) unsaturated fatty acid content in the membrane [51,55]. The elevated unsaturated content observed in UcTE-expressing cells occurred despite strong transcriptional repression of fabA and fabB, perhaps indicating a metabolic incapability to maintain membrane lipid homeostasis in the face of altered acyl-ACP pools. The degree to which a thioesterase alters membrane composition appears to be directly correlated to its substrate specificity, resulting in disparate effects (see Figure II in Box 3). For instance, expression of the FatA type thioesterase from Helianthus annuus in E. coli, which predominantly cleaves unsaturated C16 and C18 acyl-ACPs increased the saturated phospholipid acyl content by approximately 5% [67]. Overexpression of a cytosolic form of E. coli thioesterase I (TesA′), which was reported to generate a FFA distribution of approximately 54% unsaturated and 46% saturated, also resulted in a 7.5% reduction of unsaturated and cyclic phospholipid acyl group content [47]. Given the strong impact of unsaturated content on membrane properties (e.g., temperature and membrane fluidity), developing strategies for maintaining proper membrane composition may be vital for the stability of industrial FFA producers.
Concluding remarks and future perspectives
Fatty acid biosynthesis is a promising pathway for producing next-generation biofuels and high-value oleochemicals. Significant progress has been made in engineering E. coli for production of FFA, a central intermediate in the production of these targeted compounds. Although yields of FFA are not yet above desired targets (>90% TY), many strategies that combine blocking FFA consumption and optimal thioesterase expression can achieve 50% TY. Conversely, the production of reduced products has not advanced as far [8]. Lower titers are likely to be the result of new challenges such as balancing additional enzymes, energy (ATP), and reducing power (NADH) needed on top of those targeted for FFA production. Alternatively, the presence of futile cycles (e.g., thioesterase activity on acyl-CoAs), product toxicity, and/or product accumulation could reduce final yields. Despite these challenges, efforts to commercialize microbial production of reduced fatty acid derivatives are being pursued in both industrial and academic groups. Future efforts in E. coli and other organisms (e.g., cyanobacteria, yeast, Pseudomonas, Bacillus, and Lactobacillus) will benefit from further study of FAB regulation and how it compares to E. coli. Identifying metabolic bottlenecks (e.g., carbon uptake and utilization) and designing optimal cultivation strategies (e.g., fed batch strategies or designing a stable stationary phase) for producing FFA from renewable carbon sources are obvious targets for improving yields. In addition, further studies to mitigate product toxicities and enhance the catalytic activity of pathway enzymes are warranted. Many of these challenges will also be faced by groups engineering other secondary metabolite pathways such as isoprenoids and polyketides.
Update
Recently, plasmid based expression of FadR, a known repressor of βoxidation and activator of unsaturated fatty acid biosynthesis, was shown to activate additional genes in saturated fatty acid biosynthesis (Zhang et al., Metabolic Engineering, in press). Co-expression of FadR and TesA′ in E. coli DH1 resulted in FFA production greater than 70% TY in minimal media, whereas co-expression of TesA′ with individual genes upregulated by FadR did not. These findings strongly motivate further study into the modes of FadR regulation.
Acknowledgments
This work was funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Sciences DE-FC02-07ER64494). R.M.L. was supported as a trainee in the Chemistry–Biology Interface Training Program (NIH) and by the Department of Chemical and Biological Engineering Dahlke–Hougen Fellowship.
Glossary
- Acetyl-CoA carboxylase (ACC)
a multi-enzyme complex comprised of four protein subunits in E. coli (AccA, AccB, AccC, AccD). The ACC complex catalyzes the carboxylation of biotin and transfer of a carbon dioxide from carboxybiotin to acetyl-CoA to yield malonyl-CoA, the direct precursor to the elongation unit used to extend fatty acid chains
- Acyl carrier protein (ACP)
a small (78 kDa in E. coli), abundant protein that is linked to intermediates in FAB by a post-translationally added phosphopantetheine group. ACP functions as a scaffold for shepherding intermediates between catalytic enzymes in FAB
- Fatty acid biosynthesis (FAB)
used to represent the collection of reactions and enzymes responsible for synthesizing fatty acids from acetyl-CoA including synthesis of starter and elongation units, the iterative cycle of chain elongation, and reduction
- Fatty acid methyl/ethyl esters (FAMEs/FAEEs)
molecules that when blended are commonly referred to as biodiesel. FAMEs are made by esterifying a fatty acid with methanol and FAEE are made by esterifying a fatty acid with ethanol
- Free fatty acid(s) (FFA)
non-esterified carboxylic acids containing acyl chains ranging from four (butyric) to 18 (stearic) carbons. FFA are produced by enzymatic cleavage of lipids and acyl-thioesters in the cell
- Theoretical yield (TY)
the maximum possible yield of product from a given feedstock within the context of the cellular network of biochemical reactions
- Triacylglycerides (TAG)
a common energy storage molecule comprised of a central glycerol esterified to three fatty acids. TAG derived from plant, algae, and animal sources are currently used as a starting material for biodiesel synthesis
References
- 1.Ruiz N, et al. Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol. 2006;4:57–66. doi: 10.1038/nrmicro1322. [DOI] [PubMed] [Google Scholar]
- 2.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 3.Walther TC, Farese RV. Lipid droplets and cellular lipid metabolism. In: Kornberg RD, editor. Annual Review of Biochemistry. 2012. pp. 687–714. Annual Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 5.Gunstone FD, Padley FB, editors. Lipid Technologies and Applications. Marcel Dekker; 1997. [Google Scholar]
- 6.Jones AM, et al. Isolation and identification of mosquito (Aedes aegypti) biting deterrent fatty acids from male inflorescences of breadfruit (Artocarpus altilis (Parkinson) Fosberg) J Agric Food Chem. 2012;60:3867–3873. doi: 10.1021/jf300101w. [DOI] [PubMed] [Google Scholar]
- 7.Handke P, et al. Application and engineering of fatty acid biosynthesis in Escherichia coli for advanced fuels and chemicals. Metab Eng. 2011;13:28–37. doi: 10.1016/j.ymben.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Huffer S, et al. Escherichia coli for biofuel production: bridging the gap from promise to practice. Trends Biotechnol. 2012;30:538–545. doi: 10.1016/j.tibtech.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Peralta-Yahya PP, et al. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 10.Wackett LP. Engineering microbes to produce biofuels. Curr Opin Biotechnol. 2011;22:388–393. doi: 10.1016/j.copbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Durrett TP, et al. A distinct DGAT with sn-3 acetyltransferase activity that synthesizes unusual, reduced-viscosity oils in Euonymus and transgenic seeds. Proc Natl Acad Sci USA. 2010;107:9464–9469. doi: 10.1073/pnas.1001707107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosa M, Ragauskas AJ. Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol. 2011;29:53–61. doi: 10.1016/j.tibtech.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Kalscheuer R, et al. Microdiesel: Escherichia coli engineered for fuel production. Microbiology. 2006;152:2529–2536. doi: 10.1099/mic.0.29028-0. [DOI] [PubMed] [Google Scholar]
- 14.Steen EJ, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463:559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- 15.Nawabi P, et al. Engineering Escherichia coli for biodiesel production utilizing a bacterial fatty acid methyltransferase. Appl Environ Microbiol. 2011;77:8052–8061. doi: 10.1128/AEM.05046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doan TT, et al. Functional expression of five Arabidopsis fatty acyl-CoA reductase genes in Escherichia coli. J Plant Physiol. 2009;166:787–796. doi: 10.1016/j.jplph.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Schirmer A, et al. Microbial biosynthesis of alkanes. Science. 2010;329:559–562. doi: 10.1126/science.1187936. [DOI] [PubMed] [Google Scholar]
- 18.Teerawanichpan P, Qiu X. Fatty acyl-CoA reductase and wax synthase from Euglena gracilis in the biosynthesis of medium-chain wax esters. Lipids. 2010;45:263–273. doi: 10.1007/s11745-010-3395-2. [DOI] [PubMed] [Google Scholar]
- 19.Rude MA, et al. Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from Jeotgalicoccus species. Appl Environ Microbiol. 2011;77:1718–1727. doi: 10.1128/AEM.02580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez-Perez D, et al. Modular synthase-encoding gene involved in alpha-olefin biosynthesis in Synechococcus sp. strain PCC 7002. Appl Environ Microbiol. 2011;77:4264–4267. doi: 10.1128/AEM.00467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh EB, et al. Engineering of bacterial methyl ketone synthesis for biofuels. Appl Environ Microbiol. 2012;78:70–80. doi: 10.1128/AEM.06785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnew D, et al. Engineering Escherichia coli for production of mcl-PHA homopolymer from glucose. Metab Eng. 2012;14:705. doi: 10.1016/j.ymben.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukovich DJ, et al. Widespread head-to-head hydrocarbon biosynthesis in bacteria and role of OleA. Appl Environ Microbiol. 2010;76:3850–3862. doi: 10.1128/AEM.00436-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beller HR, et al. Genes involved in long-chain alkene biosynthesis in Micrococcus luteus. Appl Environ Microbiol. 2010;76:1212–1223. doi: 10.1128/AEM.02312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James OO, et al. A review on conversion of triglycerides to on-specification diesel fuels without additional inputs. Int J Energy Res. 2012;36:691–702. [Google Scholar]
- 26.Lennen RM, et al. A process for microbial hydrocarbon synthesis: overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng. 2010;106:193–202. doi: 10.1002/bit.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lestari S, et al. Transforming triglycerides and fatty acids into biofuels. ChemSusChem. 2009;2:1109–1119. doi: 10.1002/cssc.200900107. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 29.Cronan JE, Jr, Rock CO. Chapter 3.6.4: Biosynthesis of membrane lipids. In: Curtiss R III, editor. EcoSal - Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; 2008. [Google Scholar]
- 30.Clark DP, Cronan JE. Two-carbon compounds and fatty acids as carbon sources. In: Böck A, editor. EcoSal - Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; 2005. [DOI] [PubMed] [Google Scholar]
- 31.Jiang P, Cronan JE., Jr Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol. 1994;176:2814–2821. doi: 10.1128/jb.176.10.2814-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heath RJ, et al. Guanosine tetraphosphate inhibition of fatty acid and phospholipid synthesis in Escherichia coli is relieved by overexpression of glycerol-3-phosphate acyltransferase (plsB) J Biol Chem. 1994;269:26584–26590. [PubMed] [Google Scholar]
- 33.Cho H, Cronan JE., Jr Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J Biol Chem. 1993;268:9238–9245. [PubMed] [Google Scholar]
- 34.Davis MS, Cronan JE., Jr Inhibition of Escherichia coli acetyl coenzyme A carboxylase by acyl-acyl carrier protein. J Bacteriol. 2001;183:1499–1503. doi: 10.1128/JB.183.4.1499-1503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath RJ, Rock CO. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:1833–1836. doi: 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 36.Davis MS, et al. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2000;275:28593–28598. doi: 10.1074/jbc.M004756200. [DOI] [PubMed] [Google Scholar]
- 37.Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, et al. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli. Proc Natl Acad Sci USA. 2011;108:18643–18648. doi: 10.1073/pnas.1110852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu K, et al. Transcriptional regulation of membrane lipid homeostasis in Escherichia coli. J Biol Chem. 2009;284:34880–34888. doi: 10.1074/jbc.M109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita Y, et al. Regulation of fatty acid metabolism in bacteria. Mol Microbiol. 2007;66:829–839. doi: 10.1111/j.1365-2958.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 41.Li SJ, Cronan JE., Jr Growth rate regulation of Escherichia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J Bacteriol. 1993;175:332–340. doi: 10.1128/jb.175.2.332-340.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James ES, Cronan JE. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J Biol Chem. 2004;279:2520–2527. doi: 10.1074/jbc.M311584200. [DOI] [PubMed] [Google Scholar]
- 43.Meades G, Jr, et al. A tale of two functions: enzymatic activity and translational repression by carboxyltransferase. Nucleic Acids Res. 2010;38:1217–1227. doi: 10.1093/nar/gkp1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murat Sen S, et al. Catalytic conversion of lignocellulosic biomass to fuels: process development and technoeconomic evaluation. Chem Eng Sci. 2012;67:57–67. [Google Scholar]
- 45.Feist AM, et al. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol Syst Biol. 2007;3:121. doi: 10.1038/msb4100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, et al. Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng. 2008;10:333–339. doi: 10.1016/j.ymben.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Cho H, Cronan JE., Jr Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem. 1995;270:4216–4219. doi: 10.1074/jbc.270.9.4216. [DOI] [PubMed] [Google Scholar]
- 48.Yuan L, et al. Modification of the substrate specificity of an acylacyl carrier protein thioesterase by protein engineering. Proc Natl Acad Sci USA. 1995;92:10639–10643. doi: 10.1073/pnas.92.23.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, et al. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng. 2010;12:378–386. doi: 10.1016/j.ymben.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Smith S, Tsai SC. The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat Prod Rep. 2007;24:1041–1072. doi: 10.1039/b603600g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voelker TA, Davies HM. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J Bacteriol. 1994;176:7320–7327. doi: 10.1128/jb.176.23.7320-7327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youngquist JT, et al. Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol Bioeng. 2012;109:1518–1527. doi: 10.1002/bit.24420. [DOI] [PubMed] [Google Scholar]
- 53.Hoover SW, et al. Bacterial production of free fatty acids from freshwater macroalgal cellulose. Appl Microbiol Biotechnol. 2011;91:435–446. doi: 10.1007/s00253-011-3344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F, et al. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- 55.Lennen RM, et al. Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl Environ Microbiol. 2011;77:8114–8128. doi: 10.1128/AEM.05421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohlrogge J, et al. Alteration of acyl-acyl carrier protein pools and acetyl-CoA carboxylase expression in Escherichia coli by a plant medium chain acyl-acyl carrier protein thioesterase. Arch Biochem Biophys. 1995;317:185–190. doi: 10.1006/abbi.1995.1152. [DOI] [PubMed] [Google Scholar]
- 57.Miyahisa I, et al. Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl Microbiol Biotechnol. 2005;68:498–504. doi: 10.1007/s00253-005-1916-3. [DOI] [PubMed] [Google Scholar]
- 58.Leonard E, et al. Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol. 2007;73:3877–3886. doi: 10.1128/AEM.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zha W, et al. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab Eng. 2009;11:192–198. doi: 10.1016/j.ymben.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Fowler ZL, et al. Increased malonyl coenzyme A biosynthesis by tuning the Escherichia coli metabolic network and its application to flavanone production. Appl Environ Microbiol. 2009;75:5831–5839. doi: 10.1128/AEM.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu P, et al. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng. 2011;13:578–587. doi: 10.1016/j.ymben.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Li M, et al. Effect of acetate formation pathway and long chain fatty acid CoA-ligase on the free fatty acid production in E. coli expressing acy-ACP thioesterase from Ricinus communis. Metab Eng. 2012;14:380–387. doi: 10.1016/j.ymben.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Dellomonaco C, et al. Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature. 2011;476:355–359. doi: 10.1038/nature10333. [DOI] [PubMed] [Google Scholar]
- 64.Zhou S, et al. Betaine tripled the volumetric productivity of D(−)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett. 2006;28:671–676. doi: 10.1007/s10529-006-0033-4. [DOI] [PubMed] [Google Scholar]
- 65.Sikkema J, et al. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sardessai Y, Bhosle S. Tolerance of bacteria to organic solvents. Res Microbiol. 2002;153:263–268. doi: 10.1016/s0923-2508(02)01319-0. [DOI] [PubMed] [Google Scholar]
- 67.Serrano-Vega MJ, et al. Cloning, characterization and structural model of a FatA-type thioesterase from sunflower seeds (Helianthus annuus L. ) Planta. 2005;221:868–880. doi: 10.1007/s00425-005-1502-z. [DOI] [PubMed] [Google Scholar]
- 68.Benning C. Annual Review of Cell and Developmental Biology, Annual Reviews. 2009. Mechanisms of lipid transport involved in organelle biogenesis in plant cells; pp. 71–91. [DOI] [PubMed] [Google Scholar]
- 69.Cantu DC, et al. Thioesterases: a new perspective based on their primary and tertiary structures. Protein Sci. 2010;19:1281–1295. doi: 10.1002/pro.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jing F, et al. Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem. 2011;12:44. doi: 10.1186/1471-2091-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer AK, et al. Thioesterases I and II of Escherichia coli Hydrolysis of native acyl-acyl carrier protein thioesters. J Biol Chem. 1978;253:5922–5926. [PubMed] [Google Scholar]
- 72.Louis, H. et al. LS9, Inc. Methods and compositions related to thioesterase enzymes, 2010/0154293
- 73.King G, Sharom FJ. Proteins that bind and move lipids: MsbA and NPC1. Crit Rev Biochem Mol Biol. 2012;47:75–95. doi: 10.3109/10409238.2011.636505. [DOI] [PubMed] [Google Scholar]
- 74.Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta. 2009;1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunlop MJ, et al. Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol. 2011;7:487. doi: 10.1038/msb.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeon E, et al. Development of Escherichia coli MG1655 strains to produce long chain fatty acids by engineering fatty acid synthesis (FAS) metabolism. Enzyme Microb Technol. 2011;49:44–51. doi: 10.1016/j.enzmictec.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, et al. Improving fatty acid production in Escherichia coli through the overexpression of malonyl coA-acyl carrier protein transacylase. Biotechnol Prog. 2012;28:60–65. doi: 10.1002/btpr.716. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, et al. Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metab Eng. 2011;13:713–722. doi: 10.1016/j.ymben.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Liu H, et al. Production of extracellular fatty acid using engineered Escherichia coli. Microb Cell Fact. 2012;11:41. doi: 10.1186/1475-2859-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]