Abstract
Introduction
Cystic fibrosis related diabetes is the most common co-morbidity in persons with CF. ISPAD guidelines recommend annual OGTT screening starting at age 10. The OGTT might be recommended in younger children if, as in adults, it provided clinically relevant prognostic information. A database review was performed to determine whether OGTT findings in children with CF predict subsequent clinical course.
Methods
A retrospective matched-pair cohort study was based on OGTTs performed 1998–2003. Children age 6–9 were classified as normal glucose tolerance (NGT) or abnormal glucose tolerance (AGT). AGT were matched by age and gender to NGT. Clinical status was assessed at baseline and 5 years later. In a separate investigation, diabetes and prior AGT status of children aged 10–18 were used to assess predictions derived from the cohort study.
Results
In 1998–2003, 39 of 94 children had AGT. Of these, 31 had sufficient follow-up data to be included. Both at baseline and 5 years later there was no significant difference in height, weight, BMI or lung function between AGT and NGT. Diabetes developed in 13 AGT (42%) and one NGT (3%) (odds ratio (OR) 11, p= 0.0009). Age of diabetes onset was 12±1 years in boys and 11±1 in girls, compared to ~23 years in the general CF population. Fifteen current children age 10–18 who had AGT before age 10 have diabetes, close to the prediction of 19.
Conclusions
Abnormal glucose tolerance in children with CF age 6–9 years identifies those at high risk for progression to early onset diabetes.
Keywords: CFRD, prognosis
Introduction
Cystic fibrosis related diabetes (CFRD) is the most common co-morbidity in persons with CF, occurring in about 20% of adolescents and 40–50% of adult patients (1). Fibrotic damage to the pancreas leads to insulin insufficiency, which is associated with a spectrum of glucose tolerance abnormalities (Table 1). Patients can be classified by oral glucose tolerance testing (OGTT) as having normal glucose tolerance (NGT), impaired glucose tolerance (IGT), CFRD without fasting hyperglycemia (CFRD FH−) or CFRD with fasting hyperglycemia (CFRD FH+). In addition, indeterminate glycemia (INDET) occurs in individuals whose baseline and 2-hour glucose levels are normal but whose glucose level is greater than 200 mg/dl (11.1 mmol/l) in the middle of the OGTT. While INDET is not an American Diabetes Association designation, it has been recognized in major studies such as the Diabetes Prevention Trial-1 and TrialNet (2) and by the recent Consensus Conference on CFRD, sponsored by the CF Foundation, the American Diabetes Association and the Lawson Wilkins Pediatric Society (3). Impaired fasting glucose (IFG), defined as a fasting glucose level between 100–126 mg/dl (5.6–11.1 mmol/l), has also been described in CF. Generally, IGT, INDET, and CFRD FH− are clinically silent and can only be detected through screening. The International Society for Pediatric and Adolescent Diabetes (ISPAD) 2008 Consensus Guidelines recommend annual OGTT screening for all CF patients age 10 and older because it is at this age that the prevalence of diabetes steeply rises (4).
Table 1.
Classification of glucose tolerance abnormalities in CF
| Fasting Plasma Glucose mg/dl (mmol/l) | 2-Hour OGTT Glucose mg/dl (mmol/l) | |
|---|---|---|
| Normal glucose tolerance (NGT) | <100 (5.6) | <140 (7.8) |
| Abnormal glucose tolerance (AGT) | ||
| Indeterminate glycemia (INDET)* | <100 (5.6) | <140 (7.8) |
| Impaired glucose tolerance (IGT) | <100 (5.6) | 140–199 (7.8–11.1) |
| CFRD FH− | <126 (7.0) | ≥200 (11.1) |
| CFRD FH+ | ≥126 (7.0) | ≥200 (11.1) |
| Impaired fasting glucose (IFG) | 100–125 (5.6–6.9) | NA |
mid-OGTT glucose ≥200 mg/dl (11.1 mmol/l)
What about younger children with CF? Diabetes is rare prior to puberty, and when it does occur in this age group it is usually accompanied by obvious clinical signs and symptoms. Thus, if the sole purpose of the OGTT is to identify unsuspected diabetes, the yield would be too low in young children to justify routine screening. However, it could be recommended in children with CF if, as in adults, the glucose tolerance category was shown to provide clinically relevant prognostic information about future nutritional, pulmonary or metabolic risk.
OGTTs have been performed in children with CF for many years at the University of Minnesota (UM). The current retrospective chart review was performed to determine whether the baseline OGTT category in children with CF age 6–9 years predicts the subsequent clinical course.
Methods
Subjects
CF patients at the UM CF Center are routinely seen at quarterly intervals, and their clinical and laboratory data are prospectively recorded in the CF database. Annual OGTT screening is recommended starting at age 6 years for pancreatic insufficient patients not already known to have diabetes. Screening is not usually done before age 6 for practical logistical reasons (more difficult intravenous access, difficulty sitting quietly for 2 hours, decreased cooperation with test beverage consumption).
Clinical data were retrieved from patients who had an OGTT performed between January 1,1998 and December 31, 2003, to identify individuals who were between the ages of 6–9 years for a matched-pair cohort study. In a separate analysis, clinical data were subsequently retrieved from all children aged 10 to 18 followed at this center in 2008, for the purpose of validating the prediction model developed from the cohort study.
Children were characterized as having normal or abnormal glucose tolerance, summarized in Table 1. NGT was defined as a normal fasting glucose (<100 mg/dl, 5.6 mmol/l), a normal 2-hour OGTT glucose level (<140 mg/dl, 7.8 mmol/l), and no glucose level >200 mg/dl (11.1 mmol/l) during the OGTT. Abnormal glucose tolerance (AGT) was defined as IGT, where the fasting glucose level was normal and the 2-hour OGTT glucose was 140–199 mg/dl (7.8–11.1 mmol/l), or INDET, where fasting and 2-hour glucose levels were normal but the glucose level was >200 mg/dl (11.1 mmol/l) at another time point during the test. No child had IFG, so this category was not considered in the current analysis. Glucose tolerance can wax and wane in CF, but once a patient had an abnormal OGTT (IGT or INDET) they were considered to have AGT, even if subsequent tests showed NGT. In previous studies of adolescents and adults at UM, a single abnormal OGTT has been shown to correlate with future rate of pulmonary function decline (5), risk of microvascular complications (6), and survival (1; 7).
Baseline in the AGT group was defined as the age at the first abnormal OGTT. Control NGT CF subjects were matched for gender and age at the time of their own normal OGTT. Height, weight, %predicted forced expiratory volume in 1 second (FEV1), and %predicted forced vital capacity (FVC) were collected at baseline and 5 years later. All patients and their parents gave informed consent, permitting their records to be reviewed for research purposes. The protocol was approved by the Institutional Review Board of the University Of Minnesota.
Clinical Assessment
OGTTs were performed during a routine annual study visit, at a time when patients were clinically well. After an overnight fast, glucose 1.75g/kg (maximum 75g) was administered orally. Blood was sampled through an indwelling catheter for glucose and insulin levels at time 0 and every 30 minutes for 2 hours. Plasma insulin levels were measured in the UM Fairview Laboratory by radioimmunoassay using a double-antibody method (Immulite 2000, Siemens). Plasma glucose was measured in the UM Fairview Laboratory by the glucose oxidase method (Vitros, Ortho-Clinical Diagnostics). Fasting weight was measured on the same calibrated clinic scale at each visit (Stow-a-weigh model 6202, Scaletronix) and height was measured in triplicate on a wall-mounted stadiometer. The %predicted FEV1 and FVC were measured by standardized American Thoracic Society methods.
Statistical Methods
Age, height, weight, FVC, FEV1 and BMI percentile were compared between the groups at baseline and five-year follow-up using paired t-tests. Development of diabetes was compared between groups using conditional logistic regression to account for the pairing. BMI percentile was calculated using a SAS program from the Centers for Disease Control (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). Data analysis was performed on SAS 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Subjects
During 1998–2003, 114 CF children age 6–9 were treated at UM. Ninety-four (82%) had 220 OGTTs. Twenty eligible children did not have an OGTT and were not included in this study: 2 were pancreatic sufficient, 4 had just turned 6 years of age in the last year of the study and had not yet had their first OGTT, 2 developed diabetes before their first OGTT, and the reasons for no OGTT are unknown in 12.
Of the 94 children who underwent OGTT screening, 39 (41%) had at least one OGTT diagnosis of AGT while the remaining 55 (59%) had persistently NGT. Eight of the AGT children were excluded from the final analysis: 7 who did not have sufficient follow-up data (6 transferred to other institutions and one died unrelated to CF) and 1 who received a lung transplant shortly after her first OGTT. The remaining 31 children with AGT and their age- and gender-matched CF NGT controls formed the cohort-pairs for this study.
Clinical Characteristics at Baseline
At baseline, fasting blood glucose levels in both groups combined ranged from 51–97 mg/dl (2.8–5.4 mmol/l), and no child in either group had fasting hyperglycemia or IFG. Nine boys and 10 girls had IGT and 6 boys and 6 girls had INDET.
There were no significant differences in age, height, weight, BMI, FVC, or FEV1 at baseline between the children with AGT and their matched NGT controls (Table 2) or between those with IGT and those with INDET, either in the total cohort or when the data were analyzed with separate comparisons within each gender. There were no differences within genders in BMI percentile or lung function (data not shown).
Table 2.
Patient characteristics at baseline and 5 years later, mean ± S.E.M. Baseline is the time of the first abnormal glucose tolerance test in CF AGT. CF NGT were matched by gender and baseline age to CF AGT.
| At Baseline
|
Five Year Follow-Up
|
|||||
|---|---|---|---|---|---|---|
| NGT (n=31) | AGT (n=31) | P value | NGT (n=31) | AGT(n=31) | P value | |
| Age (years) | 8 ± 0 | 8 ± 0 | p = 0.07 | 13 ± 0 | 13 ± 0 | p = 0.16 |
| Height (cm) | 123 ± 1 | 122 ± 1 | p = 0.53 | 151 ± 1 | 150 ± 1 | p = 0.33 |
| Weight (kg) | 25 ± 1 | 25 ± 1 | p = 0.75 | 43± 1 | 42 ± 1 | p =0.61 |
| BMI percentile | 54 ± 4 | 58 ± 4 | p =0.50 | 49 ± 3 | 49 ± 3 | p =0.98 |
| FEV1 %predicted | 107 ± 3 | 110 ± 3 | p =0.53 | 94 ± 3 | 100 ± 3 | p =0.14 |
| FVC %predicted | 110 ± 3 | 109 ± 3 | p =0.91 | 100 ± 2 | 102 ± 2 | p =0.33 |
The magnitude of change over the five years for each parameter was also not significantly different for AGT compared to NGT.
OGTT Results
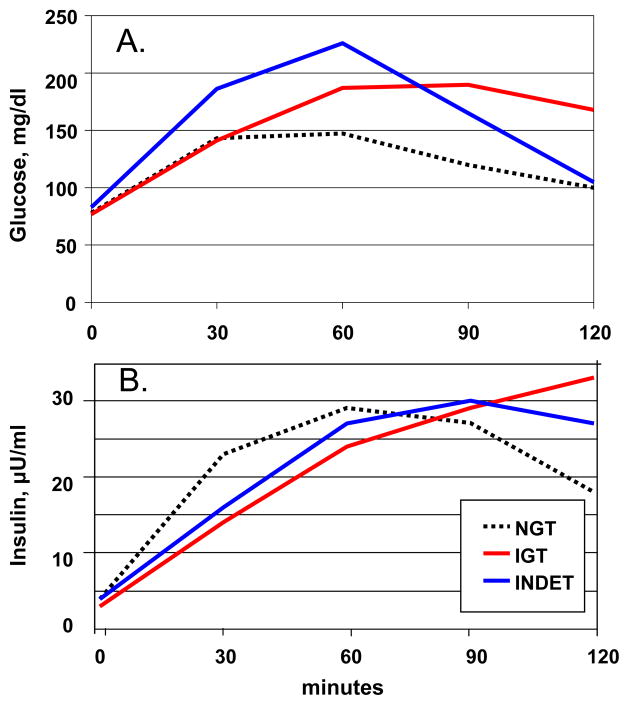
Glucose and insulin excursion during the baseline OGTT are presented in Figure 1. There was no difference in baseline fasting glucose levels between the two groups. The 2-hour area under the curve (AUC) for glucose was greater in the AGT group: NGT= 249 ± 8 mg/dl*hr, AGT= 330± 8 mg/dl*hr, p<0.0001. Neither fasting nor 2-hour AUC insulin levels differed between NGT and AGT. Fasting insulin was 4.4 ± 0.4 μU/ml (26± 2 pmol/l) for NGT and 3.6 ± 0.4 μU/ml (22 ± 2 pmol/l) for AGT, p=0.07. AUC for insulin was 43 ± 4 μU/ml*hr for NGT and 41 ± 4 μU/ml*hr for AGT, p=0.7. Peak insulin levels were 33 ± 18 in NGT, 32 ± 18 in INDET and 32 ± 20 in IGT. Insulin secretion was delayed in CF patients, with peak levels achieved at 70 ± 5 minutes in NGT, 88 ± 6 minutes in INDET, and 106 ± 6 minutes in IGT.
Figure 1.
A. Glucose and B. Insulin excursion during a standard oral glucose tolerance test in children age 6–9 years with CF. NGT=normal glucose tolerance (n=31), IGT = impaired glucose tolerance (n=19) and INDET=indeterminate glucose tolerance (n=12).
Clinical Characteristics Five Years After Baseline
At the 5 year follow-up there continued to be no significant differences between AGT and NGT children in mean age, height, weight, BMI percentile, FVC, or FEV1 (Table 2). Similarly, the 5 year magnitude of change from baseline in BMI percentile, FVC, or FEV1 was not significant between NGT and AGT (data not shown).
Development of Diabetes
During the five-year follow-up, the odds of developing diabetes were 11 times greater in children with AGT compared to those with NGT (p<0.001 for OR). Ten years after study onset, diabetes had developed in 42% of the children with AGT at baseline compared to 3% of the NGT group. Diabetes cases in the AGT group were equally divided between IGT and INDET and between boys and girls. The one NGT child who developed diabetes was an 11 year old boy who also developed liver failure and is currently awaiting liver transplantation. The average age of onset of for those who developed diabetes was 12±1 years for boys (range 9–14) and 11±1 for girls (range 8 to 14). This is considerably younger than the average age of CFRD onset of ~23 years in the total UM CF population (7). Diabetes developed on average 4±1 years after the first abnormal OGTT in boys and 3±1 years in girls.
Assessment of screening predictions using current clinical population
OGTT screening during 1998–2003 yielded 41% children with AGT, and of these 42% developed diabetes within 5 years. Among the 110 children 10–18 years followed at the UM CF Center in 2008, if all had received OGTT screening between ages 6 and 10, we would predict on average 41%*42%*110 = 19 cases of diabetes within 5 years. There were 20 children with diabetes among the 110 followed in 2008, but only 17 of them had OGTT screening before age 10. Of these current 17 diabetes cases with childhood OGTT screening results, 15 had AGT when they were 6–9 years of age, in close agreement with the prediction from the paired cohorts.
Discussion
Diabetes is common in CF starting at about 10 years of age, and is often associated with compromised nutrition and worse pulmonary status. In adolescents and adults, OGTT screening performed during a period of baseline stable health predicts the rate of pulmonary function decline over the next four years, and thus serves to identify patients at highest risk for rapid clinical deterioration (5). A multi-center, randomized, placebo-controlled intervention trial demonstrated that institution of pre-meal insulin therapy in adult patients with CFRD FH− is able to reverse chronic decline in nutritional status, making identification of these patients (which can only be done by OGTT) an important goal (8). Thus, there is clear rationale for performing OGTT screening in CF patients starting at age 10 years, as is reflected in the ISPAD consensus guidelines (4). The current study extends these observations to children younger than age 10. This retrospective matched-pair cohort study has demonstrated that a single abnormal OGTT, when performed in 6–9 year old children with CF during a period of stable baseline health, strongly predicts early progression to diabetes.
Multiple studies have shown that the diagnosis of diabetes is associated with worse lung disease and poorer survival in patients with CF (7; 9–10). Clinical decline has been reported to begin in the years prior to CFRD diagnosis, in the pre-diabetes period (5; 11–13). Survival in CF is intimately associated with attaining and maintaining normal weight and lean body mass. Insulin insufficiency, which begins long before overt diabetes develops in this population, is associated with increased protein catabolism (14) and lipid breakdown (15). Insulin replacement therapy clearly has a beneficial effect on nutritional status and lung function in adult CF patients with diabetes (1; 8; 13; 16–19), and there are small studies suggesting that these advantages may also extend to subjects with IGT treated with insulin therapy (18; 20).
Few data are available specific to the CF pediatric population. The OGTT area under the curve for glucose has been associated with decreased BMI and height (21), and the insulin area under the curve has correlated with height velocity in children with CF (22). In an early study, the insulin secretagogue tolbutamide improved glucose tolerance and growth velocity in non-diabetic children with CF (23). More recent studies using relatively low-dose therapy with the basal insulin glargine have demonstrated improved pulmonary status in children with CF and AGT (18; 20; 24). These studies have been small and uncontrolled, but suggest that actively growing CF children, with their high anabolic demands, may be particularly sensitive to the effects of insulin insufficiency.
Do CF patients who develop AGT or diabetes at an unusually early age have greater morbidity than their non-diabetic CF peers? Most of the published data suggest the answer to this question is “yes”. Large registry studies demonstrate that compared to other children and adolescents with CF, pediatric CF patients with diabetes experience greater mortality (25), reduced weight-forage (10), and poorer lung function (10). In a British cohort, adolescents who were still actively growing at the time of CFRD diagnosis demonstrated nutritional compromise, with a BMI that was 5–11% lower than non-diabetic CF controls (26). In Australian children age 10–18, hyperglycemia mid-OGTT or by home continuous glucose monitoring was associated with declining weight standard deviation scores and a greater rate of lung function decline (27). Early abnormal glucose tolerance was associated with lower survival in French CF children (21).
Our own published data show that at UM in the 1990’s, children and adolescents with CFRD were more than six times as likely to die as non-diabetic CF youth (1). However, in contrast to the world literature, more recent data from UM show this risk has disappeared. This is consistent with the current study, where there was no difference in pulmonary disease or nutritional status between children with NGT and those with AGT, either at baseline or 5 years later despite progression to diabetes in almost half of the subjects in the AGT group. This may be related to the fact that once they were identified as having AGT, these children were treated differently than their NGT peers. They were referred a to pediatric endocrinologist where they received education about glucose tolerance abnormalities in CF, risk of progression to CFRD, and the importance of early identification of symptoms such as decreased lung function or inability to gain weight. They received nutrition counseling to spread their carbohydrate intake evenly throughout the day without restricting total daily carbohydrate or calorie intake. During acute illness they received intensive glucose monitoring. Once diabetes was diagnosed, whether the hyperglycemia occurred during a stable outpatient OGTT or during acute illness in the hospital, patients were started on insulin therapy. Early risk identification coupled with patient and family education, vigilant surveillance, and aggressive treatment once diabetes was diagnosed may have ameliorated the risk of clinical deterioration in these young patients.
In summary, OGTT screening was able to identify children with CF who were at high risk for developing diabetes in the next 3–4 years. Early intervention may have helped to prevent an expected decline in pulmonary and nutritional status. In clinical practice, for every 100 children screened, it appears that about 41 will have AGT and about 17 of these will progress to early-onset diabetes. For most disease states, a 17% risk would be considered sufficient to warrant routine screening. We recommend that annual OGTT screening in the CF population begin at 6 years of age.
Acknowledgments
This project was funded in part by a grant from Pennsylvania Cystic Fibrosis Inc. The University of Minnesota Cystic Fibrosis Database is supported by a Center Grant from the Cystic Fibrosis Foundation and the University of Minnesota.
Abbreviations
- OGTT
Oral Glucose Tolerance Testing
- CF
Cystic Fibrosis
- ISPAD
International Society for Pediatric and Adolescent Diabetes
- NGT
Normal Glucose Tolerance
- AGT
Abnormal Glucose Tolerance
- OR
Odds Ratio
- BMI
Body Mass Index
- CFRD
Cystic Fibrosis Related Diabetes
- CFRD FH−
Cystic Fibrosis Related Diabetes without fasting hyperglycemia
- CFRD FH+
Cystic Fibrosis Related Diabetes with fasting hyperglycemia
- UM
University of Minnesota
- IGT
Impaired Glucose Tolerance
- INDET
Indeterminate Glycemia
- FEV1
percent predicted forced expiratory volume in 1 second
- FVC
percent predicted forced vital capacity
- AUC
area under the curve
References
- 1.Moran A, Billings J, Dunitz J, Kempainen R, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis related diabetes: modern trends in prevalence, incidence and mortality. Diabetes Care. 2009;32:1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosenko JM, Palmer MP, Rafkin-Mervis L, Krishcher JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS Group AtDPT-TS. Incident Dysglycemia and Progression to Type 1 Diabetes Among Participants in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:0603–1607. doi: 10.2337/dc08-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran A. Updates on CFRD guidelines. Pediatric Pulmonology Suppl. 2009;32:208–209. [Google Scholar]
- 4.O’Riordan SMP, Robinson PD, Donaghue KC, Moran A. ISPAD clinical practice consensus guidelines 2008: management of cystic fibrosis-related diabetes. Pediatric Diabetes. 2008;9:338–344. doi: 10.1111/j.1399-5448.2008.00437.x. [DOI] [PubMed] [Google Scholar]
- 5.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in cystic fibrosis patients correlate with the degree of glucose intolerance at baseline. Am J Resp Crit Care Med. 2001;162:891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 6.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla CE, Moran A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care. 2007;30:1056–1061. doi: 10.2337/dc06-1576. [DOI] [PubMed] [Google Scholar]
- 7.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28:2141–2144. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 8.Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H. Insulin therapy to improve BMI in cystic fibrosis related diabetes without fasting hyperglycemia: results of the CFRDT trial. Diabetes Care. 2009;32:1783–1788. doi: 10.2337/dc09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146:681–687. doi: 10.1016/j.jpeds.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Koch D, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, McKenzie SG, Navarro J, Strandvik B. Presence of cystic fibrosis-related diabetes mellitus is tightly liked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol. 2001;32:343–350. doi: 10.1002/ppul.1142. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, Klein DJ. Diabetes mellitus associated with cystic fibrosis. J Pediatr. 1988;112:373–377. doi: 10.1016/s0022-3476(88)80315-9. [DOI] [PubMed] [Google Scholar]
- 12.Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr. 1992;151:684–687. doi: 10.1007/BF01957574. [DOI] [PubMed] [Google Scholar]
- 13.Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubina-Rufi N, Paul C, Polzk M. CFRD: Clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr. 2001;90:860–867. [PubMed] [Google Scholar]
- 14.Moran A, Milla C, DuCret R, Nair KS. Protein metabolism in clinically stable adult CF patients with abnormal glucose tolerance. Diabetes. 2001;50:1336–1343. doi: 10.2337/diabetes.50.6.1336. [DOI] [PubMed] [Google Scholar]
- 15.Moran A, Basu R, Milla C, Jensen M. Insulin regulation of free fatty acid kinetics in adult cystic fibrosis patients with impaired glucose tolerance. Metabolism. 2004;53:1467–1472. doi: 10.1016/j.metabol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Lanng S, Thorsteinsson B, Nerup J, Koch C. Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatrica. 1994;83:849–853. doi: 10.1111/j.1651-2227.1994.tb13156.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ. Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration. 2008;76:181–186. doi: 10.1159/000110206. [DOI] [PubMed] [Google Scholar]
- 18.Mozzillo E, Franzese A, Valerio G, Sepe A, De Simone I, Mazzarella G, Ferri P, Raia V. One year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatric Diabetes. 2009;10:162–167. doi: 10.1111/j.1399-5448.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 19.Nousia-Arvanitakis S, Galli-Tsinopoulou A, Karamouzis M. Insulin improves clinical status of patients with cystic fibrosis-related diabetes mellitus. Acta Paediatr. 2001;90:515–519. [PubMed] [Google Scholar]
- 20.Bizzarri C, Lucidi V, Ciampalini P, Bella S, Russo B, Cappa M. Clincial effects of early treatment with insulin glargine in patients with cystic fibrosis and impaired glucose tolerance. J Endocrinol Invest. 2006;29:1–4. doi: 10.1007/BF03345538. [DOI] [PubMed] [Google Scholar]
- 21.Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, De Buc J, Lenoir G, Robert J-J. Glucose tolerance and insulin secretion, morbidity and death in patients with CF. J Pediatr. 2008;152:540–545. doi: 10.1016/j.jpeds.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Ripa P, Robertson I, Cowley D, Harris M, Master IB, Cotterill AM. The relationship between insulin secretion, the insulin-like growth factor axis and growth in children with cystic fibrosis. Clin Endocrinol. 2002;56:383–389. doi: 10.1046/j.1365-2265.2002.01484.x. [DOI] [PubMed] [Google Scholar]
- 23.Zipf WB, Kien CL, Horswill CA, McCoy KS, O’Dorisio T, Pinyerd BL. Effects of tolbutamide on growth and body composition of nondiabetic children with CF. Pediatr Res. 1991;30:309–314. doi: 10.1203/00006450-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Franzese A, Spagnuolo MI, Sepe A, Valerio G, EM, Raia V. Can glargine reduce the number of lung infections in patients with CFRD? Diabetes Care. 2005;28:2333. doi: 10.2337/diacare.28.9.2333. [DOI] [PubMed] [Google Scholar]
- 25.Chamnan P, Shine BSF, Haworth C, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010 doi: 10.2337/dc09-1215. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White H, Pollard K, CE, Clifton I, Morton AB, Owen D, Conway SP, Peckham DG. Nutritional decline in CFRD: the effect of intensive nutritional intervention. J Cyst Fibros. 2009;8:179–185. doi: 10.1016/j.jcf.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Hameed S, Morton J, Jaffe A, Field P, Belessis Y, Yoong T, Katz T, Verge C. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010 doi: 10.2337/dc09-1492. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]