Abstract
Control of osteoblastic bone formation involves the cumulative action of numerous transcription factors, including both activating and repressive functions that are important during specific stages of differentiation. The nuclear receptor retinoic acid receptor-related orphan receptor β (Rorβ) has been recently shown to suppress the osteogenic phenotype in cultured osteoblasts, and is highly upregulated in bone marrow-derived osteogenic precursors isolated from aged osteoporotic mice, suggesting Rorβ is an important regulator of osteoblast function. However the specific gene expression patterns elicited by Rorβ are unknown. Using microarray analysis, we identified 281 genes regulated by Rorβ in an MC3T3-E1 mouse osteoblast cell model (MC3T3-Rorβ-GFP). Pathway analysis revealed alterations in genes involved in MAPK signaling, genes involved in extracellular matrix (ECM) regulation, and cytokine-receptor interactions. Whereas the identified Rorβ-regulated ECM genes normally decline during osteoblastic differentiation, they were highly upregulated in this non-mineralizing MC3T3-Rorβ-GFP model system, suggesting that Rorβ may exert its anti-osteogenic effects through ECM disruption. Consistent with these in vitro findings, the expression of both RORβand a subset of RORβ-regulated genes were increased in bone biopsies from postmenopausal women (73 ± 7 years old) compared to premenopausal women (30 ± 5 years old), suggesting a role for RORβ in human age-related bone loss. Collectively, these data demonstrate that Rorβ regulates known osteogenic pathways, and may represent a novel therapeutic target for age-associated bone loss.
Keywords: Osteoblast, Rorβ, extracellular matrix, proliferation, microarray, pathway analysis
1. Introduction
The control of osteoblastic differentiation, maturation and mineralization is a highly complex process involving expression of key transcription factors in a tightly coordinated pattern. The Runx2 transcription factor is considered the most important transcription factor driving expression of the osteoblastic phenotype, as its deletion in mice results in a complete lack of an ossified skeleton [1-3]. Therefore, identification and characterization of auxiliary transcription factors which modulate the activity of Runx2 are of particular interest and may represent novel avenues to either increase or decrease the influence of Runx2, and therefore osteoblastic function.
We have recently identified retinoic acid-related orphan receptor β (Rorβ) as a transcription factor that suppresses Runx2-dependent transcriptional activation. The expression of Rorβ was originally thought to be limited to certain regions of the retina and brain [4]; however, we have detected Rorβ expression in multiple osteoblastic cell models as well as in primary mouse and human bone tissue. Rorβ expression is high in undifferentiated osteoblastic cultures and is downregulated during the process of differentiation. Constitutive Rorβ expression inhibits mineralization of mouse calvarial osteoblasts. Interestingly, Rorβ expression is highly upregulated in osteoblastic precursors cells isolated from the bone marrow of aged (18-22 month-old), osteoporotic mice [5]. Collectively, these data suggest that Rorβ may function to inhibit Runx2-dependent processes not only during differentiation but also in an aging context. However the genes and cellular pathways regulated by Rorβ are completely unknown in osteoblasts. Identification of Rorβ-dependent gene expression patterns will generate a more complete model of how Rorβ suppresses the osteoblastic phenotype, which may be exploited in the development of clinical treatments of osteoporosis.
In this study, we used microarray analysis to identify genetic targets regulated by Rorβ in the mouse MC3T3-E1 osteoblastic cell model. Using this approach we provide evidence that Rorβ regulates genes involved in proliferation and in the production and maintenance of the extracellular matrix, an essential component needed for proper bone mineralization. Finally, we provide data demonstrating that Rorβ, including select Rorβ target genes identified by this microarray analysis, are increased in needle bone biopsies from postmenopausal compared to premenopausal women, suggesting a possible role in aging.
2. Materials and Methods
2.1. Cell culture reagents
The MC3T3-GFP and MC3T3–Rorβ-green fluorescent protein (GFP) cell lines were cultured in αMEM growth medium (Invitrogen, Carlsbad, CA) supplemented with 1X antibiotic/antimycotic (Invitrogen), 10% (v/v) fetal bovine serum (Hyclone, Logan, UT) and 400 ug/mL G418 antibiotic (Invitrogen) [5]. These cells stably express either GFP (control cells) or Rorβ-GFP (experimental cells), respectively. Primary mouse calvarial osteoblasts and the U2OS human osteosarcoma cell line were cultured in the same media, minus the selection antibiotic.
2.2. RNA/cDNA isolation
MC3T3-GFP and MC3T3–Rorβ-GFP cells were plated in 10-cm culture dishes (n=6) at a density of 2 × 104 cells/cm2 and allowed to grow for 48 hrs. Total RNA was prepared from using RNeasy minicolumns (Qiagen, Valencia, CA) and treated with RNase-free DNase (Qiagen) to remove potential contaminating DNA, as previously described [6].
2.3. Human needle bone biopsies
The human bone samples used in this study were part of a larger study on age-related bone loss in humans; results of this larger study, excluding the Rorβ analysis described here, are being published separately [7, 8]. Briefly, post-menopausal (73 ± 7 years old) and pre-menopausal (30 ± 5 years old) women study subjects were admitted to the outpatient Mayo Clinical Research Unit following an overnight fast. Following local anesthesia with 1% lidocaine and monitored IV sedation using 1-3 mg of intravenous midazolam and 50-100 μg of fentanyl, needle biopsies of bone from the posterior iliac crest were obtained using an 8G needle. These biopsies contain a mixture of cortical and trabecular bone [6]. The biopsies were immediately placed in lysis buffer (Qiagen) and homogenized using Tissue Tearor™ variable speed homogenizer (Cole-Parmer, Vernon Hills, IL). All human studies were approved by the Mayo Institutional Review Board and subjects provided written, informed consent.
2.4. Microarray
One μg of RNA from the MC3T3-GFP and MC3T3–Rorβ-GFP cells (n=6) was submitted for microarray analysis using the Illumina MouseWG6-v2.0 Expression BeadChips (Illumina, San Diego, CA) array platform. The preparation of the samples and microarray hybridizations was performed by the Advanced Genomic Technology Center Microarray Shared Resource at the Mayo Clinic as previously described [9].
2.5. Quantitative Real-time PCR Analysis (QPCR)
The PCR reactions were run in the ABI Prism 7900HT Real-Time System (Applied Biosystems, Carlsbad, CA) using SYBR Green (Qiagen) as the detection method, as previously described [10]. The method for data normalization using multiple reference genes and threshold calculations are as previously described [10]. Primer sequences for individual genes were designed using the Primer Express program (Applied Biosystems) and are available on request.
2.6. Transient transfection and assays
U2OS cells were plated at a density of 2.6 × 104 cells/cm2 in 6-well plates the day before transfection. The E2F1-Luc reporter construct (SABiosciences, Valencia, CA) and either Rorβ-pCMV6 or pCMV6-Entry (OriGene, Rockville, MD) were cotransfected at a concentration of 250 ng/well/construct (n=6) using FuGENE6 transfection reagent (Promega, Madison, WI) according to the manufacturer’s instructions. Following incubation at 37°C for 48 hours, the cells were lysed in 1x Passive Lysis Buffer (Promega) and assayed using Luciferase Assay Reagent (Promega) and normalized to total protein as measured by Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL).
2.7. Cell proliferation assay
MC3T3-GFP and MC3T3-Rorβ-GFP cell lines were seeded in growth medium into 96-well plates at a density of 2 × 104 cells/cm2 (n=6) and allowed to proliferate for 48 hours. Twenty-five (25) μl of conditioned media was assayed using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions. The plate was read on a GloMax® luminometer (Promega) and data expressed as percent of MC3T3-GFP control.
2.8. Statistical analyses
Calculations and statistical analyses were performed using Microsoft Office Excel 2003 (Microsoft Corp., Redmond, WA). The data are presented as the mean ± SE. All values of p ≤ 0.05 were considered statistically significant using Student’s t-test. The microarray data was filtered based on a detection p-value (p ≤ 0.05 called “detected”), where probe sets not detected in all samples were removed. Following this noise filtering 15,860 probe sets remained. Analysis of variance (ANOVA) statistical modeling was then used to categorize differentially expressed genes between the MC3T3-GFP and MC3T3-Rorβ-GFP cell datasets. All genes regulated at p ≤ 0.05, false discovery rate (FDR; q) ≤ 0.05 and fold-change (FC) ≤ ≥1.5 were considered significant and included in this report. Only those probe sets with known annotations were included in this analysis and these were subjected to gene ontology analysis using DAVID Bioinformatics Resources Version 6.7 [11]. The O’Brien Umbrella test was used to assess the significance of pre-defined sets of genes in the QPCR analyses, rather than in individual genes [10, 12-14].
3. Results
3.1. Microarray and pathways analysis of novel Rorβ target genes
Previous examination of the role of Rorβ in MC3T3-E1 mouse osteoblastic cells demonstrated a suppression of the osteogenic phenotype in bone mineralization assays [5]; however, the molecular and cellular mechanisms of how Rorβ exerts its anti-osteogenic role are unknown. Therefore, we utilized Illumina microarray technology to identify global gene expression patterns between the MC3T3-GFP (control) and MC3T3–Rorβ-GFP (experimental) cell models [5]. The analysis identified 281 differentially expressed genes (Supplemental Table 1), of which 154 were upregulated (55%) and 127 downregulated (45%), using the statistical cutoffs delineated in the Materials and Methods. Forty-seven randomly chosen genes from this dataset were analyzed by QPCR in independent samples to validate the microarray data and regulation of all genes was confirmed (Supplemental Table 2), demonstrating a high level of accuracy of the microarray dataset.
The microarray analysis provided important information regarding the regulation of specific genes by Rorβ. To further understand the broader cellular role of Rorβ in osteoblasts, we performed gene ontology and pathway analysis on the microarray dataset using the DAVID functional annotation tool [15]. This analysis revealed numerous cellular pathways modulated by Rorβ (Supplemental Table 3). Of particular interest are the MAP-kinase signaling pathway, extracellular matrix regulation and cytokine-receptor interactions, since these processes have been implicated osteoblast biology.
3.2. Rorβ regulates genes involved in osteoblastic extracellular matrix (ECM) production
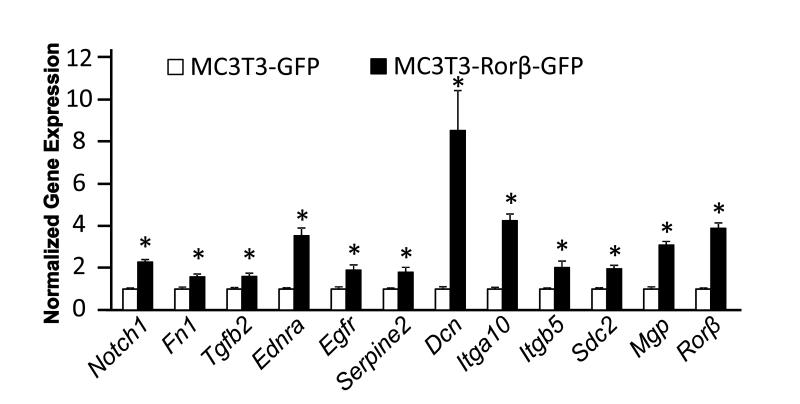
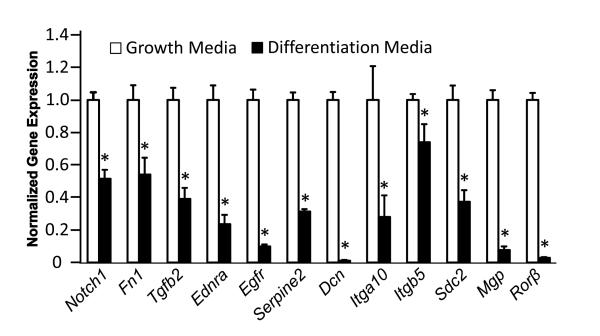
Our previous work demonstrated that the MC3T3–Rorβ-GFP cell line fails to form mineralized nodules using standard in vitro mineralization assays [5]. We were intrigued to discover that genes involved in ECM-receptor and cytokine-receptor interactions were regulated by Rorβ (Supplemental Table 3), suggesting that disruption of the ECM may be involved in the mineralization defect of MC3T3–Rorβ-GFP cells. Therefore, we chose a subset of these genes and performed QPCR analysis (Fig. 1). Interestingly, all of these genes are upregulated in MC3T3–Rorβ-GFP cells. To validate the regulation of these genes during normal osteoblastic differentiation, we measured the expression of these genes during differentiation of parental MC3T3-E1 cells, where Rorβ mRNA levels are known to decline (Fig. 2). If these genes are truly dependent on Rorβ expression, similar decreases in these genes would be expected in normal, differentiated osteoblasts. Indeed, all of these genes were downregulated in cells cultured in differentiation media, as compared to growth media, suggesting that these genes are coordinately regulated with Rorβ.
Fig. 1.
Regulation of select Rorβ-regulated genes identified by microarray analysis in MC3T3-Rorβ-GFP cells. MC3T3-GFP and MC3T3-Rorβ-GFP cell lines were cultured for 48 h and QPCR analysis was performed. Data are expressed relative to the MC3T3-GFP cells for each gene, *p<0.01.
Fig. 2.
Regulation of select Rorβ-regulated genes during normal MC3T3-E1 differentiation. MC3T3-E1 cells were cultured in either normal growth (open bars) or differentiation media (closed bars) for 14 days and QPCR analysis was performed. Data are expressed as in Fig 1., *p<0.01.
3.3. Rorβ affects proliferation in osteoblasts
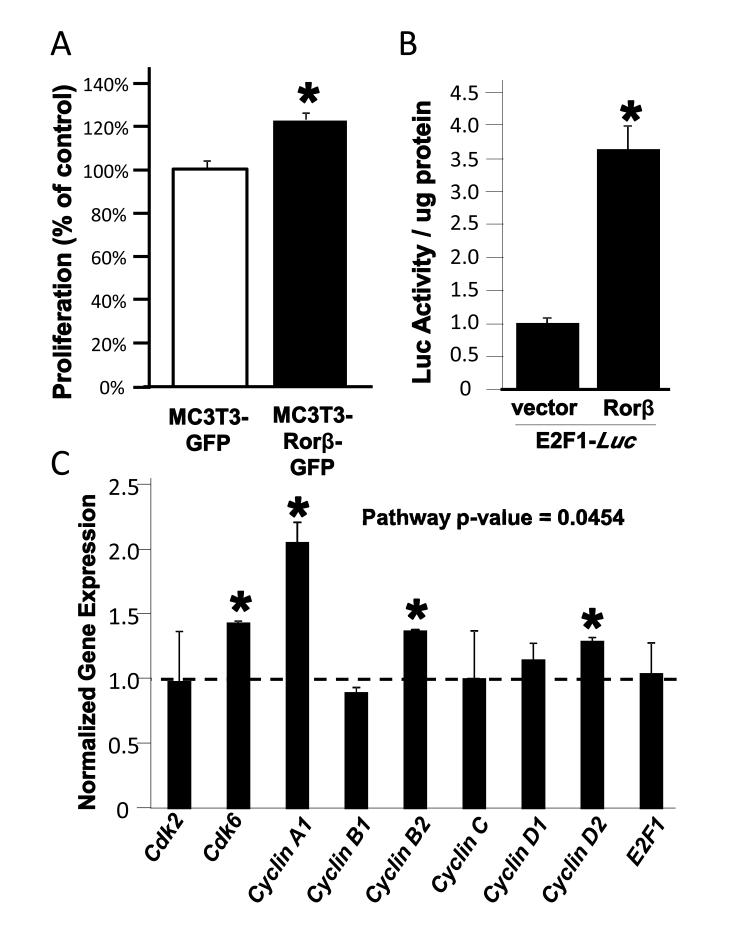
The top Rorβ-regulated pathway was mitogen-activated protein kinase (MAPK) signaling. Examination of the individual genes regulating MAPKs in the microarray dataset revealed downregulation of Dual-specificity phosphatases−1, −5, and −8 (Dusp- 1,−5,−8), which repress MAPKs through dephosphorylation [16]. This suggests that Rorβ stimulates MAPK activity and possibly cellular proliferation. We therefore examined the proliferation rates of our cell models and found a 20% increase in proliferation in the MC3T3–Rorβ-GFP cell line (Fig. 3A). Further experiments demonstrated that Rorβ stimulates an E2F1-Luciferase reporter construct (Fig. 3B) and increases select genes involved in proliferation (Fig. 3C). Overall this data suggests that Rorβ stimulates proliferation, possibly through the activation of the MAPK pathway in osteoblasts.
Fig. 3.
Rorβ regulates cellular proliferation. (A) MC3T3-GFP and MC3T3-Rorβ-GFP cell lines were cultured for 24 h and a proliferation assay was performed. Data are presented as % of MC3T3-GFP control. (B) Cells were transiently transfected with the E2F1-Luc reporter either in the presence of vector control or a Rorβ expression plasmid. Cells were harvested 48 h later and a luciferase assay was performed. (C) MC3T3-GFP and MC3T3-Rorβ-GFP cell lines were cultured and analyzed as in Fig 3A. The overall pathway p-value was determined using the O’Brien Umbrella test, *p<0.01.
3.4. Validation of select Rorβ targets in human bone biopsies
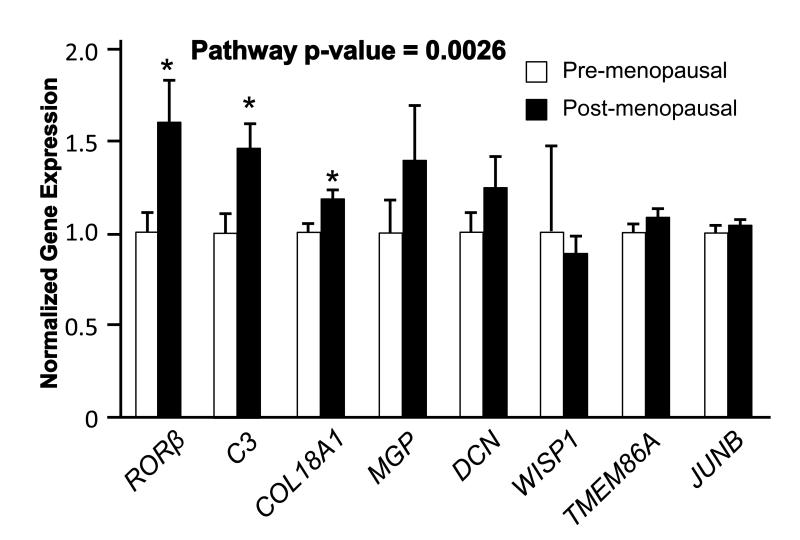
Our previous work demonstrated that Rorβ expression levels are greatly upregulated (>50-fold) in the osteogenic cell population derived from the bone marrow of aged mice (18-22 month old)[5]. We examined RORβ expression in primary human biopsies from young and old individuals, and found that like in the mouse system, RORβ expression was upregulated in bone biopsies from aged humans (Fig. 4). Examination of a subset of RORβ-regulated genes also revealed a statistically significant increase in the transcription of these genes.
Fig. 4.
Regulation of RORβ and RORβ targets in needle bone biopsies from pre- and post-menopausal women. Data are expressed relative to pre-menopausal woman. The overall pathway p-value was determined using the O’Brien Umbrella test, *p<0.01.
4. Discussion
Previous studies from our laboratory have demonstrated that Rorβ is an important regulator of osteoblast differentiation and may play a role during aging in bone [5]. Other experiments suggested that Rorβ may exert its anti-osteogenic function through repression of Runx2-dependent transcription, the requisite transcription factor for osteoblastic differentiation [1-3]. However, the role of Rorβ in regulation of other processes/pathways important in osteoblast differentiation remains unknown. In the present study, we examined the Rorβ-dependent expression profile in the MC3T3-E1 mouse osteoblast model and found highly significant regulation of proliferative pathways and in regulation of the extracellular matrix (ECM), two processes implicated in osteoblast metabolism. We further extended our previous findings of increased RORβ expression in a primary, human bone biopsy model, suggesting that RORβ plays an important and unexplored role during aging in humans.
The identification of numerous genes involved in the production, deposition and organization of the ECM was of particular interest since proper regulation of the ECM is requisite for osteoblast differentiation and mineralization [17]. The ECM provides not only a structural support for the deposition of bone mineral, but also is implicated in directing the activities of a number of growth factors and cytokines, such as transforming growth factor (Tgf)-β and bone morphogenetic proteins (Bmps) [18]. Indeed, we found that the Tgfβ inhibitor decorin (Dcn) [19], is highly upregulated in Rorβ-expressing cells, suggesting that one mechanism by which Rorβ exerts its anti-osteogenic effects may be through suppression of Tgfβ activation. We also found that matrix gla protein (Mgp), another known inhibitor of bone formation through sequestration of Bmp2 [20], was also upregulated. These data suggest that downregulation of these ECM inhibitors may be partly controlled through the suppression of Rorβ expression during normal osteoblastic differentiation.
Interestingly, genes involved in cellular proliferation were highly regulated in the microarray dataset. We observed downregulation of dual-specificity phosphatases−1, −5, and −8 (Dusp−1,−5,−8), which repress MAPKs through dephosphorylation [16], suggesting that Rorβ may indirectly stimulate MAPK activity and therefore increase cellular proliferation. Indeed, Rorβ-dependent increases in proliferation were observed using a number of different measures. It is well known that osteoblasts must exit from the cell cycle to properly differentiate. These data suggest that another mechanism by which Rorβ elicits it anti-osteogenic function is through maintenance of cellular proliferation.
We have previously demonstrated that Rorβ expression is highly upregulated in osteoblastic precursors cells isolated from the bone marrow of aged, osteoporotic mice [5]. Although this fits with our hypothesis that Rorβ is an osteogenic inhibitor, any human data on the importance of RORβ was lacking. In this study we extended our findings in mice and demonstrated that RORβ and several of its target genes are also upregulated in human bone biopsies isolated from aged individuals, lending further support to the notion that RORβ may play an important role in age-related bone loss not only in mice, but also in humans.
A limitation of this study is that not all the Rorβ-dependent genes identified in the microarray study were regulated in the aged human bone biopsies. This could be due to a number of reasons, aside from the obvious differences in both species and cellular system. The microarray study provides an idealized system where Rorα levels were constitutively fixed, leading to the identification of “possible” Rorβ targets under strictly controlled conditions, whereas the human bone biopsies represent a more physiologically relevant system that is not as controlled and significantly more heterogeneous. Even considering these differences, it is striking that not only RORβ, but a few select RORβ-dependent genes were upregulated in the aged human bone biopsies. Applying the O’Brien’s Umbrella Test [12], which analyzes the changes in expression of select genes as a group, demonstrates that RORβ-regulated genes are statistically significant as a group. Thus, the data generated in the microarray samples give a reasonably reliable estimate to the identity of potential RORβ-dependent genes. Examination of these genes in other physiologically relevant, bone-related systems, should provide us with a more accurate picture of the true role of RORβ in osteoblasts/bone.
In conclusion, we have for the first time identified the transcriptome of Rorβ- regulated genes in osteoblasts. These data suggest that the role of Rorβ in the suppression of the osteogenic phenotype may not only include repression of Runx2 activity [5], but also through the abnormal regulation of the ECM and in regulation of cellular proliferation. Recently, a Rorβ knockout mouse model has been described and examination of its bone phenotype will aid in the understanding of Rorβ in bone [21]. Future studies involving this mouse model will uncover how Rorβ-regulated pathways function in vivo to elicit proper control of osteoblast and bone biology.
Supplementary Material
We examine the gene expression patterns controlled by Rorβ in osteoblasts.
Genes involved in extracellular matrix regulation and proliferation are affected.
Rorβ mRNA levels increase in aged, human bone biopsies.
Rorβ may affect osteoblast activity by modulation of these pathways.
Acknowledgements
Work was supported by NIH Grant R01 AG004875 and the Mayo Kogod Center on Aging.
Funding: Supported by NIH Grant R01 AG004875, UL1 TR000135 (Mayo Clinic Center for Clinical and Translational Science), and the Mayo Kogod Center on Aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 2.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 3.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 4.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roforth MM, Liu G, Khosla S, Monroe DG. Examination of nuclear receptor expression in osteoblasts reveals rorb as an important regulator of osteogenesis. J Bone Miner Res. 2012;27:891–901. doi: 10.1002/jbmr.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804–810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita K, Roforth MM, Demaray S, McGregor UI, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S. Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women. doi: 10.1210/jc.2013-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S. Efftects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. doi: 10.1016/j.bone.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chokalingam J, Roforth MM, Nicks KM, McGregor U, Fraser D, Khosla S, Monroe DG. Examination of ERa signaling pathways in bone of mutant mouse models reveals the importance of ERE-dependent signaling. Endocrinology. 2012;153:5325–5333. doi: 10.1210/en.2012-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. Effects of estrogen on osteoprogenitor cells and cytokines/bone regulatory factors in postmenopausal women. Bone. 2011;49:202–207. doi: 10.1016/j.bone.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 13.Syed FA, Modder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, Oursler MJ, Khosla S. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res. 2010;25:2438–2446. doi: 10.1002/jbmr.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peris P, Atkinson EJ, Gossl M, Kane TL, McCready LK, Lerman A, Khosla S, McGregor UI. Effects of bisphosphonate treatment on circulating osteogenic endothelial progenitor cells in postmenopausal women. Mayo Clin Proc. 2013;88:46–55. doi: 10.1016/j.mayocp.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D.W. H, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 16.Huang CY, Tan TH. DUSPs to MAP kinases and beyong. Cell Biosci. 2012;2:24. doi: 10.1186/2045-3701-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 18.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 20.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–1044. [PubMed] [Google Scholar]
- 21.Liu H, KIm SY, Fu Y, Wu X, Ng L, Swaroop A, Forrest D. An isoform of retinoid-related orphan receptor b directs differentiation of retinal amacrine and horizontal interneurons. Nat Commun. 2013;4:1813. doi: 10.1038/ncomms2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.