Abstract
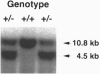
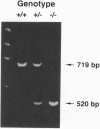
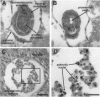
The DNA-binding activity of AP-1 proteins is modulated, in vitro, by a posttranslational mechanism involving reduction oxidation. This mode of regulation has been proposed to control both the transcriptional activity and the oncogenic potential of Fos and Jun. Previous studies revealed that reduction of oxidized Fos and Jun by a cellular protein, Ref-1, stimulates sequence-specific AP-1 DNA-binding activity. Ref-1, a bifunctional protein, is also capable of initiating the repair of apurinic/apyrymidinic sites in damaged DNA. The relationship between the redox and DNA repair activities of Ref-1 is intriguing; both activities have been suggested to play an important role in the cellular response to oxidative stress. To investigate the physiological function of Ref-1, we used a gene targeting strategy to generate mice lacking a functional ref-1 gene. We report here that heterozygous mutant mice develop into adulthood without any apparent abnormalities. In contrast, homozygous mutant mice, lacking a functional ref-1 gene, die during embryonic development. Detailed analysis indicates that death occurs following blastocyst formation, shortly after the time of implantation. Degeneration of the mutant embryos is clearly evident at embryonic day 5.5. These findings demonstrate that Ref-1 is essential for early embryonic development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Aboussekhra A., Wood R. D. Repair of UV-damaged DNA by mammalian cells and Saccharomyces cerevisiae. Curr Opin Genet Dev. 1994 Apr;4(2):212–220. doi: 10.1016/s0959-437x(05)80047-4. [DOI] [PubMed] [Google Scholar]
- Babiychuk E., Kushnir S., Van Montagu M., Inzé D. The Arabidopsis thaliana apurinic endonuclease Arp reduces human transcription factors Fos and Jun. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3299–3303. doi: 10.1073/pnas.91.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. M., Bronner C. E., Zhang L., Plug A. W., Robatzek M., Warren G., Elliott E. A., Yu J., Ashley T., Arnheim N. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995 Jul 28;82(2):309–319. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]
- Chen D. S., Herman T., Demple B. Two distinct human DNA diesterases that hydrolyze 3'-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res. 1991 Nov 11;19(21):5907–5914. doi: 10.1093/nar/19.21.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. R., Edbauer-Nechamen C. A., Lowry C. V., Salmon S. L., Kim Y. K., Davies J. M., Davies K. J. Assessing gene expression during oxidative stress. Methods Enzymol. 1994;234:175–217. doi: 10.1016/0076-6879(94)34087-0. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Demple B., Herman T., Chen D. S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Cunningham R. P. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Reardon J. T., Ansari A., Huang J. C., Zawel L., Ahn K., Sancar A., Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Sancar A., Reinberg D. Where transcription meets repair. Cell. 1994 Apr 8;77(1):9–12. doi: 10.1016/0092-8674(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Gardiner C. S., Reed D. J. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod. 1994 Dec;51(6):1307–1314. doi: 10.1095/biolreprod51.6.1307. [DOI] [PubMed] [Google Scholar]
- Gardiner C. S., Reed D. J. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys. 1995 Apr 1;318(1):30–36. doi: 10.1006/abbi.1995.1200. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A. E., Wang Z. Q., Cecchini M. G., Hofstetter W., Felix R., Fleisch H. A., Wagner E. F. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994 Oct 21;266(5184):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Guillouf C., Rosselli F., Krishnaraju K., Moustacchi E., Hoffman B., Liebermann D. A. p53 involvement in control of G2 exit of the cell cycle: role in DNA damage-induced apoptosis. Oncogene. 1995 Jun 1;10(11):2263–2270. [PubMed] [Google Scholar]
- Gujuluva C. N., Baek J. H., Shin K. H., Cherrick H. M., Park N. H. Effect of UV-irradiation on cell cycle, viability and the expression of p53, gadd153 and gadd45 genes in normal and HPV-immortalized human oral keratinocytes. Oncogene. 1994 Jul;9(7):1819–1827. [PubMed] [Google Scholar]
- Hainaut P., Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993 Oct 1;53(19):4469–4473. [PubMed] [Google Scholar]
- Hainaut P. The tumor suppressor protein p53: a receptor to genotoxic stress that controls cell growth and survival. Curr Opin Oncol. 1995 Jan;7(1):76–82. [PubMed] [Google Scholar]
- Hanawalt P. C., Donahue B. A., Sweder K. S. Repair and transcription. Collision or collusion? Curr Biol. 1994 Jun 1;4(6):518–521. doi: 10.1016/s0960-9822(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Transcription-coupled repair and human disease. Science. 1994 Dec 23;266(5193):1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- Harrison L., Galanopoulos T., Ascione A. G., Antoniades H. N., Demple B. Regulated expression of APE apurinic endonuclease mRNA during wound healing in porcine epidermis. Carcinogenesis. 1996 Feb;17(2):377–381. doi: 10.1093/carcin/17.2.377. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Huang R. P., Adamson E. D. Characterization of the DNA-binding properties of the early growth response-1 (Egr-1) transcription factor: evidence for modulation by a redox mechanism. DNA Cell Biol. 1993 Apr;12(3):265–273. doi: 10.1089/dna.1993.12.265. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Van Houten B., Borm P. J., Mossman B. T. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., van Lingen B., Papaioannou V. E., Spiegelman B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993 Jul;7(7B):1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K. Cell cycle checkpoints and DNA repair preserve the stability of the human genome. Cancer Metastasis Rev. 1995 Mar;14(1):31–41. doi: 10.1007/BF00690209. [DOI] [PubMed] [Google Scholar]
- Kornhauser J. M., Nelson D. E., Mayo K. E., Takahashi J. S. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992 Mar 20;255(5051):1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Midgley C. A., Hupp T. R., Lu X., Vojtesek B., Picksley S. M. On the regulation of the p53 tumour suppressor, and its role in the cellular response to DNA damage. Philos Trans R Soc Lond B Biol Sci. 1995 Jan 30;347(1319):83–87. doi: 10.1098/rstb.1995.0013. [DOI] [PubMed] [Google Scholar]
- Levin J. D., Demple B. Analysis of class II (hydrolytic) and class I (beta-lyase) apurinic/apyrimidinic endonucleases with a synthetic DNA substrate. Nucleic Acids Res. 1990 Sep 11;18(17):5069–5075. doi: 10.1093/nar/18.17.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Ono Y., Furuta T., Ohmoto T., Akiyama K., Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994 Jul;315(1):55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Watanabe M., Inoue Y., Ohmoto T., Akiyama K., Tsutsui K., Seki S. Developmental expression of APEX nuclease, a multifunctional DNA repair enzyme, in mouse brains. Brain Res Dev Brain Res. 1995 May 26;86(1-2):1–6. doi: 10.1016/0165-3806(94)00212-i. [DOI] [PubMed] [Google Scholar]
- Powell S. N., Abraham E. H. The biology of radioresistance: similarities, differences and interactions with drug resistance. Cytotechnology. 1993;12(1-3):325–345. doi: 10.1007/BF00744671. [DOI] [PubMed] [Google Scholar]
- Ramotar D., Popoff S. C., Demple B. Complementation of DNA repair-deficient Escherichia coli by the yeast Apn1 apurinic/apyrimidinic endonuclease gene. Mol Microbiol. 1991 Jan;5(1):149–155. doi: 10.1111/j.1365-2958.1991.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Ramotar D., Popoff S. C., Gralla E. B., Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3'-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991 Sep;11(9):4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Solis R., Rivera-Pérez J., Wallace J. D., Wims M., Zheng H., Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992 Mar;201(2):331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- Rivkees S. A., Kelley M. R. Expression of a multifunctional DNA repair enzyme gene, apurinic/apyrimidinic endonuclease (APE; Ref-1) in the suprachiasmatic, supraoptic and paraventricular nuclei. Brain Res. 1994 Dec 12;666(1):137–142. doi: 10.1016/0006-8993(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Hickson I. D. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991 Oct 25;19(20):5519–5523. doi: 10.1093/nar/19.20.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Akiyama K., Watanabe S., Hatsushika M., Ikeda S., Tsutsui K. cDNA and deduced amino acid sequence of a mouse DNA repair enzyme (APEX nuclease) with significant homology to Escherichia coli exonuclease III. J Biol Chem. 1991 Nov 5;266(31):20797–20802. [PubMed] [Google Scholar]
- Seroz T., Hwang J. R., Moncollin V., Egly J. M. TFIIH: a link between transcription, DNA repair and cell cycle regulation. Curr Opin Genet Dev. 1995 Apr;5(2):217–221. doi: 10.1016/0959-437x(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Takiguchi Y., Chen D. J. Genomic structure of the mouse apurinic/apyrimidinic endonuclease gene. Mamm Genome. 1994 Nov;5(11):717–722. doi: 10.1007/BF00426079. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., McConville C. M., Byrd P. J. Cancer and DNA processing disorders. Br Med Bull. 1994 Jul;50(3):708–717. doi: 10.1093/oxfordjournals.bmb.a072919. [DOI] [PubMed] [Google Scholar]
- Walker L. J., Craig R. B., Harris A. L., Hickson I. D. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994 Nov 25;22(23):4884–4889. doi: 10.1093/nar/22.23.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. J., Robson C. N., Black E., Gillespie D., Hickson I. D. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993 Sep;13(9):5370–5376. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn L. M., Wells P. G. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995 Jul;48(1):112–120. [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G. G., Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K. S., Xanthoudakis S., Curran T., O'Dwyer P. J. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol Cell Biol. 1994 Sep;14(9):5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N., Dekker M., Berns A., Radman M., te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995 Jul 28;82(2):321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]