Contrast echocardiography encompasses a broad variety of different applications whereby cardiovascular ultrasound is used to detect microbubble contrast agents that produce a strong acoustic signal. Ultrasound contrast agents are currently approved for use in cardiology for the purposes of opacifying the left ventricular chamber in order to aid interpretation of echocardiography, yet their off-label use has been applied for myocardial perfusion imaging in ischaemic heart disease for over a decade. This article provides a broad overview of some of the future uses of contrast ultrasound in patients with cardiovascular disease that are on the horizon, including: (1) evaluating vascular anatomy and plaque neovascularisation; (2) detecting abnormal microvascular function; (3) assessing peripheral vascular disease with stress/rest limb perfusion imaging; (4) molecular imaging of cardiovascular disease; (5) site targeted delivery of therapeutic genes or drugs; and (6) ultrasound facilitated thrombolysis.
THE ACOUSTIC BUBBLE
Signal enhancement during contrast enhanced ultrasound (CEU) relies on the detection of the acoustic energy produced by encapsulated micro-bubbles or other acoustically active particles. The basis for signal generation from these contrast agents is their compressibility.w1 w2 Ultrasound contrast agents contain gases or, less commonly, emulsions with a low vapour pressure. Hence, they are several orders of magnitude more compressible than water or tissue and, because they are also smaller than the wavelength of diagnostic ultrasound, undergo volumetric oscillation in the pressure fluctuations of an ultrasound field.w1 The ‘vibration’ of microbubbles produces, among other forms of energy (light, heat), strong backscattered acoustic pressure signals that produce contrast enhancement on ultrasound imaging.
As predicted by the Rayleigh–Plesset principles, the magnitude of bubble oscillation and, hence, signal generation is influenced by the compressibility and density of the gas, the frequency and power of ultrasound applied, and the bubble radius.w3 These principles have governed the design of ultrasound contrast agents for human use through the following considerations: (1) signal generation within the diagnostic frequency range will increase with microbubble size, although microbubbles must still be smaller than capillary dimension in order to avoid lodging; (2) contrast agent signal will increase with the ultrasound pressure, although at high power, exaggerated microbubble oscillation produces bubble instability and destruction, also called inertial cavitation; and (3) microbubbles must retain a stable gas volume for a period of time after their injection in order to produce strong contrast signal enhancement. With regard to the latter issue, stability for micro-bubble agents that have been approved for use in humans has been optimised by using high molecular weight gases with low diffusivity, and also by encapsulation with shells composed of lipid or albumin, which reduce gas diffusion and surface tension, and help control microbubble size distribution. However, the encapsulation of micro-bubbles introduces another factor which is that the shell component can influence the vibration of microbubbles.w3
Microbubble specific imaging methods have been developed specifically to enhance signal from microbubbles and to suppress signal from tissue. These techniques have been described in detail elsewhere but many involve either: (1) filtering for harmonic signals and off-harmonic signals produced preferentially by microbubbles when they oscillate in a non-linear fashion; and (2) multipulse techniques that eliminate or minimise linear signal from tissue but retain the non-linear microbubble component.1 The improved signal-to-noise ratio for microbubble contrast agents provided by these new technologies has paved the way for new cardiovascular applications for contrast ultrasound.
PERFUSION IMAGING
The only cardiovascular indication approved by North American and European regulatory agencies for commercially produced microbubbles is for left ventricular cavity opacification, whereby contrast is administered to delineate ventricular geometry and endocardial borders better. Multiple agents have been approved for this application. Although none of these agents is specifically approved for microvascular perfusion imaging in the heart, myocardial contrast echocardiography (MCE) perfusion imaging has been used for over a decade in patients to assess the presence and severity of coronary artery disease (CAD) during stress testing, myocardial viability, and microvascular reflow in acute myocardial infarction. Accordingly, we will not consider these applications as ‘future’ for the purposes of this article and will instead refer the reader to published reviews on these subjects.w4 There are, however, several new potential cardiovascular diagnostic applications for MCE that have emerged in the past several years.
The ability to perform MCE at the patient bedside and to obtain data within several minutes has led to its application in the emergency department as a tool to confirm or exclude acute coronary syndrome rapidly in patients with chest pain.2 w5 In this setting, the addition of perfusion information to wall motion improves diagnostic accuracy and also provides valuable prognostic information (figure 1). For those individuals in whom acute coronary syndrome has been excluded and who have been referred for immediate stress echocardiography, myocardial perfusion imaging provides incremental benefit to wall motion assessment for identifying patients with CAD severe enough to produce symptoms.3 In those with recognised STelevation myocardial infarction, MCE perfusion imaging also provides potentially valuable information on whether there is perfusion within the risk area that occurs from either spontaneous recanalisation or collateral flow.w6 In the past couple of years, regulatory concerns regarding the safety of ultrasound contrast agents in unstable patients placed these applications in jeopardy, although these concerns have been largely refuted by several large studies establishing safety even in high risk patient populations.w7 w8
Figure 1.
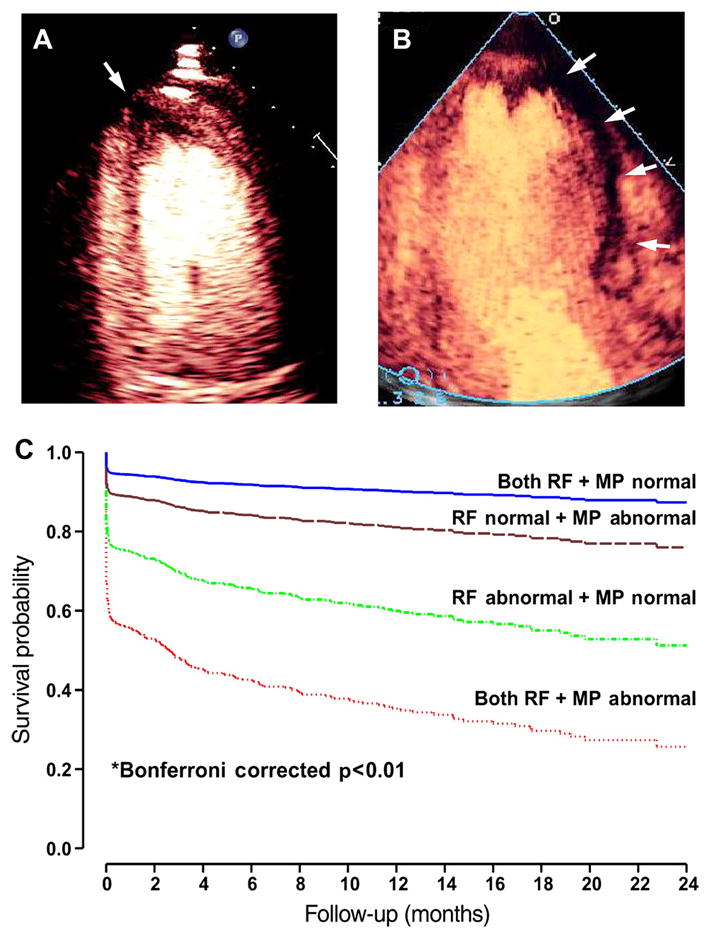
Bedside diagnosis of acute coronary syndrome. The top images are examples of acute myocardial infarction from two patients detected by bedside myocardial contrast echocardiography perfusion imaging where regions of hypoperfusion (arrows) are depicted by lack of myocardial opacification. The images illustrate (A) a small risk area in the apical four chamber view, and (B) a large risk area in the apical three chamber view. (C) Adjusted event-free survival (myocardial infarction, death, and heart failure) in 1017 patients presenting to the emergency department with chest pain undergoing myocardial contrast echocardiography.2 Data are stratified according to whether regional function (RF) and myocardial perfusion (MP) were normal or abnormal and comparisons made with Cox proportional hazards model.
The ability of MCE to provide quantitative information on perfusion has led to new diagnostic applications in cardiovascular medicine. MCE has recently been applied to evaluate microvascular function in patients who have ischaemic symptoms that may be secondary to abnormal vasoregulatory mechanisms rather than severe obstructive disease in epicardial vessels. The evaluation of perfusion by MCE relies on measuring the product of micro-vascular blood volume and the microvascular blood flux rate (an index of capillary blood velocity), which are derived from the kinetics of signal reappearance after microbubble destruction within the ultrasound beam.w9 The ability to quantify rapidly microvascular flow or even simply flux rate allows one to detect abnormal responses to endothelial dependent and independent substances that are typically used to unmask abnormal microvascular function often seen in diabetes, hypertension, dilated cardiomyopathy, or patients with angina and positive stress tests but no epicardial disease (figure 2).4 w10 Whether this information provides prognostic information that can influence therapy is not yet proven. Similarly, MCE has been used to assess abnormal microvascular response to catecholamine excess in stress cardiomyopathy,w11 although it has not yet been used to assess prognosis as has been done with epicardial coronary flow reserve (CFR) measurement.w12
Figure 2.
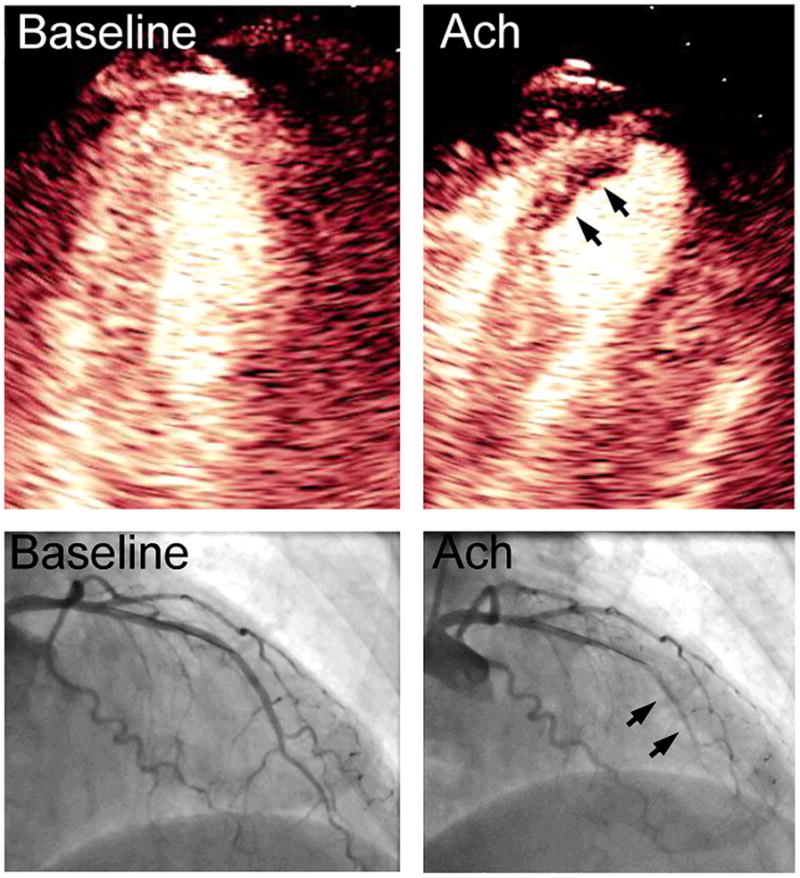
Apical four chamber myocardial contrast echocardiography images and left coronary arteriography from a right anterior oblique projection at baseline and after intracoronary injection of acetylcholine (Ach) to test for endothelial function through nitric oxide mediated vasodilation. Acetylcholine resulted in a paradoxical vasoconstriction of the left anterior descending artery (arrows on arteriography), which could be detected non-invasively by a severe subendocardial perfusion defect in the distal anterior septum on myocardial contrast echocardiography (arrows).
Ultrasound contrast agents are able to enhance signals on spectral Doppler examination. Although routine use of contrast solely for Doppler enhancement in valve disease is uncommon in clinical practice, it has been used for evaluating epicardial CFR using a non-invasive imaging approach. CFR is performed by measuring coronary artery flow velocities with transthoracic pulsed wave Doppler at rest and during hyperaemia, and has been used to evaluate the presence and functional severity of CAD. The ability to identify the major coronary arteries and reliably produce crisp spectral patterns is improved with contrast, thereby improving the likelihood for more widespread use of this approach.5 w13
PERIPHERAL VASCULAR IMAGING
The ability to enhance blood pool with ultrasound contrast agents has led to its use for identifying not only ventricular borders but vascular borders as well. It has been shown that contrast ultrasound agents facilitate measurement of near-wall carotid intima–media thickness (IMT).w14 However, because carotid IMT is a rapid and inexpensive screening technique, it is unlikely that there will be widespread adoption of contrast for this purpose, particularly since there are few data showing that contrast enhances the predictive power of IMT. Contrast has also been used to improve detection of high risk features of carotid stenosis such as ulceration, but again there is currently little evidence that routine use of contrast during carotid ultrasound can improve patient outcomes and is worth the added expense and effort.
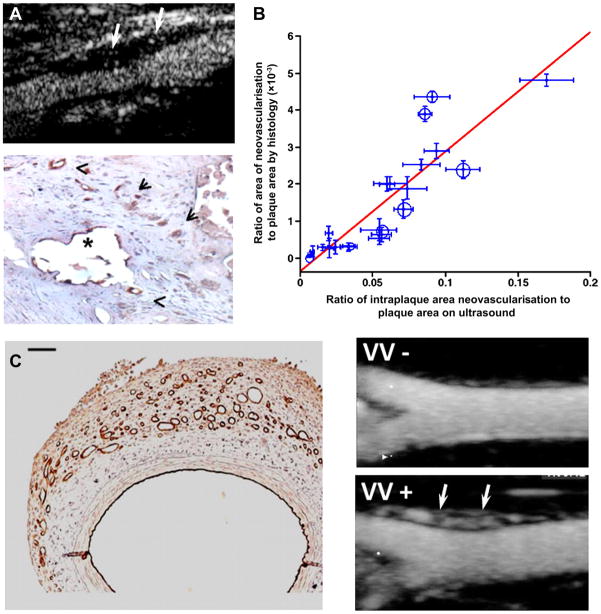
One unique application for contrast enhanced ultrasound perfusion imaging in carotid disease is its ability to evaluate the vasa vasorum (VV). In atherosclerotic disease, expansion of the VV and development of plaque neovessels that penetrate through the elastic lamina have been causatively linked with plaque progression, expansion of the necrotic core, macrophage accumulation, and plaque rupture.w15 w16 Plaque neovascularisation has been causatively linked to high risk plaques because they are a portal of entry for leucocytes and lipoproteins, are prone to haemorrhage, and require matrix protease activity for their formation which can have deleterious effects on the fibrous cap. These mural vessels can be detected by CEU (figure 3) and their presence has been correlated with high risk features such plaque echolucency (a feature of lipid-rich plaque) and unstable cerebrovascular events.6 w17 Because a low proportion of VV vessels have active flow at any time, quantitation of perfusion with conventional CEU is difficult, as illustrated by the images in figure 3 which indicate very low contrast intensity at any one instance. This limitation has been overcome by CEU with maximum intensity projection which provides a much more robust method for evaluating the density of functional VV vessels (figure 3C).7 Because of the pathophysiologic role of neovascularisation in plaque instability, in the future CEU is likely to be an important tool for evaluating new therapies designed to quiesce late stage atherosclerotic disease or, quite possibly, to stratify patients to these therapies.
Figure 3.
Contrast enhance ultrasound (CEU) imaging of vasa vasorum (VV) and plaque neovascularisation. (A) Examples of plaque neovascularisation detected by CEU in a patient with severe carotid stenosis (top) and also by histology with dual CD31/CD34 immunostaining after endarterectomy (bottom).6 (B) Relation between plaque neovascularisation by histology of carotid endarterectomy specimens and by CEU before surgery.w17 Black circles are from symptomatic patients and scales for x and y axis data are different. (C) Examples of VV proliferation produced by a model of vascular haemorrhage detected by CD31 immunohistology (left) and CEU with maximal intensity projection imaging of a rabbit femoral artery in long axis without (VV−) and with (VV+) VV proliferation (arrows) produced by haemorrhage.7
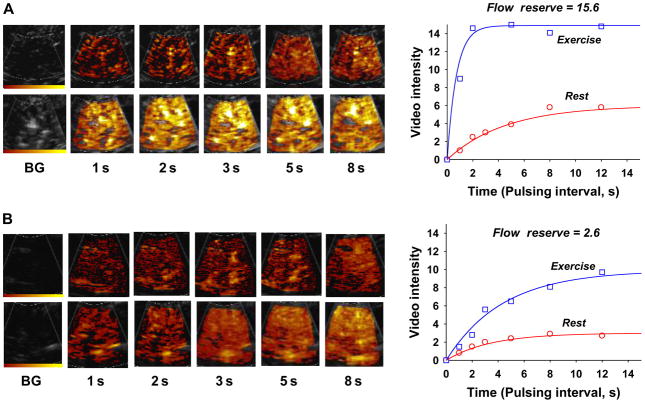
For decades there has been interest in using tissue perfusion imaging techniques to evaluate patients with peripheral arterial disease (PAD). Current diagnostic approaches that rely on measurement of pressure gradients are not well-suited to detecting moderate disease or quantifying response to therapy. Non-invasive perfusion imaging techniques commonly used to evaluate myocardial perfusion have not been routinely applied to evaluate PAD because of practical issues such as cost and time. More importantly, unlike CAD, PAD requires techniques that can assess flow quantitatively rather than measuring spatial heterogeneity of flow. Interest in using CEU to evaluate PAD is based on its ability to quantify perfusion rapidly and the fact that it requires equipment that is already present in most vascular laboratories. In animal models and humans, limb skeletal muscle CEU perfusion imaging at rest and during stress can detect and quantify PAD (figure 4).8,9 w18 Either bolus transit rate kinetics or post-destruction replenishment techniques have been applied for this application. Importantly, perfusion data have been shown to be more predictive for the severity of symptoms than standard pressure based techniques, particularly in patients with diabetes in whom functional abnormalities are also a prominent feature.8 Potential clinical uses of CEU perfusion imaging in PAD include early detection of disease, better quantitation of disease therapy, and guiding revascularisation strategies according to spatial patterns of flow. The technique may also be an important tool for quantifying the impact of investigational therapies for severe chronic PAD, including cell and gene therapy.
Figure 4.
Contrast enhanced ultrasound (CEU) images and corresponding time-intensity data obtained from the plantar flexor calf muscles at rest and during plantar flexion exercise in (A) a control subject and (B) a patient with moderate peripheral arterial disease in whom there is a pronounced reduction in hyperaemic flow and flow reserve.8 Images and data were obtained after a destructive pulse sequence. BG, background.
MOLECULAR IMAGING
Techniques for molecular and cellular imaging of disease have been developed for essentially all forms of medical imaging. This process often involves the development of site targeted contrast agents that are retained or activated in regions of disease. In its simplest form, CEU molecular imaging can be achieved by modification of the components of the microbubble shell which will amplify the endogenous ability of lipid or albumin membranes to interact with activated inflammatory cells or with activated vascular endothelium.10 w19 Specific shell components have, in the past, been used to amplify surface activation of serum complement which appears to be responsible for many of these interactions. A more specific approach to CEU molecular imaging has been to conjugate ligands that recognise disease related molecules to the surface of lipid-shelled microbubbles.w20 In general, ligand density is generally several thousand per μm2 surface area and ligands are placed at the end of a molecular spacer, taking a lesson from nature where the binding domain for cell adhesion molecules are usually projected away from the cell membrane to increase bond formation and tether efficiency. The result is a ‘multivalent’ ultrasound contrast that is able to attach to disease related targets within the vascular space where microbubbles reside. The binding efficiency of targeted microbubbles is influenced both by the density of the target molecule and the vascular shear forces.
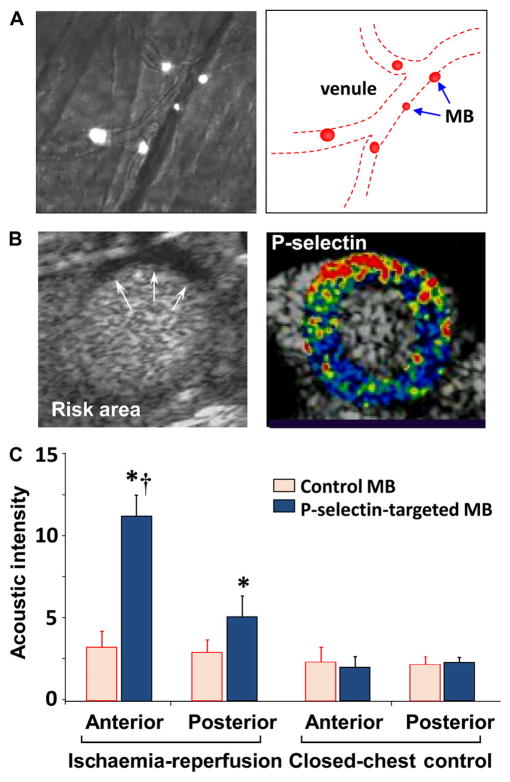
Proof-of-concept studies performed in animal models of disease have demonstrated that CEU with targeted microbubbles can detect the presence of many different cardiovascular conditions in which disease pathophysiology occurs within the vascular compartment where microbubbles reside. The transition of this technology to the clinical setting is most likely to occur in applications where there are distinct advantages of using an ultrasound based approach—namely cost, portability, and time required for image acquisition and interpretation. One such application is ischaemic memory imaging which is based on the idea that imaging the molecular sequelae of ischaemia that can persist for hours can be used to diagnose and assess the extent of recent myocardial ischaemia long after resolution. This approach may be particularly useful for diagnosing active ischaemia in those who have pre-existing wall motion abnormalities and perfusion defects from remote events. For radionuclide ischaemic memory imaging, radiolabelled long chain fatty acid analogues have been used to detect the switch away from fatty acid metabolism caused by active or recent myocyte ischaemia.w21 An ultrasound based approach may be more practical due to its portability and speed (<10 min). Microbubbles have been developed against selectins which are expressed for hours after tissue ischaemia and are involved in the initial phases of leucocyte recruitment by interacting with glycoprotein counter-ligands on the leucocyte surface.w22 Although the first description of this technology was with renal ischaemia–reperfusion injury,w22 CEU molecular imaging of P-selectin has been shown to detect myocardial ischaemia without infarction even after post-ischaemic stunning has resolved (figure 5).11,12
Figure 5.
Detection of recent myocardial ischaemia with myocardial contrast echocardiography (MCE) molecular imaging.11 (A) Intravital microscopy of the cremaster muscle in mice illustrating attachment of intravenously administered fluorescently labelled P-selectin targeted microbubbles (MB) to the venular surface after brief ischaemia–reperfusion injury of the muscle. (B) MCE perfusion imaging in a mouse illustrating an ischaemic risk area (arrows) during brief occlusion of the left anterior descending artery (LAD) and molecular imaging with P-selectin targeted microbubbles 45 min after reflow showing enhancement in the previously ischaemic risk area. Colour scale is at bottom. (C) Quantitative data from MCE P-selectin molecular imaging 45 min after ischaemia–reperfusion of the anterior LAD supplied territory. Data from closed chest control animals are also shown. *p<0.05 versus control microbubbles; †p<0.05 versus posterior non-ischaemic territory.
Methods for molecular imaging of atherosclerosis are currently being developed as tools to be used in both the research and clinical settings. For the latter, it is thought that molecular imaging could be used to ascertain better early risk for developing aggressive atherosclerotic disease or risk for adverse events in those with later stages of disease. In animal models of atherosclerosis, CEU molecular imaging has been able to detect high risk features of disease through microbubble targeting to endothelial cell adhesion molecules involved in leucocyte recruitment (selectins, vascular cell adhesion molecule 1 (VCAM-1)) and to molecules involved in thrombus formation or platelet adhesion (fibrin, von Willebrand factor, glycoprotein IIb/IIIa and 1bα).13–15 Interestingly, CEU imaging of endothelial activation has been able to detect the earliest stages of disease, suggesting that it could have a role for guiding therapy by early risk assessment.13 There are certainly advantages in terms of safety and cost of using an ultrasound based approach for screening purposes. Because of the difficulties of imaging coronary vessels non-invasively with ultrasound, the technique is most likely to have a clinical impact through imaging a surrogate vessel (eg, carotid artery) as a reflection of vascular phenotype. Molecular imaging of vascular phenotype holds promise in pharmaceutical development as a means for high throughput evaluation of new therapies before investing in expensive clinical trials. Many of the serum biomarkers currently used for this purpose are specific enough to identify vascular inflammation, and markers such as IMT and coronary calcium do not necessarily depict inflammatory burden.
THERAPEUTIC APPLICATIONS
When microbubbles are exposed to high pressure ultrasound, they undergo inertial cavitation whereby they are rapidly destroyed from exaggerated oscillation. The energy released by inertial cavitation can produce bioeffects that can be entrained for therapeutic purposes. One application is drug delivery whereby focused ultrasound can be used for site targeted drug release as well as augmenting vascular permeability for delivery. Unfortunately, this application is far from entering clinical use, largely because of the complexity in material design of a microbubble agent that is capable of carrying sufficient therapeutic payload while maintaining acoustic responsiveness. Gene delivery with contrast ultrasound is a more developed concept. Ultrasound energy in combination with microbubble contrast has been shown to enhance delivery of cDNA to tissue and to enhance transfection with plasmid cDNA safely (figure 6).16,17 Either commercially produced microbubbles or custom designed cationic lipid microbubbles that are capable of charge-coupling cDNA to their surface have been used for gene delivery. Potential mechanisms by which microbubble cavitation enhances transfection have included transient endothelial microporation caused by pressure microstreaming, ballistic effects, and enhanced clathrin or caveolin dependent cellular uptake.18 w23 Because vascular cells are the primary source of transfection with contrast ultrasound mediated gene delievery, one of the most promising applications has been pro-angiogenic gene delivery for potential use in severe ischaemic CAD or PAD (figure 6).w24 w25 However, there are many hurdles for eventual clinical translation, not least of which is the understanding of optimal conditions for ultrasound mediated transfection.
Figure 6.
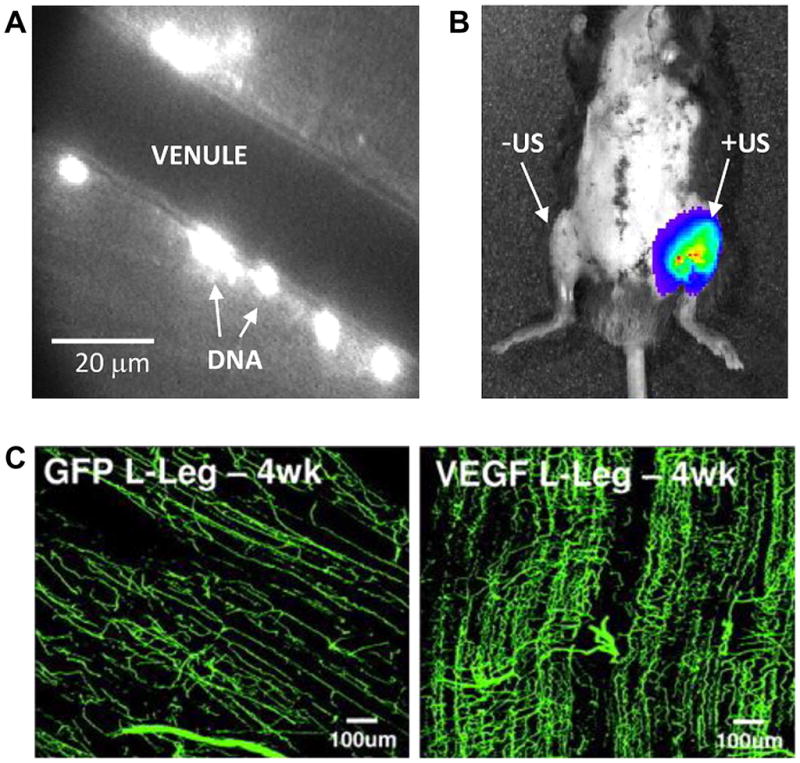
Vascular gene delivery and transfection. (A) Fluorescent labelling of plasmid cDNA that has been charge-coupled to cationic microbubbles and intravital microscopy of the muscle, demonstrating perivascular delivery to the microcirculation after intravenous injection of plasmid conjugated microbubbles and ultrasound exposure of the muscle.16 (B) In vivo optical imaging demonstrating firefly luciferase activity solely at the site of ultrasound (US) exposure (left hindlimb) 3 days after intravenous administration of cationic microbubbles bearing luciferase plasmid cDNA in a mouse. (C) Fluorescent microangiography in a rat ischaemic hindlimb model 4 days after contrast ultrasound demonstrating vascular remodelling when using vascular endothelial growth factor (VEGF) plasmid coupled microbubbles compared to microbubbles with control plasmid (GFP).w25
Exposure of microbubbles to ultrasound has also been used for accelerating endogenous or therapeutic thrombolysis. The exact mechanism(s) responsible for sonothrombolysis are unknown but probably involve high energy pressure microstreams that occur upon exaggerated microbubble oscillation and disruption with release of free gas. Preclinical studies have been performed by exposing microbubbles to ultrasound at frequencies within or just below the diagnostic range and at acoustic powers that can be achieved on clinical scanners. In animal models, sonothrombolysis has been shown to accelerate clot lysis in experimentally produced thrombus in coronary and peripheral arteries (figure 7).19,20 Interestingly, for coronary sonothrombolysis, an improvement in myocardial microvascular perfusion and amelioration of ischaemia can occur even in the absence of epicardial recanalisation. The exact mechanism for this is still not well understood. Clinical trials using sonothrombolysis with contrast ultrasound have been successfully performed in patients with acute stroke.w26 However, more recent studies have suggested an increased risk of intracerebral bleeding from using microbubbles together with unfocused 2 MHz ultrasound in patients undergoing plasminogen activator therapy for acute stroke.w27 Accordingly, a cautious approach is warranted until further research is performed on optimal acoustic conditions for achieving therapeutic effects with adverse bioeffects.
Figure 7.
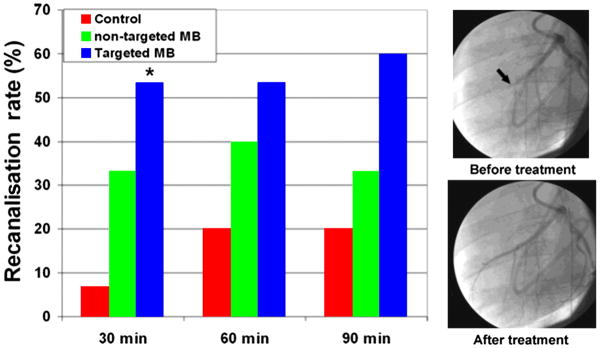
Sonothrombolysis by cavitation of microbubbles (MB).19 Data demonstrating recanalisation rate in a porcine model of acute left anterior descending artery (LAD) thrombotic occlusion using half-dose thrombolytic therapy alone (control) or with cardiac ultrasound exposure of non-targeted or platelet targeted microbubbles. Images illustrate angiographic LAD (arrow) recanalisation 30 min after contrast ultrasound exposure of targeted microbubbles.
REGULATORY ISSUES AND CONCLUSIONS
Although the only clinical indication approved by regulatory agencies for the use of ultrasound contrast agents in cardiovascular disease is for ventricular opacification, there are a host of diagnostic and therapeutic applications that are being explored. Some of these applications, such as myocardial perfusion imaging, enhancement for CFR measurement, and VV imaging, are already being used off-label for either clinical or research purposes. The likelihood that any new drug application to regulatory agencies for these uses will occur is very low given the current stall in even more established uses for MCE such as for detection of CAD. Other truly novel applications, such as molecular imaging, gene delivery, and coronary sonothrombolysis, will require substantial effort in the future with regard to probe development and testing, and determining optimal acoustic conditions. For molecular imaging, there will be challenges ahead for transition of these technologies to clinical practice, not least of which is the development of novel agents with ligands and surface conjugation strategies with proven safety and efficacy in humans. Most importantly, there must be firm evidence for clinical scenarios where these technologies can be applied to improve patient care.
Supplementary Material
Future applications of contrast echocardiography: key points.
Contrast enhanced ultrasound (CEU) relies on the generation of acoustic signals produced by cavitation (oscillation) of encapsulated micro-bubbles within the ultrasound field.
Microbubble contrast agents are approved for use in patients with cardiovascular disease, primarily for the purpose of ventricular cavity opacification and endocardial definition.
Myocardial microvascular perfusion imaging has been used off-label to evaluate patients with chest pain and microvascular dysfunction.
Vascular applications of CEU include detection of plaque neovascularisation and assessment of the severity of peripheral vascular disease.
Molecular imaging of atherosclerosis, thrombus, and recent myocardial ischaemia is possible using microbubbles that bear targeting ligands for disease related molecules.
The local bioeffects produced by acoustic disruption of microbubble contrast agents can be entrained in order to amplify thrombolysis and to deliver drugs/genes to target tissues.
You can get CPD/CME credits for Education in Heart.
Education in Heart articles are accredited by both the UK Royal College of Physicians (London) and the European Board for Accreditation in Cardiology— you need to answer the accompanying multiple choice questions (MCQs). To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl
RCP credits: Log your activity in your CPD diary online (http://www.rcplondon.ac.uk/members/CPDdiary/index.asp)—pass mark is 80%.
EBAC credits: Print out and retain the BMJ Learning certificate once you have completed the MCQs—pass mark is 60%. EBAC/EACCME Credits can now be converted to AMA PRA Category 1 CME Credits and are recognised by all National Accreditation Authorities in Europe (http://www.ebac-cme.org/newsite/?hit=men02).
Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group. If prompted, subscribers must sign into Heart with their journal’s username and password. All users must also complete a one-time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Footnotes
Competing interests: In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article. Dr Lindner has received a research grant from GE Medical.
Contributors: Dr Lindner and Dr Davidson together planned the content of the article and participated in writing the manuscript.
Provenance and peer review: Commissioned; internally peer reviewed.
References
▶ Additional references are published online only. To view these references please visit the journal online (http://heart.bmj.com/content/98/3.toc).
- 1.Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32:51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2▶.Rinkevich D, Kaul S, Wang XQ, et al. Regional left ventricular perfusion and function in patients presenting to the emergency department with chest pain and no ST-segment elevation. Eur Heart J. 2005;26:1606–11. doi: 10.1093/eurheartj/ehi335. This was a very large single centre study that established the incremental value of performing point-of-care myocardial perfusion imaging at the bedside in patients presenting to the emergency department with chest pain. [DOI] [PubMed] [Google Scholar]
- 3.Galbazzi N, Reverberi C, Squeri A, et al. Contrast stress echocardiography for the diagnosis of coronary artery disease in patients with chest pain but without acute coronary syndrome: incremental value of myocardial perfusion. J Am Soc Echocardiogr. 2009;22:404–10. doi: 10.1016/j.echo.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Moir S, Hanekom L, Fang ZY, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart. 2006;92:1414–19. doi: 10.1136/hrt.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigo F, Richieri M, Pasanisi E, et al. Usefulness of coronary flow reserve over regional wall motion when added to dual-imaging dipyridamole echocardiography. Am J Cardiol. 2003;91:269–73. doi: 10.1016/s0002-9149(02)03153-3. [DOI] [PubMed] [Google Scholar]
- 6▶.Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52:223–30. doi: 10.1016/j.jacc.2008.02.082. Although non-quantitative techniques were used, this study was one of the first to demonstrate that contrast ultrasound imaging could detect the presence of intraplaque flow in the carotid artery of patients. [DOI] [PubMed] [Google Scholar]
- 7.Lee SC, Carr CL, Davidson BP, et al. Temporal characterization of the functional density of the vasa vasorum by contrast-enhanced ultrasonography maximum intensity projection imaging. JACC Cardiovasc Imaging. 2010;3:1265–72. doi: 10.1016/j.jcmg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▶.Lindner JR, Womack L, Barrett EJ, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–50. doi: 10.1016/j.jcmg.2008.04.001. Results from this study indicate that stress–rest perfusion imaging may provide more accurate evaluation of the total impact of peripheral arterial disease in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duerschmied D, Olson L, Olschewski M, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J. 2006;27:310–15. doi: 10.1093/eurheartj/ehi636. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen JP, Leong-Poi H, Klibanov AL, et al. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002;105:1764–7. doi: 10.1161/01.cir.0000015466.89771.e2. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann BA, Lewis C, Xie A, et al. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28:2011–17. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115:345–52. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▶.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–9. doi: 10.1161/ATVBAHA.109.196386. This study demonstrated for the first time that any molecular imaging technique could detect the earliest stages of atherosclerosis. These results are important when considering the potential role of molecular imaging in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty OJ, Conley RB, Shentu W, et al. Molecular imaging of activated von Willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc Imaging. 2010;3:947–55. doi: 10.1016/j.jcmg.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton AJ, Huang SL, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–60. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen JP, French BA, Klibanov AL, et al. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003;29:1759–67. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 17.Bekeredjian R, Chen S, Frenkel PA, et al. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–6. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 18.Taniyama Y, Tachibana K, Hiraoka K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–9. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 19▶.Xie F, Lof J, Matsunaga T, et al. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation. 2009;119:1378–85. doi: 10.1161/CIRCULATIONAHA.108.825067. The results of this study formed the basis for planned clinical studies using ultrasound and microbubbles to facilitate reperfusion in patients with acute myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnbaum Y, Luo H, Nagai T, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug: recanalisation of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–4. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.