Abstract
Responses during a simple reaction time task are influenced by temporal expectation, or the ability to anticipate when a stimulus occurs in time. Here, we test the hypothesis that prefrontal D1 dopamine signaling is necessary for temporal expectation during simple reaction time task performance. We depleted dopamine projections to the medial prefrontal circuits by infusing 6-hydroxidopamine, a selective neurotoxin, into the ventral tegmental area (VTA) of rats, and studied their performance on a simple reaction time task with two delays. VTA dopamine depletion did not change movements or learning of the reaction time task. However, VTA dopamine-depleted animals did not develop delay-dependent speeding of reaction times, suggesting that mesocortical dopamine signaling is required for temporal expectation. Next, we manipulated dopamine signaling within the medial prefrontal cortex using local pharmacology. We found that SCH23390, a D1-type dopamine receptor antagonist, specifically attenuated delay-dependent speeding, while sulpiride, a D2-type receptor antagonist, did not. These data suggest that prefrontal D1 dopamine signaling is necessary for temporal expectation during performance of a simple reaction time task. Our findings provide insight into temporal processing of the prefrontal cortex, and how dopamine signaling influences prefrontal circuits that guide goal-directed behavior.
Keywords: Frontal cortex, dopamine, mesocortical, ventral tegmental area, response preparation, foreperiod
Introduction
Organizing behavior in time is a key feature of goal-directed behavior (Coull et al., 2011; Merchant et al., 2013). Humans suffering from a variety of diseases, such as schizophrenia, Parkinson’s disease, and attention deficit hyperactivity disorder, can develop difficulties organizing their movements in time (Barkley, 1997; Jahanshahi et al., 2010; Ngan and Liddle, 2000). While frontostriatal regions are known to be involved in organizing behavior (Coull et al., 2011; Nomoto et al., 1997; Stuss et al., 2005), the precise neural circuits remain unclear. Characterizing these circuits could be important in the development of therapies for diseases involving the frontal cortex and striatum (Arnsten et al., 2012).
One paradigm that has been used to study temporal expectation is the simple reaction time task (Luce, 1991; Fig. 1A). In this task, subjects learn to anticipate a stimulus at long waiting times and universally exhibit delay-dependent or foreperiod-dependent speeding of reaction times (Naatanen, 1972, 1970). That is, with long delay times subjects exhibit faster reaction times as temporal expectation is high, meaning that subjects are maximally prepared to respond. On the other hand, with short delay times, temporal expectation is low and subjects are minimally prepared to respond, making reaction times slower (Ollman and Billington, 1972). Delay-dependent speeding is heavily influenced by the ‘hazard function’ representing the conditional probability that an event will occur at a given time (Nomoto et al., 1997).
Figure 1.
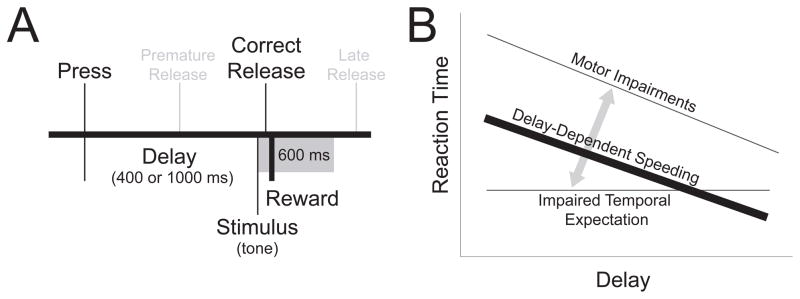
The simple reaction time task measures temporal expectation. (A) In the simple reaction time task, animals were trained to press and hold a lever until the onset of a tone (stimulus) at which point a lever release was rewarded with water (correct release). If lever release occurred prior to the stimulus onset (premature release) or greater than 600 ms after tone offset (late release), no reward was delivered. Following initial training with a 1000 ms fixed interval, animals were trained in a two-delay task (short delay - 400 ms, long delay - 1000 ms). (B) Schematic representation of delay-dependent speeding measured by reaction time (thick black line). The longer the delay, the more time the subject has to prepare to respond, leading to faster reaction times. When the delay is short and unexpected, the subject has less time to prepare for their response and correspondingly exhibit a slower reaction time. With motor impairments, reaction times are slower at both short and long delays. With impaired temporal expectation, reaction times do not change with increasing delay length. For a detailed discussion of this model, see Smith et al., 2010.
Prior work has shown that prefrontal cortex is required for temporal expectation during simple reaction time performance (Stuss et al., 2005; Vallesi et al., 2007). In rodents, disrupting the medial prefrontal cortex consistently impairs temporal expectation during a simple reaction time task (Narayanan and Laubach, 2006; Narayanan et al., 2006), as well as the ability to inhibit responding (Risterucci et al., 2003). Furthermore, prefrontal neurons are strongly modulated by temporal processing (Narayanan and Laubach, 2009; Niki and Watanabe, 1979, 1976) and control other brain areas according to temporal rules (Narayanan and Laubach, 2006). While prefrontal networks are modulated by several ascending projection systems, dopamine projections exert unique and cognitively specific modulation of prefrontal networks (Goldman-Rakic et al., 2000; Lapish et al., 2007).
The role of prefrontal dopamine signaling in reaction time performance remains unclear. Thus far, impairments of dopamine signaling in the striatum, such as those seen in Parkinson’s disease (Evarts et al., 1981) or by disrupting striatal dopamine (Amalric and Koob, 1987; Hauber et al., 2000) have led to motor impairments, evidenced by slowed reaction time without influencing temporal expectation (Fig. 1B; for more detail see Smith et al., 2010). However, manipulations of prefrontal dopamine signaling selectively impaired timing behavior in timing tasks (Narayanan et al., 2012). These data lead to the hypothesis that prefrontal dopamine signaling is involved in temporal expectation during simple reaction time performance (Fig. 1B). Specifically, disrupting prefrontal dopamine signaling should impair an animal’s ability to anticipate stimuli close to the response deadline and attenuate delay-dependent speeding.
Notably, prior work has suggested that disrupting mesolimbic projections from the ventral tegmental area (VTA) does not influence temporal expectation (Amalric and Koob, 1987; Hauber et al., 2000). However, in the present study, we tested the hypothesis that disruption of prefrontal dopamine would affect an animal’s temporal expectancy in two ways. First, we deprived the medial prefrontal cortex of dopamine input using targeted 6-hydroxydopamine (6-OHDA) lesions of the VTA. Second, we disrupted dopamine signaling within the medial prefrontal cortex during reaction time performance via infusion of pharmacological agents. We found that prefrontal dopamine is necessary for temporal expectation during reaction time performance, implicating the involvement of D1-type dopamine receptors in this process. These data provide novel evidence that prefrontal dopamine systems are required to organize behavior in time.
Experimental Procedures
Animals
Long Evans rats (Rattus norvegicus, N=27), weighing 250–300 grams were used in this study. All animal procedures were performed in accordance with the protocol approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC). Animals were housed individually following surgery with food ad libitum and had a 12 hour light-on light-off schedule. Animals were deprived of water 24 hours prior to behavioral testing.
Behavioral apparatus
Operant sound-attenuating chambers housing behavioral arenas (MedAssociates, St Albans, VT) were equipped with a lever, a drinking tube, and a speaker driven to produce an 8 kHz tone at 70 dB. Water was delivered via a pump (MedAssociates) connected to a standard metal drinking tube (AnCare) via Tygon tubing. Each correct response in the task activated the pump at 0.03 mL/s for 1000–3000 ms, depending on the stage of training. Behavior was monitored during all sessions via a closed circuit video camera mounted in each chamber and was recorded in some sessions.
Behavioral training
Well-handled animals were trained using the method of successive approximation to depress the response lever (~0.5 N). Each lever press activated the pump for 3000 ms (volume per trial: 0.075 mL), during which time additional lever presses were not rewarded. Animals that successfully learned the lever press protocol were trained to ‘wait’ for the stimulus using a simple reaction time task with a fixed delay of 1000 ms (Fig. 1A). On each training day, the pump time was decreased by 500 ms until it reached 1000 s and the response window was shortened until it reached 600 ms. Lever presses shorter than the 1000 ms delay or longer than the 600 ms response window were followed by a timeout period (4000–8000 ms). Once animals performed over 70% correct responses in a session (i.e., waited for the full delay and responded before the end of the response window), they were trained on the simple reaction time task with two delays (400 ms and 1000 ms; Fig. 1A).
Surgery
Animals were initially anesthetized with 4% isoflurane followed by intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg). A surgical level of anesthesia was maintained over the course of surgery with supplements (30 mg/kg) of ketamine every 45–60 minutes if necessary. Under aseptic conditions, the scalp was retracted, and the skull was leveled between bregma and lambda.
Experiment 1
Bilateral craniotomies were stereotaxically made above target sites (AP −5.6, ML ±2.3, DV −8.0 at 12 degrees laterally; Fig. 2A). A Hamilton syringe was lowered into the target site and 1 μL of either 6-OHDA or saline was slowly infused over 2 minutes at 15 μL/hour. Syringes were kept in place for 2 minutes to allow drug diffusion after which the needle was withdrawn, craniotomies were sealed and scalps sutured closed. After 1 week recovery, animals were trained in the simple reaction time task.
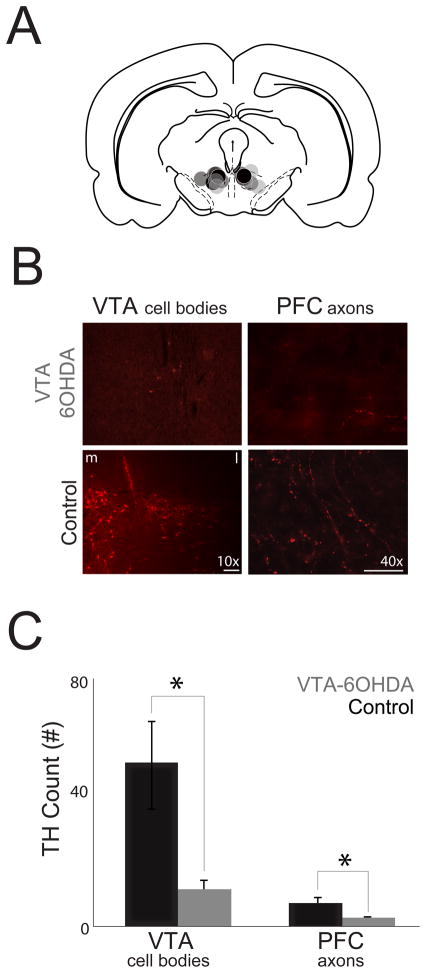
Figure 2.
VTA 6OHDA depletes dopamine in mesocortical circuits. (A) Stereotaxic injections targeting the bilateral VTA; control animals - black (
 ) and VTA-6OHDA - gray (
) and VTA-6OHDA - gray (
 ). (B) Representative images of TH+ (red) cells in the VTA (10X) and axons in the prefrontal cortex (PFC) (40X) in controls and VTA dopamine depleted animals (VTA 6OHDA). (C) VTA-6OHDA significantly decreased TH+ cells in the VTA and the number of TH+ projection axons in the prefrontal cortex (PFC) when compared with controls. * indicates statistical significance, p<0.05.
). (B) Representative images of TH+ (red) cells in the VTA (10X) and axons in the prefrontal cortex (PFC) (40X) in controls and VTA dopamine depleted animals (VTA 6OHDA). (C) VTA-6OHDA significantly decreased TH+ cells in the VTA and the number of TH+ projection axons in the prefrontal cortex (PFC) when compared with controls. * indicates statistical significance, p<0.05.
Experiment 2
Trained rats were anesthetized and 22-gauge guide cannulae (Plastics One) were implanted bilaterally in the medial prefrontal cortex using coordinates (AP +3.2, ML ±1.2, DV −3.6 at 10 degrees laterally; Fig. 6A) and procedures described previously (Narayanan et al., 2006). Following one week of recovery, animals were acclimated to infusion procedures and briefly anesthetized with isoflurane. A 33-gauge injector cannula (Plastics One) that protruded 0.2 mm from the tip of the guide cannula was infused with vehicle or drug (0.5 μL of sulpiride (1 μg/μL), 0.5 μL of SCH23390 (0.1 μg/μL or 1 μg/μL) dissolved in PBS with sufficient drops of hydrochloric acid, pH ~ 7.4; less than 5 total infusions per animal). The injector was inserted into the guide cannula and 0.5 μL of infusion fluid was delivered per site at a rate of 0.5 μL/min via a syringe infusion pump (KDS Scientific, Holliston, MA). Fluid was infused via 0.46-mm diameter polyethylene tubing (Intramedic, New York, NY) that connected the injector to a 5 μL Hamilton syringe (Hamilton, Reno, NV). Injections were confirmed by monitoring movement of fluid in the tubing via a small air bubble. After the injection was completed, the injector was left in place for 2 minutes to allow diffusion. Thirty minutes following infusion, animals underwent the simple reaction time task.
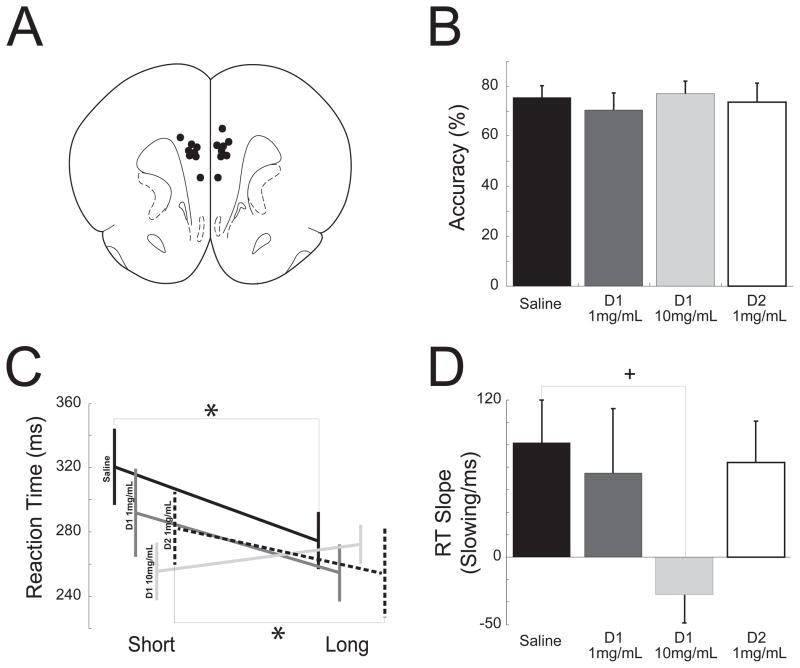
Figure 6.
Medial prefrontal D1 dopamine blockade attenuated temporal expectation. (A) Stereotaxic injections targeting the medial prefrontal cortex. Black dots (
 ) illustrate guide cannula locations. (B) There was no effect on accuracy following saline infusions, 1 mg/ml SCH23390 (D1 1 mg/ml), 10mg/ml SCH23390 (D1 10mg/ml), and 1 mg/ml sulpiride (D2 1 mg/ml) into the medial prefrontal cortex. (C) Following saline (black line) and sulpiride (dotted line), infusions to the medial prefrontal cortex, animals exhibited delay-dependent speeding as shown by faster reaction times (ms) during the long delay period (long) when compared to the short delay (short) in the simple reaction time task. However, following SCH23390 (D1; gray lines), animals had slower reaction times during the longer delays. (D) D1 blockade via SCH23390 attenuated the slope of delay-dependent slope while D2 blockade via sulpiride did not. * indicates significant differences via a t-test at p<0.05; + indicates a significant interaction between SCH23390 dose and delay-length in a linear random-effects model.
) illustrate guide cannula locations. (B) There was no effect on accuracy following saline infusions, 1 mg/ml SCH23390 (D1 1 mg/ml), 10mg/ml SCH23390 (D1 10mg/ml), and 1 mg/ml sulpiride (D2 1 mg/ml) into the medial prefrontal cortex. (C) Following saline (black line) and sulpiride (dotted line), infusions to the medial prefrontal cortex, animals exhibited delay-dependent speeding as shown by faster reaction times (ms) during the long delay period (long) when compared to the short delay (short) in the simple reaction time task. However, following SCH23390 (D1; gray lines), animals had slower reaction times during the longer delays. (D) D1 blockade via SCH23390 attenuated the slope of delay-dependent slope while D2 blockade via sulpiride did not. * indicates significant differences via a t-test at p<0.05; + indicates a significant interaction between SCH23390 dose and delay-length in a linear random-effects model.
Histology
Animals were anesthetized with 100 mg/kg sodium pentobarbital, transcardially perfused with 4% paraformaldehyde, and brains were coronally sectioned on a sliding microtome.
Experiment 1
Slices were free-floated in 1xPBS with 0.01% sodium azide while immunohistochemistry was performed according to methods described previously (Hommel et al., 2003). Immunofluorescent staining for tyrosine hydroxylase (TH) (Rabbit anti-TH; Millipore; 1:2000), with secondary antibodies (Alexa Flour 568 goat anti-rabbit IgG, 1:400, Invitrogen) was performed in normal goat serum and tissues were mounted on slides coated with Superfrost Plus and coverslipped with DAPI staining. Tissue was visualized using a fluorescent microscope (Zeiss) using standard FITC and TRITC filters. TH was quantified by counting TH+ cell bodies in the VTA and axons in the prefrontal cortex for both 6-OHDA and control animals. These data were statistically compared by performing a standard between group t-test.
Experiment 2
Guide cannulae locations were noted during histology, slices were mounted on slides coated with Superfronst Plus, and visualized under a microspoce following DAPI staining. Their placement was translated to a coronal brain atlas.
Data analysis
All behavioral data were loaded into MATLAB for exploratory data analysis and plotting. Reaction times were compared via two-tailed t-tests between groups. For trial-by-trial analysis, reaction time data were loaded into R and a linear-mixed effects model with trials and animals as a random effect was performed.
Results
Experiment 1: VTA dopamine depletion and temporal expectation
In the present study, our goal was to test the hypothesis that prefrontal dopamine influences temporal expectation (Fig. 1). To test this hypothesis, in experiment 1 we depleted the source of prefrontal dopamine from the VTA (Fig. 2) using stereotaxic 6-OHDA injections into the VTA. These injections significantly decreased the number of TH+ cell bodies in the VTA (t(12)=2.78, p=0.02; Fig. 2B–C). Notably, VTA dopamine depletion also significantly decreased the number of axons stained with TH in the prefrontal cortex (t(11)=3.31, p=0.007; Fig. 2B–C). These data suggest that we successfully depleted dopamine in mesocortical circuits.
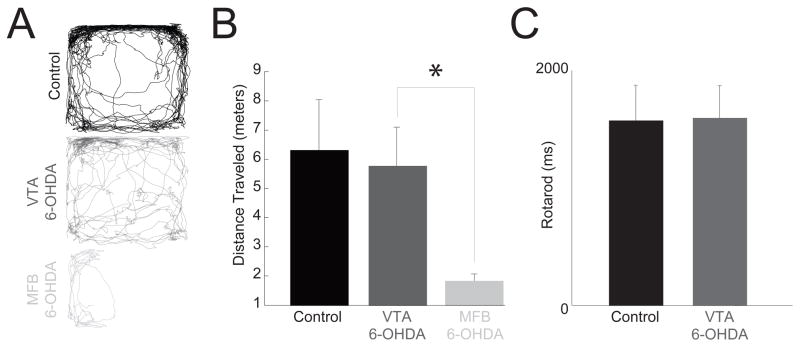
To ensure that our dopamine depletions did not influence movement, we measured two movement parameters. In open field testing, we found that while classic unilateral (right) median-forebrain-bundle dopamine depletions decreased movement (t(18)=4.2, p=0.0006; Fig. 3A–B), depleting VTA dopamine had no effect on open-field movement (t(12)=0.28, p=0.78; median-forebrain bundle: t(12)=5.0, p=0.0003; Fig. 3A–B). Furthermore, we found that depleting VTA dopamine did not influence rotarod performance (t (13)=−0.06, p=0.93; Fig. 3C). No impairment on these gross measures of movement suggests that VTA dopamine depletion does not impair movement.
Figure 3.
VTA dopamine depletion did not influence movement. (A) Individual examples of locomotor activity during an open field test showing no motor impairments in controls and VTA 6OHDA, while right median-forebrain bundle dopamine depletion (MFB 6-OHDA) caused circling and decreased movement. (B) Group analyses revealed that in comparison to controls, VTA 6-OHDA had no effect on the distance traveled during an open field test while MFB 6-OHDA exhibited significantly impaired movement. (C) There were no significant differences between controls and VTA 6-OHDA animals in rotarod performance. * indicates statistical significance, p<0.05.
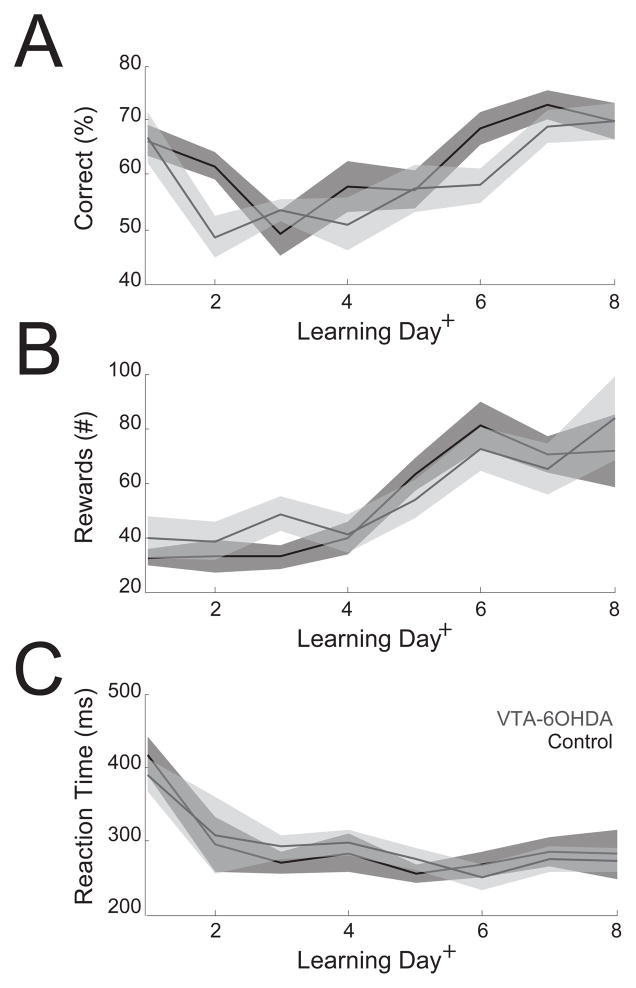
Previous work suggests that VTA dopamine signaling can influence motor learning (Hosp et al., 2011) as well as motivation (Wise, 2004). We investigated this issue in the learning rates of our animals (Fig. 4). We used a 3-way analysis of variance modeling the effect of learning on task performance, trials, and reaction time as a function of VTA dopamine depletion. There was a main effect of learning on performance (F(104)=26.5, p<10−5; Fig. 4A), rewards collected (F(104)=16.1, p=0.0001; Fig. 4B), and reaction time (F(104)=29.0, p<10−6; Fig. 4C). We found that VTA dopamine depletion did not significantly interact with performance (F(104)=0.01, p=0.76), rewards collected (F(104)=1.2, p=0.28) or reaction time (F(104)=0.8, p=0.37). These data suggest that VTA dopamine depletion does not impair learning or performance of the simple reaction time task, as animals with depleted VTA dopamine learned similarly when compared to controls.
Figure 4.
VTA dopamine depletion did not impair learning the simple reaction time task. Across days of learning in the simple reaction time task, the (A) rates of learning (%), (B) number of rewards, and (C) reaction times (ms) did not differ significantly between VTA-6OHDA animals and controls. Shaded areas represent SEM; control – black; gray – VTA dopamine depletion. + indicates a main effect of learning in a linear random-effects model. No consistent differences between VTA dopamine depletion and control animals were found.
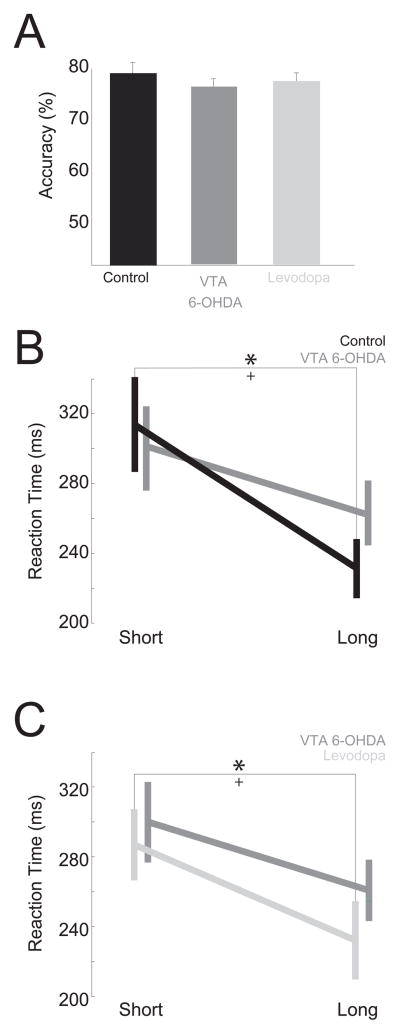
We then investigated the hypothesis that mesocortical dopamine projections are involved in temporal expectation by testing animals with VTA dopamine depletion in a simple reaction time task with two delays. This manipulation allowed us to directly investigate whether VTA dopamine depletion influences temporal expectation. VTA dopamine-depleted animals had similar correct responses (Short: 77±2%, Long: 79±2% - mean±SEM; t(13)=1, p=0.33; Fig. 5A) and similar overall reaction times when compared to controls (Short: 275±19 ms, Long: 259±17 ms; t(13)=1.21, p=0.25; Fig. 5B). However, whereas control animals demonstrated robust delay-dependent speeding in their first session (Short: 312±27 ms, Long: 230±17 ms; paired t(7)=4.37, p=0.003), animals with VTA dopamine depletion did not (Short: 300±25 ms, Long: 261±18 ms; paired t(7)=1.62; p=0.16; Fig. 5B). Furthermore, a linear mixed effects model with animals and trials as random effects revealed that there was a main effect of delay length on reaction time (F(15,1496)=5.69, p<10−10) and no main effect of VTA dopamine depletion. Crucially, there was a significant interaction between VTA dopamine depletion, delay, and reaction time (F(15,1496)=3.10, p<10−5) suggesting that VTA dopamine depletion attenuates delay-dependent speeding and impairs temporal expectation.
Figure 5.
VTA dopamine depletion attenuates temporal expectation in a simple reaction time task with two-delays. While VTA dopamine depletion did not change accuracy (A), control animals developed delay-dependent speeding and (B), animals with VTA dopamine depletion did not. They did nvvot learn delay-dependent speeding with further training. (C) Levodopa administration in VTA dopamine-depleted animals restored delay-dependent speeding. * indicates significant differences via a t-test at p<0.05; + indicates a significant interaction between dopamine depletion and delay-length in a linear random-effects model.
Next, we trained the animals for 5 days to investigate if VTA dopamine depleted animals learned temporal expectancy. After training, VTA dopamine depleted animals did not learn delay-dependent speeding (Short: 304±31 ms, Long: 260±27 ms; paired t(7)=1.1; p=0.30), whereas control animals consistently demonstrated delay-dependent speeding (Short: 313±27 ms, Long: 223±15 ms; paired t(7)=2.7; p=0.02). While VTA dopamine depleted animals learned to wait for an imperative stimulus in the simple reaction time task, they did not learn to speed their reaction times during longer delays. These data provide evidence that mesocortical dopamine signaling is required for temporal expectation.
To confirm that these effects were truly dopamine dependent, we administered levodopa (15 mg/kg intraperitoneal) to VTA dopamine-depleted animals. We found that levodopa administration to VTA dopamine-depleted animals restored delay-dependent speeding (Short: 294±21 ms, Long: 239±22 ms; paired t(6)=3.8; p=0.009; linear mixed-effects interaction between delay and levodopa: F(7,1435)=2.3, p=0.004; Fig. 5C). Taken together, these data support the idea that VTA dopamine projections are necessary for temporal expectation during simple reaction time performance.
Experiment 2: Prefrontal dopamine and temporal expectation
As described in experiment 1 above, VTA dopamine projections are involved in temporal expectation. The VTA projects to two major targets: 1) the medial prefrontal cortex, and 2) the nucleus accumbens (Lapish et al., 2007; Narayanan et al., 2010). Previous work has demonstrated that dopamine disruption in the nucleus accumbens does not influence reaction time performance (Amalric and Koob, 1987; Hauber et al., 2000). However, the role of the VTA projection to the medial prefrontal cortex is unknown. In experiment 2 we test the hypothesis that dopamine signaling within the medial prefrontal cortex is also necessary for temporal expectation.
To investigate this idea, we trained 8 animals to perform a simple reaction time task. After they were fully trained, we implanted cannula in the medial prefrontal cortex targeting the medial prefrontal cortex bilaterally (Fig. 6A), and infused drugs 30 min prior performance in the simple reaction time task.
We found that infusing saline into the prefrontal cortex did not appreciably influence behavior; animals performed at established high levels of accuracy (75±5%) and exhibited delay-dependent speeding (Short: 321±67 ms, Long: 274±29 ms; paired t(7)=2.4, p=0.04). Animals infused with the D1-type dopamine receptor blocker SCH23390 into the prefrontal cortex retained accurate simple reaction-timing (10 mg/mL SCH23390; 77±7%; paired t(5)=0.3, p=0.81; Fig. 6B); however, they did not exhibit delay-dependent speeding at low doses (0.5 μg SCH23390: Short: 288±37 ms, Long: 244±29 ms; paired t(4)=1.3, p=0.28) or at high doses (5 μg SCH23390: Short: 258±22 ms, Long: 271±16 ms; paired t(4)=0.5, p=0.68; Fig. 6C). Interestingly, high doses of SCH23390 resulted in a slight speeding of reaction time at short delays (258±22 ms, 321±67 ms in saline sessions; paired t(4)=2.6, p=0.05). At 5.0 μg of SCH23390, animals performed less trials (63±15 rewards, 45.7±14 rewards in saline sessions; paired t(5)=3.65, p=0.02); however, at lower doses they performed a similar number of trials when compared to control sessions (0.5 μg SCH23390: paired t(4)=0.12, p=0.91).
A linear mixed-effects model with trials and animals as random effects revealed main effects of SCH23390 dose (F(5,662)=3.4, p=0.004) as well as delay (F(5,662)=3.2, p=0.007) on reaction time. Notably, there was a significant interaction between dose and delay-dependent speeding (F(5,662)=4.0, p=0.001; Fig. 6C–D). To confirm specificity of this effect, we infused a D2-type dopamine receptor blocker, sulpiride, and found no effect on temporal expectation. That is, animals with prefrontal D2 blockade had similar accuracy (73.5±9.6%) and exhibited delay-dependent speeding similar to saline sessions (7 animals; Short: 294 ±67 ms, Long: 274±29 ms; paired t(6)=2.61, p=0.04). These data suggest that prefrontal D1 blockade attenuates reaction time delay-dependent. Combined with the results from experiment 1, this study provides specific evidence that mesocortical dopamine signaling is selectively involved in temporal expectation during simple reaction time performance.
Discussion
The present study examined the hypothesis that prefrontal dopamine signaling is involved in temporal expectation during simple reaction time performance. We tested this idea using neurotoxin-induced depletion of dopamine input from the VTA to the medial prefrontal cortex and local pharmacological blockade of prefrontal dopamine receptors. We report that VTA dopamine depletion specifically impaired delay-dependent speeding without affecting learning or performance of the simple reaction time task or motor behavior. Furthermore, we found that prefrontal D1 but not D2-type receptor blockade selectively impaired delay-dependent speeding. Taken together, these data provide novel evidence that prefrontal D1 dopamine signaling is involved in temporal expectation during simple reaction time tasks.
These findings are novel as the medial prefrontal cortex has been shown to be involved in temporal processing during a simple reaction time task (Narayanan and Laubach, 2006; Narayanan et al., 2006; Risterucci et al., 2003), and prefrontal D1 dopamine signaling is required perceptual timing (Narayanan et al., 2012). These results expand the scope of prefrontal D1 signaling in temporal processing to temporal expectation during a simple reaction time task (Luce, 1991; Naatanen, 1970), and provide a pharmacological window into how prefrontal networks organize behavior in time (Fuster, 2008; Stuss and Alexander, 2000).
Although the simple-reaction time task can be used to study temporal processing (Naatanen et al., 1974; Narayanan et al., 2006), in this study, temporal expectation is confounded with attention, arousal (Eason et al., 1969) and motor preparation (Niemi and Näätänen, 1981). As such, we cannot rule out a role for attention or motor preparation in our study. Future studies will independently manipulate temporal expectation using a cue or blocked design (Los, 1999) to present stimuli at the same time but with different temporal expectation. Such studies require extensive training in rats, but could further illuminate the neural circuitry of temporal processing.
Prior work has consistently demonstrated that striatal dopamine signaling does not influence temporal expectation. For instance, patients with Parkinson’s disease have dramatically decreased striatal dopamine (Chinaglia et al., 1992) and impaired response initiation (Evarts et al., 1981; Gauntlett-Gilbert and Brown, 1998). These patients tend to exhibit temporal expectation and delay-dependent speeding despite having slow overall reaction times. Multiple studies have demonstrated that blocking dopamine signaling within the nucleus accumbens does not influence reaction time performance or delay-dependent speeding (Amalric and Koob, 1987; Hauber et al., 2000). Although the VTA sends strong dopaminergic projections to the nucleus accumbens (Narayanan et al., 2010), these studies suggest that this projection is not consistently involved reaction time performance or temporal expectation. Importantly, a subset of patients with Parkinson’s disease have impaired executive function at incident diagnosis (Aarsland et al., 2009; Williams-Gray et al., 2007) and likely have impaired prefrontal dopamine signaling (Cools et al., 2002; Narayanan et al., 2013). Our data lead to the hypothesis that only those patients with executive dysfunction would have impaired temporal expectation.
In our study, neither dopamine manipulation (SCH23390 or sulpiride) increased premature responding or influenced waiting. Our results are quite distinct from prefrontal lesions (Risterucci et al., 2003) or inactivation (Narayanan and Laubach, 2006; Narayanan et al., 2006), and instead suggest that prefrontal D1 signaling is not involved in impulsivity or inhibitory control (Jacobsen, 1936). Furthermore, we found no evidence that mesocortical dopamine signaling is involved in motivation (Wise, 2004) or motor control (Hosp et al., 2011), supporting the idea that these critical functions involve other circuits. We did find a specific effect of prefrontal D1 signaling on temporal expectation, suggesting an involvement in computation of the ‘hazard function’ (Nobre et al., 2007) and conditional probability of reward availability with time (Watanabe, 1996). In light of previous research, these data suggest that prefrontal networks responsive to D1 dopamine signaling may inhibit reaction times only during a temporally relevant window (i.e., after the animal knows a reward is available but before the response ‘deadline’ (Ollman and Billington, 1972), or the time by which animals must respond and may explicitly be involved in encoding of the hazard function. Although there was no difference in overall reaction time following VTA dopamine depletion, animals with prefrontal D1 dopamine blockade were marginally faster, from which we infer that prefrontal influence might be inhibitory (Fig. 6C–D). A recent study (Smith et al., 2010) reported that such a pattern of reaction times with delay-length could be explained by proactive inhibition (Bari et al., 2011). One additional possibility for differences in reaction times between VTA dopamine depletion and prefrontal dopamine blockade could include cortical reorganization as seen in lesion studies (Bao et al., 2001). VTA dopamine depletions should affect all targets of the VTA, which include other cortical regions. A final possibility is that additional areas, such as the premotor cortex (Smith et al., 2010), may be affected by VTA dopamine depletion and influence reaction time performance. Future studies could systematically deplete dopamine in several motor regions (Neafsey et al., 1986) to explore this issue.
Prefrontal D1 signaling has traditionally been associated with high level cognitive behaviors, such as working memory (Goldman-Rakic et al., 2004), risk-based decision making (St Onge et al., 2011), and attention (Granon et al., 2000). Prefrontal D1 microcircuits are extraordinarily specific in such behaviors (Constantinidis et al., 2001; Goldman-Rakic et al., 2000; Williams and Goldman-Rakic, 1995). Prefrontal D1 signaling has also been shown to be required for perceptual timing (Narayanan et al., 2012) using selective optogenetic manipulations in transgenic animals. The present study supports previous data implicating prefrontal D1 signaling in organizing goal-directed behavior (Miller and Cohen, 2001). Yet, our data are the first to link prefrontal D1 signaling to a simple reaction time task, supporting the idea that performance on this task can benefit from cognitive judgment of time.
Conclusion
In summary, we tested the idea that mesocortical dopamine projections are involved in temporal expectation during simple reaction time performance. We found that rats with VTA dopamine depletion did not exhibit delay-dependent speeding. Furthermore, we found that rats with specific blockade of D1 but not D2-type dopamine receptors in the prefrontal cortex also did not exhibit delay-dependent speeding. Our findings suggest that prefrontal D1 receptors are involved in temporal expectation, and provide evidence that mesocortical dopamine signaling is involved in organizing behavior in time.
Highlights.
Prefrontal dopamine signaling is involved in temporal expectation during a simple reaction time task.
VTA dopamine depletion did not change movements or learning of the reaction time task.
VTA dopamine depletion animals did not develop delay-dependent speeding in a two delay task.
Prefrontal D1, not D2, dopamine receptor block slowed simple reaction time performance.
Prefrontal D1 dopamine is necessary for temporal expectation in a simple reaction time task.
Acknowledgments
NIH K08 and Aging Mind and Brain Initiative to NSN
Abbreviations
- 6OHDA
6-hydroxydopamine
- VTA
Ventral tegmental area
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Depletion of dopamine in the caudate nucleus but not in nucleus accumbens impairs reaction-time performance in rats. J Neurosci. 1987;7:2129–2134. doi: 10.1523/JNEUROSCI.07-07-02129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KCNA, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. ADHD and the Nature of Self-control. The Guilford Press; New York, NY: 1997. [Google Scholar]
- Chinaglia G, Alvarez FJ, Probst A, Palacios JM. Mesostriatal and mesolimbic dopamine uptake binding sites are reduced in Parkinson’s disease and progressive supranuclear palsy: a quantitative autoradiographic study using [3H]mazindol. Neuroscience. 1992;49:317–327. doi: 10.1016/0306-4522(92)90099-n. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. Coding specificity in cortical microcircuits: a multiple-electrode analysis of primate prefrontal cortex. J Neurosci. 2001;21:3646–55. doi: 10.1523/JNEUROSCI.21-10-03646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason RG, Harter MR, White CT. Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiology & Behavior. 1969;4:283–289. [Google Scholar]
- Evarts EV, Teräväinen H, Calne DB. Reaction time in Parkinson’s disease. Brain. 1981;104:167–186. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex. 4. Academic Press; New York, NY: 2008. [Google Scholar]
- Gauntlett-Gilbert J, Brown VJ. Reaction time deficits and Parkinson’s disease. Neurosci Biobehav Rev. 1998;22:865–81. doi: 10.1016/s0149-7634(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Bohn I, Giertler C. NMDA, but not dopamine D(2), receptors in the rat nucleus accumbens areinvolved in guidance of instrumental behavior by stimuli predicting reward magnitude. J Neurosci. 2000;20:6282–6288. doi: 10.1523/JNEUROSCI.20-16-06282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen C. I. The functions of the frontal association areas in monkeys. Comparative Psychology Monographs. 1936;13:1–60. [Google Scholar]
- Jahanshahi M, Wilkinson L, Gahir H, Dharmaindra A, Dharmarinda A, Dharminda A, Lagnado DA. Medication impairs probabilistic classification learning in Parkinson’s disease. Neuropsychologia. 2010;48:1096–1103. doi: 10.1016/j.neuropsychologia.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los SA. Identifying stimuli of different perceptual categories in pure and mixed blocks of trials: evidence for stimulus-driven switch costs. Acta Psychol (Amst) 1999;103:173–205. doi: 10.1016/s0001-6918(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response Times: Their Role in Inferring Elementary Mental Organization. Oxford University Press; 1991. [Google Scholar]
- Merchant H, Harrington DL, Meck WH. Neural Basis of the Perception and Estimation of Time. Annu Rev Neurosci. 2013 doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Naatanen R. The diminishing time-uncertainty with the lapse of of time after the warning signal in reaction-time experiments with varying foreperiods. Acta Psychologia. 1970;1970:399–419. doi: 10.1016/0001-6918(70)90035-1. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Time uncertainty and occurrence uncertainty of the stimulus in a simple reaction time task. Acta Psychologia. 1972;36:492–503. [Google Scholar]
- Naatanen R, Muranen V, Merisalo A. Timing of expectance peak in simple reaction time situation. Acta Psychol (Amst) 1974;38:461–70. doi: 10.1016/0001-6918(74)90006-7. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, Dileone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 2013;24:267–278. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Ngan ET, Liddle PF. Reaction time, symptom profiles and course of illness in schizophrenia. Schizophr Res. 2000;46:195–201. doi: 10.1016/s0920-9964(00)00027-x. [DOI] [PubMed] [Google Scholar]
- Niemi P, Näätänen R. Foreperiod and simple reaction time. Psychological Bulletin. 1981;89:133–162. [Google Scholar]
- Niki H, Watanabe M. Prefrontal unit activity and delayed response: relation to cue location versus direction of response. Brain Res. 1976;105:79–88. doi: 10.1016/0006-8993(76)90924-0. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Nobre A, Correa A, Coull J. The hazards of time. Curr Opin Neurobiol. 2007;17:465–470. doi: 10.1016/j.conb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Nomoto M, Kaseda S, Iwata S, Osame M, Fukuda T. Levodopa in pregnancy. Mov Disord. 1997;12:261. [PubMed] [Google Scholar]
- Ollman RT, Billington MJ. The deadline model for simple reaction times. Cognitive Psychology. 1972;3:311–336. [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Smith NJ, Horst NK, Liu B, Caetano MS, Laubach M. Reversible Inactivation of Rat Premotor Cortex Impairs Temporal Preparation, but not Inhibitory Control, During Simple Reaction-Time Performance. Front Integr Neurosci. 2010;4:124. doi: 10.3389/fnint.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Mussoni A, Mondani M, Budai R, Skrap M, Shallice T. The neural basis of temporal preparation: Insights from brain tumor patients. Neuropsychologia. 2007;45:2755–2763. doi: 10.1016/j.neuropsychologia.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]