Abstract
Poor vascularization is the key limitation for long-term acceptance of large three-dimensional (3D) tissue engineering constructs in regenerative medicine. 45S5 Bioglass® was investigated given its potential for applications in bone engineering. Since native Bioglass® shows insufficient angiogenic properties, we used a collagen coating, to seed human adipose tissue-derived stem cells (hASC) confluently onto 3D 45S5 Bioglass®-based scaffolds. To investigate vascularization by semiquantitative analyses, these biofunctionalized scaffolds were then subjected to in vitro human umbilical vein endothelial cells formation assays, and were also investigated in the chorioallantoic membrane (CAM) angiogenesis model, an in vivo angiogenesis assay, which uses the CAM of the hen's egg. In their native, nonbiofunctionalized state, neither Bioglass®-based nor biologically inert fibrous polypropylene control scaffolds showed angiogenic properties. However, significant vascularization was induced by hASC-seeded scaffolds (Bioglass® and polypropylene) in the CAM angiogenesis assay. Biofunctionalized scaffolds also showed enhanced tube lengths, compared to unmodified scaffolds or constructs seeded with fibroblasts. In case of biologically inert hernia meshes, the quantification of vascular endothelial growth factor secretion as the key angiogenic stimulus strongly correlated to the tube lengths and vessel numbers in all models. This correlation proved the CAM angiogenesis assay to be a suitable semiquantitative tool to characterize angiogenic effects of larger 3D implants. In addition, our results suggest that combinations of suitable scaffold materials, such as 45S5 Bioglass®, with hASC could be a promising approach for future tissue engineering applications.
Introduction
Scaffolds for bone tissue engineering must fulfil several requirements, including adequate mechanical strength and stiffness, interconnected pore structure, bioactive behaviour, and bioresorbability in predetermined rates.1,2 Furthermore, scaffolds for bone tissue should enable cells to attach and proliferate and also promote the vascularization of the whole construct, while new bone tissue is growing. Preferably, the scaffold material itself ought to induce angiogenesis.3,4 Bioactive glass, for example, the composition 45S5 Bioglass®,5 is a candidate material being considered for the development of bone scaffolds based on its proved bioactive behaviour, for example, strong interaction with bone tissue, as well as the known effect of ion dissolution products of bioactive glass inducing the up-regulation of genes in bone cells and their effect in enhancing bone formation.6–9 More recently, studies have shown that bioactive glasses may also be effective in promoting the vascularization of tissue constructs in different combinations, for example, as sintered scaffolds and composites.10–17 The use of Bioglass® to fabricate scaffolds using a foam replica method was introduced in 2006 followed by studies on the possible angiogenic effect of such scaffolds.18 Similar bioactive glass scaffolds were investigated in coculture studies using human umbilical vein endothelial cells (HUVEC) and human osteoblasts,14 and it was shown that the scaffolds were able to support both endothelial and human osteoblast proliferation.
Considering contradictory data presented in the literature on the putative angiogenic potential of 45S5 Bioglass®-based scaffolds, there is a need for further investigations. Recent in vivo studies using different animal models described an effect of 45S5 Bioglass® on the angiogenic response in soft connective and bone tissue.16 Ghosh et al.19 and Nandi et al.20 implanted bioactive glass blocks in the lateral aspect of diaphysis of radius bone from black Bengal goats and observed a well-formed vascularization towards the blocks, as well as organized trans-implant angiogenesis. Ross et al.21 obtained similar results with 45S5 Bioglass®-coated silicon tubes implanted subcutaneously in a rat model, whereas other rat model studies, performed with PLGA–Bioglass® foam composites, did not result in any significant increase in angiogenesis towards the implant.16
Animal experiments are still the in vivo gold standard to characterize angiogenesis. However, due to ethical aspects and expenses, this approach is unfortunate for screening of materials or cell-scaffold-combinations. On the other hand, standard in vitro angiogenesis tests, such as tube formation assays, cannot be conducted with every material due to technical obstacles. In addition, there is another drawback, especially with three-dimensional (3D) scaffolds, as they cannot be characterized without cracking them into small pieces. A promising method to address angiogenesis is the chorioallantoic membrane (CAM) angiogenesis assay, which uses the CAM of fertilized chicken eggs. Based on the rapid growth of its capillary network and a larger application area, the CAM angiogenesis assay allows the in vivo testing of multiple samples at the same time within just a few days.22–24 Although the CAM angiogenesis assay is an interesting in vivo alternative to study angiogenic and antiangiogenic responses to tissues, cells, or soluble factors, quantification of vascularization is still difficult in this model.23–25
To our knowledge, there is only one study which used the CAM angiogenesis assay for the investigation of the angiogenic response of 45S5 Bioglass®-based glass-ceramic scaffolds.18 In this work the material itself did not induce vessel ingrowth. On the other hand a topic application of small amounts of positive regulators of angiogenesis directly onto scaffolds, for example, vascular endothelial growth factor (VEGF), has been shown to promote angiogenesis in the CAM assay.26 However, this approach was unable to initiate a long-term angiogenic stimulus. Once the applied amount of growth factor was utilized, the angiogenic stimulus stopped.
To overcome the drawbacks of angiogenic burst effects by pharmacological application of growth factors onto scaffolds, a more promising approach to induce durable angiogenesis by scaffolds is generally seen in the use of autologous progenitor cells. This approach bears the chance that the cells constantly secrete capillary recruiting mediators27–29 and therefore, induce long-term vessel growth. To biofunctionalize scaffolds, high numbers of adult progenitor cells can easily be isolated, for example, from human adipose tissue- derived stem cells (hASC), but they are present and responsible for tissue repair and tissue regeneration in every adult tissue.30 In addition, hASC are able to both differentiate into various specific cell types (e.g., chondrocytes, adipocytes and osteocytes) and to secrete different growth factors, including VEGF, which stimulates repair and regeneration processes, including angiogenesis.28
In this study, we examined for the first time 45S5-Bioglass®-based 3D-scaffolds seeded with hASC for their effects on in vivo vascularization using the CAM angiogenesis assay. Commercial fibrous scaffolds (polypropylene hernia meshes) were used as biologically inert control material. For further investigation of the angiogenic effects, the biofunctionalized scaffolds were subjected to in vitro HUVEC formation assays. Since VEGF is a potent and the most likely proangiogenic stimulus,31 we followed VEGF-secretion of hASC via enzyme immunoassay and correlated all in vitro data with the effects observed in the CAM angiogenesis assay.
Materials and Methods
All experiments were run in triplicate with human primary cells. hASC were purchased from Invitrogen Life Technologies GmbH. For the isolation of human fibroblasts (HF) from adult skin tissue (AST) and HUVEC, written informed consent was obtained from all tissue donors. HUVEC were characterized by flow cytometry and showed >98% PECAM-1 positive cells directly after isolation.
Bioglass®-based scaffolds
The scaffolds for this study were fabricated using 45S5 Bioglass® powder (nominal composition in wt.%: 45 SiO2, 24.5 CaO, 24.5 Na2O, 6 P2O5) using the foam replica technique.9 The scaffolds exhibited porosity in the range of 90%–95%. The fabrication involves the coating of a polyurethane (PU) foam with 45 ppi (Recticel), cut to size 10×10×10 mm3, acting as a sacrificial template, with a Bioglass® slurry, which infiltrates the pore structure. The slurry is fabricated incorporating Bioglass® powder, including 9.14 and 1.3 g of Bioglass® powder with particle sizes of ∼11 and 5 μm, respectively, in 25 mL deionized water and the addition of 1.1 g of poly(vinyl alcohol) (PVA) (∼Mw 30,000; Merck) as binder. Upon immersion of the PU foam in this slurry for 1 min, Bioglass® particles adhere on the polymer surface forming a homogeneous coating. After drying at 60°C for at least 12 h, the coated polymer foam is placed in a furnace where the PU template is burned out slowly at 400°C for 1 h. Once the PU sacrificial template has been removed the scaffold is sintered to the desired density at 1050°C for 2 h. The heating and cooling rates are 2°C/min and 5°C/min, respectively. At the sintered temperature, the Bioglass® structure densifies and crystallizes leading to relatively robust but brittle foam struts.9
Fibrous polypropylene scaffolds
Fibrous polypropylene scaffolds [hernia meshes (Optilene® mesh)] were purchased from Aesculap (B. Braun Melsungen AG) and were used as biologically inert control materials since cell behaviour (e.g., proliferation or VEGF secretion) on these fibrous scaffolds can be compared to monolayer culture conditions (vgl. Figs. 1 and 4). These elastic meshes are commonly used for open or laparoscopic inguinal hernia surgery and for open umbilical hernia surgery. They are made of polypropylene monofilaments with a pore size of 1.5 mm and a weight of 60 g/m2.
FIG. 1.
(A) Proliferation analysis of human adipose tissue-derived stem cells (hASC) seeded on 45S5 Bioglass®-based and hernia meshes showed no further proliferation after seeding hASC onto 45S5 Bioglass®-based scaffolds. Statistical analysis (α=0.05) showed significant higher proliferation rates of hASC seeded on hernia meshes compared to hASC seeded on 45S5 Bioglass®-based scaffolds (*). (B) Fluorescence microscopy image of hASC seeded on a 45S5 Bioglass®-based scaffold. Nuclei stained with 4′,6-diamidino-2-phenylindole, actin cytoskeleton stained with rhodamine phalloidin. (C) Scanning electron microscopy (SEM) image of hASC. Note dense seeding of cells on the hernia mesh fiber. Color images available online at www.liebertpub.com/tea
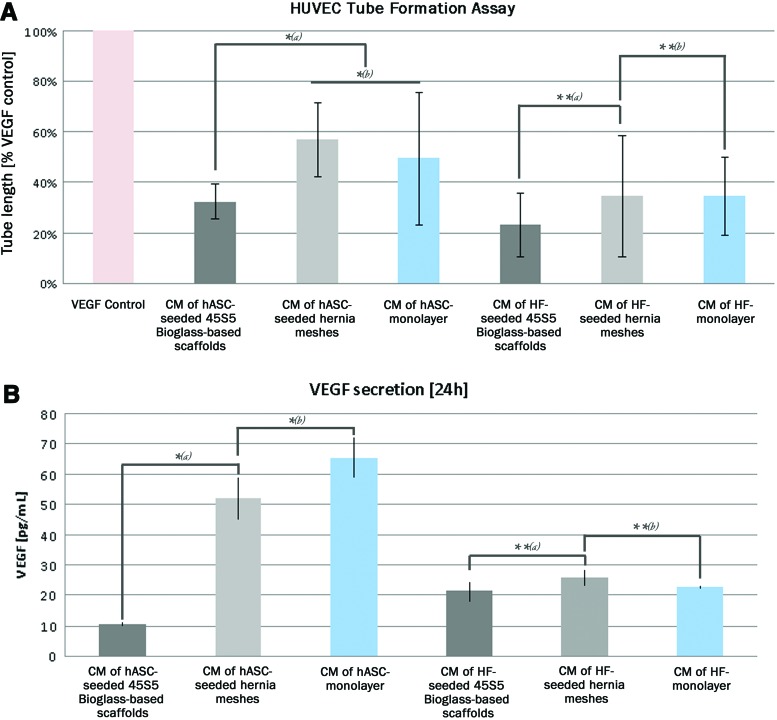
FIG. 4.
(A) Tube formation assay with hASC and HF conditioned media. Statistical analysis (α=0.05) proved significant shorter tube lengths of “conditioned medium (CM) of hASC-seeded 45S5 Bioglass®-based scaffolds” compared to “CM of hASC seeded hernia meshes” and “CM of hASC monolayer” [A, *(a)]. “CM of hASC seeded hernia meshes” and “CM of hASC monolayer” showed equal tube lengths [A, *(b)]. “CM of HF on 45S5 Bioglass®-based Scaffolds,” “CM of HF-seeded hernia meshes and “CM of HF (monolayer)” showed no significant differences (A, **). (B) Quantification of vascular endothelial growth factor (VEGF) in these hASC conditioned media showed a matching trend: “hASC seeded on 45S5 Bioglass®-based scaffolds” showed significant less VEGF secretion into the cell culture medium than “hASC seeded on hernia meshes” and “hASC (monolayer)” [B, *(a)], whereas “hASC seeded on hernia meshes” and “hASC (monolayer)” [B, *(b)] showed equal VEGF secretion rates. “HF seeded on45S5 Bioglass®-based scaffolds,” “HF seeded on hernia meshes” and “HF (monolayer) showed similar VEGF secretion rates (B, **). Color images available online at www.liebertpub.com/tea
Cell culture
Adipose tissue-derived stem cells (Invitrogen Life Technologies GmbH) were cultured according to the manufacturer's instructions at 5% CO2 and 37°C in MesenProRS stem cell medium supplemented with MesenProRS stem cell supplement (Invitrogen Life Technologies GmbH) and 1% l-Glutamine (Sigma-Aldrich) to prevent unintended differentiation. Cell culture medium was changed every third day and cells were subcultured before confluency after a short treatment with 0.05% trypsin, 0.02% ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline (PBS) (Sigma-Aldrich). Primary HUVEC were cultured in M200 supplemented with defined M200 medium supplement (GIBCO; Invitrogen Life Technologies GmbH). Primary HF isolated from AST were cultivated in Dulbecco's modified Eagle's medium (DMEM) (Lonza) supplemented with 10% fetal bovine serum (FBS) (Biochrom) and 2% glutamine.
Scaffold preconditioning
All scaffolds were sterilized by steam sterilization and placed in 24-well cell culture plates (Greiner Bio-One). As the ion release of 45S5 Bioglass®-based scaffolds and the resulting pH shift towards basic pH showed negative effects on cultured cells, whole 45S5 Bioglass®-based scaffolds were conditioned with cell culture medium containing phenol red as pH indicator in cell culture medium in such a way that after six washing cycles, cell culture medium remained in a neutral pH. As soon as the cell culture medium remained in a neutral pH, scaffolds were used for the following experiments, for example, cell seedinig or grinding.
Seeding cells on scaffolds
hASC, an HF was treated with 0.05% trypsin and 0.02% EDTA in PBS to obtain a cell suspension containing 106 cells/mL. A 0.5% collagen solution (collagen from rat tail, Sigma-Aldrich) was added to the cell suspension in equal parts. One hundred microliter of this cell/collagen mixture were then applied to each scaffold. After 30 min of incubation at 37°C and 5% CO2 to allow cell attachment and collagen coagulation, 1 mL of DMEM supplemented with 5% FBS and 2% l-glutamine was added. For control experiments HF were treated equally.
Conditioning cell culture medium
For HUVEC proliferation and HUVEC tube formation, minimal medium was conditioned for 72 h on the respective hASC monolayer cultures or hASC-containing tissue engineered constructs (TECs) for hASC-specific protein enrichment of the culture medium.
Grinding of 45S5 Bioglass®-based scaffolds
Preconditioned 45S5 Bioglass®-based scaffolds were dried at 37°C and ground up using a Precellys homgenizer (Peqlab) with magnetic grinding beads. Afterwards the beads were magnetically removed.
Rhodamine phalloidin/DAPI staining and fluorescence microscopy
Scaffolds (45S5 Bioglass®-based scaffolds or fibrous polypropylene hernia meshes) were fixed with a buffered 4% paraformaldehyde solution and washed twice with PBS. Rhodamine phalloidin (Cytosceleton) was used for staining actin cytoskeleton of the cells (red). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes) in PBS (blue). Fluorescence microscopy was performed using an AX-70 fluorescence microscope (Olympus) and the associated software (analySIS®; soft imaging systems).
Scanning electron microscopy
Scaffolds (45S5 Bioglass®-based scaffolds and fibrous polypropylene scaffolds) were fixed with a buffered 4% paraformaldehyde solution and dehydrated with a graded ethanol series. Subsequently the samples were dried using a critical point drier (Polaron CPD7501; GaLa Instruments). The dried samples were mounted on aluminium devices and sputter-coated (Ion Sputter SCD/040) with platinum before scanning electron microscopy (SEM) observation, which was performed using JSM-6100 (JEOL) at 10 kV.
MTT cell proliferation assay
MTT cell proliferation assay was performed to study cell proliferation as a response to applied test media (i.e., conditioned cell culture media or media containing ground Bioglass®-powder), as well as to determine cell proliferation on fibrous and 45S5 Bioglass®-based scaffolds. The yellow tetrazolium MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is reduced by metabolically active cells during 2 h of incubation at 37°C and 5% CO. The resulting intracellular purple formazan was then solubilized using 100% 2-Propanol and quantified with a TECAN GENios multiwell plate reader at 570/650 nm.
CAM angiogenesis assay
Fertilized Lohmann LSL white leghorn eggs were incubated at 37°C and 60% relative humidity in a standard incubator. Samples and controls were placed on the CAM at 7 days of total incubation for further 72 h. On day 10, scaffolds and surrounding CAM were explanted. Images were captured using a stereomicroscope SZ11 (Olympus) equipped with a digital camera (Color View II; Olympus) and the corresponding image analysis software (analySIS; Soft Imaging Systems). To quantify the angiogenic response of the CAM to the scaffold material, digital images were overlaid with a standardized frame. The interconnecting nodes or bifurcations of the blood vessels within this frame were counted using the image analysis software ImageJ (Freeware of the NIH).
HUVEC tube formation assay
For the in vitro study of angiogenesis, BD Matrigel™ was thawed on ice. Then 10 μL were applied to each well of an “IBIDI μSlide Angiogenesis.” After 30 min of incubation at 37°C and 5% CO2 to allow Matrigel gelation, 50 μL of a cell suspension containing 105 cells/mL HUVEC were added to each well followed by additional 30 min of incubation at 37°C and 5% CO2 to allow cell attachment. Subsequently, cell culture medium was replaced by the test media. After 18 h of cultivation, 2 μg/mL Calcein-AM was added to each well. After 20 min of further incubation, digital images were captured using a fluorescence microscope AX 70 (Olympus) and the corresponding image analysis software (analySIS; Soft Imaging Systems). To quantify the angiogenic effect of the applied test media, total number, and average length of all tubes were evaluated using the image analysis software ImageJ (Freeware NIH).
Human VEGF ELISA
For quantification of human VEGF-A in cell culture medium, the human VEGF (Hu VEGF) ELISA (Invitrogen Life Technologies GmbH) was used. ELISA was performed according to the manufactorer's instructions.
Statistical analysis
Analysis of variance (ANOVA) was used to compare the differences in proliferation, CAM angiogenesis, and HUVEC tube formation assays, as well as in VEGF secretion studies. p<0.05 was considered significant (α=0.05). Statistical analysis was performed using MS Excel (Microsoft Corporation).
Results
hASC adhesion and proliferation on fibrous and 45S5 Bioglass®-based scaffolds
In a previous study, we were able to show that adhesion of hASCs on scaffolds can be improved by mixing the cell suspension with collagen before cell seeding onto the scaffolds.32 To control negative effects of ion dissolution products of 45S5 Bioglass®, we began by preconditioning of the scaffolds and hernia meshes. When the cell suspension of hASC finally was mixed with collagen and seeded onto the preconditioned scaffolds, cell seeding resulted in a dense layer of hASCs (Fig. 1).
The stem cells displayed an elongated and well spread cell morphology on both scaffold materials as shown by fluorescence and scanning electron microscopy (Fig. 1B, C). Proliferation analysis revealed constant cell numbers after seeding hASC onto 45S5 Bioglass®-based scaffolds. In contrast to that, hASC seeded on conventional hernia meshes tripled their cell number after 72 h. Furthermore, hASC seeded on hernia meshes showed significant higher cell numbers and formed a nearly confluent cell layer, compared to hASC seeded on 45S5 Bioglass®-based scaffolds (Fig. 1A).
CAM angiogenesis assay
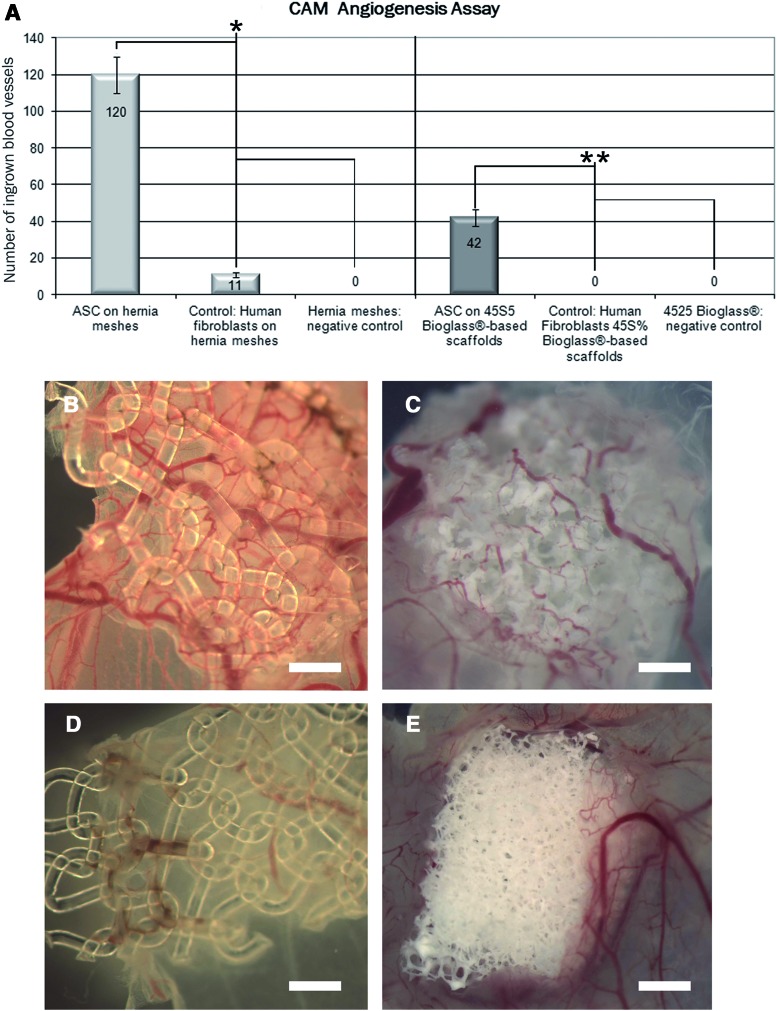
To examine vascularization of whole 3D 45S5 Bioglass®-based scaffolds and hernia meshes, we performed in vivo CAM angiogenesis assays with hASC-seeded scaffolds, HF seeded scaffolds and blank controls without hASC. Blank 3D scaffolds were unable to induce an angiogenic response of the CAM vessels (Fig. 2A). In contrast to that, all scaffolds coated with hASC showed significant higher numbers of ingrown blood vessels in both, fibrous and 45S5 Bioglass®-based scaffolds (Fig. 2A–C). hASC seeded on hernia meshes induced a significant higher number of blood vessels than hASC seeded on 45S5 Bioglass®-based scaffolds (Fig. 2A, C). Biofunctionalization of the scaffolds with HF did not result in a significant ingrowth of blood vessels (Fig. 2D, E).
FIG. 2.
Fibrous (B) and 45S5 Bioglass®-based (C) scaffolds seeded with hASC after investigation in the chorioallantoic membrane angiogenesis assay. Poor angiogenic responses could be detected with cell free scaffolds and with hernia meshes and 45S5 Bioglass®-based scaffolds seeded with human fibroblasts (HF) (A, D, E). Statistical analysis of the quantification of ingrown blood vessels proved a significant higher number of ingrown blood vessels with hASC seeded on hernia meshes compared to HF seeded on hernia meshes (*). hASC seeded on 45S5 Bioglass®-based scaffolds showed similar angiogenic effects (**): compared to HF seeded onto 45S5 Bioglass®-based scaffolds, hASC seeded onto 45S5 Bioglass®-based scaffolds showed significant higher number of ingrown blood vessels. Scale bars represent 1 mm. CAM, chorioallantoic membrane. Color images available online at www.liebertpub.com/tea
45S5 Bioglass® and hASC-induced HUVEC proliferation
To discriminate the effects of 45S5 Bioglass® and hASCs on the endothelial cell proliferation in more detail, minimal culture medium was supplemented with different concentrations [0.01%, 0.1% and 1% (w/v)] of the ground biomaterial and compared to the effect of 45S5 Bioglass®-free but hASC-conditioned minimal medium. 45S5 Bioglass®-based scaffolds were finely grounded, and then added to minimal medium. After 24 h, HUVEC proliferation was examined via the MTT assays and the proliferation rate of the cells within the different test media evaluated in relation to unconditioned minimal medium (Fig. 3).
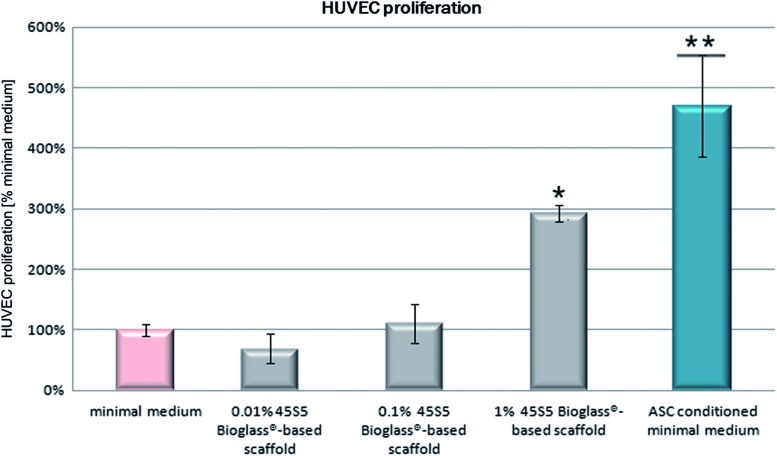
FIG. 3.
Effect of 0.01%, 0.1%, and 1% 45S5 Bioglass® containing minimal medium and hASC conditioned minimal medium on human umbilical vein endothelial cells (HUVEC) proliferation. Statistical analysis (α=0.05) proved significantly higher proliferation rates of HUVEC within 1% 45S5 Bioglass® containing minimal medium (*) compared to minimal medium. In contrast, hASC conditioned minimal medium (**) showed significantly higher proliferation rates than minimal medium, as well as significantly higher proliferation rates than 1% 45S5 Bioglass® containing minimal medium. Color images available online at www.liebertpub.com/tea
Proliferation rates of HUVEC within 1% 45S5 Bioglass® containing minimal medium were significantly higher compared to unmodified minimal medium. In contrast, hASC conditioned minimal medium showed significant higher proliferation rates than unconditioned minimal medium or 1% 45S5 Bioglass® containing minimal medium (Fig. 3).
HUVEC tube formation angiogenesis assay and VEGF secretion
Since hASC are known to secrete cytokines, which promote wound healing and tissue regeneration, including angiogenesis, cell proliferation and cell migration; we investigated minimal media that were conditioned with either cells or cell-scaffold combinations using HUVEC tube formation assays. In parallel the amount of VEGF was quantified using a human VEGF immunoassay. HF seeded on 45S5 Bioglass®-based scaffolds and hernia meshes, as well as HF monolayer-cultures were used as controls (Fig. 4).
HUVEC tube formation assay proved significant lower tube lengths of “conditioned medium (CM) of hASC-seeded 45S5 Bioglass®-based scaffolds” and equal tube lengths of “CM of hASC seeded hernia meshes” and “CM of hASC monolayer.” HF showed similar tube lengths in all approaches (Fig. 4A). Quantification of VEGF in these conditioned media resulted in correlating measurements, that is, “hASC seeded on 45S5 Bioglass®-based scaffolds” showed significant less VEGF secretion into the cell culture medium, whereas “hASC seeded on hernia meshes” and “hASC (monolayer)” showed similar VEGF secretion rates. HF showed equal VEGF secretion without reference to the used scaffold or culture method (Fig. 4B).
Discussion
Since poor vascularization is still a key limitation for the long-term acceptance especially of larger TECs in regenerative medicine, we investigated in this study the biological performance of 45S5 Bioglass®-based scaffolds by different direct and indirect in vitro angiogenesis assays. As it is known that experimental results differ between different approaches and depend on the applied concentration of the biomaterial—whether it is applied as suspension within cell culture medium and whether the cells are seeded directly on the bioactive material or exposed to liquid extracts6—we intended to investigate a direct effect of the scaffold materials and TECs on angiogenesis.
The CAM model is currently used with increasing frequency as a tool for the prediction and broad screening of direct pro- and antiangiogenic,22–24 as well as for biocompatibility tests.18 For instance, Gorustovich et al.17 and Vargas et al.18 evaluated the biocompatibility and bone mineralization potential of 45S5 Bioglass®-based scaffolds using the chick embryo CAM culture system ex ovo. They proved the 45S5 Bioglass®-based scaffolds to be biocompatible in terms of the absence of inflammatory responses of the CAM, but were unable to observe vascular reactions of CAM vessels around the scaffolds. In a first set of our in ovo CAM experiments, neither the native ion releasing 45S5 Bioglass®-based scaffolds nor the biologically inert polypropylene hernia meshes were able to induce ingrowth of blood vessels. This result is therefore, in line with the results of Gorustovich et al.17 and Vargas et al.18
Stimulated by the insufficient vascularization of the plain scaffolds and by various studies using hASC for engineering TECs to improve angiogenesis,27,30,33–35 we biofunctionalized for the first time 45S5 Bioglass®-based scaffolds with hASC and HF as control. Biologically inert polypropylene hernia meshes were treated equally and used as controls. hASC have been shown to influence tissue interaction by secretion of VEGF or bFGF, proangiogenic growth factors, which induce vessel ingrowth,36 and could therefore, also promote the blood supply of TECs.34,35 A variety of studies revealed that both, the scaffold material,6 as well as scaffold-bound cells have their own, discrete bioactive properties, which may influence the scaffold/tissue interaction, respectively.28,35,36 Bioactive glasses, as reviewed by Hoppe et al.,6 are known to release various ion products. The ion dissolution products of 45S5 Bioglass®, are presumed to comprise Si, Ca, Na, and P.6 The subsequent release of these ions after exposure to a physiological environment is then believed to favorably affect the behavior of human cells and to enhance the bioactivity of the scaffolds related to both osteogenesis and angiogenesis.6 To determine whether potential effects in our CAM angiogenesis assay were due to these ion dissolution products or other material-specific characteristics, we used conventional biologically inert polypropylene hernia meshes as a control material. For a plain biofunctionalization of the scaffold materials, we referred to a previously published technique,32 where cell adhesion on the polypropylene meshes could be clearly enhanced by coating them before seeding with collagen containing extracellular matrix-like coating. Leu and Leach,13 as well as Yao et al.37 already showed that collagen does not produce false-positive angiogenic side effects: Sponges made of collagen type-1 improved neither endothelial cell proliferation, HUVEC tube formation or an upregulation of VEGF production in coculture experiments in vitro13 nor an ingrowth of blood vessels in the CAM angiogenesis assay in vivo.37
This time, the evaluation of angiogenic effects of the CAM was slightly changed. Whole mounts of the CAM and implant materials were explanted after 72 h incubation on the CAM, and then observed. In contrast to our first cell-free CAM angiogenesis screenings, where both scaffold materials were not angiogenic, all hASC-seeded scaffolds showed a significant number of ingrown new vessels, whereas cell-free control scaffolds again did not show any angiogenic effects. In this context, the question arises whether this effect is induced by growth factors secreted by the hASC, or a simple result of an unspecific inflammatory process, for example, a consequence of a chemical or mechanical irritation of the CAM.22,23,38,39 To prove this, one possibility would be an extended histological examination of the TEC after its investigation in the CAM angiogenesis assay. However, due to the size of the scaffold material and the small area of CAM tissue, in our hands it was impossible to prepare adequate semithin sections for histological analysis. Nevertheless, in contrast to other evaluation methods, which simply focus on the assessment of blood vessel growth around the implant,38,40 our whole mount/stereomicroscopic modification of the CAM angiogenesis assay allowed the evaluation and quantification of those blood vessels, which grew directly into the implant material. Since ingrown blood vessels within 0.5 cm2 of the scaffold were counted, a direct comparison between different implants could be achieved.
To verify the observed vessel ingrowth, we correlated our CAM data with standardized and approved in vitro angiogenesis models,38 that is, HUVEC proliferation and the HUVEC tube formation assay, where endothelial cells solely respond to soluble factors within the culture medium with a formation of tubular structures.41 In a first set of in vitro experiments and with regard to the angiogenic stimuli of TECs, we compared the endothelial cell proliferation capacity of the ion dissolution products of 45S5 Bioglass®-based scaffolds versus secreted growth factors of hASC using a direct in vitro model.6 The minimal medium containing different concentrations of crushed 45S5 Bioglass®-based scaffolds induced a significant proliferation of HUVECs. This observation is in line with previous studies,6,10,11,14,16,42 which found a stimulating effect of 45S5 Bioglass® on endothelial cell proliferation. For example, Day10 found that CM, produced by CCD-18Co fibroblasts grown on 45S5 Bioglass®, produced a significant increase in the number of endothelial cells after 24 h compared with endothelial cells grown in basal medium. The fibroblasts themselves, however, showed a decrease in the number of metabolically active cells when cultured on surfaces coated with 45S5 Bioglass®, a result that is consistent with our study of hASC (Fig. 1) and HF (data not shown) cell proliferation on 45S5 Bioglass®-based scaffolds. In accordance with these studies, we determined an enhanced HUVEC proliferation within 45S5 Bioglass®-containing culture medium. Furthermore, we observed an additional, significant boost in the endothelial cell proliferation when HUVECs were cultured within hASC-CM. At first sight the absence of an angiogenic response in our CAM investigation; thus, seems to be contradictory to the observed in vitro proliferation rates of HUVEC endothelial cells induced by 45S5 Bioglass® containing culture medium. Nevertheless, we presume that this observation is easily explained by an insufficient ion release of the 45S5 Bioglass®-based scaffolds grafted on the CAM (smaller contact area of the 3D-scaffold to the CAM), in contrast to the ion release of the crushed biomaterial within cell culture medium (higher surface area and consequently greater ion release). In our hands, hASC CM showed a higher proliferation rate of endothelial cells than crushed 45S5 Bioglass®, a result most likely due to the secretion of growth factors by hASCs.36,43
One of the most widely used in vitro assays to model the reorganization stage of angiogenesis is the tube formation assay,41 which measures the ability of endothelial cells, plated at sub-confluent densities with the appropriate extracellular matrix support, to form capillary-like structures. In a next step, our in vitro investigations involved HUVEC tube formation assays to verify angiogenic effects of the crushed 45S5 Bioglass®-based scaffolds. We observed that the density of Bioglass® particles within the cell culture medium considerably impeded the microscopic evaluation of the tube formation assay due to a strong adherence to the Matrigel coating within the cell culture dishes (data not shown). Furthermore, the sharp pieces of the (crushed) biomaterial also led to abrasive effects of the endothelial cells. Since both observations thus, made it impossible to evaluate any kind of tube formation, it is likely that these technical obstructions are likewise also problematic for testing other porous biomaterials in culture medium suspensions. Additionally, it must be considered that adding glass extracts or crushed scaffold material to cells in vitro is not suitable for the investigation of scaffold-related characteristics, such as surface roughness, surface stiffness, or pore size.6
Still, the in vitro screening of whole scaffolds, as well as TECs in cell culture assays poses some difficulties in feasibility, evaluability, and reproducibility. To overcome these obstacles we performed an indirect approach and investigated conditioned cell culture media of TECs in HUVEC tube formation assays. For the relative quantification, tube length and tube numbers were calculated in relation to the positive control containing VEGF, the key factor in blood vessel formation.44 With this experimental setup, we were able to observe significant more and longer tubes within the cell culture medium of hASC seeded on hernia meshes, compared to the culture medium of hASC seeded on 45S5 Bioglass®-based scaffolds.
It is tempting to speculate that the differences in the angiogenic effects of TECs with hASC/45S5 Bioglass® versus hASC/hernia meshes is up to the secretion of different amounts of growth factors. Therefore, we quantified VEGF within the conditioned culture media and were able to prove considerable higher concentrations of VEGF within the culture medium of hASCs seeded on hernia meshes, compared to the culture medium of hASC seeded on 45S5 Bioglass®-based scaffolds. This observation goes along with the observed lower total cell number after 72 h on the 45S5 Bioglass®-based scaffolds, compared to the biologically inert polypropylene hernia meshes. As described above, previous studies of Day et al. already showed a similar inhibition of cell proliferation when fibroblasts were seeded directly on 45S5 Bioglass®-coated cell culture plastics.10,11
Conclusions
Taken together, our results show that the angiogenic effects, observed in the CAM angiogenesis assay, as well as in the HUVEC tube formation assay, are most probably caused by soluble factors, such as the VEGF, secreted by hASC. As we used biologically inert polypropylene hernia meshes as control scaffold material, we were able to show that the ion release of 45S5 Bioglass® is negligible as an angiogenic stimulus in the CAM angiogenesis assay. Neither the hASCs showed an improved VEGF secretion in presence of 45S5 Bioglass®-based scaffolds, nor the CAM showed vascular reactions. Even though it is now well accepted that ion dissolution products of bioactive silicate glasses are able to stimulate osteogenesis, as well as angiogenesis,6 further detailed investigations are required to find out whether and how the ion release of 45S5 Bioglass®-based scaffolds or likewise the surface properties of the scaffold material affect the proliferation and tube formation of HUVECs, as well as the growth factor secretion of cells. Concededly, further in vitro and in vivo investigations are required to generate relevant information on the angiogenic potential of cell seeded 45S5 Bioglass®-based scaffolds and other bioactive glass compositions were specific (metal) ions with possible angiogenic effect (e.g., Cu)6,45 are included in the silicate matrix.
Our results suggest that the combination of suitable scaffold materials, such as 45S5 Bioglass® seeded with hASC could be a promising approach for future tissue engineering application. The recommendation is based on the secretion of VEGF and proliferative mediators as feasible angiogenic stimuli. Indeed further in vivo investigations are necessary to correlate the results of this study with descriptive animal experiments. Nevertheless, the correlation of the in vivo CAM angiogenesis assay and in vitro data shows this semiquantitative method to be a suitable tool to characterise angiogenic effects of 3D implants and TECs.
Acknowledgments
Marina Handel acknowledges scientific support by Prof. Dr. Klaus Pfizenmaier (University Stuttgart, Germany). Patcharakamon Nooeaid acknowledges financial support by the Thai Government Science and Technology Scholarship, granted by the Office of the Civil Service Commission tissue engine, Bangkok, Thailand. We thank Dr. I.D. Thompson (King's College London, United Kingdom) for providing the glass powder used.
Disclosure Statement
No competing financial interests exist.
References
- 1.Guarino V. Causa F. Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 2007;4:405. doi: 10.1586/17434440.4.3.405. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher D.W. Schantz J.T. Lam C.X. Tan K.C. Lim T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 3.Lovett M. Lee K. Edwards A. Kaplan D.L. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghanaati S. Unger R.E. Webber M.J. Barbeck M. Orth C. Kirkpatrick J.A., et al. Scaffold vascularization in vivo driven by primary human osteoblasts in concert with host inflammatory cells. Biomaterials. 2011;32:8150. doi: 10.1016/j.biomaterials.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Hench L.L. Bioceramics and the origin of life. J Biomed Mater Res. 1989;23:685. doi: 10.1002/jbm.820230703. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe A. Guldal N.S. Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Xynos I.D. Edgar A.J. Buttery L.D. Hench L.L. Polak J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J Biomed Mater Res. 2001;55:151. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q.Z. Efthymiou A. Salih V. Boccaccini A.R. Bioglass®-derived glass-ceramic scaffolds: study of cell proliferation and scaffold degradation in vitro. J Biomed Mater Res A. 2008;84:1049. doi: 10.1002/jbm.a.31512. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q.Z. Thompson I.D. Boccaccini A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414. doi: 10.1016/j.biomaterials.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Day R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005;11:768. doi: 10.1089/ten.2005.11.768. [DOI] [PubMed] [Google Scholar]
- 11.Day R.M. Boccaccini A.R. Shurey S. Roether J.A. Forbes A. Hench L.L., et al. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials. 2004;25:5857. doi: 10.1016/j.biomaterials.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Leach J.K. Kaigler D. Wang Z. Krebsbach P.H. Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Leu A. Leach J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25:1222. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 14.Deb S. Mandegaran R. Di Silvio L. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci Mater Med. 2010;21:893. doi: 10.1007/s10856-009-3936-5. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt L.C. Widdows K.L. Erol M.M. Burch C.W. Sanz-Herrera J.A. Ochoa I., et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32:4096. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Gorustovich A.A. Roether J.A. Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B Rev. 2010;16:199. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 17.Gorustovich A.A. Vargas G.E. Bretcanu O. Mesones R.V. Porto Lopez J.M. Boccaccini A.R. Novel bioassay to evaluate biocompatibility of bioactive glass scaffolds for tissue engineering. Adv Appl Ceram. 2008;107:274. [Google Scholar]
- 18.Vargas G.E. Mesones R.V. Bretcanu O. Lopez J.M. Boccaccini A.R. Gorustovich A. Biocompatibility and bone mineralization potential of 45S5 Bioglass®-derived glass-ceramic scaffolds in chick embryos. Acta Biomater. 2009;5:374. doi: 10.1016/j.actbio.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S.K. Nandi S.K. Kundu B. Datta S. De D.K. Roy S.K., et al. In vivo response of porous hydroxyapatite and beta-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J Biomed Mater Res B Appl Biomater. 2008;86:217. doi: 10.1002/jbm.b.31009. [DOI] [PubMed] [Google Scholar]
- 20.Nandi S.K. Kundu B. Datta S. De D.K. Basu D. The repair of segmental bone defects with porous bioglass: an experimental study in goat. Res Vet Sci. 2009;86:162. doi: 10.1016/j.rvsc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Ross E.A. Batich C.D. Clapp W.L. Sallustio J.E. Lee N.C. Tissue adhesion to bioactive glass-coated silicone tubing in a rat model of peritoneal dialysis catheters and catheter tunnels. Kidney Int. 2003;63:702. doi: 10.1046/j.1523-1755.2003.00764.x. [DOI] [PubMed] [Google Scholar]
- 22.Auerbach R. Akhtar N. Lewis R.L. Shinners B.L. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000;19:167. doi: 10.1023/a:1026574416001. [DOI] [PubMed] [Google Scholar]
- 23.Ribatti D. Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol. 2008;270:181. doi: 10.1016/S1937-6448(08)01405-6. [DOI] [PubMed] [Google Scholar]
- 24.Ribatti D. Nico B. Vacca A. Roncali L. Burri P.H. Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat Rec. 2001;264:317. doi: 10.1002/ar.10021. [DOI] [PubMed] [Google Scholar]
- 25.Ribatti D. Presta M. The role of fibroblast growth factor-2 in the vascularization of the chick embryo chorioallantoic membrane. J Cell Mol Med. 2002;6:439. doi: 10.1111/j.1582-4934.2002.tb00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribatti D. Nico B. Morbidelli L. Donnini S. Ziche M. Vacca A., et al. Cell-mediated delivery of fibroblast growth factor-2 and vascular endothelial growth factor onto the chick chorioallantoic membrane: endothelial fenestration and angiogenesis. J Vasc Res. 2001;38:389. doi: 10.1159/000051070. [DOI] [PubMed] [Google Scholar]
- 27.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckermann B.M. Kallifatidis G. Groth A. Frommhold D. Apel A. Mattern J., et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M. Liu Z. Liu C. Jiang X. Wei Z. Qiao W., et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res B Appl Biomater. 2012;100:111. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y. Chen L. Scott P.G. Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 31.Nagy J.A. Vasile E. Feng D. Sundberg C. Brown L.F. Manseau E.J., et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 32.Handel M. Hammer T.R. Hoefer D. Adipogenic differentiation of scaffold-bound human adipose tissue-derived stem cells (hASC) for soft tissue engineering. Biomed Mater. 2012;7:054107. doi: 10.1088/1748-6041/7/5/054107. [DOI] [PubMed] [Google Scholar]
- 33.Gimble J.M. Guilak F. Bunnell B.A. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterodimas A. de Faria J. Nicaretta B. Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63:1886. doi: 10.1016/j.bjps.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Nakagami H. Morishita R. Maeda K. Kikuchi Y. Ogihara T. Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 36.Lee E.Y. Xia Y. Kim W.S. Kim M.H. Kim T.H. Kim K.J., et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 37.Yao C. Markowicz M. Pallua N. Noah E.M. Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29:66. doi: 10.1016/j.biomaterials.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Auerbach R. Lewis R. Shinners B. Kubai L. Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 39.Oates M. Chen R. Duncan M. Hunt J.A. The angiogenic potential of three-dimensional open porous synthetic matrix materials. Biomaterials. 2007;28:3679. doi: 10.1016/j.biomaterials.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Ribatti D. Nico B. Vacca A. Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- 41.Arnaoutova I. George J. Kleinman H.K. Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 42.Xynos I.D. Edgar A.J. Buttery L.D. Hench L.L. Polak J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000;276:461. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 43.Chung H.M. Won C.H. Sung J.H. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9:1499. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 44.Hagedorn M. Balke M. Schmidt A. Bloch W. Kurz H. Javerzat S., et al. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dyn. 2004;230:23. doi: 10.1002/dvdy.20020. [DOI] [PubMed] [Google Scholar]
- 45.Mourino V. Cattalini J.P. Boccaccini A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface. 2012;9:401. doi: 10.1098/rsif.2011.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]