Abstract
In this work, human mesenchymal stem cells (hMSCs) and their osteogenically precultured derivatives were directly cocultured with human umbilical vein endothelial cells (HUVECs) on electrospun three-dimensional poly(ɛ-caprolactone) microfiber scaffolds to evaluate the coculture's effect on the generation of osteogenic constructs. Specifically, cells were cultured on scaffolds for up to 3 weeks, and the cellularity, alkaline phosphatase (ALP) activity, and bone-like matrix formation were assessed. Constructs with cocultures and monocultures had almost identical cellularity after the first week, however, lower cellularity was observed in cocultures compared to monocultures during the subsequent 2 weeks of culture. Scaffolds with cocultures showed a significantly higher ALP activity, glycosaminoglycan and collagen production, as well as greater calcium deposition over the course of study compared to monocultures of hMSCs. Furthermore, the osteogenic outcome was equally robust in cocultures containing osteogenically precultured and non-precultured hMSCs. The results demonstrate that the combination of MSC and HUVEC populations within a porous scaffold material under osteogenic culture conditions is an effective strategy to promote osteogenesis.
Introduction
A major challenge in current bone tissue engineering strategies is the lack of vascular ingrowth.1–3 Native bone tissue has an abundant vascular network, without which, engineered tissue must rely primarily on diffusion for oxygen and nutrient transport, which is effective only over distances of 100–200 μm.4 One strategy for improving the survival and osteogenesis of tissue-engineered bone grafts involves the addition of endothelial cells (ECs) to cultures containing mesenchymal stem cells (MSCs).5,6 MSCs are promising candidates for tissue engineering applications7,8 because they have a capacity to differentiate along bone, cartilage, and adipose lineages.9–12 Additionally, MSCs reside in the bone marrow perivascular niche,13,14 which would facilitate paracrine communication between MSCs and ECs. Studies evaluating the use of ECs in MSC or osteoblast cultures have observed the formation of microvessels in the engineered construct.15–19
Furthermore, previous studies have demonstrated that ECs are capable of enhancing the proliferation and osteogenic differentiation of MSCs.20–24 Thus, cocultures of MSCs and ECs are currently being investigated for their ability to enhance bone formation and have shown that trophic regulation from ECs can provide necessary components for MSC osteogenic differentiation.5,6 The addition of ECs to MSC or osteoblast cultures has been shown to enhance both proangiogenic and pro-osteogenic gene expression, stimulate the alkaline phosphatase (ALP) activity, and increase mineralization.15–17,20–23,25–27 While numerous studies have evaluated such cocultures in two dimensions, data obtained from three-dimensional (3D) conditions are still very limited, as studies emphasizing the osteogenic outcome have primarily been performed in monolayer19,21,23,24 or pellet cultures,20,22,26 but not on porous scaffolds. Studies performed with cocultures of ECs and bone-forming cells on porous scaffolds have investigated primary osteoblasts16,17,28 or an osteoblast cancer cell line,27,28 and have focused on the survival of the ECs,28 angiogenic gene expression, the development of scaffold vascularization with ECs16,17,28 or the properties of the scaffold material,27 but not on the osteogenic differentiation of bone-forming cells (e.g., by quantifying bone-like matrix production and maturation). As such, previous coculture studies have often utilized culture conditions favoring the angiogenic,16,17,27,28 over the osteogenic outcome.
A variety of scaffold materials have been investigated for EC and MSC cocultures, including poly(ɛ-caprolactone) (PCL), starch-based scaffolds with a fiber size of more than 200 μm,16,17 hydroxyapatite (HA) and β-TCP (Ca3(PO4)2) porous discs,28 hydrophilic and hydrophobic titanium surfaces,27 collagen mesh scaffolds,29 and PCL-HA composite membranes.30 Recently, porous microfiber mesh scaffolds made of biodegradable PCL have been developed using electrospinning.31 Electrospun PCL scaffolds with 5- or 10-μm fiber diameters used in our laboratory have been shown to successfully support MSC proliferation and osteogenic differentiation.32,33
In this study, it was hypothesized that cocultures of human mesenchymal stem cells (hMSCs) and/or osteogenically precultured hMSCs and human umbilical vein endothelial cells (HUVECs) on 3D scaffolds would lead to enhanced osteogenesis over hMSCs or their derivatives alone. To test this hypothesis, hMSCs were cocultured with HUVECs on electrospun PCL microfiber scaffolds under osteogenic culture conditions, and the effect on osteogenic differentiation was quantified with a variety of biochemical assays.
Materials and Methods
Experimental design
This study investigated a total of eight groups, seeded onto electrospun PCL scaffolds and cultured in an osteogenic medium for 7, 14, or 21 days. Three coculture groups were investigated with the total number of cells used to seed each scaffold remaining constant (3.0×105 cells per scaffold). In the first group, hMSCs and HUVECs were cultured in a 1:1 ratio (MH). In the second group, hMSCs were precultured for 7 days in the osteogenic medium before being combined at a 1:1 ratio with HUVECs (OH). In the third group, hMSCs, osteogenically precultured for 7 days, were mixed with non-precultured hMSCs and HUVECs at a 1:1:1 ratio (OMH). Additionally, five monoculture groups were investigated, which consisted of a high density (3.0×105 cells per scaffold) of hMSCs with the same total number of cells as the coculture (M2); a high density (3.0×105 cells per scaffold) of osteogenically precultured hMSCs (O2); a low density (1.5×105 cells per scaffold) of hMSCs with the same total number of hMSCs as the coculture groups (M1); a low density (1.5×105 cells per scaffold) of osteogenically precultured hMSCs (O1); and a monoculture of HUVECs at a density of 1.5×105 cells per scaffold (H).
hMSC culture and HUVEC culture
Frozen bone marrow-derived hMSCs were kindly provided by Dr. Darwin Prockop of the Texas A&M University System Health Science Center (Temple, TX). A specification certificate provided with the cells indicated that they possessed widely accepted CD markers, including CD90, CD105, and CD7312 and were tested for the capability to differentiate toward osteogenic and adipogenic lineages until passage 4. The hMSCs were thawed and cultured for 1–2 passages in a general expansion medium: minimum essential medium α without nucleosides and ribonucleosides (Gibco, Grand Island, NY) with 13% v/v fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco), and 2–4 mM l-glutamine (Sigma, St. Louis, MO). Osteogenically precultured cells were expanded for 1 passage in general media and an additional 7 days in the osteogenic medium containing 10 nM dexamethasone, 10 mM β-glycerol-phosphate, and 0.2 mM ascorbic acid (all from Sigma). Cells at passage 3 were used for this study.
Frozen, pooled, primary HUVECs (ATCC, Manassas, VA), which were certified by the vendor as von Willebrand factor positive and smooth muscle α-actin negative were expanded before use in our studies. The HUVECs were thawed and cultured in the vascular cell basal medium containing 0.2% bovine brain extract (BBE), 5 ng/mL recombinant human epidermal growth factor, 10 mM l-glutamine, 0.75 U/mL heparin sulfate, 1 μg/mL hydrocortisone, 50 μg/mL ascorbic acid, 2% v/v FBS (Endothelial Cell Growth Kit—BBE) (all from ATCC) and 10 U/mL penicillin, 10 μg/mL streptomycin and 25 ng/mL amphotericin B (Gibco). Cells at passage 4 were used for this study.
Scaffold fabrication and characterization
Nonwoven PCL microfiber mats were fabricated using a horizontal electrospinning apparatus as previously described.31 Mats were electrospun to achieve an average fiber diameter of ∼10 μm using 18 wt% PCL (inherent viscosity 1.0–1.3 dL/g; Lactel, Birmingham, AL) in a solution of 5:1 v/v chloroform:methanol. The resulting PCL mats of ∼1 mm thickness were stored in a desiccator until use. Five PCL microfiber mats were electrospun to generate a sufficient number of discoid scaffolds for this study (average scaffold thickness 1.05±0.05 mm). For cell seeding, discoid PCL scaffolds 8 mm in diameter were punched from the electrospun mats using a dermal biopsy punch (Miltex, Inc. York, PA), and the microfiber morphology was assessed via scanning electron microscopy (SEM) (mean diameter 10.6±1.6 μm). The scaffolds were then sterilized by exposure to ethylene oxide (AN74i; Andersen Sterilizers, Haw River, NC) at room temperature for 14 h and aerated overnight.
Cell seeding of scaffolds
To facilitate cell adhesion, prewetting of scaffolds was performed in a gradient series of ethanol (100-25%; centrifugation for 15 min each step at 2000 rpm), followed by two rinses in phosphate-buffered saline (PBS) and one rinse in a general expansion medium. Scaffolds were maintained in the culture medium overnight at 37°C before cell seeding. Prewetted scaffolds were press-fitted into polymethylmethacrylate cylindrical holders specifically designed to fit the 8-mm scaffolds and localize the cell suspension above the scaffolds for the time required for cell adhesion. Cell suspensions were slowly pipetted onto the scaffolds at a density of 1.5×105 cells per scaffold for monocultures of hMSCs (M1), preosteoblasts (O1), and pure HUVECs (H) or 3.0×105 cells per scaffold for monocultures of hMSCs (M2), preosteoblasts (O2), and cocultures (MH, OH, and OMH) in 200 μL of the culture medium and allowed to adhere for 4 h before the general expansion medium was added to completely fill the holders. Scaffolds were transferred after 24 h into 12-well culture plates (Becton Dickinson, Franklin Lake, NJ) with 3 mL of a fresh osteogenic medium. Thereafter, the medium was changed twice a week for up to 21 days. Four scaffolds from each group were harvested the day after seeding to assess the initial cellularity and ALP activity (day 1). Twelve scaffolds from each experimental cellular group were harvested after 7, 14, and 21 days of culture.
Biochemical assays
After harvesting, the constructs were washed with PBS and frozen at −20°C until use for the biochemical assays. For extraction of DNA, glycosaminoglycan (GAG) and collagen, thawed samples were digested in 500 mL of proteinase K solution [1 mg/mL proteinase K, 0.01 mg/mL pepstatin A and 0.185 mg/mL iodoacetamide in a 50 mM tris-(hydroxymethyl-aminomethane)—1 mM ethylene-diamine-tetraacetic acid buffer, pH 7.6] in a 56°C water bath for 16 h followed by two additional freeze–thaw cycles and 10-min sonication. For measuring of the ALP activity and calcium (Ca) content, scaffolds were placed into 1 mL of milliQ water after thawing, and then subjected to two additional freeze–thaw cycles followed by 10-min sonication.32
The DNA content of each cell lysate solution was quantified using the fluorometric PicoGreen assay (Invitrogen, Eugene, OR) with an excitation wavelength of 490 nm and an emission wavelength of 520 nm (BioTek FLX800, Winooski, VT), as described elsewhere.34,35 The ALP activity was performed according to an established colorimetric protocol.35 The ALP activity of samples was determined relative to a p-nitrophenol standard curve and presented as activity per scaffold. For measuring the calcium content, each scaffold was removed from the aqueous cell lysates, placed into 1 mL 0.5 N acetic acid, and left on a shaker table at 200 rpm overnight to dissolve the calcium on the scaffolds. A colorimetric assay was used to quantify the calcium content based on the color change that occurs when a calcium reagent (Arsenazo III; Diagnostic Chemicals, Oxford, CT) binds to free calcium.32 The GAG content was determined using the colorimetric dimethylmethylene blue assay.36 GAG concentrations were determined relative to a chondroitin sulfate standard curve. Total collagen was extracted from scaffolds via basic hydrolysis of a 100 μL sample solution (obtained after proteinase K digestion) with 100 μL 4 N NaOH for 20 min at 120°C. After cooling, samples were neutralized with acid, and the resulting hydrolyzed collagen was determined by measuring hydroxyproline in a colorimetric assay as previously described.37 The resulting hydroxyproline concentrations were then converted to collagen contents for each scaffold following a 1:10 ratio of hydroxyproline to collagen.32 All colorimetric assays were performed on PowerWaveX340 Microplate Reader (BioTek Instruments, Winooski, VT).
Histology
At each time point, two scaffolds from each group were rinsed with PBS and fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA). Cryosections of 7 μm thickness were cut using a cryostat (Leica CM 1850 UV; Leica Biosystems, Nussloch GmbH, Germany). Sections were stained with Safranin-O, von Kossa, and Picrosirius Red to visualize GAG, mineralized matrix, and collagen fibers, respectively. Imaging was performed using a ZeissAxio Imager Z2 microscope (equipped with Axi-oCam MRc5; Carl Zeiss MicroImaging GmbH, Jena, Germany).
Scanning electron microscopy
Extracellular matrix (ECM) morphology of cell–scaffold constructs was evaluated at each time point via SEM (FEI Quanta 400 Environmental, Hillsboro, OR). Two scaffolds from each group were rinsed with PBS, fixed in 2.5% v/v glutaraldehyde in PBS for 30 min at room temperature, and dehydrated via an alcohol gradient (50–100%) and dried overnight in ambient conditions.
Statistical analysis
Results are presented as mean±standard deviation for n=4. The differences were analyzed using one-way analysis of variance followed by the Tukey's post hoc test using the JMP 10 software package (SAS Institute, Cary, NC). Differences were considered significant at p<0.05.
Results
Scaffold cellularity
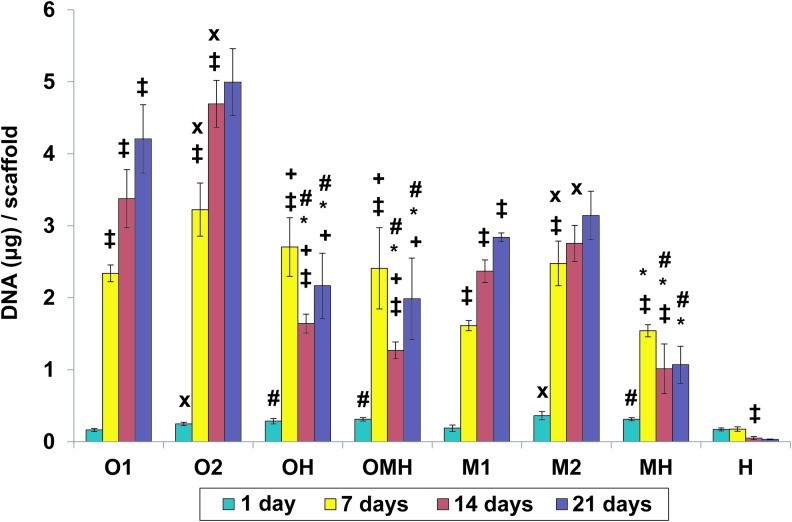
As seen in Figure 1, an increase in scaffold cellularity over time was observed in all monoculture groups (M1, O1, M2, O2), with the monoculture containing osteogenically precultured cells (O2) displaying the highest cellularity at day 14 and 21. All cocultures containing HUVECs (OH, OMH, and MH) were seeded with the same total number of cells as high-density monocultures (M2, O2). In the coculture groups, cell proliferation took place primarily in the first 7 days. At 14 days, the DNA content in the cocultures was significantly lower than either seeding density of the corresponding monocultures (O1 and O2 for OH and OMH; M1 and M2 for MH).
FIG. 1.
The DNA content at 1, 7, 14, and 21 days of culture in (O1) osteogenically precultured human mesenchymal stem cells (hMSCs) seeded at 1.5×105 cells per scaffold, (O2) osteogenically precultured hMSCs seeded at 3.0×105 cells per scaffold, (OH) 1:1 ratio of osteogenically precultured hMSCs and human umbilical vein endothelial cells (HUVECs) seeded at 3.0×105 cells per scaffold, (OMH) 1:1:1 ratio of osteogenically precultured hMSCs, hMSCs, and HUVECs seeded at 3.0×105 cells per scaffold, (M1) hMSCs seeded at 1.5×105 cells per scaffold, (M2) hMSCs seeded at 3.0×105 cells per scaffold, (MH) 1:1 ratio of hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, and (H) HUVECs seeded at 1.5×105 cells per scaffold. The results are presented as mean±standard deviation (SD) for n=4. #, *Denote significant difference of cocultures from the corresponding monocultures (with lower and higher densities, respectively),+indicates significant difference of precultured cocultures (OH, OMH) versus coculture of non-precultured cells (MH),×denotes significant difference between monocultures with lower and higher seeding density. ‡Reflects significant difference to the previous time point for all groups (p<0.05). Color images available online at www.liebertpub.com/tea
ALP activity
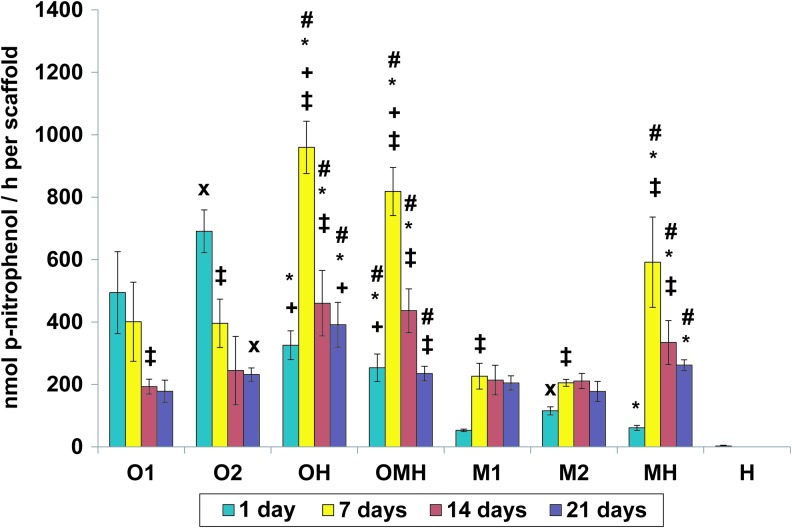
The ALP activity generally peaked at day 7 and then decreased over time in the case of cocultures (OH, OMH, and MH). However, the ALP activity remained relatively unchanged after day 7 for monocultures of non-precultured cells (M1, M2) and decreased in the osteogenically precultured monoculture groups at day 7 or later (O1, O2) (Fig. 2). Despite lower scaffold cellularity, all cocultures showed a significantly higher total ALP activity per scaffold compared to the corresponding monocultures (O1 and O2 for OH and OMH; M1 and M2 for MH) from day 7 onward (Fig. 2). The only exception was the coculture group with mixed precultured and non-precultured hMSCs (OMH) and the monoculture group with osteogenically precultured cells at the higher seeding density (O2); these were not significantly different at day 21 (Fig. 2).
FIG. 2.
Alkaline phosphatase activity at 1, 7, 14, and 21 days of culture in (O1) osteogenically precultured hMSCs seeded at 1.5×105 cells per scaffold, (O2) osteogenically precultured hMSCs seeded at 3.0×105 cells per scaffold, (OH) 1:1 ratio of osteogenically precultured hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, (OMH) 1:1:1 ratio of osteogenically precultured hMSCs, hMSCs, and HUVECs seeded at 3.0×105 cells per scaffold, (M1) hMSCs seeded at 1.5×105 cells per scaffold, (M2) hMSCs seeded at 3.0×105 cells per scaffold, (MH) 1:1 ratio of hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, and (H) HUVECs seeded at 1.5×105 cells per scaffold. The results are presented as mean±SD for n=4. #,*Denote significant difference of cocultures from the corresponding monocultures (with lower and higher densities, respectively),+indicates significant difference of precultured cocultures (OH, OMH) versus coculture of non-precultured cells (MH),×denotes significant difference between monocultures with lower and higher seeding density. ‡Reflects significant difference to the previous time point for all groups (p<0.05). Color images available online at www.liebertpub.com/tea
Collagen synthesis
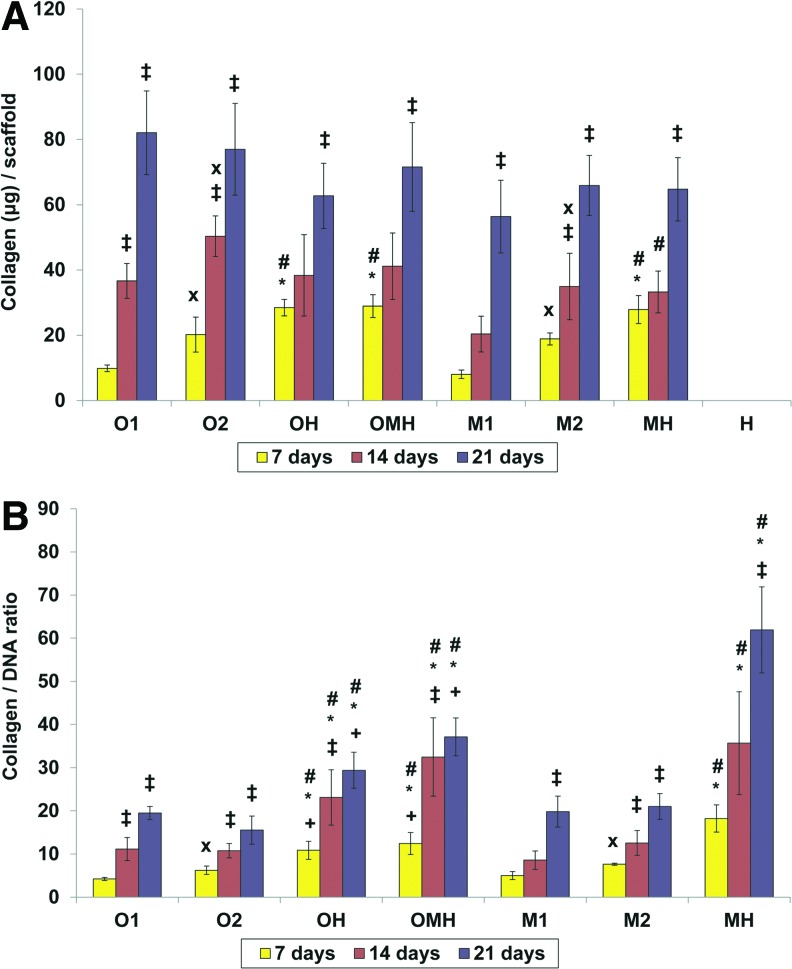
All groups showed increasing collagen per scaffold, as seen in Figure 3A, as well as collagen content normalized to DNA (Fig. 3B). In the cocultures, significantly increased collagen per scaffold was observed from day 14 to 21. The cocultures with osteogenically precultured cells (OH, OMH) had significantly increasing the normalized collagen content during the first 14 days of culture, whereas cocultures with hMSCs (MH) showed significantly increased collagen normalized to DNA from day 14 to 21. All cocultures with either osteogenically precultured cells or hMSCs showed a significantly higher collagen production per scaffold compared to all corresponding monocultures at day 7 of culture, as shown in Figure 3A. The differences between groups were not significant at days 14 and 21, excluding the coculture with hMSCs (MH), which presented a higher collagen content per scaffold in comparison to the hMSC monoculture with the lower seeding density (M1) at day 14. In contrast to the total collagen content, normalized collagen content was significantly elevated for all coculture groups compared to the corresponding monocultures at all investigated time points (Fig. 3B). Among the cocultures, the highest collagen to the DNA ratio was observed in the group with non-precultured cells (MH) at days 7 and 21.
FIG. 3.
Collagen content (A) or normalized collagen content (B) at 7, 14, and 21 days of culture in (O1) osteogenically precultured hMSCs seeded at 1.5×105 cells per scaffold, (O2) osteogenically precultured hMSCs seeded at 3.0×105 cells per scaffold, (OH) 1:1 ratio of osteogenically precultured hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, (OMH) 1:1:1 ratio of osteogenically precultured hMSCs, hMSCs, and HUVECs seeded at 3.0×105 cells per scaffold, (M1) hMSCs seeded at 1.5×105 cells per scaffold, (M2) hMSCs seeded at 3.0×105 cells per scaffold, (MH) 1:1 ratio of hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, and (H) HUVECs seeded at 1.5×105 cells per scaffold. The results are presented as mean±SD for n=4. #,*Denote significant difference of cocultures from the corresponding monocultures (with lower and higher densities, respectively),+indicates significant difference of precultured cocultures (OH, OMH) versus coculture of non-precultured cells (MH),×denotes significant difference between monocultures with lower and higher seeding density. ‡Reflects significant difference to the previous time point for all groups (p<0.05). Color images available online at www.liebertpub.com/tea
GAG synthesis
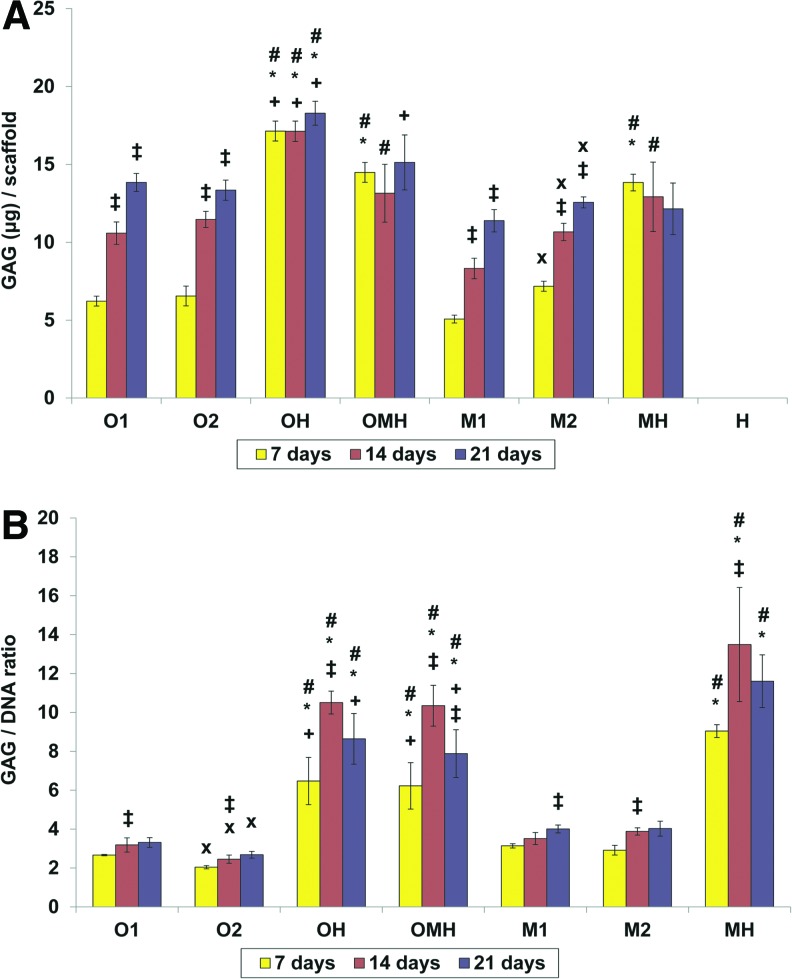
All monocultures of either osteogenically precultured or non-precultured hMSCs (O1, O2 and M1, M2, respectively), showed continuously increasing GAG contents per scaffold, as seen in Figure 4A. Cocultures demonstrated completely different behavior in terms of GAG production and synthetic activity. The GAG production per scaffold remained relatively unchanged with time for all cocultures (Fig. 4A). The GAG synthetic activity of cells significantly increased only from day 7 to 14 in all investigated cocultures (Fig. 4B). Total GAG production per scaffold over time was the strongest in the coculture group with osteogenically precultured cells (OH) among all tested groups, whereas synthetic GAG activity (GAG/DNA) was similar between OH and OMH groups and was the strongest in the MH group at days 7 and 21. With regard to the GAG to DNA ratio, all cocultures had a significantly higher synthetic activity in comparison to corresponding monocultures.
FIG. 4.
Glycosaminoglycan (GAG) content (A) or normalized GAG content (B) at 7, 14, and 21 days of culture in (O1) osteogenically precultured hMSCs seeded at 1.5×105 cells per scaffold, (O2) osteogenically precultured hMSCs seeded at 3.0×105 cells per scaffold, (OH) 1:1 ratio of osteogenically precultured hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, (OMH) 1:1:1 ratio of osteogenically precultured hMSCs, hMSCs, and HUVECs seeded at 3.0×105 cells per scaffold, (M1) hMSCs seeded at 1.5×105 cells per scaffold, (M2) hMSCs seeded at 3.0×105 cells per scaffold, (MH) 1:1 ratio of hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, and (H) HUVECs seeded at 1.5×105 cells per scaffold. The results are presented as mean±SD for n=4. #, *Denote significant difference of cocultures from the corresponding monocultures (with lower and higher densities, respectively),+indicates significant difference of precultured cocultures (OH, OMH) versus coculture of non-precultured cells (MH),×denotes significant difference between monocultures with lower and higher seeding density. ‡Reflects significant difference to the previous time point for all groups (p<0.05). Color images available online at www.liebertpub.com/tea
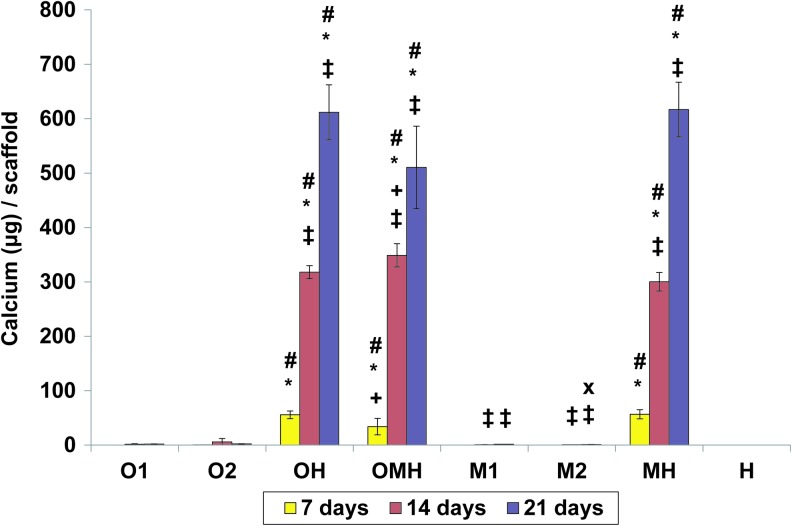
Calcium deposition
The amount of calcium deposited on scaffolds was minimal over time for all monoculture groups (Fig. 5). On the contrary, all investigated coculture groups (OH, OMH, MH) had continuously increasing calcium deposition over time (Fig. 5). All cocultures with either osteogenically precultured cells or hMSCs showed significantly higher calcium per scaffold compared to the corresponding monocultures at all tested time points, as shown in Figure 5. There were no differences in calcium deposited between cocultures with osteogenically precultured cells (OH) and hMSCs (MH) over time, but the coculture with both cell types (OMH) showed the lowest calcium deposition per scaffold among these groups at day 7 and the highest at day 14.
FIG. 5.
Calcium contents at 7, 14, and 21 days of culture in (O1) osteogenically precultured hMSCs seeded at 1.5×105 cells per scaffold, (O2) osteogenically precultured hMSCs seeded at 3.0×105 cells per scaffold, (OH) 1:1 ratio of osteogenically precultured hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, (OMH) 1:1:1 ratio of osteogenically precultured hMSCs, hMSCs, and HUVECs seeded at 3.0×105 cells per scaffold, (M1) hMSCs seeded at 1.5×105 cells per scaffold, (M2) hMSCs seeded at 3.0×105 cells per scaffold, (MH) 1:1 ratio of hMSCs and HUVECS seeded at 3.0×105 cells per scaffold, and (H) HUVECs seeded at 1.5×105 cells per scaffold. The results are presented as mean±SD for n=4. #, *Denote significant difference of cocultures from the corresponding monocultures (with lower and higher densities, respectively),+indicates significant difference of precultured cocultures (OH, OMH) versus coculture of non-precultured cells (MH),×denotes significant difference between monocultures with lower and higher seeding density. ‡Reflects significant difference to the previous time point for all groups (p<0.05). Color images available online at www.liebertpub.com/tea
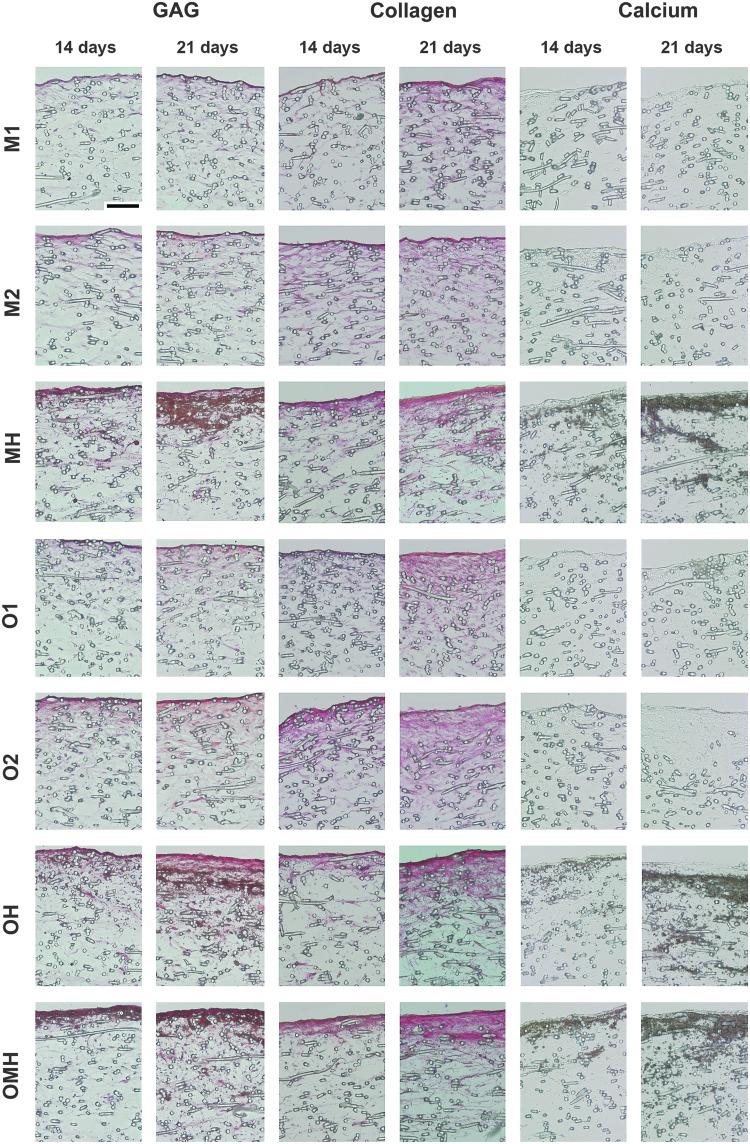
Histology
Histology corroborated the results of calcium, collagen, and GAG biochemical assays (Fig. 6). Histological analysis showed cells and ECM accumulated near the top surface of the scaffolds, with increasing cellular penetration and matrix distribution with time. A clear difference between all cocultures and monocultures was observed for calcium mineralization (Fig. 6). Specifically, cocultures showed abundant deposits of mineralized matrix that penetrated deeper into the scaffolds with time, while monocultures displayed relatively little mineral deposition. Similarly, the coculture groups showed greater staining for GAGs relative to the monoculture groups (Fig. 6). The cocultures also displayed more abundant collagen-rich matrix deposition, especially on the top scaffold surface (Fig. 6).
FIG. 6.
Bone-like matrix production, maturation, and mineralization within the poly(ɛ-caprolactone) scaffolds with monocultures of hMSCs (M1, M2, O1, O2) and osteogenically precultured cocultures (OH, OMH) or non-precultured coculture hMSCs (MH) with HUVECs. Three panels display Safranin O staining for sulfated GAGs (red), Picrosirius Red staining for total collagen (pink), and von Kossa staining for calcium salts (brown). Scale bar represents 100 μm in all images.
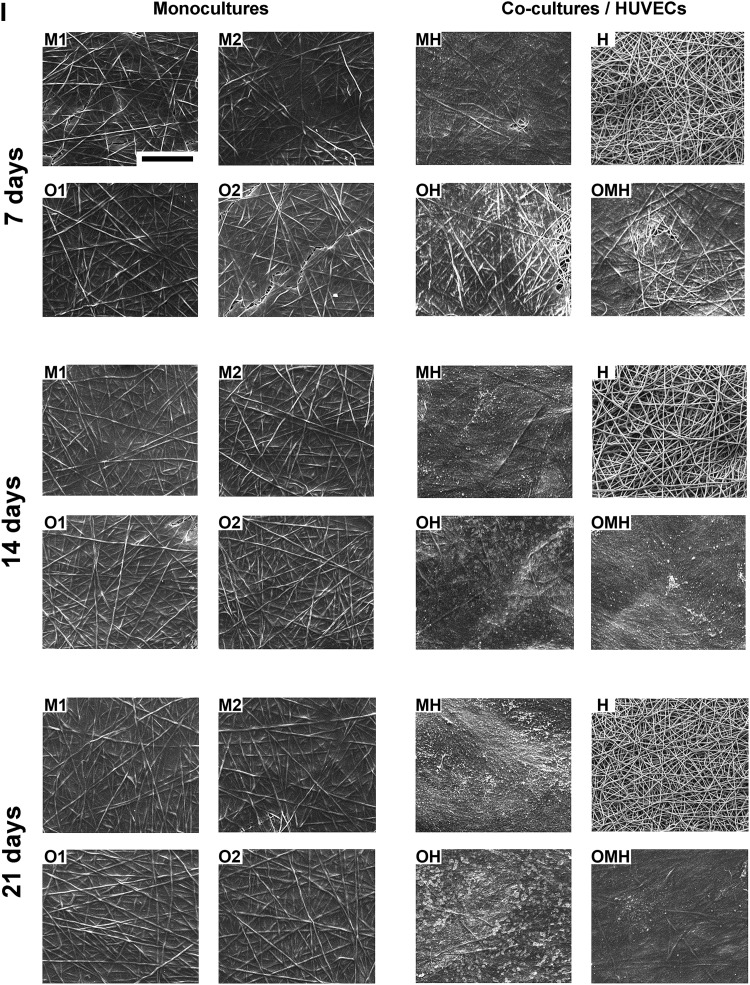
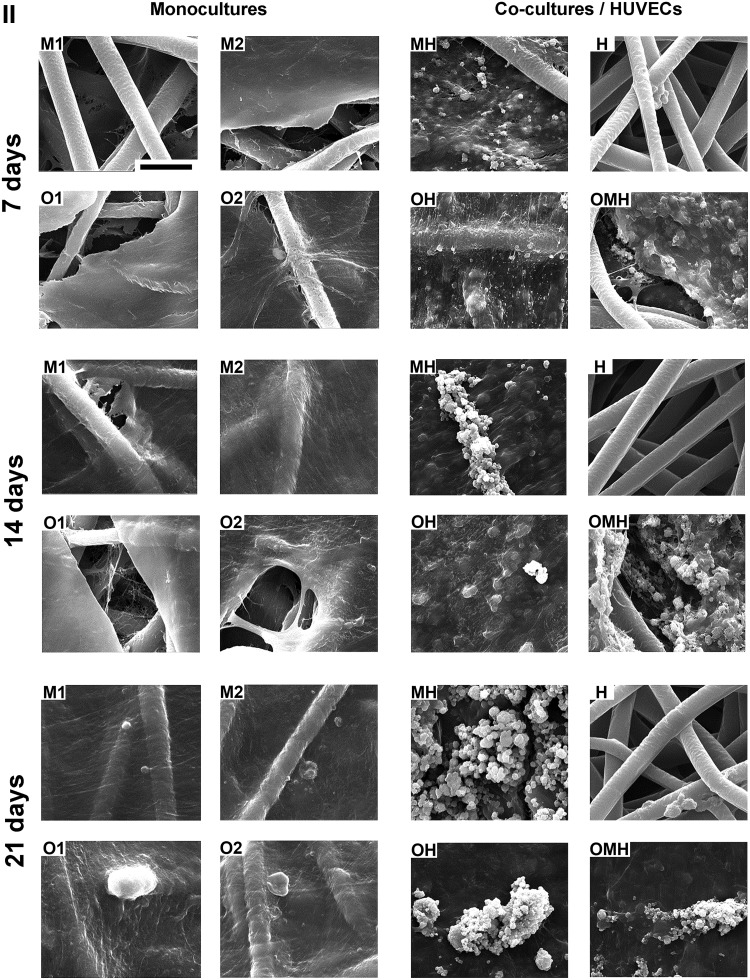
Scanning electron microscopy
SEM images (Appendix Fig. A1) generally showed the ECM completely covering the scaffold surfaces and cells infiltrating the pores between PCL fibers, with the only exception being the HUVEC only group (H) (Appendix Fig. A2).
Discussion
The main objective of this study was to determine whether coculturing hMSCs with ECs on PCL microfiber scaffolds could enhance osteogenesis (as determined by ALP activity and calcium deposition) and the production of bone-like ECM components (as determined by collagen and GAG matrix production). HUVECs were selected for this study because they are an established model of ECs and have been shown to stimulate osteogenesis in vitro.20–23,27 A general hMSC expansion medium with osteogenic supplements was used because of its proven ability to support osteogensis in cocultures of HUVECs and hMSCs,20 as opposed to other media formulations that have demonstrated greater utility in maintaining and promoting angiogenesis of ECs in coculture.17,20,28 While the absence of angiogenic growth factors in osteogenic media limits the EC metabolic activity and proliferation,20 it is still sufficient for manifestation of the angiogenic potential of HUVECs in cocultures with hMSCs.15,20,22
From previous studies, it is known that coculturing ECs with bone-forming cells promotes cellular proliferation in monolayer21,23 as well as in 3D.27 However, since most of these findings were obtained using endothelial growth media, we evaluated the effect of 3D culture in osteogenic media. In the present study, the DNA content increased after 7 days of coculture in the osteogenic medium, but then decreased by 14 days. This is consistent with literature data, where similar cocultures led to a decrease in the DNA content after 14 days of 2D culture in osteogenic conditions.20 Indeed, the balance between cell proliferation and differentiation during development of the osteoblastic phenotype is well established.38 Consequently, the lower DNA content observed in cocultures at 14 days relative to 7 days of culture could reflect a decrease in proliferation corresponding to osteogenic differentiation. This finding, together with the significantly increased ALP activity, as well as matrix production and maturation (collagen and GAG production and calcium deposition) observed in cocultures at the earlier time points, supports the hypothesis that osteogenesis is enhanced in cocultures of hMSCs and ECs on microfiber scaffolds.
The osteogenic preculture conditions used for the hMSCs in this study typically result in committed osteoprogenitor cells.39 In this study, it was observed that precultured and non-precultured cocultures presented an ALP activity significantly higher than corresponding monocultures. The enhancement of ALP activity in cocultures may be mediated by p38 mitogen-activated protein kinase-dependent stabilization of mRNA for ALP.40 This type of stimulation has been shown in series of studies.20–23,25–27,40 One novel aspect of this study was that, in addition to the elevated ALP activity, all cocultures were shown to have a significantly higher production of the main nonmineral components of bone ECM, namely, collagen and GAGs. The levels of these ECM components (as reflected by the collagen and GAG contents of scaffolds normalized to DNA) in monocultures at each time point were always lower than those observed in cocultures. The promotion of increased collagen matrix production by cocultures is supported by several studies.16,23,25 For instance, upregulated collagen type 1 gene expression was found in cocultures of human osteoblasts and dermal endothelial microvascular cells16 and in cocultures of HUVECs and hMSCs.23,25 However, no upregulation in collagen expression was found in the coculture of outgrowth ECs and MSCs.15 To our knowledge, no other studies have reported increased GAG production in cocultures of MSCs and ECs in either 2D or 3D. GAGs have a variety of biological functions necessary for osteogenesis and cell-to-cell communications.41 One possible reason for increased GAG production observed in cocultures in the present study could be residual heparin sulfate, which is a component of vascular basal media commonly used for HUVEC cultivation and the media used for HUVEC culture in the present study. However, no GAGs were detected in HUVEC monoculture (Fig. 4) Interestingly, it is thought that heparan sulfate proteoglycans assist in mediating the interactions between marrow stromal cells and hematopoietic cells,42,43 and thus, the upregulation of GAG production observed in the present study potentially could have further enhanced the effect of the cocultures.
All cocultures presented increased mineralized matrix deposition compared to monocultures. This result was consistent with the observation in cocultures of an enhanced ALP activity, which hydrolyzes the phosphate source for mineralization, as well as increased collagen matrix production, which provides the structural support necessary for calcium deposition.38 The observed increased mineralization in cocultures is consistent with literature data.15,20,21 Furthermore, the very low calcium deposition found in monocultures is consistent with data obtained in a recent study in which, hMSCs from several donors demonstrated minimal calcium deposition for up to 28 days of culture, while cocultures of HUVECs and hMSCs exhibited a significant mineralized matrix much earlier in culture.20 This result emphasizes the role of ECs as osteogenic mediators in coculture models.
To address the question of what stage of maturity favors the promotion of osteogenesis in this study, we tested three different cocultures, including two groups with a 1:1 ratio of either osteogenically precultured or non-precultured hMSCs together with HUVECs, as well as a third group combining all three cell types (OMH). The latter group was included to investigate the simultaneous cross talk between various cell types that exist together in the bone marrow niche and to investigate the possibility of decreasing the number of HUVECs needed while maintaining a suitable osteogenic outcome. MSCs reside in the perivascular niche,13,14 and may be recruited to the site of bone regeneration by cues from endosteal (pre)osteoblasts as well as through signaling from the surrounding endothelium. Several studies investigating cocultures of nonhuman MSCs with osteoblasts or osteoblast/osteocyte murine lines44,45 have shown the potential of directing osteogenic differentiation of MSCs in vitro by cues from more mature cell types. However, the effects were different depending on whether terminally or partially differentiated cells were applied. Osteoblasts were shown to be more favorable for proliferation, but not the differentiation of MSCs, while osteocytes significantly stimulated the osteogenic outcome.45 Studies have not been conducted with hMSCs cocultured with their osteogenically committed derivative together with ECs. Although in the current study there were no control groups consisting of hMSCs cocultured with preosteoblasts without HUVECs, the data obtained were compared with other cocultures and monocultures used (as the amount of cells were the same) and support a beneficial effect of such a coculture. Comparing the osteogenically precultured to non-precultured cocultures, the present study found that the OH and OMH cocultures resulted in higher levels of proliferation than the nonosteogenically precultured cocultures (MH). Preculture also affected GAG and collagen matrix production and calcium content at early time points; however, at later time points, the calcium content of the constructs, which is the primary marker of osteogenesis, was equal among all cocultured populations, indicating that the preculture period did not significantly affect the coculture construct mineralization.
The cell proportions in the cocultures with 1:1 ratios were selected based on the literature,20,22,40 and the current study resulted in comparable osteogenic outcomes. Additionally, for comparison of the results, several control groups with monocultures of either osteogenically precultured cells or hMSCs at lower and higher densities were included. Importantly, both seeding densities resulted in inferior osteogenic outcomes than the corresponding cocultures. This suggests that stimulation of osteogenesis observed in cocultures most likely does not reflect the effects of cell density; although it is evidently dependent on cell-to-cell contacts16,25 and trophic cross talk.6 In direct cocultures, tight heterotypic cell-to-cell contacts (gap-junctions) were found between ECs and hMSCs and were thought to contribute toward activation of angiogenic and osteogenic gene expression in the cell types, respectively.16,25
Overall, with regard to construct cellularity and ALP activity, the best outcome (indicated by the highest ALP production on a per cell basis) was achieved in the cocultures with osteogenically precultured cells (OH, OMH), which is consistent with longer exposure to osteogenic supplements. However, diverse effects were observed in matrix production (collagen, GAGs) and maturation (calcium deposition) among the cocultures investigated. The result that collagen and GAG synthetic activity both achieved their maximum observed value in the present study in the coculture with non-precultured cells (MH) was unexpected, as it indicates that osteogenically precultured cells effected cell osteogenic synthetic activity in cocultures relatively less than hMSCs. Previously, it was shown that the preculture period of MSCs in the osteogenic medium influences their in vivo bone forming potential.39 Perhaps, the preculture period of 7 days chosen in the present study was not appropriate to achieve positive osteogenic effects exceeding that of non-precultured hMSCs. Alternatively, it is possible that the outcome of cocultures of HUVECs and hMSCs depends mostly on the EC activity and their trophic communication with progenitor cells, which has not been characterized with respect to the stage of cell maturity. Therefore, these data demonstrate that supplementation with ECs promotes osteogenic differentiation of hMSCs cultured on 3D electrospun PCL microfiber scaffolds regardless of their stage of maturity.
Conclusions
In this work, we investigated osteogenesis in polymeric microfiber scaffolds using osteogenically committed or noncommitted hMSCs alone and in combined coculture with ECs with the goal of generating engineered bone constructs. Cocultures showed a lower proliferation, but a more pronounced bone-like matrix formation and mineralization in comparison to hMSC monocultures. All cocultures showed greater degrees of osteogenic differentiation compared to monocultures, which suggests that the cellular cross talk in cocultures enhances the osteogenic potential of hMSCs in microfiber scaffolds. Based on these results, we conclude that cocultures of ECs and hMSCs are a promising strategy to improve osteogenesis in engineered bone constructs.
Appendix
Appendix FIG. A1.
Extracellular matrix organization of scaffolds with monocultures of human mesenchymal stem cells (hMSCs; M1, M2, O1, O2) and cocultures of hMSCs with human umbilical vein endothelial cells (HUVECs; MH, OH, and OMH) imaged via scanning electron microscopy (SEM). Magnification 100×, scale bar is 500 μm and applies for all images. Note the absence of the matrix and the lack of any mineralization on the scaffolds with endothelial cells (H) although cells are still present on the poly(ɛ-caprolactone) (PCL) fibers.
Appendix FIG. A2.
Extracellular matrix organization of scaffolds with monocultures of hMSCs (M1, M2, O1, O2) and cocultures of hMSCs with HUVECs (MH, OH, and OMH) imaged via SEM. Magnification 2000×, scale bar is 30 μm and applies for all images. Note the absence of the matrix and the lack of any mineralization on the scaffolds with endothelial cells (H) although cells are still present on the PCL fibers.
Acknowledgments
This work was supported by grants from the National Space Biomedical Research Institute Postdoctoral Fellowship Program through NCC 9-58 (J.G.G.) and the National Institutes of Health R01 AR057083 (A.G.M.). The authors acknowledge the editorial assistance of Dr. Paschalia M. Mountziaris in the preparation of this manuscript.
Disclosure Statement
The authors declare no conflict of interests.
References
- 1.Bauer T.W. Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10. [PubMed] [Google Scholar]
- 2.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R., et al. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon J.J. West J.L. Vascularization of engineered tissues: approaches to promote angiogenesis in biomaterials. Curr Top Med Chem. 2008;8:300. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouwkema J. Rivron N.C. van Blitterswijk C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Kanczler J.M. Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 6.Grellier M. Bordenave L. Amédée J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. 2009;27:562. doi: 10.1016/j.tibtech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Ingber D.E. Levin M. What lies at the interface of regenerative medicine and developmental biology. Development. 2007;134:2541. doi: 10.1242/dev.003707. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein A.J. Piatetzky-Shapiro I.I. Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381. [PubMed] [Google Scholar]
- 10.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 11.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 14.Shi S. Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 15.Kolbe M. Xiang Z. Dohle E. Tonak M. Kirkpatrick C.J. Fuchs S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A. 2011;17:2199. doi: 10.1089/ten.TEA.2010.0474. [DOI] [PubMed] [Google Scholar]
- 16.Santos M.I. Unger R.E. Sousa R.A. Reis R.L. Kirkpatrick C.J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs S. Ghanaati S. Orth C. Barbeck M. Kolbe M. Hofmann A., et al. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Unger R.E. Ghanaati S. Orth C. Sartoris A. Barbeck M. Halstenberg S., et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31:6959. doi: 10.1016/j.biomaterials.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 19.Dohle E. Fuchs S. Kolbe M. Hofmann A. Schmidt H. Kirkpatrick C.J. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A. 2010;16:1235. doi: 10.1089/ten.tea.2009.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J. van den Beucken J.J. Yang F. Both S.K. Cui F.Z. Pan J., et al. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng Part C Methods. 2011;17:349. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 21.Saleh F.A. Whyte M. Ashton P. Genever P.G. Regulation of mesenchymal stem cell activity by endothelial cells. Stem Cells Dev. 2011;20:391. doi: 10.1089/scd.2010.0168. [DOI] [PubMed] [Google Scholar]
- 22.Saleh F.A. Whyte M. Genever P.G. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242. doi: 10.22203/ecm.v022a19. [DOI] [PubMed] [Google Scholar]
- 23.Bidarra S.J. Barrias C.C. Barbosa M.A. Soares R. Amédée J. Granja P.L. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011;7:186. doi: 10.1016/j.scr.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Kaiger D. Krebsbach P.H. West E.R. Horger K. Huang Y.C. Mooney D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 25.Villars F. Guillotin B. Amédée T. Dutoya S. Bordenave L. Bareille R., et al. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 26.Stahl A. Wenger A. Weber H. Stark G.B. Augustin H.G. Finkenzeller G. Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun. 2004;322:684. doi: 10.1016/j.bbrc.2004.07.175. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y. Andrukhov O. Berner S. Matejka M. Wieland M. Rausch-Fan X., et al. Osteogenic properties of hydrophilic and hydrophobic titanium surfaces evaluated with osteoblast-like cells (MG63) in coculture with human umbilical vein endothelial cells (HUVEC) Dent Mater. 2010;26:1043. doi: 10.1016/j.dental.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Unger R.E. Sartoris A. Peters K. Motta A. Migliaresi C. Kunkel M., et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Usami K. Mizuno H. Okada K. Narita Y. Aoki M. Kondo T., et al. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2009;90:730. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- 30.Yu H. VandeVord P.J. Mao L. Matthew H.W. Wooley P.H. Yang S.Y. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 31.Pham Q.P. Sharma U. Mikos A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 32.Liao J. Guo X. Nelson D. Kasper F.K. Mikos A.G. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta Biomater. 2010;6:2386. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibault R.A. Baggett L.S. Mikos A.G. Kasper F.K. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A. 2010;16:431. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer V.L. Jones L.J. Yue S.T. Haugland R.P. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 35.Datta N. Holtorf H.L. Sikavitsas V.I. Jansen J.A. Mikos A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Liao J. Guo X. Grande-Allen K.J. Kasper F.K. Mikos A.G. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31:8911. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy G.K. Enwemeka C.S. Simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 38.Lian J.B. Stein G.S. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118. [PMC free article] [PubMed] [Google Scholar]
- 39.Castano-Izquierdo H. Alvarez-Barreto J. van den Dolder J. Jansen J.A. Mikos A.G. Sikavitsas V.I. Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A. 2007;82:129. doi: 10.1002/jbm.a.31082. [DOI] [PubMed] [Google Scholar]
- 40.Hager S. Lampert F.M. Orimo H. Stark G.B. Fainkenzeller G. Up-regulation of alkaline phosphatase expression in human primary osteoblasts by cocultivation with primary endothelial cells is mediated by p38 mitogen-activated protein kinase-dependent mRNA stabilization. Tissue Eng Part A. 2009;15:3437. doi: 10.1089/ten.TEA.2009.0133. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers K.D. San Antonio J.D. Jacenko O. Heparan sulfate proteoglycans: a GAGgle of skeletal-hematopoietic regulators. Dev Dyn. 2008;237:2622. doi: 10.1002/dvdy.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris A.J. Turnbull J.E. Riley G.P. Gordon M.Y. Gallagher J.T. Production of heparan sulphate proteoglycans by human bone marrow stromal cells. J Cell Sci. 1991;99:149. doi: 10.1242/jcs.99.1.149. [DOI] [PubMed] [Google Scholar]
- 43.Drzeniek Z. Siebertz B. Stöcker G. Just U. Ostertag W. Greiling H., et al. Proteoglycan synthesis in haematopoietic cells: isolation and characterization of heparan sulphate proteoglycans expressed by the bone-marrow stromal cell line MS-5. Biochem J. 1997;327:473. doi: 10.1042/bj3270473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H. Lee J.H. Suh H. Interaction of mesenchymal stem cells and osteoblasts for in vitro osteogenesis. Yonsei Med J. 2003;44:187. doi: 10.3349/ymj.2003.44.2.187. [DOI] [PubMed] [Google Scholar]
- 45.Birmingham E. Niebur G.L. McHugh P.E. Shaw G. Barry F.P. McNamara L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]