Abstract
Superficial zone protein (SZP) functions as a boundary lubricant in articular cartilage and decreases the coefficient of friction. As lubrication of articular cartilage is critical for normal joint function, the ability to secrete SZP at the surface of tissue-engineered cartilage is a prerequisite for optimal lubrication. Synovium-derived mesenchymal stem cells (MSCs) are thought to be an attractive cell source for cartilage regeneration. However, optimization of a three-dimensional environment is necessary for tissue engineering. In this study, we investigated whether synovial explants, which would preserve the physiologic microenvironment for MSCs therein, have the potential of SZP secretion after chondrogenic differentiation by treatment with transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-7 (BMP-7). Immunostaining and enzyme-linked immunosorbent assay analysis demonstrated that synovial explants can synthesize and secrete SZP following chondrogenic differentiation in response to TGF-β1 and BMP-7. Interestingly, the combined treatment with TGF-β1 and BMP-7 or treatment first with TGF-β1 followed by BMP-7 was more effective than other treatment groups in both chondrogenic differentiation and SZP secretion. In conclusion, synovial explants represent not only a superb source of progenitors/stem cells for the regeneration of the surface zone of articular cartilage, but also a useful model system for the in vitro differentiation into mature articular cartilage phenotypes in response to morphogens for tissue engineering of articular cartilage.
Introduction
Normal articular cartilage maintains a well-lubricated surface with an extremely low coefficient of friction for joint mobility during locomotion.1 Superficial zone protein (SZP), also known as lubricin and PRG4,2,3 is a mucinous glycoprotein that is synthesized and secreted into the synovial fluid by the surface zone articular chondrocytes and synovial membrane lining the joint cavity.4–7 SZP plays an important role in lubrication of articular cartilage and reduces the coefficient of friction.8–10 The loss of SZP influences the functional properties of synovial joints, and the focal decrease in SZP in early osteoarthritis (OA) could have a role in the pathogenesis of cartilage degeneration.11,12
Articular cartilage is an avascular tissue with limited innate potential for repair and regeneration.13 A number of therapeutic strategies, including autologous chondrocyte implantation, microfracture, and mosaicplasty, have been introduced to induce the repair of articular damage due to injuries or arthritis, but these treatments do not regenerate tissue that resembles its native form.14 On the other hand, attention has been focused on stem/progenitor cells for articular cartilage tissue engineering, as it presents a promising potential for the biological repair of articular cartilage.15 Mesenchymal stem cells (MSCs) that can be used for cartilage regeneration have been isolated from various tissues, such as bone marrow (BM),16,17 adipose,18 synovium,19 muscle,20 and periosteum.21 As both synovium and cartilage are known to originate from a common pool of progenitor cells,22 it has been suggested that synovium-derived MSCs (SMSCs) may be tissue specific for articular cartilage tissue regeneration.14,23 In fact, SMSCs are reported to have a greater chondrogenic potential than other MSCs derived from BM, adipose, muscle, and periosteum.24,25 In addition, it is noteworthy that SMSCs have a high ability to synthesize and secrete SZP after chondrogenic differentiation,26 because SZP is a key mediator in boundary lubrication and the ability to secrete SZP at the surface of tissue-engineered cartilage may be a prerequisite for proper lubrication.27 Therefore, SMSCs are thought to be an attractive cell source for cartilage regeneration.
SMSCs can undergo chondrogenic differentiation in a three-dimensional (3D) environment with optimal growth factors.19,24,28 It is suggested that synovial tissue itself might also provide an optimum environment for chondrogenic differentiation of SMSCs, as synovium is known to produce hyaline cartilage in synovial chondromatosis and rheumatoid pannus,14 and the physiologic microenvironment of the SMSCs would be preserved.29 However, the ability of synovial explants to secrete SZP after chondrogenic differentiation has not been characterized. Therefore, we hypothesized that synovial explants may have high SZP secretion potential after chondrogenic differentiation and could be used as an optimal source for the regeneration of the surface zone of articular cartilage. In this study, we investigated the potential of SZP secretion after chondrogenic differentiation of synovial explants by transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-7 (BMP-7).
Material and Methods
Acquisition and culture of synovial explants
Stifle (knee) joints from 3-month-old calves were obtained within 6 h of slaughter and dissected under aseptic conditions. The synovium was harvested from the suprapatellar pouch, rinsed in sterile phosphate-buffered saline, and cut into small pieces (approximately 2×2 mm). After determining the wet weight of synovial explants, they were sandwiched between two layers of agarose to maintain them for an extended period and to minimize the outgrowth of chondroprogenitor cells,29–31 in which the nutritional and oxygen-tension conditions are similar to those operating physiologically.29 Initially, each well of 24-well plates was precoated with 250 μL of 1% low-melting agarose (Bio-Rad) in the chondrogenic medium consisting of high-glucose Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 1% ITS+ Premix (BD Biosciences), 100 nM dexamethasone (Sigma-Aldrich), 0.4 mM proline (Sigma-Aldrich), 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich), and antibiotics. After gelation, one explant was introduced and covered with 750 μL of 0.5% agarose in the chondrogenic medium. Each agarose-sandwiched explant was then covered with 1 mL of chondrogenic medium. TGF-β1 (10 ng/mL; R&D Systems) and/or BMP-7 (3000 ng/mL; a generous gift from Dr. D. Rueger; Stryker Biotech) were supplied to the medium for 3 weeks as following six groups; (1) no growth factors (control), (2) TGF-β1, (3) BMP-7, (4) 1-week TGF-β1 followed by 2-week BMP-7, (5) 1-week BMP-7 followed by 2-week TGF-β1, and (6) a combination of TGF-β1 and BMP-7. The medium was changed every 3–4 days. All cultures were incubated at 37°C in a moist atmosphere of 5% carbon dioxide and 95% air.
Histology and immunohistochemistry
After 3 weeks of culture, synovial explants were fixed in 4% paraformaldehyde, followed by paraffin embedding and sectioning at 5 μm. Toluidine blue staining and immunostaining for collagen type II (Col II) and SZP were performed. For immunohistochemistry, sections were deparaffinized and endogenous peroxidase was blocked with 3% H2O2. Thereafter, sections were blocked with normal blocking serum. The sections were incubated overnight at 4°C with a mouse monoclonal antibody (mAb) for Col II (1:200; Thermo Fisher Scientific) or mouse mAb S6.7932 (a generous gift from Dr. T. Schmid, Rush Medical Collage, Chicago, IL) for SZP (1:5000), or without a primary antibody as a negative control. The sections were incubated with a biotinylated horse anti-mouse immunoglobulin G for 30 min, followed by a 30-min incubation with Vecatatin® ABC reagent (Vector Laboratories). Observation was achieved using diaminobenzine (DAB)/peroxidase reaction (ImmPACT™ DAB peroxidase substrate; Vector Laboratories) resulting in brown precipitate.
Measurement of tissue weight and glycosaminoglycan content
The wet weight of synovial explants was determined before and after culturing. After 21 days of chondrogenic differentiation, the explants were digested with 0.1% papain (Sigma-Aldrich), 0.1% proteinase K (Sigma-Aldrich), and 5 mM L-cysteine (Sigma-Aldrich) in 10 mM Tris HCl for 18 h at 60°C. The concentration of glycosaminoglycan (GAG) of the digests was determined using a dimethylmethylene blue (Sigma-Aldrich).29 Chondroitin sulfate was used as a standard. All GAG values were normalized to the total amount of DNA, which was determined using a Quanti-iT PicoGreen dsDNA Assay Kit (Life Technologies).
Enzyme-linked immunosorbent assay for SZP
To investigate the SZP-secretion potential of synovial explants after chondrogenic differentiation, the medium between days 18 and 21 from synovial explant cultures was collected, and quantitatively analyzed for SZP by sandwich enzyme-linked immunosorbent assay (ELISA) using purified SZP as the standard.7 Each well of 96-well MaxiSorp plates (Nalge Nunc International) was coated with 1 μg/mL peanut lectin (EY Laboratories) in a 50 mM sodium carbonate buffer (pH 9.5). Then, the wells were blocked with 1% BSA in the same buffer. Aliquots of the culture medium were incubated in the wells. The wells were incubated with mAb S6.79 (1:5000) as the primary antibody and goat anti-mouse IgG conjugated with horseradish peroxidase (1:3000; Bio-Rad) as the second antibody. Finally, SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) was used and quantified by measuring relative light units in a luminometer. Wells were washed with phosphate-buffered saline containing 0.05% Tween 20 (Sigma-Aldrich) between all steps. SZP levels were calculated using a SZP standard, which was purified by affinity chromatography on a peanut lectin column;4,32 purity was verified by immunoblot analysis and quantified using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific).6,31,33
Real-time polymerase chain reaction analysis
Before the gene expression analysis, each cultured synovial explant was stored in RNAlater reagent (Qiagen). The samples were homogenized in QIAzol lysis reagent (Qiagen), and RNA was extracted using the RNeasy lipid tissue mini kit (Qiagen) with on-membrane DNase I (Qiagen) digestion to avoid genomic DNA contamination. Total RNA was reverse transcribed into single-strand cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time polymerase chain reaction (PCR) was performed in triplicate on the cDNA with an Applied Biosystems 7900HT Fast Real-Time PCR System and Fast SYBR® Green Master Mix (Applied Biosystems) following the recommended protocols. Results were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels and expressed relative to the control culture levels (ΔΔCT methods; Applied Biosystems). Primers for bovine GAPDH, type II collagen (Col II), aggrecan, and Sox9 were generated as previously described.34
Statistical analysis
All the quantitative data are presented as mean±standard deviation of six or eight individual experiments using six or eight different animals. The paired t-test was performed using StatView statistics (SAS Institute, Inc.) to determine the difference between the control and treated groups. p Values less than 0.05 were considered significant.
Results
Histological and immunohistochemical analysis for GAG and Col II after 3-week chondrogenic differentiation
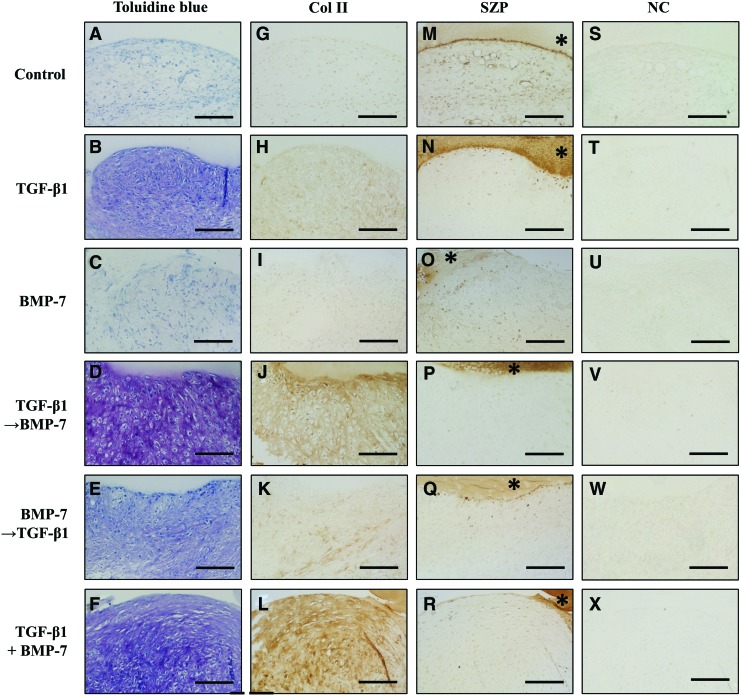
The chondrogenic potential of synovial explants was evaluated histologically with toluidine blue staining for metachromasia in the extracellular matrix and immunohistochemical staining for Col II (Fig. 1). Toluidine blue staining demonstrated the production and accumulation of GAGs in the groups treated with TGF-β1 followed by BMP-7 and the combination of TGF-β1 and BMP-7 was highest among six groups, while the groups treated with TGF-β1 and BMP-7 followed by TGF-β1 showed less production of GAGs. There was no production of GAGs in the control and BMP-7 alone. The differentiation of synovial cells into a chondrocytic phenotype with lacunae was most abundant in the group of TGF-β1 followed by BMP-7, followed by the group with a combination of TGF-β1 and BMP-7. Immunohistochemical staining revealed that Col II was produced in the groups treated with TGF-β1 followed by BMP-7 and the combination of TGF-β1 and BMP-7. On the other hand, the untreated control and BMP-7 alone showed no deposition of Col II.
FIG. 1.
Toluidine blue staining (A–F) and immunostaining with a monoclonal antibody against collagen type II (Col II) (G–L) or superficial zone protein (SZP) (M–R), or without the primary antibody as negative control (NC; S–X) of synovial explants cultured in the chondrogenic medium by treatment with transforming growth factor-β1 (TGF-β1) (10 ng/mL) and/or bone morphogenetic protein-7 (BMP-7) (3000 ng/mL) for 21 days. *agarose. Scale bar=100 μm. Col II, collagen type II; SZP, superficial zone protein; TGF-β→BMP-7, 1-week TGF-β1 followed by 2-week BMP-7; BMP-7→TGF-β1, 1-week BMP-7 followed by 2-week TGF-β1; combined treatment of TGF-β1 and BMP-7, combination of TGF-β1 and BMP-7. Color images available online at www.liebertpub.com/tea
Analysis of the wet weight change and GAG content in the synovial explant after 3-week chondrogenic differentiation
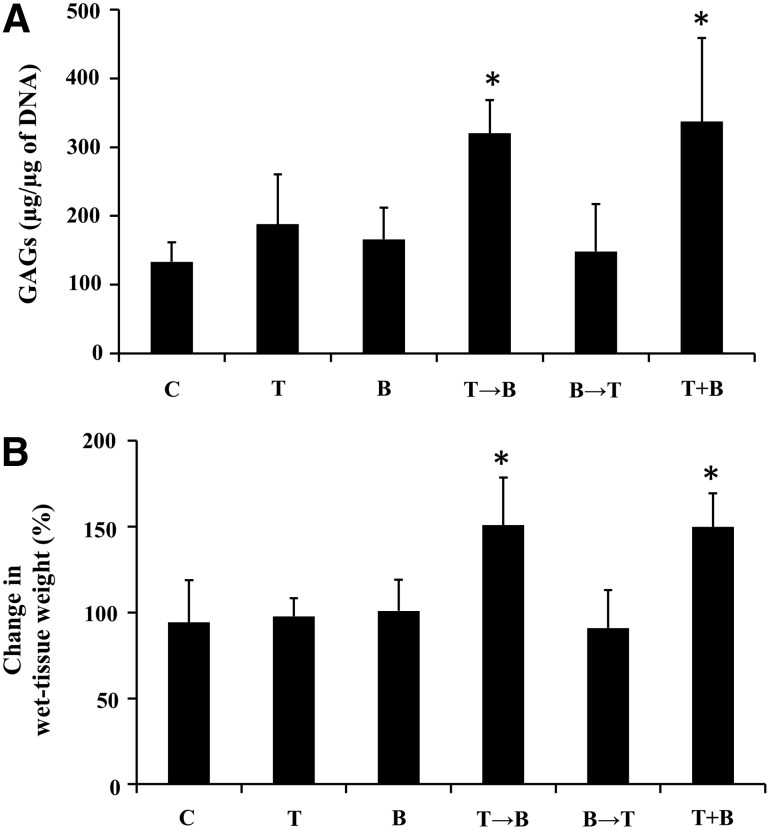
The GAG content of synovial explants after 3-week chondrogenic differentiation was evaluated (Fig. 2A). The GAG content significantly increased in the groups treated by TGF-β1 followed by BMP-7 and the combination of TGF-β1 and BMP-7 compared to the control, while other groups showed no influence on GAG content. The wet-tissue weight of synovial explants was significantly increased in the groups treated with TGF-β1 followed by BMP-7 and the combination of TGF-β1 and BMP-7 after 3-week chondrogenic differentiation (Fig. 2B).
FIG. 2.
GAG content (A) and the change in wet-tissue weight (B) after 3-week chondrogenic differentiation by treatment with TGF-β1 (10 ng/mL) and/or BMP-7 (3000 ng/mL). Values are mean±standard deviation (n=8). *p<0.01 compared to the control. GAG, glycosaminoglycan; C, control; T, TGF-β1; B, BMP-7; T→B, 1-week TGF-β1 followed by 2-week BMP-7; B→T, 1-week BMP-7 followed by 2-week TGF-β1; T+B, combination of TGF-β1 and BMP-7.
Analysis of chondrogenic gene expression after 3-week chondrogenic differentiation
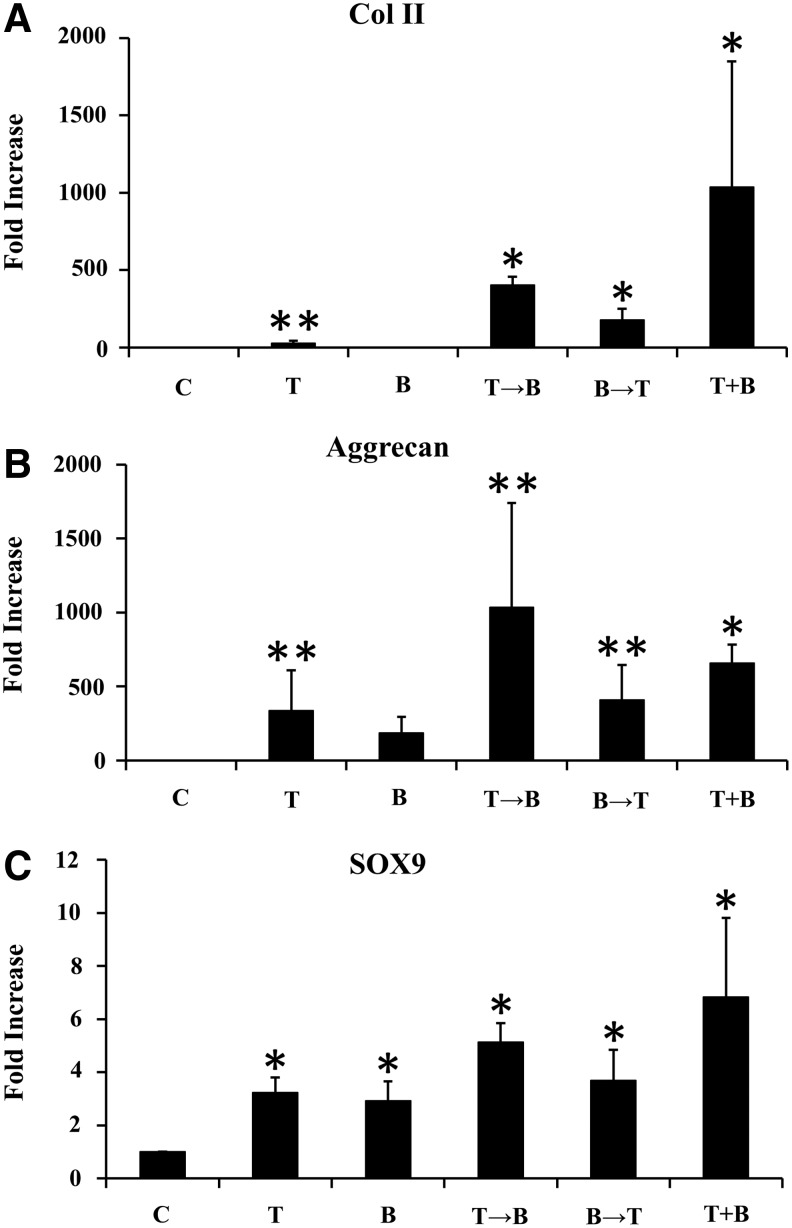
Chondrogenic differentiation of synovial explants after 3-week chondrogenic differentiation was further confirmed by real-time PCR (Fig. 3). The gene expression of Col II and aggrecan was dramatically increased in the groups treated with TGF-β1, TGF-β1 followed by BMP-7, and the combination of TGF-β1 and BMP-7 compared to the control. The expression of Col II was highest in the group with the combination of TGF-β1 and BMP-7, while the expression of aggrecan was highest in the group of TGF-β1 followed by BMP-7. The gene expression of Sox9 was higher in all groups treated with growth factors compared to control.
FIG. 3.
Quantitative real-time polymerase chain reaction analysis of synovium explants after 3-week chondrogenic differentiation by treatment with TGF-β1 (10 ng/mL) and/or BMP-7 (3000 ng/mL). The mRNA expression levels of Col II (A), aggrecan (B), and Sox9 (C) are expressed relative to those for the control and presented as a fold increase. Values are mean±standard deviation (n=6). *p<0.01 and **p<0.05 compared to control. GAG, glycosaminoglycan; C, control; T, TGF-β1; B, BMP-7; T→B, 1-week TGF-β1 followed by 2-week BMP-7; B→T, 1-week BMP-7 followed by 2-week TGF-β1; T+B, combination of TGF-β1 and BMP-7.
SZP expression after 3-week chondrogenic differentiation
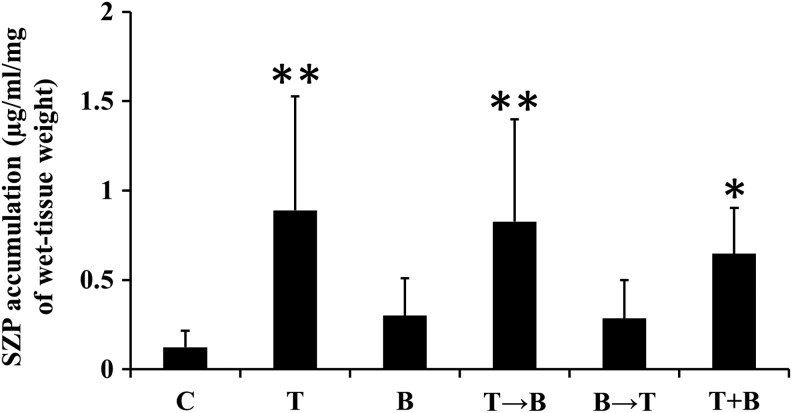
We next evaluated SZP expression in synovial explants after 3-week chondrogenic differentiation by immunohistochemical analysis. The control group showed slightly positive staining for SZP in synovial explants, while other groups showed less SZP deposition (Fig. 1). On the other hand, the agarose in contact with the synovial explant (*agarose in Fig. 1) showed strong SZP staining in the groups treated with TGF-β1, TGF-β1 followed by BMP-7, and the combination of TGF-β1 and BMP-7. As SZP was reported to be not retained in the extracellular matrix, but is mostly secreted into the synovial fluid,4 we investigated the SZP accumulation in the media from synovial explant cultures between days 18 and 21 of culture (Fig. 4). SZP accumulation in the media was robustly upregulated in the groups treated with TGF-β1, TGF-β1 followed by BMP-7, and the combination of TGF-β1 and BMP-7 compared to control.
FIG. 4.
SZP accumulation in the media after 3-week chondrogenic differentiation by treatment with TGF-β1 (10 ng/mL) and/or BMP-7 (3000 ng/mL). SZP accumulation was quantified by ELISA and expressed per milligram of preculture wet-tissue weight. Values are mean±standard deviation (n=8). *p<0.01 and **p<0.05 compared to the control. SZP, superficial zone protein; C, control; T, TGF-β1; B, BMP-7; T→B, 1-week TGF-β1 followed by 2-week BMP-7; B→T, 1-week BMP-7 followed by 2-week TGF-β1; T+B, combination of TGF-β1 and BMP-7.
Discussion
It is well established that stem cells reside, proliferate, and differentiate within a 3D environment that surrounds them in native tissue, and these niches provide the correct biochemical signals to the stem cells in response to a physiological change.35–37 Therefore, optimization of a 3D environment is necessary for tissue engineering of articular cartilage, including surface zone cartilage. In this study, we utilized synovial explants, which would preserve the physiologic microenvironment for MSCs therein, and investigated their ability to secrete SZP after chondrogenic differentiation with TGF-β1 and BMP-7. Our results indicated that synovial explants had a high ability to synthesize and secrete SZP after chondrogenic differentiation in response to TGF-β1 and BMP-7.
The chondrogenesis of SMSCs or synovial explants has been induced by using the members of the TGF-β superfamily, including TGF-β1, TGF-β2, TGF-β3, BMP-2, and BMP-7.24,26,28,29,31,38 However, the effects of the combinatorial treatment with TGF-β isoform and BMP isoform on chondrogenic differentiation of synovial explants have not been investigated. In the current study, the production of GAGs in the synovial explants was highest in the groups of TGF-β→BMP-7 and combined treatment of TGF-β1 and BMP-7, and the gene expressions of Col II, aggrecan, and SOX9 were most upregulated in the same groups, indicating that treatment with TGF-β1 followed by BMP-7 and the combination of TGF-β1 and BMP-7 have profound effects on chondrogenic differentiation of synovial explant, similar to the pellet culture as reported.26,38
The ability to synthesize and secrete SZP was determined after chondrogenic differentiation of synovial explant. The ELISA analysis of the media between days 18 to 21 showed that SZP accumulation was robustly upregulated in the groups of TGF-β1, TGF-β→BMP-7, and combined treatment of TGF-β1 and BMP-7. Immunohistochemical analysis also showed strong SZP staining of the agarose in the groups of TGF-β1, TGF-β1 followed by BMP-7, and combined treatment of TGF-β1 and BMP-7. These results are consistent with the findings that TGF-β isoforms robustly stimulate SZP secretion in monolayer culture of synoviocytes or pellet culture of SMSCs after chondrogenic differentiation, described in previous studies.7,26 In contrast, immunohistochemical staining revealed that the retention of SZP in the synovium in all treated groups was limited compared to that in the control. This might be because SZP was not retained in the extracellular matrix, but is mostly secreted into the synovial fluid.4
This study has also shown the new patterns of order of addition of growth factors and morphogens in chondrogenic differentiation; TGF-β1 followed by BMP-7, and BMP-7 followed by TGF-β1. Our results demonstrated that the ability of chondrogenic differentiation and SZP secretion in synovial explants was significantly stimulated by the treatment with TGF-β1 followed by BMP-7 compared to BMP-7 followed by TGF-β1. These findings suggested that in the initial stages of chondrogenic differentiation, TGF-β1 is indispensable for chondrogenic differentiation of MSCs in the synovial explant, which is supported by previous reports that TGF-beta isoforms regulate chondrogenesis at early stages of chondrocyte differentiation.39
It is reported that synovium contains MSCs with high potential of chondrogenic differentiation and SZP secretion in 3D culture systems, including micromass culture and pellet culture, which is thought to be crucial for the chondrogenic differentiation of SMSCs.24–26 However, these culture environments cannot be directly used in most clinical therapies because of the limitations in their mass size.40 In addition, there was very little knowledge about the properties of MSCs in its native environment, despite the emerging information about MSCs and their use in cell-based strategies. In this study, we utilized the explant culture system, where the physiologic environment of MSCs would be preserved, and demonstrated that synovial explants are useful for the in vitro culture of MSCs therein to form articular cartilage with SZP-producing potential. As synovium have the potential of self-regeneration after removal from joints,41,42 synovium might be an attractive source for the treatment of a large size of cartilage defect.
A potential limitation of synovial explants is that they are a mixed population of various cell types and produce large amount of type I collagen (Col I). The change of Col I amount was not investigated in this study, Shintani et al. reported that Col I mRNA expression was not appreciably lowered even after 6 weeks of culturing in chondrogenic induction.29 Therefore, the hyaline-like qualities of the cartilage, including Col I expression must be improved for clinical use. Another limitation is that this study does not include the comparison between the synovial explant and other culture conditions, including the 3D pellet culture system. Therefore, further investigation is needed to discuss the superiority of the synovial explant culture system to other culture systems.
In conclusion, this investigation demonstrated that synovial explants had the potential to synthesize and secrete SZP after chondrogenic differentiation in response to TGF-β1 and BMP-7. Synovial explants represent not only a superb source of progenitors/stem cells for the regeneration of surface zone of articular cartilage, but also a useful model system to investigate and gain mechanistic insights into the terminal differentiation into articular cartilage phenotypes, a topic at the crux of regeneration and tissue engineering of cartilage in OA.
Acknowledgments
Dr. Reddi is supported, in part, by the NIH grant R01-AR-061496-01. This research is also supported by Lawrence J. Ellison Endowed Chair in Musculoskeletal Molecular Biology. We thank Dr. T. Schmid (Department of Biochemistry, Rush Medical College, Chicago, IL) for his generous gift of the mAb S6.79, and Dr. D. Rueger (Stryker Biotech, Hopkinton, MA) for his generous gift of BMP-7.
Disclosure Statement
No competing financial interests exist.
References
- 1.Charnley J. The lubrication of animal joints in relation to surgical reconstruction by arthroplasty. Ann Rheum Dis. 1960;19:10. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jay G.D. Tantravahi U. Britt D.E. Barrach H.J. Cha C.J. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 3.Ikegawa S. Sano M. Koshizuka Y. Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90:291. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher B.L. Block J.A. Schmid T.M. Aydelotte M.B. Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher B.L. Hughes C.E. Kuettner K.E. Caterson B. Aydelotte M.B. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 6.Khalafi A. Schmid T.M. Neu C. Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 7.Niikura T. Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 8.Flannery C.R. Hughes C.E. Schumacher B.L. Tudor D. Aydelotte M.B. Kuettner K.E. Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 9.Swann D.A. Hendren R.B. Radin E.L. Sotman S.L. Duda E.A. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981;24:22. doi: 10.1002/art.1780240104. [DOI] [PubMed] [Google Scholar]
- 10.Neu C.P. Khalafi A. Komvopoulos K. Schmid T.M. Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 11.Young A.A. McLennan S. Smith M.M. Smith S.M. Cake M.A. Read R.A. Melrose J. Sonnabend D.H. Flannery C.R. Little C.B. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee D.K. Marcelino J. Baker M. Gong Y. Smits P. Lefebvre V. Jay G.D. Stewart M. Wang H. Warman M.L. Carpten J.D. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerra J. Andrades J.A. Guerado E. Zamora-Navas P. Lopez-Puertas J.M. Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 14.Jones B.A. Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda R. Ishida K. Matsumoto T. Akisue T. Fujioka H. Mizuno K. Ohgushi H. Wakitani S. Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 19.De Bari C. Dell'Accio F. Tylzanowski P. Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Mastrogiacomo M. Derubeis A.R. Cancedda R. Bone and cartilage formation by skeletal muscle derived cells. J Cell Physiol. 2005;204:594. doi: 10.1002/jcp.20325. [DOI] [PubMed] [Google Scholar]
- 21.De Bari C. Dell'Accio F. Vanlauwe J. Eyckmans J. Khan I.M. Archer C.W. Jones E.A. McGonagle D. Mitsiadis T.A. Pitzalis C. Luyten F.P. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 22.Archer C.W. Dowthwaite G.P. Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 23.Segawa Y. Muneta T. Makino H. Nimura A. Mochizuki T. Ju Y.J. Ezura Y. Umezawa A. Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura H. Muneta T. Nimura A. Yokoyama A. Koga H. Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.Y. Nakagawa T. Reddi A.H. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: analysis of superficial zone protein/lubricin expression. Tissue Eng Part A. 2010;16:317. doi: 10.1089/ten.TEA.2009.0104. [DOI] [PubMed] [Google Scholar]
- 27.Gleghorn J.P. Jones A.R. Flannery C.R. Bonassar L.J. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20. doi: 10.22203/ecm.v014a02. [DOI] [PubMed] [Google Scholar]
- 28.Park Y. Sugimoto M. Watrin A. Chiquet M. Hunziker E.B. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13:527. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Shintani N. Hunziker E.B. Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007;56:1869. doi: 10.1002/art.22701. [DOI] [PubMed] [Google Scholar]
- 30.O'Driscoll S.W. Recklies A.D. Poole A.R. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994;76:1042. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura K. Solchaga L.A. Caplan A.I. Yoo J.U. Goldberg V.M. Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999;42:2631. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Su J.L. Schumacher B.L. Lindley K.M. Soloveychik V. Burkhart W. Triantafillou J.A. Kuettner K. Schmid T. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20:149. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt T.A. Schumacher B.L. Klein T.J. Voegtline M.S. Sah R.L. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum. 2004;50:2849. doi: 10.1002/art.20480. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.Y. Nakagawa T. Reddi A.H. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376:148. doi: 10.1016/j.bbrc.2008.08.138. [DOI] [PubMed] [Google Scholar]
- 35.Lund A.W. Yener B. Stegemann J.P. Plopper G.E. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15:371. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison S.J. Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchs E. Tumbar T. Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 38.Shirasawa S. Sekiya I. Sakaguchi Y. Yagishita K. Ichinose S. Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 39.Chimal-Monroy J. Diaz de Leon L. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41:91. [PubMed] [Google Scholar]
- 40.De Bari C. Dell'Accio F. Luyten F.P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 41.Bentley G. Kreutner A. Ferguson A.B. Synovial regeneration and articular cartilage changes after synovectomy in normal and steroid-treated rabbits. J Bone Joint Surg Br. 1975;57:454. [PubMed] [Google Scholar]
- 42.Theoret C.L. Barber S.M. Moyana T. Townsend H.G. Archer J.F. Repair and function of synovium after arthroscopic synovectomy of the dorsal compartment of the equine antebrachiocarpal joint. Vet Surg. 1996;25:142. doi: 10.1111/j.1532-950x.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]