Abstract
4H-Pyrano-[2,3-b]naphthoquinone is a structural motif commonly found in natural products manifesting anticancer activities. As part of a program aimed at structural simplification of bioactive natural products utilizing multicomponent synthetic processes, we developed a compound library based on this heterocyclic scaffold. We found that several library members displayed low micromolar antiproliferative activity and induced apoptosis in human cancer cells. Selected compounds showed promising activity against cancer cell lines resistant to proapoptotic stimuli, demonstrating their potential in treating cancers with dismal prognoses.
Keywords: Multicomponent synthesis, Antiproliferative, Apoptosis, Heterocycles
Although natural products have traditionally been an excellent source of new medicinal leads, their structural complexity and poor availability greatly impede natural product-based drug discovery.1 To address this challenge we have advocated the utilization of natural product-mimetic scaffolds, which can be synthetically accessed in one step by utilizing multicomponent reactions (MCR).2 for example, we showed that the stereochemically complex structure of an important anticancer lead podophyllotoxin can be efficiently simplified to fused dihydropyridine scaffolds, efficiently prepared with MCRs.2a–c Compounds based on this heterocyclic scaffold retain the antitubulin mode of action of the natural product and rival podophyllotoxin in their antiproliferative potency and apoptosis inducing potential. Other successful applications of this approach involve the discovery of pyranoquinolones, designed to be mimetics of pyranoquinolone alkaloids,2d,e and topoisomerase-targeting indenopyridines inspired by the structure of camptothecin.2f,g
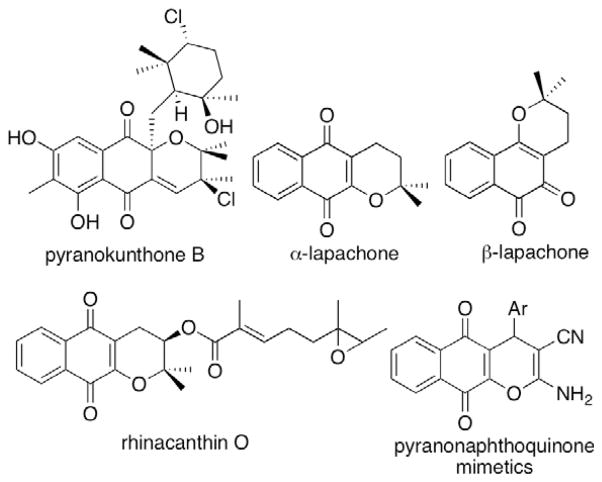
In continuation of these efforts we have been investigating compounds based on the 4H-pyrano-[2,3-b]naphthoquinone skeleton as mimetics of a diverse group of naturally occurring pyranonaphthoquinones and their synthetic analogues with promising anticancer activities.3 Selected examples of these natural products include pyranokunthone B from a marine actinomycete,3a rhinacanthin O from the Asian medicinal plant Rhinocanthus nasutus,3b α- and β-lapachones isolated from the heartwood of the trees of Bignoniaceae family,3c among others (Fig. 1).3d,e β-Lapachone is investigated for the treatment of cancers associated with elevated NADH quinone oxidoreductase (NQO1) levels and it is currently in phase II clinical trials for the treatment of pancreatic cancer.4 In this paper we present a one-step preparation of a small library of simple pyranonaphthoquinones and evaluation of these compounds for antiproliferative and apoptosis-inducing properties in human cancer cell lines.
Figure 1.
Structures of selected pyranonaphthoquinone natural products with anticancer activity and the mimetic scaffold utilized in this work.
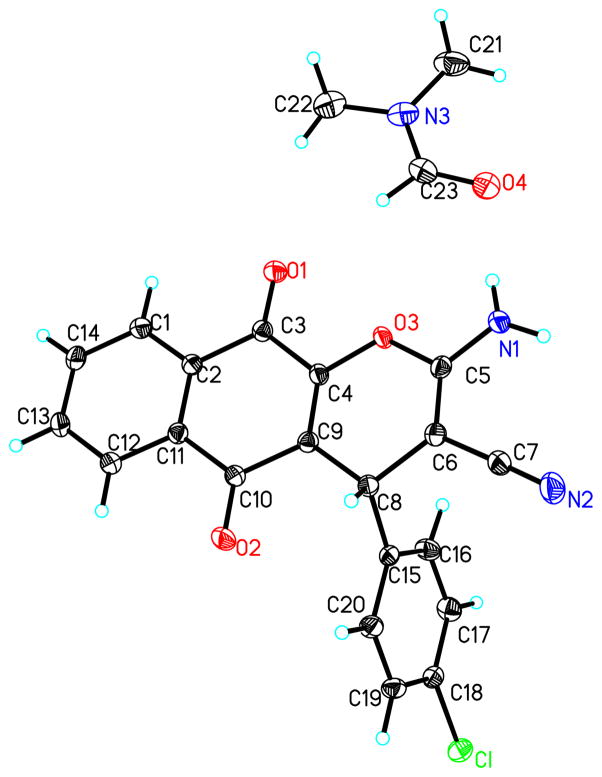
As a rapid entry to the 4H-pyrano-[2,3-b]naphthoquinone skeleton, we adapted the previously reported cyclocondensation of arylidenemalononitriles with 2-hydroxy-1,4-naphthoquinone5 to a multicomponent process, in which the Knoevenagel intermediates are formed in situ from malononitrile and aromatic aldehydes (Fig. 2).6 Although both 1,4- (C) and 1,2-naphthoquinones (D) resulting from the corresponding pyran ring closures A and B could be expected, only 1,4-products were formed. This was unambiguously established by obtaining an X-ray crystal structure of pyranonaphthoquinone 5 (Fig. 3). In agreement with these experimental findings is a theoretical analysis, which indicates that the predominant formation of 1,4-naphthoquinones should be favored on the basis of both thermodynamic and kinetic considerations. Thus, both semiempirical (PM6) and Density Functional Theory (DFT) computations reveal the higher stability of 1,4-naphthoquinone C over its 1,2-counterpart D (Fig. 2). In addition, the analysis of O(2) versus O(4) ambident nucleophilic sites in the model structure E indicates an equal charge distribution between the oxygens and a larger HOMO coefficient on O(2) favoring the attack by this oxygen (A vs B).
Figure 2.
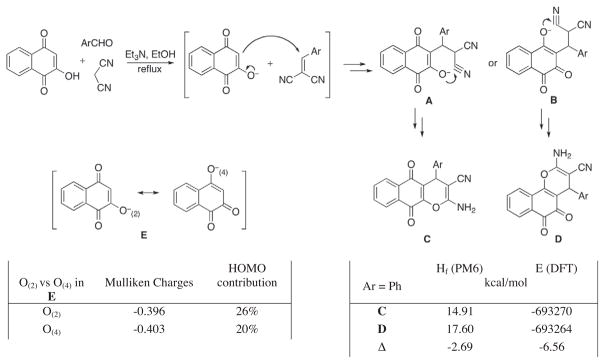
Mechanistic details of the MCR and computed characteristics of relevant molecules.
Figure 3.
X-ray structure of pyranonaphthoquinone 5 (thermal ellipsoids are shown at 50% probability, CCDC 884823).
We found that both substituted benzaldehydes and heteroaromatic aldehydes reacted with equal facility to give moderate to good yields of the desired products 1–23 (Table 1). In all cases the MCR products precipitated as the reaction mixtures were allowed to cool to room temperature and were isolated by simple filtration. A single recrystallization was sufficient in all cases to achieve product purity of >98% as judged by NMR analysis.7,8
Table 1.
MCR products: synthetic yields, antiproliferative and apoptosis-inducing properties
| Compound |
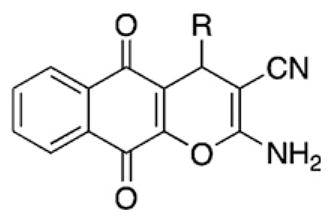 R |
Synthesis % yield | GI50a (μM)
|
% Apoptosisb Jurkat | ||
|---|---|---|---|---|---|---|
| HeLa | MCF-7/AZ | Jurkat | ||||
| 1 | 3-PhO-C6H4- | 82 | 4.0 ± 2.9 | 2.2 ± 0.4 | 17.9 ± 1.8 | 50 ± 1.9 |
| 2 | 4-F3CO-C6H4- | 57 | 4.3 ± 0.3 | 7.1 ± 1.1 | 105 ± 27 | 13.5 ± 0.9 |
| 3 | 3-Cl-C6H4- | 76 | 77.5 ± 1.7 | 59.8 ± 3.1 | 108 ± 28 | 22.0 ± 0.7 |
| 4 | 2-Cl-C6H4- | 80 | 20.5 ± 4.8 | 19 ± 0.9 | 185 ± 76 | 6.3 ± 1.6 |
| 5 | 4-Cl-C6H4- | 88 | 31.7 ± 0.6 | 24 ± 2.2 | 105 ± 5 | 23.8 ± 3.5 |
| 6 | 2,6-di-Cl-C6H3- | 90 | 5.3 ± 0.1 | 3.1 ± 0.9 | 171.9 ± 9.1 | 5.1 ± 4.2 |
| 7 | 3-Br-C6H4- | 85 | 16.1 ± 2.2 | 28.6 ± 5.6 | 20 ± 0.1 | 48.4 ± 2.5 |
| 8 | 2-Br-C6H4- | 83 | 31.6 ± 2.8 | 26.3 ± 5.6 | 30.7 ± 1.8 | 41.7 ± 1.8 |
| 9 | 4-Br-C6H4- | 79 | 30 ± 3.3 | 22.8 ± 1.4 | 38.3 ± 2.4 | 40.1 ± 3.5 |
| 10 | 2-F-6-Cl-C6H3- | 82 | 34.4 ± 1.8 | 8 ± 5.6 | 52.3 ± 6.7 | 21.3 ± 4.3 |
| 11 | 4-F3C-C6H4- | 74 | 11.8 ± 1.8 | 5.6 ± 1.8 | 80.5 ± 0.4 | 15.5 ± 1.5 |
| 12 | 4-Ph-C6H4- | 70 | 23.6 ± 7.0 | 26 ± 2.9 | 122.2 ± 3.1 | 11.2 ± 2.4 |
| 13 | Ph | 77 | 23.5 ± 5.9 | 18.5 ± 0.8 | 43.3 ± 4.8 | 16.7 ± 2.0 |
| 14 | 3-O2N-C6H4- | 62 | 9.1 ± 0.7 | 10.8 ± 4.7 | 19.1 ± 2.8 | 33.6 ± 3.5 |
| 15 | 2-O2N-C6H4- | 76 | 11.2 ± 2.6 | 8.3 ± 1.9 | 16.8 ± 2.4 | 25 ± 1.1 |
| 16 | 4-O2N-C6H4- | 84 | 16.8 ± 1.5 | 9.8 ± 1.0 | 24.3 ± 1.0 | 23.1 ± 1.1 |
| 17 | 4-HOOC-C6H4- | 86 | 119 ± 49 | 94.4 ± 3.1 | 332 ± 55 | 4.1 ± 1.8 |
| 18 | 4-HO-C6H4- | 69 | 30.6 ± 2.0 | 8.6 ± 0.6 | 85 ± 20 | 16.7 ± 0.9 |
| 19 | 4-iPr-C6H4- | 73 | 62.5 ± 13.6 | 34 ± 7.4 | 80.6 ± 18.8 | 2.9 ± 4.3 |
| 20 |
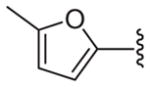
|
55 | 54.3 ± 7.3 | 51.6 ± 1.4 | 47.8 ± 3.7 | 57.8 ± 0.5 |
| 21 |
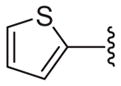
|
51 | 94 ± 5.3 | 36.9 ± 1.7 | 82.4 ± 3.4 | 11.2 ± 0.7 |
| 22 |

|
63 | 14.5 ± 0.8 | 6.0 ± 1.7 | 23.9 ± 1.2 | 28.6 ± 3.3 |
| 23 |
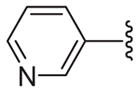
|
63 | 14.2 ± 1.0 | 3.4 ± 0.8 | 20.4 ± 5.8 | 20.8 ± 0.4 |
| α-Lapachone | N/A | N/A | 25 ± 3.8 | 16.7 ± 3.1 | 75 ± 1.4 | 7.4 ± 2.3 |
| β-Lapachone | N/A | N/A | 4.6 ± 0.5 | 6.0 ± 2.2 | 6.1 ± 0.5 | 51 ± 1.6 |
Concentration required to reduce the viability of cancer cells by 50% after 48 h of treatment with indicated compounds relative to DMSO control ± SD from two independent experiments, each performed in 8 replicates. Determined by MTT assay.
% Apoptotic Jurkat cells after 24 h of treatment with indicated compounds at the concentration of 50 μM relative to DMSO control ± SD from two independent experiments, each performed in 4 replicates. Determined by flow cytometric Annexin-V/propidium iodide assay.
Analogues 1–23 were evaluated for antiproliferative activity against three cancer cell lines, HeLa, MCF-7/AZ and Jurkat as models for human cervical and breast adenocarcinomas and T-cell leukemia, using the MTT method.9 In addition, the same compounds were tested for their ability to induce apoptosis in Jurkat cells in the flow cytometric Annexin-V/propidium iodide assay10 at a concentration of 50 μM and the percentages of apoptotic cells after 24 hours of treatment are shown in the last column of Table 1. The analysis of these data indicates that although a polar 4-HOOC-Ph analogue 17 lacks any significant anticancer activity, most of the other library members have notable antiproliferative properties and several compounds exhibit good apoptosis-inducing potential. The most potent compounds appear to be 3-substituted phenyl analogues (1, 7, 14) and 3-PhO-Ph pyranonaphthoquinone 1 is clearly superior to all the other members of the library. Its antiproliferative and apoptosis-inducing effects surpass those of α-lapachone and rival those of clinically relevant β-lapachone, both synthesized using literature methods11 and used as positive controls. Also, the low micromolar antiproliferative activity of 2,6-di-Cl-Ph analogue 6 toward HeLa and MCF-7/AZ cell lines is noteworthy, although this compound fails to maintain its potency or induce apoptosis in the Jurkat model.
Based on their promising activities pyranonaphthoquinones 1 and 6 were selected for further testing. Glioma, melanoma, oesophageal, non-small-cell lung, and a number of other types of cancer known to be associated with dismal prognoses, all have the characteristic of being intrinsically resistant to pro-apoptotic stimuli.12 Moreover, more than 90% of cancer patients die from tumor metastases, which are incompetent in initiating the apoptotic program as well. Thus, the rise in incidence of gliomas and melanomas has not been paralleled by improved therapeutic options over the years. New types of drugs are, therefore, urgently needed to combat cancers that are resistant to proapoptotic stimuli and poorly responsive to current therapies. We selected seven human cell lines (Table 2) representing cancers associated with dismal prognoses (U373 glioblastoma (GBM), A549 non-small-cell-lung cancer (NSCLC), Hs683 anaplastic oligodendroglioma, OE21 oesophageal cancer, SKMEL-28 melanoma as well as LoVo colon and PC-3 prostate cancers) and evaluated compounds 1 and 6, together with α- and β-lapachone controls, against this challenging panel. The results (Table 2) indicate that analogue 1 maintains its potent activity against the cell lines in this panel. Its antiproliferative effects are superior to those of α-lapachone but secondary to the regioisomeric β-lapachone by an order of magnitude (see the mean values in Table 2).
Table 2.
Antiproliferative properties of analogues 1 and 6 against a panel of cancer cell lines representing cancers with dismal prognoses
| Compound | GI50a (lM)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| U373 (GBM) | A549 (NSCLC) | HS683 (glioma) | OE21 (esophageal cancer) | SKMEL-28 (melanoma) | LoVo (colon cancer) | PC-3 (prostate cancer) | mean | |
| 1 | 3.4 | 5.0 | 5.0 | 2.2 | 4.5 | 3.4 | 6.2 | 4.2 |
| 6 | 12.8 | 40.6 | 19.3 | 17.4 | 17.1 | 4.3 | 6.2 | 16.8 |
| α-Lapachone | 23.1 | 16.3 | 9.0 | 22.7 | 4.7 | 24.4 | 13.0 | 16.2 |
| β-Lapachone | 0.4 | 0.8 | 0.4 | 0.6 | 0.6 | 0.3 | 0.01 | 0.44 |
Concentration required to reduce the viability of cancer cells by 50% after 72 h of treatment with indicated compounds relative to DMSO control. Determined by MTT assay.
In conclusion, as part of a program involving the simplification of bioactive natural products with mimetic scaffolds accessible via one-step multicomponent synthetic processes, we synthesized a small library of pyrano-1,4-naphthoquinones inspired by the common occurrence of this structural motif in natural products possessing promising anticancer activities. Several library members inhibit the proliferation of cancer cells at single-digit micromolar concentrations and induce apoptosis in the human Jurkat leukemia model. Particularly noteworthy is the promising activity of these compounds against human cell lines representing cancers with dismal prognoses. Further work in this area focuses on modifying the MCR conditions to access the analogous 1,2-naphthoquinone scaffold, which would be mimetic of the β-lapachone-type natural products appearing to have superior anticancer properties.
Acknowledgments
This project was supported by Grants from the National Center for Research Resources (5P20RR016480-12), National Institute of General Medical Sciences (8 P20 GM103451-12) and National Cancer Institute (CA-135579). X-ray structural studies are funded by the NSF PREM program (DMR-0934212). We thank Dr. Thierry Gras, Dr. Liliya Frolova and Professors Robert Kiss, Tatiana V. Timofeeva and Snezna Rogelj for their kind technical assistance.
References and notes
- 1.Li JWH, Vederas JC. Science. 2009;325:161. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 2.(a) Magedov IV, Manpadi M, Rozhkova E, Przheval’skii NM, Rogelj S, Shors ST, Steelant WFA, Van Slambrouck S, Kornienko A. Bioorg Med Chem Lett. 2007;17:1381. doi: 10.1016/j.bmcl.2006.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Magedov IV, Manpadi M, Van Slambrouck S, Steelant WFA, Rozhkova E, Przhevalískii NM, Rogelj S, Kornienko A. J Med Chem. 2007;50:5183. doi: 10.1021/jm070528f. [DOI] [PubMed] [Google Scholar]; (c) Magedov IV, Frolova L, Manpadi M, Bhoga UD, Tang H, Evdokimov NM, George O, Georgiou KH, Renner S, Getlik M, Kinnibrugh TL, Fernandes MA, Van slambrouck S, Steelant WFA, Shuster CB, Rogelj S, van Otterlo WAL, Kornienko A. J Med Chem. 2011;54:4234. doi: 10.1021/jm200410r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Magedov IV, Manpadi M, Evdokimov NM, Elias EM, Rozhkova E, Ogasawara MA, Bettale JD, Przeval’skii NM, Rogelj S, Kornienko A. Bioorg Med Chem Lett. 2007;17:3872. doi: 10.1016/j.bmcl.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Magedov IV, Manpadi M, Ogasawara MA, Dhawan AS, Rogelj S, Van slambrouck S, Steelant WFA, Evdokimov NM, Uglinskii PY, Elias EM, Knee EJ, Tongwa P, Antipin MY, Kornienko A. J Med Chem. 2008;51:2561. doi: 10.1021/jm701499n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong Y-SC, Anderson LA, Ortega AJ, Van slambrouck S, Steelant WFA, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Kornienko A. Org Biomol Chem. 2007;5:3865. doi: 10.1039/b713820b. [DOI] [PubMed] [Google Scholar]; (g) Evdokimov NM, Van slambrouck S, Heffeter P, Tu L, Le Calve B, Lamoral-Theys D, Hooten CJ, Uglinskii PY, Rogelj S, Kiss R, Steelant WFA, Berger W, Bologa CJ, Yang JJ, Kornienko A, Magedov IV. J Med Chem. 2011;54:2012. doi: 10.1021/jm1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Soria-Mercado I, Prieto-Davo A, Jensen P, Fenical W. J Nat Prod. 2005;68:904. doi: 10.1021/np058011z. [DOI] [PubMed] [Google Scholar]; (b) Siripong P, Kanokmedakul K, Piyaviriyagul S, Yahuafai J, Chanpai R, Ruchirawat S, Oku N. J Trad Med. 2006;23:166. [Google Scholar]; (c) Hussain H, Krohn K, Uddin Ahmad VU, Miana GA, Greend IR. Arkivoc. 2007;2:145. [Google Scholar]; (d) da Silva AJM, Netto CD, Pacienza-Lima W, Torres-Santos EC, Rossi-Bergmann B, Maurel S, Valentin A, Costa PRR. J Braz Chem Soc. 2009;20:176. [Google Scholar]; (e) Jiinnez-Alonso S, Orellana HC, Estevez-Braun A, Ravelo AG, Perez-Sacau E, Machin F. J Med Chem. 2008;51:6761. doi: 10.1021/jm800499x. [DOI] [PubMed] [Google Scholar]
- 4.Bentle MS, Bey EA, Dong Y, Reinicke KE, Boothman DE. J Mol Histol. 2006;37:203. doi: 10.1007/s10735-006-9043-8. [DOI] [PubMed] [Google Scholar]
- 5.Khodeir M, El-Taweel F, Elagamey A. Pharmazie. 1992;47:486. [Google Scholar]
- 6.As this work was in progress a number of researchers reported the development of this multicomponent reaction, see: Mobinikhaledi A, Foroughifar N, Fard MAB. Synth Commun. 2011;41:441.Khurana JM, Nand B, Saluja P. Tetrahedron. 2010;66:5637.Shaabani A, Ghadari R, Ghasemi S, Pedarpour M, Rezayan AH, Sarvary A, Ng SW. J Comb Chem. 2009;11:956. doi: 10.1021/cc900101w.Yao C, Yu C, Li T, Tu S. Chin J Chem. 1989;2009:27.Yu Y, Guo H, Li X. J Heterocyclic Chem. 2011;48:1264.
- 7.General procedure for the synthesis of pyranonaphthoquinones 1–23: To a solution of selected aldehyde (1.0 mmol) and malononitrile (1.0 mmol) in 4 mL of ethanol was added Et3N (0.01 mmol) dropwise at room temperature. The resulting mixture was heated to 50 °C and stirred for 5 min followed by the addition of hydroxynaphthoquinone (1.0 mmol). The reaction mixture was refluxed for 2–3 h and then allowed to cool to room temperature. The formed precipitate (a structurally similar analogue can be added to facilitate crystallization if no precipitation occurs) was isolated by filtration and the filtrate was concentrated under reduced pressure. To the dark residue was added methanol (2 mL), which resulted in the crystallization of an additional 5–10% of the product. The solids were combined and recrystallized from methanol (3 mL) to yield a corresponding pure naphthoquinone.
- 8.Selected characterization data: 2-amino-5,10-dioxo-4-(3-phenoxyphenyl)-5,10-dihydro-4H-benzo[g]chromene-3-carbonitrile (1). 82%; 1H NMR (DMSO-d6) δ 8.06 (dd, J = 2.3, 3.6 Hz, 1H), 7.91 (m, 1H), 7.86 (m, 2H), 7.35 (m, 4H), 7.31 (dd, J = 8.9, 9.0 Hz, 1H), 7.14 (d, J = 7.5 Hz, 1H), 7.09 (d, J = 7.5 Hz, 1H), 7.04 (s, 1H), 7.00 (d, J = 7.2 Hz, 2H), 6.80 (d, J = 8.0 Hz, 1H), 4.63 (s, 1H); 13C NMR (DMSO-d6) δ 183.1, 177.3, 159.0, 157.3, 156.9, 149.6, 146.3, 135.2, 131.6, 131.2, 130.8, 130.6, 130.5, 126.7, 126.5, 124.1, 123.2, 122.3, 119.7, 119.2, 118.6, 117.3, 58.0, 37.1; HRMS m/z (ESI) calcd for C26H16N2O4Na (M+Na)+ 443.1008, found 443.1009.
- 9.Mosmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 10.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger CJ. Immunol Methods. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Sacau E, Diaz-Peñate RG, Estévez-Braun A, Ravelo AG, García-Castellano JM, Pardo L, Campillo M. J Med Chem. 2007;50:696. doi: 10.1021/jm060849b. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lefranc F, Brotchi J, Kiss R. J Clin Oncol. 2005;23:2411. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]; (b) La Porta CA. Curr Med Chem. 2007;14:387. doi: 10.2174/092986707779941078. [DOI] [PubMed] [Google Scholar]; (c) D’Amico TA, Harpole DH., Jr Chest Surg Clin N Am. 2000;10:451. [PubMed] [Google Scholar]; (d) Fenell DA. Clin Cancer Res. 2005;11:2097. doi: 10.1158/1078-0432.CCR-04-1482. [DOI] [PubMed] [Google Scholar]