Abstract
Adipose-derived stem cells (ASCs) can alleviate acute kidney injury and promote kidney cell regeneration and repair. To investigate the role of ASCs in diabetic nephropathy (DN), Sprague-Dawley rats were made diabetic by intraperitoneal injection of streptozotocin (STZ) after uninephrectomy. After 12 weeks, proteinuria was well established. Five times of 5×106 human ASCs repeatedly injected through a tail vein at 4 weekly intervals. A reduction in proteinuria was not observed in diabetic rats until 24 weeks. However, urinary protein excretion was significantly suppressed at 28 weeks and persisted up to 32 weeks after STZ treatment. ASC treatment significantly attenuated glomerulus hypertrophy and tubular interstitial injury, and led to the downregulation of WT-1 and synaptopodin expression. CFSE labeled ASCs were injected into DN rats via the tail vein. Within 24 h after injection, the cells were detected in lung, spleen, and peritubular regions, but rarely in pancreas. Human Alu gene expression was detected in lung and spleen up to 4 weeks after ASCs injection. ASC treatment did not improve hyperglycemia or pancreatic damage. In vitro, recombinant human glial cell line–derived neurotrophic factor (GDNF) prevented podocyte injury by high glucose similarly to ASC-conditioned medium. After blocking GDNF in ASC-CM with neutralizing antibody, the therapeutic effect of ASC-CM was significantly decreased. ASCs cocultured with podocytes restored the downregulation of synaptopodin expression, which was weakened by GDNF-RNA interfering. These findings indicate that repeated intravenous ASC can reduce diabetic kidney damage in rats even at the progressive stage, and promote podocyte recovery via GDNF secretion.
Introduction
More than 371 million people had diabetes mellitus (DM) in 2012, a number expected to rise to 552 million by 2030, which corresponds to a global prevalence of 9.9% [1]. China has one of the largest numbers of people suffering from diabetes. The estimated age-standardized prevalence of total diabetes was 9.7% among adults in China according to a national study from June 2007 through May 2008 [2]. About 25% to 40% of patients with DM will develop diabetic nephropathy (DN), which represents the leading cause of end-stage renal disease worldwide [3]. Unfortunately, once patients develop overt proteinuria, there is no cure for DN. Therefore, the availability of a strategy aimed at delaying or reversing DN is highly desirable [4–7].
Multipotent stem cells within adipose tissue, termed adipose-derived stem cells (ASCs), are one of the most promising stem cell populations identified thus far. ASCs are of increasing interest to biologists and clinicians because of their potential to differentiate into adipogenic, osteogenic, chondrogenic, and other cell types of mesenchymal lineage, in addition to possessing other clinically useful properties [8–10]. Since human adipose tissue is easily obtained in large quantities with little donor site morbidity or patient discomfort, the use of autologous ASCs as cellular therapeutics is feasible and has been shown to be safe and efficacious in preclinical and clinical studies of injury and disease [11–14].
While data supporting the contribution of mesenchymal stem cells (MSCs) in the management of renal lesions in diabetic individuals has been generated in several animal models of DN [15–18], few reports explored the mechanisms behind renoprotection. Due to the low numbers of donor cells found in recipient kidneys, it is expected that mechanisms different from cell differentiation are relevant. DN is typically chronic and progressive. If the therapeutic effect of MSCs involves endocrine effects or inflammatory regulation, one-dose MSC treatment may not achieve the therapeutic goal. We hypothesized that ASCs protect against kidney damage in DM rats via an endocrine role; however, this effect requires repeated injections of stem cells. In this study, we assessed the therapeutic effects of ASCs on type 1 DN in a rat model and investigated the underlying mechanism.
Materials and Methods
Isolation, expansion and labeling of ASCs
Human adipose tissue was obtained from patients undergoing tumescent liposuction according to procedures approved by the Ethics Committee at the Chinese Academy of Medical Sciences and Peking Union Medical College. The isolation procedure was reported by Li [19]. After isolation, 30 mL of resuspended cells were seeded in expansion medium at a density of 5×106 nucleated cells/100-mm tissue culture dish, and incubated at 37°C in a humidified environment containing 5% CO2. The expansion medium contained 58% Dulbecco's modified Eagle's medium/Ham's F-12 medium (DF-12 medium; Gibco, Life Technologies), 40% medium complete with trace elements-201 (MCDB; Sigma), 2% fetal calf serum (FCS; Gibco), 1×insulin–transferrin–selenium (ITS; Gibco), 1×10−9 M dexamethasone (Sigma), 1×10−4 M ascorbic acid 2-phosphate (Sigma), 10 ng/mL epidermal growth factor (EGF), 10 ng/mL platelet-derived growth factor-BB (PDGF-BB; Sigma), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). The third to fifth passages of human ASCs were used. The immunologic phenotypes of hAD-MSCs were positive for CD29, CD44, CD105, Flk-1, and negative for CD31, CD34, CD45, and HLA-DR, as described before [20]. Works of our laboratory have demonstrated multilineage plasticity potential of the hAD-MSCs, and their immune regulatory effect and low immunogenicity [21,22]. For fluorescence labeling of ASCs before in vivo injection, cells were labeled using carboxyfluorescein succinimidyl ester (CFSE; Sigma).
Transfection of ASCs with human glial cell line-derived neurotrophic factor small interfering RNA
When ASCs reached 50% confluence, they were transfected with 20 nM human glial cell line-derived neurotrophic factor (hGDNF) small interfering RNA (siRNA) or a siRNA-negative control (Shanghai GenePharma) using INTERFERinTM (Polyplus-transfection SA, Bioparc). Twenty-four hours after the start of transfection, ASCs were fed with serum-free medium for 24 h. To confirm GDNF knockdown, RNA was extracted from cells and assayed for GDNF by reverse transcriptase polymerase chain reaction (RT-PCR; GDNF forward primer: TGGGCTATGAAACCAAGGAG, reverse primer: CAACATGCCTGCCCTACTTT; product length=143 bp; SBS Genetech). Conditioned medium (CM) was also collected, concentrated and assayed for GDNF by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Inc.).
Cell culture
A mouse podocyte clone 5 (MPC5) which was provided by Professor Peter Mundel was cultured as described by Susztak et al. [23]. Briefly, podocytes were cultured and amplified in 11 mM D-glucose Roswell Park Memorial Institute (RPMI)-1640 medium containing 20 units/mL interferon (IFN)-γ (Invitrogen) and 10% FCS at 33°C. After passage at 37°C, the podocytes were culture in IFN-γ-free medium for 10 days to induce differentiation. The cells were cultured synchronously in medium containing 0.2% FCS and 5.5 mM D-glucose RPMI-1640 medium for 24 h before the experiment. After 10 days, MPC5 cells were seeded at a density of 5×104 cells/well in six-well plates. The podocytes were divided into six groups according to treatment: normal glucose (5.5 mM), high glucose (30 mM), ASC-CM treatment group (4×), human embryonic lung fibroblast cell line (WI38)-CM treatment group (4×), recombinant human GDNF (rhGDNF, 3 ng/mL) treatment group, and ASC-CM neutralizing antibody (ASC-CM+NtAb) group.
WI38 was purchased from the American Type Culture Collection (ATCC). The cells were cultured in RPMI-1640 medium containing 10% FCS and passaged when confluence reached 80%.
Preparation of CM
Eighty percent confluent ASCs and WI-38 cells were washed three times with phosphate buffered saline (PBS; ZSGB-Bio), and then fed with serum-free medium for 24 h. Then the culture supernatants were collected. The supernatants were centrifuged at 1500 rpm for 10 min at 4°C and transferred to a centrifugal column with a 3 kDa cut-off (Millipore).
Coculture protocol
After 10 days, podocytes were seeded at a density of 5×104 cells/well in six-well transwell plates (3-μm pore-size membrane insert; Corning). The cells were treated with glucose (Sigma). Human ASCs were cultured in the upper chamber of the coculture system for 2 days. Human ASCs were divided into three groups according to treatment: ASCs coculture group, hGDNF siRNA transfected ASCs coculture group, and siRNA-negative control group. All the cells were maintained and incubated at 37°C in humidified 5% CO2 in air.
Experimental animals and cell transplantation
Adult male Sprague-Dawley (SD) rats (2 months old, 160–200 g) were used in this study. Rats were housed at 20–24°C with relative air humidity at 40%–70%, and under a 12/12-h light/dark cycle. All animals had free access to tap water. All animal handling and experimental procedures were approved by the Animal Care and Use Committee of the Chinese PLA General Hospital (Permit Number: 09123).
All rats were subjected to right nephrectomy under sodium pentobarbital anesthesia (45 mg/kg, i.p.) to hasten the development of diabetic microglomerulopathy, and were rendered diabetic 2 weeks later by streptozotocin (STZ; 65 mg/kg, i.v.; Sigma). Four weeks after the STZ injection, the rats were fasted for 6 h. Blood glucose (BG) level from the tail vein sample was determined using a One-Touch Basic BG monitoring system to confirm STZ injection-induced hyperglycemia. Diabetic rats received daily injections of neutral protamine Hagedorn (NPH) insulin, in individually adjusted doses (ranging from two to four units), to maintain BG levels between 16.7 and 33.3 mM during the study (Fig. 1).
FIG. 1.
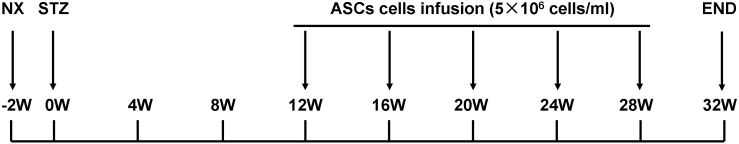
Experimental design. All rats were subjected to right nephrectomy to hasten the development of diabetic microglomerulopathy, and were rendered diabetic 2 weeks later by streptozotocin (STZ) 65 mg/kg, i.p. Twelve weeks after STZ injection (to allow the development of overt proteinuria), diabetic (DN) rats were randomly divided into two groups: DN rats treated with physiologic saline (DN, n=11) and DN rats treated with adipose-derived stem cells (DN+ASCs, n=11). Rats that received right nephrectomy without STZ were used as the nephrectomy control group (NX, n=8). Rats were weighed and blood glucose levels were measured every 4 weeks. Urine samples were collected over a 24-h period in individual metabolic cages to measure protein in urine every 4 weeks, using the Bradford method. At the end of the study, 32 weeks after STZ injection, rats were anesthetized with pentobarbital, 50 mg/kg, i.p., and blood samples were drawn from the abdominal aorta for serum creatinine determination.
Twelve weeks after STZ injection (to allow for the development of overt proteinuria), DN rats were randomly divided into two groups: DN rats treated with physiologic saline (PhyS) (DN, n=11) and DN rats treated with ASCs (DN+ASC, n=11). DN rats received 5×106 ASCs in 1.0 mL PhyS via tail vein injection every 4 weeks in the treatment groups. Rats that received right nephrectomy without STZ injection were used as the nephrectomy control group (NX, n=8). Rats were weighed and their BG levels were measured every 4 weeks. Also every 4 weeks, urine samples were collected over a 24-h period in individual metabolic cages to measure creatinine clearance and urine protein content, which was determined using the Bradford method. At the end of the study, 32 weeks after STZ injection, rats were anesthetized with pentobarbital, 50 mg/kg i.p., and blood samples were drawn from the abdominal aorta to determine serum creatinine levels.
Histology and immunohistochemistry
The kidney and pancreas were fixed in 10% formalin for 24 h for routine dehydration and paraffin embedding. Renal tissue coronal sections (2 μm thick) were stained with periodic acid-Schiff (PAS) stain, and were examined by light microscopy in a blinded fashion. Mean glomerular area measurements were acquired by digital image analysis with the Image-Pro Plus (version 5.0) software system (Media Cybernetics, Inc.). Thirty glomeruli were randomly selected for morphometric analysis. The glomerular area, defined as the cross-sectional area of the renal corpuscle bounded by Bowman's capsule, was determined by manually outlining Bowman's capsule on the image screen with the cursor, and the area was automatically calculated by the computer. The index of tubulointerstitial fibrosis was assessed in 10 different Masson's trichrome stained sections using a light microscope on a scale of 0 to 4 (grade 0, normal; grade 1, affected area <10%; grade 2, affected area 10%–25%; grade 3, affected area 25%–75%; grade 4, affected area greater than 75%) [24]. The Wilms tumor protein-1 (WT-1) was assessed by immunohistochemistry using a rabbit polyclonal anti-WT-1 antibody (Santa Cruz Biotechnology).
Immunofluorescence staining
Fresh-harvested kidney tissue was snap-frozen in Optimal Cutting Temperature compound and stored at −80°C until use. Frozen tissue was sectioned at a 4-μm thickness and placed on poly-L-lysine precoated slides. Sections were fixed in paraformaldehyde for 10 min, washed three times with PBS, and then blocked with 1% bovine serum albumin for 15 min at room temperature. A rabbit polyclonal primary antibody specific for synaptopodin (1:100; Santa Cruz Biotechnology) was added and tissue was incubated overnight at 4°C. After washing with PBS, secondary antibodies conjugated with FITC or Cy3 (Jackson ImmunoResearch Laboratories) were applied for 1 h at room temperature in a darkened humidified chamber. Negative controls were not incubated with a primary antibody. Finally, the preparations were washed with PBS and mounted in fluorescent mounting medium with DAPI (ZSGB-Bio). Each tissue section was observed under a confocal laser scanning microscope (Olympus FluoView 1000) at a magnification of 400×.
Differentiated and mature podocytes were seeded onto six-well plates. Plates covered by a cover glass that was pretreated with collagen I (Sigma). The cells were cultured at 37°C for 48 h, respectively. The cells were fixed with 4% paraformaldehyde for 5 min at room temperature followed by incubation at 4°C for 10 min. Then, 0.2% Triton X-100 was added for 5 min at room temperature to permeabilize the cells. 1×casin was sealed up for 30 min at room temperature. A primary rabbit polyclonal antibody specific for synaptopodin (Santa Cruz Biotechnology) was diluted 1:50, added to the cells, and incubated overnight at 4°C. A secondary anti-rabbit antibody conjugated to Cy3 (1:2,000; Jackson ImmunoResearch Laboratories) was incubated with the cells away from light for 1 h at room temperature. Finally, a fluorescent sealing liquid (ZSGB-BIO) containing DAPI was added, and confocal laser scanning microscopy (FluoView FV1000; Olympus America, Inc.) was used to determine the expression level and structural arrangement of podocytic synaptopodin. Ten visual fields were observed at random in each culture well.
Western blotting
Rat kidney tissue lysate was prepared by homogenization in RIPA buffer composed of 50 mM Tris–HCl (pH 7.6), 5 mM EDTA, 150 mM NaCl, 0.5% NP-40, 0.5% Triton-X 100, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 2 μg/mL antipain, 1 mM sodium orthovanadate, and 0.5 mM phenylmethylsulfonyl fluoride. Tissue debris was removed by centrifugation at 12,000 g for 20 min at 4°C. Protein concentration was measured using the Bradford assay. A total of 100 μg protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to a membrane, which was then blocked with 5% skim milk, probed with a primary antibody at 4°C overnight, and then incubated with a horseradish peroxidase-conjugated secondary antibody. Antibodies for synaptopodin and β-actin were from Santa Cruz Biotechnology (Santa Cruz Biotechnology). Immunoreactive bands were visualized by means of enhanced chemiluminescence.
Detection of human cells by PCR
To determine if hASCs were present in rat organs, DNA was extracted from rat tissues using a TIANamp Genomic DNA Kit (Tiangen Biotech) according to the manufacturer's protocol and amplified by PCR using primers specific for human Alu repeat sequences (forward, CTGGGCGACAGAACGAGATTCTAT; reverse, CTCACTACTTGGGTGACAGGTTCA; 224 bp, 54°C), and GAPDH (forward, TATTGGGCGCCTGGTCACCA; reverse, TAGTGGGGTCTCGCTCCTGGAAG, 186 bp, 58°C). DNA extracted from hASCs and rat MSCs served as controls.
ELISA of the cytokine content of CM
Quantification of GDNF in CM was determined by ELISA according to the manufacturer's instructions. Absorbance was measured at 450 nm using a microplate reader (MULTISKAN MK3; Thermo Fisher Scientific). GDNF concentration was determined with a standard curve constructed by titrating a GDNF standard.
Statistical analysis
All data are expressed as means±standard deviations (SD). Continuous variables between groups at each time point were compared using a one-way analysis of variance (ANOVA) followed by the least significant difference test when the ANOVA P<0.05. Differences between two time points were determined by independent samples t-tests. Statistical analyses were conducted with SPSS ver. 13.0 for Windows (SPSS). Significance was defined as P<0.05.
Results
Repeated intravenous administration of hASCs attenuated overt proteinuria
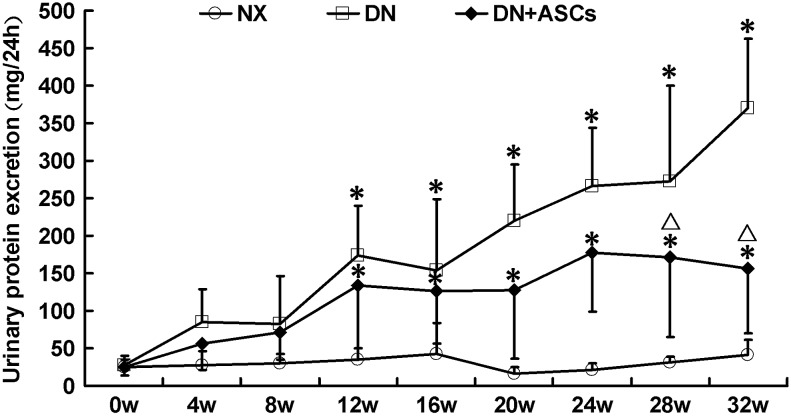
Excretion of urinary protein was markedly increased in diabetic rats 12 weeks after intraperitoneal injection of STZ as compared with right nephrectomy rats. The first dose of ASCs (5×106 cells/1 mL physiological saline) was then administered via tail vein injection and repeated every 4 weeks. There was no significant reduction in urinary protein excretion at 16, 20 or 24 weeks after three ASC doses as compared with the untreated group. Proteinuria in untreated rats rose steadily until 32 weeks, while ASC treatment significantly decreased urinary protein excretion from 28 to 32 weeks (369.4±193.7 mg/24 h vs. 156.1±85.9 mg/24 h, P<0.05; Fig. 2). The serum creatinine, blood urea nitrogen, creatinine clearance, albumin, cholesterol, and triglyceride levels in the experimental animals are shown in Table 1. Diabetic rats developed hypoalbuminemia and hyperlipidemia at 32 weeks after STZ administration. Compared with untreated DN rats, ASC treatment protected the renal function and reduced the serum cholesterol level. Although serum albumin and triglyceride levels also improved, the differences did not reach statistical significance.
FIG. 2.
Therapeutic effects of human adipose-derived stem cells (hASCs) on urine protein excretion in diabetic rats. Proteinuria was well established at 12 weeks after intraperitoneal injection of STZ. Reduction of proteinuria was not observed in diabetic rats until 24 weeks after three doses of ASCs. Beginning at 28 weeks, urinary protein excretion was significantly suppressed, which persisted up to 32 weeks after STZ injection. Data are means±standard deviations (SD; n=8–9). *P<0.05 versus NX; ▵P<0.05 versus DN.
Table 1.
Clinical and Biochemical Parameters in Each Experimental Group
| NX | DN | DN+ASCs | |
|---|---|---|---|
| Body weight(g) | 656.4±64.0 | 360.6±57.9a | 381.4±57.6a |
| Kidney weight(g) | 2.7±0.2 | 4.5±1.3a | 3.4±0.8a,b |
| Kidney/body weight(%) | 0.4±0.1 | 1.1±0.4a | 0.9±0.2a,b |
| Serum albumin level (g/L) | 36.5±2.3 | 29.7±8.5a | 32.1±5.6 |
| Serum creatinine (μM) | 39.0±2.0 | 36.9±7.0 | 27.2±3.7a,b |
| Blood urea nitrogen (mM) | 7.4±0.6 | 13.5±3.2a | 8.2±1.4b |
| Creatinine clearance (mL/min) | 1.4±0.3 | 1.1±0.2a | 1.4±0.2b |
| Serum triglyceride level (mM) | 1.6±0.7 | 3.1±1.5a | 2.1±1.4 |
| Serum cholesterol level (mM) | 2.2±0.3 | 3.4±0.8a | 2.4±0.7b |
Data are means±SD (n=8–9). NX=Rats received uninephrectomy, DN=STZ-induced diabetic rat with NX, DN+ASCs=DN rats treated with human adipose-derived stem cells.
P<0.05 versus NX group.
P<0.05 versus DN group.
ASCs, adipose-derived stem cells; NX, nephrectomy control; DN, diabetic nephropathy.
ASC treatment reduced glomerular hypertrophy and renal tubular interstitial injury
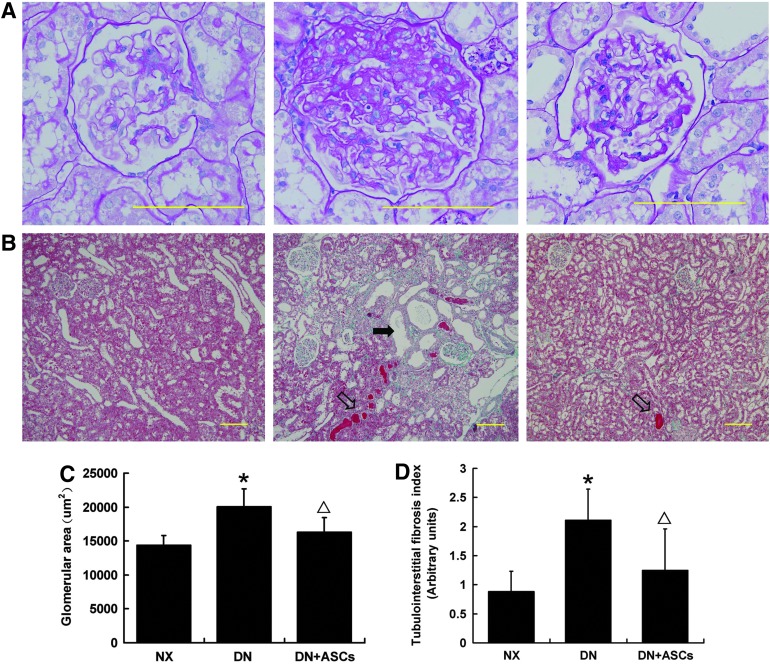
A marked increase in left kidney weight was observed in DM rats as compared with controls. Less pronounced renal hypertrophy was noted in the ASC-treated group (Table 1). Histological examination revealed glomerular enlargement, moderate mesangial proliferation, and matrix expansion in diabetic rats (Fig. 3A). Quantitative examination of PAS-stained sections revealed significantly increased glomerular volume in the DN group as compared with control rats. The glomerular area was significantly decreased in ASC-treated DN rats (Fig. 3C). The diabetic rats also showed focal dilatation of tubules and the presence of protein casts in the distal segments of the nephron. Focal tubule atrophy and fibrosis of the interstitium was often seen in the untreated group. In contrast, control animals and ASC-treated diabetic rats had only minimal disruption of the tubules and interstitium (Fig. 3B, D).
FIG. 3.
Effects of ASC treatment on glomerular hypertrophy. Periodic acid-Schiff (PAS) staining of kidney sections was used to evaluate pathologic changes. (A) Representative glomeruli. Original magnification, 400×. (B) Masson's trichrome-stained sections of the renal cortex. The diabetic rats showed focal dilatation of tubules (solid arrow) and the presence of protein casts (hollow arrow). Focal tubule atrophy and fibrosis of the interstitium was often seen in the untreated group. ASC-treated diabetic rats had only minimal disruption of the tubules and interstitium. Original magnification, 100×. (C) Quantitative analysis of glomerular area was performed in the kidney. The glomerular area was significantly less in the ASC-treated group, indicating that ASC treatment promoted regression of mesangial expansion. (D) Tubuloin-terstitial fibrosis index (TIFI). The diabetic rats exhibited increase in the degree of TIFI, which was inhibited by ASC treatment. Data are means±SD (n=8–9). *P<0.05 versus NX; ▵P<0.05 versus DN.
ASC treatment attenuated diabetic podocyte injury
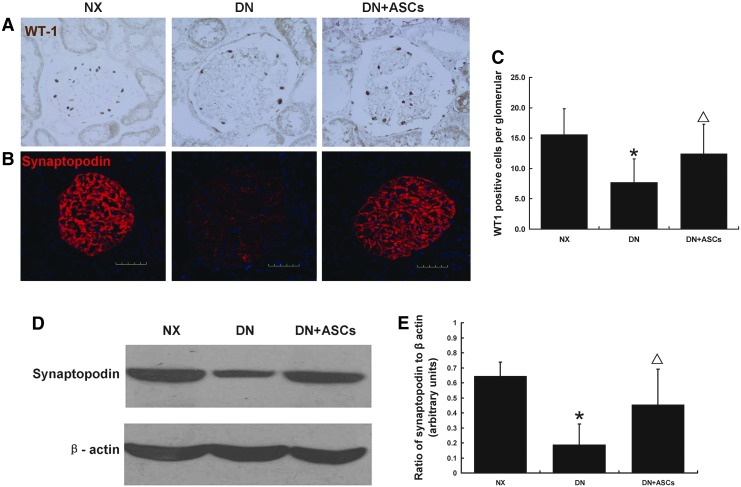
To identify the mechanisms underlying the ASC treatment-induced renoprotection, podocyte injury markers WT1, and synaptopodin were investigated in diabetic rats. Nuclear staining of the podocyte marker WT1, a transcription factor involved in podocyte differentiation and maintenance of the mature phenotype, showed a reduction in WT-1-positive nuclei per glomerulus in diabetic rats as compared to controls. This decrease was inhibited by ASC treatment (Fig. 4A, C). Immunofluorescence staining for synaptopodin protein in control and diabetic rats at 32 weeks after DM induction revealed a significant decrease in glomerular synaptopodin expression in diabetic rats as compared with controls. The decrease in synaptopodin expression in diabetic glomeruli was markedly ameliorated by ASC treatment (Fig. 4B). This result was confirmed by western blotting (Fig. 4D, E).
FIG. 4.
Effects of ASC treatment on podocyte injury in diabetic rats. Nuclear staining for the podocyte marker WT1, a transcription factor involved in podocyte differentiation and maintenance of a mature podocyte phenotype, in control and diabetic rats showed a reduction in WT-1-positive nuclei number per glomerulus in DN rats; this decrease was inhibited by ASC treatment (A, C). Immunofluorescent staining for synaptopodin protein in nephrectomy control (NX), DN, and DN+ASCs groups at 32 weeks after diabetes induction revealed a significant decrease in glomerular synaptopodin expression in DM rats. The decrease in synaptopodin expression in diabetic glomeruli was ameliorated by ASC treatment (B). This result was confirmed by western blotting (D, E). Original magnification, 400×. Data are means±SD (n=8–9). *P<0.05 versus NX; ▵P<0.05 versus DN.
ASC localization
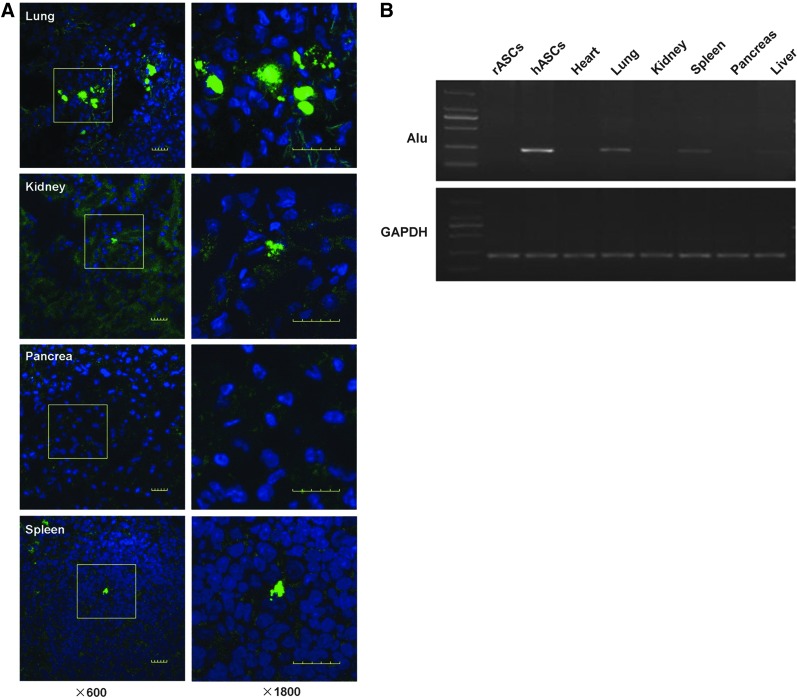
To evaluate the engraftment of CFSE-labeled hASCs after tail vein injection into DN rats, cells were detected in frozen samples by confocal laser microscopy. Imaging was performed at 4 h, 24 h, 72 h, and 7 days after injection of labeled cells. Within 24 h after injection, signals were detected in lung, peritubular regions, and spleen, but rarely in glomeruli and pancreas (Fig. 5A). Four or 32 weeks after STZ injection, tissues from the hASC-treated diabetic rats were assayed for engraftment by real-time PCR for human Alu sequences. Human Alu gene expression was detected in rat lung and spleen until 28 days after ASCs treatment (Fig. 5B). Human DNA was also detected in the rat lung and spleen at 32 weeks after STZ injection. Human Alu sequences were not detected in the kidney, heart, liver, or pancreas at either 28 days or 32 weeks.
FIG. 5.
ASC localization. ASCs were labeled with CFSE (green). ASCs (5×106 cells/1 mL saline) were infused into the tail vein 12 weeks after intraperitoneal injection of STZ. Animals were sacrificed after 24 h, 48 h, 72 h, 1 week and 4 weeks. CFSE-labeled MSCs (green) were detected by laser confocal microscopy. The cells were detected in the lung, peritubular regions, and the spleen, but rarely in the pancreas within 24 h after injection. Original magnification, 600× and high resolution for the insets, 1800× (A). Reverse transcriptase polymerase chain reaction results of heart, lung, kidney, spleen, pancreas, and liver samples of diabetic rats that received ASC injection showed detection of human Alu gene expression in rat lung and spleen up to 4 weeks after ASCs injection (B).
ASC-secreted GDNF was involved in podocyte injury reduction
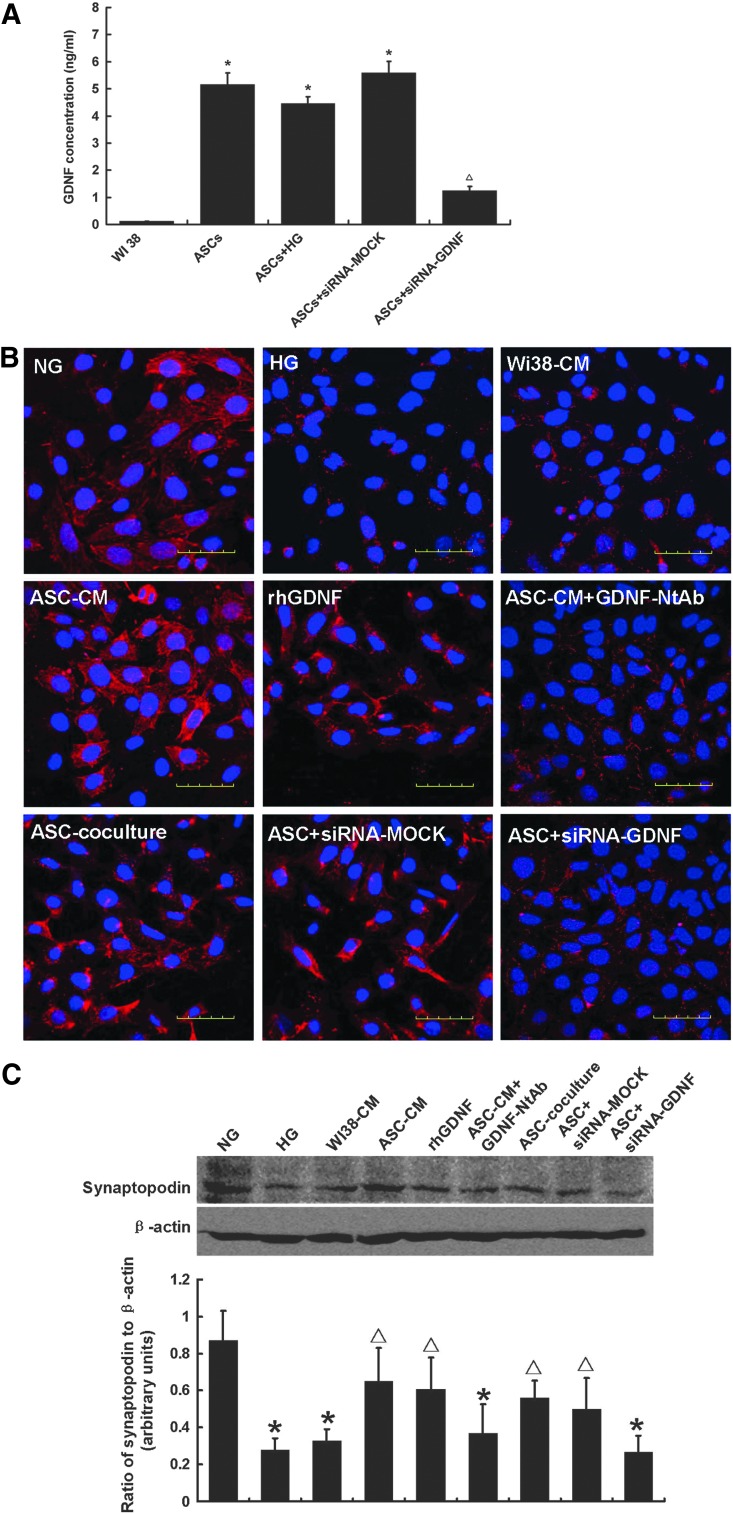
In a preliminary experiment, we evaluated cytokines released by ASCs after 24 h serum-free culture using antibody-based protein array analysis and found that a series of growth factors were released, including FGF2, EGF, and GDNF (data not shown). GDNF concentration was measured in culture supernatant by ELISA. The GDNF level in the ASC group was 5.16±0.43 ng/mL, 44.6-fold the amount released from WI-38 cells (as a negative control). We then tested whether high glucose treatment decreased the GDNF secretion in ASCs. When ASCs were exposed to high glucose (HG; 30 mM), the culture supernatant GDNF level was 4.46±0.25 ng/mL, and there was no significant difference between the two groups (Fig. 6A). To evaluate the role of GDNF secreted by ASCs in reducing podocyte injury, we cultured MPC5 in the presence of HG plus ASC-CM, ASC-CM with neutralizing antibody to block the effects of GDNF, or recombinant human GDNF one-component culture medium. The expression of podocytic synaptopodin was reduced and the localizations differed among the high glucose group. ASC-CM and rhGDNF one-component culture medium groups showed a significant up-regulation of synaptopodin expression. After blocking GDNF in ASC-CM with NtAb, the therapeutic effect of ASC-CM was partly abolished. To further assess the role of GDNF secreted by ASCs in the reduction of podocyte injury, we knocked down GDNF expression in ASCs by transient transfection with GDNF-siRNA. ASCs cocultured with podocytes injured by high glucose treatment significantly restored the down-regulation of synaptopodin expression. There was a significant reduction in podocytic synaptopodin expression in the GDNF-siRNA group compared with negative control group (Fig. 6B). This result was confirmed by western blotting (Fig. 6C).
FIG. 6.
Glial cell line-derived neurotrophic growth factor (GDNF) secreted by hASCs ameliorated podocyte injury. (A) GDNF concentration was measured in conditioned hASCs culture media (CM) by enzyme-linked immunosorbent assays (ELISA). The results revealed that 24-h ASC-CM contained a 44.6-fold increased GDNF level as compared with CM from WI38 cells. High glucose did not affect GDNF secretion. Three independent samples were measured in triplicate. *P<0.05 as compared with WI38-CM; ▵P<0.05 as compared with ASC-CM. The expression and location of the podocytic cytoskeletal protein synaptopodin (red) were evaluated by confocal microscopy (B). Nuclei were stained with DAPI (blue). The expression of podocytic synaptopodin in the high-glucose group was reduced and rearranged as compared with normal glucose group. ASC-CM and recombinant human GDNF (rhGDNF) rescued the synaptopodin downregulation, but WI38-CM, neutralizing antibody of GDNF (GDNF-NtAb) did not. To further assess the role of GDNF secreted by ASCs in the reduction of podocyte injury, GDNF expression in ASCs was knocked down by transient transfection with GDNF-siRNA. The GDNF siRNA knockdown efficiency was approximately 67.6% (A). ASCs cocultured with podocytes injured by high glucose treatment significantly restored the down-regulation of synaptopodin expression. There was a significant reduction in podocytic synaptopodin expression in the GDNF-siRNA group as compared with negative control group. Original magnification, 600×. These results were confirmed by western blotting (C). Data are means±SD (n=3–5). *P<0.05 versus NG; ▵P<0.05 versus HG.
No improvement in endocrine pancreas function in hASC-treated DN rats
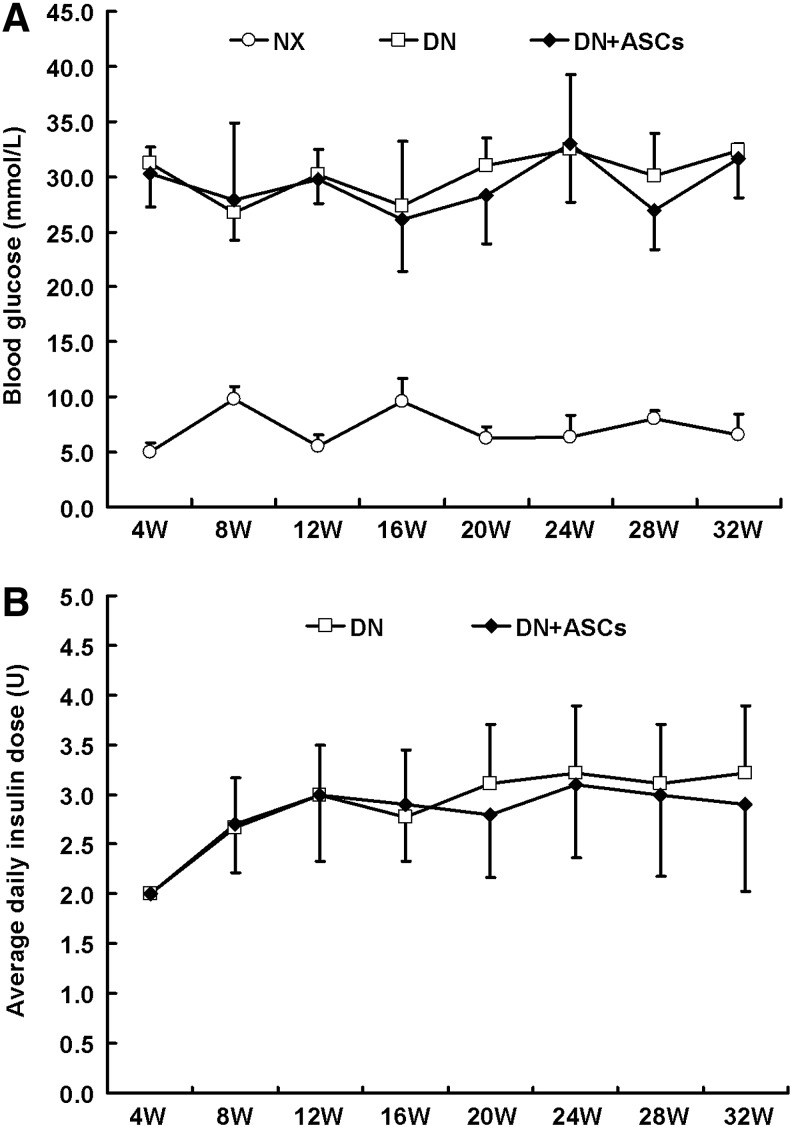
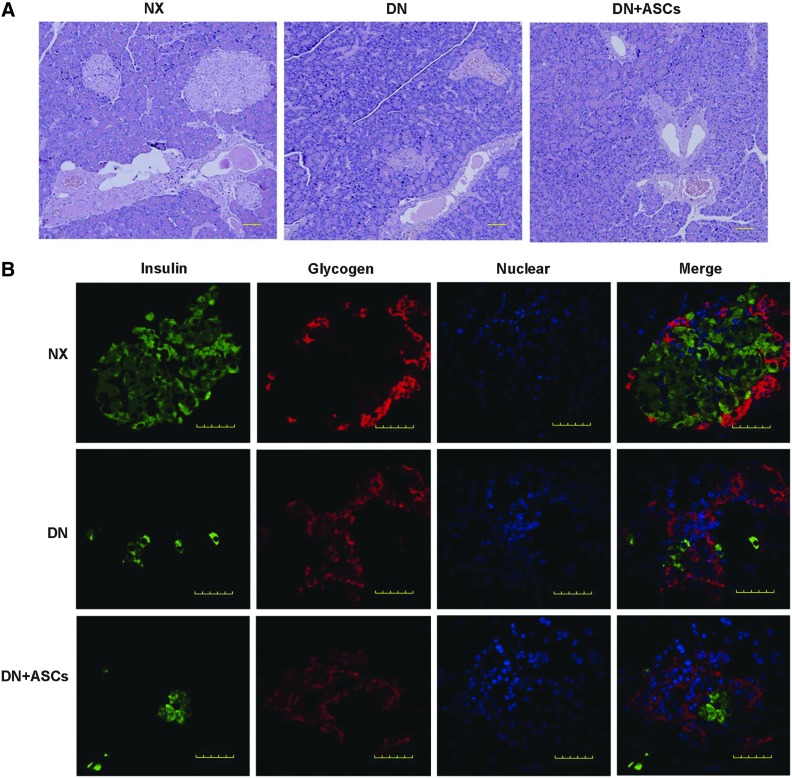
BG was significantly higher in both the DN group and the DN+ASC group as compared with the control group (P<0.05). There was no significant difference in BG between the DN group and the DN+ASC group (Fig. 7A). Also, the insulin injection doses were not significantly different between the DN group and the DN+ASC group (Fig. 7B). The pancreas was removed from rats in all three groups for histopathological evaluation. Pancreas H&E staining in diabetic rats showed that the majority of pancreatic islets were damaged. The remaining islets were small and had abnormal architecture. No significant improvement was observed in the transplantation group (Fig. 8A). β-pancreatic islets evaluated by immunofluorescence staining showed a significant reduction in islet β-cells and aberrant distribution of glucagon-positive cells in the pancreatic islets of diabetic rats. No obvious differences were found between the diabetic rats and the ASC-treated groups, implying that ASC treatment had no significant effects in STZ-induced diabetic rats (Fig. 8B).
FIG. 7.
Effects of ASC treatment on hyperglycemia. (A) Four weeks after the STZ injection, the rats were fasted for 6 h. Blood glucose (BG) level from the tail vein sample was determined using a One-Touch Basic blood glucose monitoring system to confirm STZ injection-induced hyperglycemia. (B) Diabetic rats received daily injections of neutral protamine Hagedorn (NPH) insulin ranging from two to four units to maintain blood glucose levels between 16.7 and 33.3 mM during the study. Blood glucose was significantly higher in the diabetic rats (DN) and DN+ASCs groups as compared to the control group (NX) (P<0.05). There were no significant differences in blood glucose or insulin dose between the DN and the DN+ASC groups.
FIG. 8.
Effects of ASC treatment on pancreatic injury. (A) Representative light micrographs of hematoxylin and eosin-stained pancreas sections taken from control rats or diabetic rats 32 weeks after STZ injection. Most pancreas islets were damaged in the diabetic rats. The remaining islets were small, with an abnormal architecture. Original magnification, 100×. (B) Double immunofluorescence staining for insulin (green) and glucagon (red) revealed few insulin-producing cells and aberrant distribution of glucagon-positive cells in the pancreatic islets in diabetic rats. ASC treatment did not improve STZ-induced pancreas lesions. Nuclei were visualized by DAPI staining (blue). Original magnification, 400×.
Discussion
The major findings of our study were as follows: (i) hASC administration in diabetic rats via tail vein injection led to decreased proteinuria even at the overt stage, but required repeated treatment; (ii) hASC transplantation inhibited glomerulus hypotrophy and tubular interstitium lesions; (iii) hASC transplantation reduced podocyte injury; (iv) GDNF secreted from ASCs might play an important role in the protection of synaptopodin expression; and (v) therapeutic effects were independent of blood sugar control.
Although MSCs may have therapeutic potential in several chronic kidney disease models [25–28], the underlying mechanism is still under debate. Besides the potential for multilineage differentiation, MSCs exert therapeutic effects through complex paracrine and endocrine actions [29]. Several reports showed that bone marrow stem cell transplantation reduces proteinuria and glomerular hypertrophy in diabetic mice and rats [14–17]. MSCs localized in glomeruli can express CD31, suggesting that stem cells that differentiate into endothelial cells may be involved in kidney repair [15]. MSCs can significantly reduce urine protein excretion and improve renal pathological changes, including glomerular sclerosis, mesangial proliferation, tubule dilatation, and protein casts [15]. However, a very small number of MSCs were detected in the kidney, which was not sufficient to explain kidney disease improvement. Our study showed that systemic administration of ASCs significantly reduced proteinuria in diabetic rats; however, no signal was detected in the tubules or glomeruli within 24 h after ASC injection, eliminating the possibility that ASCs homed to the injured kidney and differentiated into kidney lineage cells. Hence, our results do not support the theory that stem cell differentiation plays a major role in protection against diabetic kidney injury. Our preliminary study found that after rat tail vein injection of bone marrow MSCs, most stem cells presented in the lung and promoted the repair of peritoneal injury through secretion of TSG-6 [30]. We found human Alu expression in the lungs and spleen after 4 weeks, suggesting that rather than engraftment, secretion of growth factors by ASCs makes a major contribution to the therapeutic benefits of ASCs [31–33].
Administration dosage and frequency may be critical for paracrine effects of MSCs in the treatment of chronic disease, as well as in DN treatment. Weekly MSC administration, as opposed to a single dose, may significantly improve protective effects on remnant kidney injury [34]. In this study, proteinuria did not improve until 24 weeks after three ASC doses. Since 28 weeks (16 weeks after the injection of ASC), urinary protein excretion was significantly reduced and persisted up to 32 weeks. Further preclinical studies should assess the best dosage and frequency of ASC transplantation in individuals with DN.
Proteinuria is a manifestation of glomerular filtration barrier dysfunction. Podocytes, glomerular basement membrane, and glomerular endothelial cells constitute the glomerular filtration barrier [35–38]. Studies have suggested a relationship between the development of diabetic proteinuria and podocytic injury [39–41]. Dalla [42] found that in patients with DN, the urinary albumin excretion rate was inversely related to podocyte density and positively correlated with the width of the foot processes. Our results showed that ASC treatment decreased urine protein in diabetic rats, which might be related to the amelioration of podocyte injury. To verify this, podocyte markers were examined. The WT1 gene encodes the WT1 protein, which is highly expressed in podocytes and plays an important role in the maintenance of podocyte function [43]. Synaptopodin is essential for the integrity of the podocyte actin cytoskeleton and for the regulation of podocyte cell migration. It is also a marker of podocytic differentiation and maturation [44,45]. Recent studies of both patients and diabetic animal models reported down regulation of both WT1 and synaptopodin in DN, and so these lesions may be related to podocyte loss [23,46–47]. In the present study, levels of both proteins were decreased in diabetic rats at 32 weeks after DM, and ASC treatment reduced this damage. In vitro studies showed that high glucose leads to reduced synaptopodin in podocytes. ASC coculture markedly prevented downregulation of the podocyte cytoskeletal protein. Our results suggest that stem cells improve podocyte injury by endocrine mechanisms.
We further determined the specific soluble factors responsible for the effects of ASCs on podocyte injury. We previously explored the profile of cytokines released by 24 h serum-starved ASCs using antibody-based protein array analysis and found that a variety of growth factors were released, including FGF2, EGF, and GDNF. GDNF, a member of the transforming growth factor beta (TGF-β) family, is essential for neuronal survival, as well as development of renal morphology [48,49]. GDNF is an inducible factor that promotes ureteric bud generation and is secreted by renal mesenchyme during kidney development. GDNF is also a podocyte survival factor. Both in vitro and in vivo studies showed that the GDNF receptor, RET mRNA and protein levels are significantly upregulated after podocyte injury. Exogenous GDNF inhibits podocyte apoptosis through the PI3-K/AKT pathway [50,51]. GDNF can also increase the regenerative potential of stem cells themselves. GDNF preconditioning can improve islet survival and function [52]. GDNF pretreatment significantly enhances human amniotic fluid stem cell renewal potential. GDNF increased human amniotic fluid stem cell production of growth factors, motility, and expression of receptors involved in cell homing and survival [53]. Our in vitro study showed that ASCs secreted high levels of GDNF in comparison with control cells, and a high-sugar environment did not affect the ability of ASCs to secrete GDNF. GDNF application can reduce cytoskeletal damage, and this protective role was eliminated by a neutralizing antibody. We knocked down GDNF expression in ASCs using GDNF siRNA. GDNF-siRNA ASCs failed to protect podocytes from high-glucose injury. Our data demonstrated that GDNF secretion by ASCs might play an important role in promoting the repair of podocyte injury induced by high glucose.
Several studies showed that MSC treatment could alleviate pancreatic lesions and lower blood sugar. Lee [15] induced diabetes in NOD/SCID mice with low repeated doses of STZ (35 mg/kg, daily for 4 days). MSCs were injected into the left cardiac ventricle. Three percent of the infused hMSCs engrafted into the pancreas, which increased the number of islets and insulin-producing cells, improved hyperglycemia and increased blood levels of mouse insulin in the diabetic mice. Ezquer [16] reported that bone marrow MSC treatment could significantly lower blood sugar and alleviate pancreas lesions in diabetic mice after low-dose STZ injection (40 mg/kg, for 5 days). Ho [54] found that MSC treatment could restore BG in diabetic mice. Liver engraftment and differentiation into insulin-producing cells accounted for the therapeutic effects. In our study, ASC therapy had no significant effect on the BG levels of diabetic rats. A possible explanation is that ASCs affect islet destruction by an immune-mediated pathway. MSCs did not reduce blood sugar levels in mice when diabetes was induced with high-dose (200 mg/kg) STZ injection [17]. In addition, ASC treatment at a relatively later stage at which islet damage becomes irreversible may explain ineffective ASC treatment. Our data showed that the vast majority of islets were destroyed 32 weeks after STZ injection. Furth more, human Alu sequences were not detected in the liver, which excludes the possibility of ASC differentiation into insulin-producing cells after engrafting into liver tissues. However, this result indicates that the role of stem cells in the protection of diabetic renal damage is independent of glycemic control.
In summary, our findings indicate that repeated systemic administration of human ASCs attenuated DN, in a manner independent of an improvement in blood sugar level. GDNF secreted by ASCs might play an important role in the amelioration of diabetic podocyte injury.
Acknowledgments
This research was supported by a grant from the National Basic Research Program of China (2011CB964904), the Fund of Chinese PLA 12th Five-Year Plan for Medical Sciences (BWS11J027), and a grant from the National Basic Research Program of China (2011CB944000).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.IDF Diabetes Atlas Update. 2012. www.idf.org/diabetesatlas/5e/Update2012 www.idf.org/diabetesatlas/5e/Update2012
- 2.Yang W. Lu J. Weng J. Jia W. Ji L. Xiao J. Shan Z. Liu J. Tian H, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE. Sicree RA. Zimmet PZ. Global estimates of the prevalence of diabetes for 2010, 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME. DeFronzo RA. Franz MJ. Keane WF. Mogensen CE. Parving HH. Steffes MW American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 5.Reutens AT. Atkins RC. Epidemiology of diabetic nephropathy. Contrib Nephrol. 2011;170:1–7. doi: 10.1159/000324934. [DOI] [PubMed] [Google Scholar]
- 6.Ezquer ME. Ezquer FE. Arango-Rodríguez ML. Conget PA. MSC transplantation: a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res. 2012;45:289–296. doi: 10.4067/S0716-97602012000300010. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno H. Tobita M. Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 8.Locke M. Feisst V. Dunbar PR. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells. 2011;29:404–411. doi: 10.1002/stem.593. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Bendala J. Lanzoni G. Inverardi L. Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Wickham MQ. Erickson GR. Gimble JM. Vail TP. Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 12.Gimble JM. Katz AJ. Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobita M. Orbay H. Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–170. [PubMed] [Google Scholar]
- 14.Locke M. Windsor J. Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. Anz J Surg. 2009;79:235–244. doi: 10.1111/j.1445-2197.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee RH. Seo MJ. Reger RL. Spees JL. Pulin AA. Olson SD. Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezquer FE. Ezquer ME. Parrau DB. Carpio D. Yáñez AJ. Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Ezquer F. Ezquer M. Simon V. Pardo F. Yáñez A. Carpio D. Conget P. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15:1354–1365. doi: 10.1016/j.bbmt.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H. Tian HM. Long Y. Zhang XX. Zhong L. Deng L. Chen XH. Li XQ. Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J. 2009;122:2573–2579. [PubMed] [Google Scholar]
- 19.Li K. Han Q. Yan X. Liao L. Zhao RC. Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem Cells Dev. 2010;19:1267–1275. doi: 10.1089/scd.2009.0196. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 21.Shi D. Liao L. Zhang B. Liu R. Dou X. Li J. Zhu X. Yu L. Chen D. Zhao RC. Human adipose tissue-derived mesenchymal stem cells facilitate the immunosuppressive effect of cyclosporin A on T lymphocytes through Jagged-1-mediated inhibition of NF-κB signaling. Exp Hematol. 2011;39:214–224. doi: 10.1016/j.exphem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Deng W. Han Q. Liao L. You S. Deng H. Zhao RC. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24:458–463. doi: 10.1089/dna.2005.24.458. [DOI] [PubMed] [Google Scholar]
- 23.Susztak K. Raff AC. Schiffer M. Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 24.Mankhey RW. Bhatti F. Maric C. 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399–F405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ninichuk V. Gross O. Segerer S. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2007;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 26.Caldas HC. Fernandes IM. Gerbi F. Souza AC. Baptista MA. Ramalho HJ. Kawasaki-Oyama RS. Goloni-Bertollo EM. Pavarino-Bertelli EC. Braile DM. Abbud-Filho M. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–855. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Choi S. Park M. Kim J. Hwang S. Park S. Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells. 2009;Dev18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre CS. Volpini RA. Shimizu MH, et al. Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27:682–692. doi: 10.1634/stemcells.2008-0496. [DOI] [PubMed] [Google Scholar]
- 29.Pino CJ. Humes HD. Stem cell technology for the treatment of acute and chronic renal failure. Transl Res. 2010;156:161–168. doi: 10.1016/j.trsl.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N. Li Q. Zhang L. Lin H. Hu J. Li D. Shi S. Cui S. Zhou J, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012;7:e43768. doi: 10.1371/journal.pone.0043768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meirelles Lda S. Fontes AM. Covas DT. Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Mias C. Lairez O. Trouche E. Roncalli J. Calise D, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 33.Yoon BS. Moon JH. Jun EK. Kim J. Maeng I, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 34.Lee SR. Lee SH. Moon JY. Park JY. Lee D. Lim SJ. Jeong KH. Park JK. Lee TW. Ihm CG. Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail. 2010;32:840–848. doi: 10.3109/0886022X.2010.494803. [DOI] [PubMed] [Google Scholar]
- 35.Thomas MC. Pathogenesis and progression of proteinuria. Contrib Nephrol. 2011;170:48–56. doi: 10.1159/000324943. [DOI] [PubMed] [Google Scholar]
- 36.Sakoda M. Itoh H. Ichihara A. Podocytes as a target of prorenin in diabetes. Curr Diabetes Rev. 2011;7:17–21. doi: 10.2174/157339911794273955. .32. [DOI] [PubMed] [Google Scholar]
- 37.White KE. Research into the glomerular podocyte-is it relevant to diabetic nephropathy? Diabet Med. 2006;23:715–719. doi: 10.1111/j.1464-5491.2006.01790.x. [DOI] [PubMed] [Google Scholar]
- 38.Durvasula RV. Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 39.Yanagida-Asanuma E. Asanuma K. Kim K. Donnelly M. Young Choi H. Hyung Chang J. Suetsugu S. Tomino Y. Takenawa T. Faul C. Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53: mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross ML. Dikow R. Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int. 2005;94:S50–S53. doi: 10.1111/j.1523-1755.2005.09412.x. [DOI] [PubMed] [Google Scholar]
- 41.Saleem MA. Biology of the human podocyte. Nephron Exp Nephrol. 2003;95:e87–e92. doi: 10.1159/000074324. [DOI] [PubMed] [Google Scholar]
- 42.Dalla Vestra M. Arboit M. Bruseghin M. Fioretto P. Ihalmo P. Wessman M. Kaunisto MA. Association analysis of podocyte slit diaphragm genes as candidates for diabetic nephropathy. Diabetologia. 2008;51:86–90. doi: 10.1007/s00125-007-0854-2. [DOI] [PubMed] [Google Scholar]
- 43.Srichai MB. Konieczkowski M. Padiyar A. Konieczkowski DJ. Mukherjee A. Hayden PS. Kamat S. El-Meanawy MA. Khan S, et al. A WT1 coregulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem. 2004;279:14398–14408. doi: 10.1074/jbc.M314155200. [DOI] [PubMed] [Google Scholar]
- 44.Kawachi H. Miyauchi N. Suzuki K. Han GD. Orikasa M. Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton) 2006;11:274–281. doi: 10.1111/j.1440-1797.2006.00583.x. [DOI] [PubMed] [Google Scholar]
- 45.Asanuma K. Yanagida-Asanuma E. Faul C. Tomino Y. Kim K. Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 46.Menini S. Iacobini C. Oddi G. Ricci C. Simonelli P. Fallucca S. Grattarola M. Pugliese F. Pesce C. Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007;50:2591–2599. doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- 47.Miyauchi M. Toyoda M. Kobayashi K. Abe M. Kobayashi T. Kato M. Yamamoto N. Kimura M. Umezono T. Suzuki D. Hypertrophy and loss of podocytes in diabetic nephropathy. Intern Med. 2009;48:1615–1620. doi: 10.2169/internalmedicine.48.2137. [DOI] [PubMed] [Google Scholar]
- 48.Costantini F. GDNF/Ret signaling and renal branching morphogenesis: from mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6:252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costantini F. Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 50.Tsui CC. Shankland SJ. Pierchala BA. Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J Am Soc Nephrol. 2006;17:1543–1552. doi: 10.1681/ASN.2005080835. [DOI] [PubMed] [Google Scholar]
- 51.Huang ZY. Hong LQ. Na N. Luo Y. Miao B. Chen J. Infusion of mesenchymal stem cells overexpressing GDNF ameliorates renal function in nephrotoxic serum nephritis. Cell Biochem Funct. 2012;30:139–144. doi: 10.1002/cbf.1827. [DOI] [PubMed] [Google Scholar]
- 52.Shi H. Patschan D. Dietz GP. Bähr M. Plotkin M. Goligorsky MS. Glial cell line-derived neurotrophic growth factor increases motility and survival of cultured mesenchymal stem cells and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F229–F235. doi: 10.1152/ajprenal.00386.2007. [DOI] [PubMed] [Google Scholar]
- 53.Rota C. Imberti B. Pozzobon M. Piccoli M. De Coppi P. Atala A. Gagliardini E. Xinaris C. Benedetti VM, et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21:1911–1923. doi: 10.1089/scd.2011.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho JH. Tseng TC. Ma WH. Ong WK. Chen YF. Chen MH. Lin MW. Hong CY. Lee OK. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and β-cell differentiation in streptozocin-induced diabetic mice. Cell Transplant. 2012;21:997–1009. doi: 10.3727/096368911X603611. [DOI] [PubMed] [Google Scholar]