Abstract
COMFORT-I is a randomized, double-blind, placebo-controlled trial of the Janus kinase 1/Janus kinase 2 inhibitor ruxolitinib in 309 patients with intermediate-2 or high-risk myelofibrosis. This analysis of COMFORT-I describes the long-term efficacy and safety of ruxolitinib (median follow-up, 2 years). Spleen volume was measured by magnetic resonance imaging, and quality of life was evaluated using the EORTC QLQ-C30. Overall survival was determined according to randomized treatment group. At the time of this analysis, 100 of 155 patients randomized to ruxolitinib were still receiving treatment. All patients randomized to placebo crossed over to ruxolitinib or discontinued within 3 months of the primary analysis (median time to crossover, 41 weeks). Mean spleen volume reductions in the ruxolitinib group were 31.6% at week 24 and 34.9% at week 96; improvements in quality of life measures were also maintained. Improved survival was observed for ruxolitinib (n=27 deaths) versus placebo (n=41 deaths) (hazard ratio=0.58; 95% confidence interval: 0.36, 0.95; P=0.03). The incidence of new-onset grade 3 or 4 anemia and thrombocytopenia decreased over time to levels observed in patients receiving placebo. These data indicate that ruxolitinib treatment provides durable reductions in spleen volume and improvements in quality of life and suggest a continued survival advantage for ruxolitinib over placebo.
Introduction
Myeloproliferative neoplasms include primary myelofibrosis, as well as polycythemia vera and essential thrombocythemia – both of which can progress to myelofibrosis (i.e. post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis).1,2 Patients with myelofibrosis often present with splenomegaly, burdensome symptoms (e.g. night sweats, fever, fatigue, bone pain and pruritus) and cytopenias.3,4
The pathogenesis of myelofibrosis is linked, in part, to overactive signaling of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, resulting from various mechanisms and mutations. Although the most common and most recognized mutation in myelofibrosis is JAK2V617F, this mutation is not required for the disease or overactive JAK-STAT activity. Some patients have other mutations that activate JAK-STAT signaling (such as MPL and mutations in exon 12 of JAK2), and it is thought that as yet unidentified mechanisms are responsible for JAK-STAT activation in the remaining patients. Elevated levels of pro-inflammatory cytokines (signaling through JAK1 and JAK2) are a consistent feature in patients with myelofibrosis.5,6 Consequently, the development of agents that inhibit the JAK-STAT pathway has been a focus of current research in myelofibrosis.
Until recently, no approved treatments were available for myelofibrosis and, with the exception of allogeneic stem cell transplantation, treatment involved non-specific management of symptoms and signs with limited benefit.2 Ruxolitinib is an oral JAK1 and JAK2 inhibitor approved by the USA Food and Drug Administration for the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis. Approval was based in part on the results of two phase III clinical trials – the COntrolled MyeloFibrosis Study with ORal JAK Inhibitor Treatment (COMFORT)-I (www.clinicaltrials.gov NCT00952289)7 and COMFORT-II (www.clinicaltrials.gov NCT00934544).8 These studies compared ruxolitinib treatment with placebo and best available therapy, respectively, and both achieved their primary endpoint: significantly more patients receiving ruxolitinib experienced a ≥35% reduction in spleen volume from baseline as measured by magnetic resonance imaging or computed tomography at week 24 in COMFORT-I and at week 48 in COMFORT-II. In addition, ruxolitinib was superior to placebo and best available therapy in improving myelofibrosis-related symptoms and measures of quality of life. Improved survival for patients treated with ruxolitinib over placebo was also observed at a median follow-up of 51 weeks [hazard ratio (HR)=0.50; 95% confidence interval (CI): 0.25, 0.98; P=0.04).7 The objective of the current analysis was to describe the longer-term outcomes associated with ruxolitinib treatment in COMFORT-I, with 1 year of additional follow-up beyond previously published data.
Methods
Patients
The inclusion and exclusion criteria for COMFORT-1 have been described elsewhere.7 Briefly, eligible patients were ≥18 years of age and had primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis according to the 2008 World Health Organization criteria1 and intermediate-2 or high-risk myelofibrosis as determined by the International Prognostic Scoring System.3
The protocol was approved by the institutional review board at each participating site. The study was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice. All patients provided written informed consent. Data were collected by the investigators and analyzed by the sponsor, Incyte Corporation. All authors had access to the data.
Study design
Patients were randomized 1:1 to receive ruxolitinib or placebo orally twice daily (bid). Ruxolitinib starting doses were determined according to baseline platelet count: for patients with a baseline platelet between 100×109/L and 200×109/L, the starting dose of ruxolitinib was 15 mg bid; whereas for patients with a baseline platelet count >200×109/L, the starting dose of ruxolitinib was 20 mg bid. Doses were individualized during the study to ensure safety and enhance efficacy.7
Patients receiving placebo were eligible for crossover to ruxolitinib during the primary analysis period based on specific criteria as previously described.7 All patients were eligible for crossover following completion of the primary analysis, when all patients had completed 24 weeks and at least half had completed 36 weeks of randomized treatment, at which time the study was unblinded.
Evaluations
Imaging (magnetic resonance imaging or computed tomography) to assess spleen volume was performed at baseline and in weeks 12, 24, 36, 48, 60 and 72, and every 24 weeks thereafter.7 The myelofibrosis symptom burden was measured daily up to week 24 with the modified Myelofibrosis Symptom Assessment Form version 2.0 electronic diary. Quality of life was evaluated with the self-administered European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) at baseline and at each study visit.7 Adverse events were reported using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
The data cut-off for this analysis of the ongoing COMFORT-I study was March 1, 2012 (1 year after a prospectively defined safety follow-up). Kaplan-Meier analysis was used to evaluate the durability of the spleen response and to assess overall survival (intention-to-treat analysis). The Cox proportional hazards model was used to calculate hazard ratios and 95% confidence intervals and the log-rank test to determine P values (unadjusted for repeat analyses).
Percentage changes in spleen volume from baseline to weeks 24 and 48 and percentage change in total symptom score from baseline to week 24 were evaluated by titrated dose, which was the average dose in the last 12 weeks prior to the assessment: <10 mg bid (average total daily dose ≤15 mg), 10 mg bid (>15–25 mg), 15 mg bid (>25–35 mg), 20 mg bid (>35–45 mg) and >20 mg bid (>45 mg).
Percentage changes from baseline in hemoglobin, platelet count and the proportion of patients who received red blood cell (RBC) transfusions during the previous 4 weeks were assessed. The incidence of worsening grade 3 and grade 4 anemia and thrombocytopenia, as defined by laboratory values, was assessed at 6-month intervals.
Results
Patients
The patients’ baseline characteristics have been reported previously; all were similar between randomized groups with the exception of age (median age: ruxolitinib group, 66 years; placebo group, 70 years; P<0.05).7 The patients’ disposition is shown in Online Supplementary Figure S1. At the time of this analysis (median follow-up, 102 weeks), 100 of the 155 (64.5%) patients originally randomized to receive ruxolitinib were still receiving treatment, 111 of the 154 (72.1%) patients originally randomized to receive placebo had crossed over to ruxolitinib, and no patient was receiving placebo. The median follow-up for the 100 patients in the ruxolitinib arm who were still on treatment was 103 weeks (range, 94–126 weeks), and 90% had a follow-up of more than 96 weeks. All patients randomized to receive placebo crossed over to ruxolitinib or discontinued the study within 3 months of the primary analysis, with a median time to crossover of 41.1 weeks. The discontinuation rate in the placebo group was 16.3% in the first 6 months. In the ruxolitinib group, rates of discontinuation decreased over time (10.3% in the first 6 months and 6.0% in the last 6 months of the 2-year follow-up).
Starting and titrated doses
Most ruxolitinib dose adjustments were implemented because of changes in platelet counts and occurred early in the course of treatment. Seventy percent of patients had at least one dose adjustment (increase or decrease) in the first 12 weeks of ruxolitinib therapy; the majority of patients had a dose reduction (52%) within this time frame. By week 24, patients initiating ruxolitinib at doses of 15 mg bid (n=55) and 20 mg bid (n=100) were titrated to a mean dose of ~10 and 15–20 mg bid, respectively (median doses, 10 mg and 20 mg bid), at which time mean doses began to stabilize (Figure 1).
Figure 1.
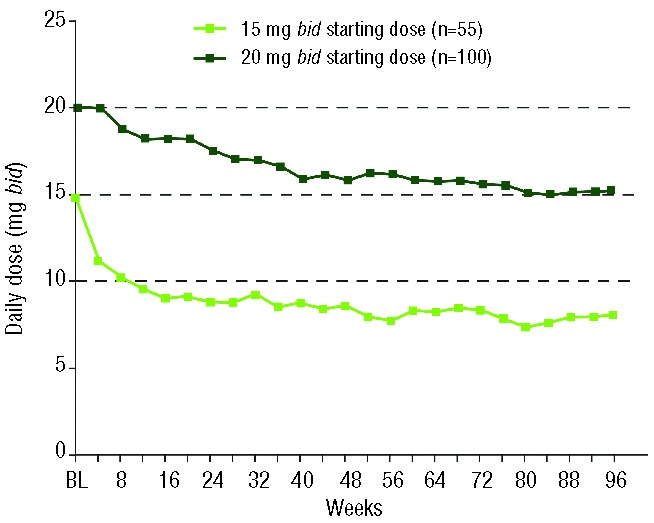
Mean daily dose of ruxolitinib over time in all patients randomized to ruxolitinib and by initial ruxolitinib dose; bid: twice daily; BL; baseline.
Efficacy
Mean spleen volume reduction in patients randomized to receive ruxolitinib observed at week 24 was maintained over time: 31.6% reduction at week 24 and 34.9% at week 96. Among the ruxolitinib-treated patients who achieved at least a 35% reduction in spleen volume at some point during the study [90/155 patients; 58%), 64% maintained this response for at least 2 years (Figure 2A). Most patients (>80%) who reached the threshold of a 35% reduction maintained at least a 10% reduction in spleen volume throughout the follow-up period – a reduction that was associated with clinically meaningful improvements in myelofibrosis-related symptoms and quality of life measures.9 Consistent with this observation, improvements in functional and symptom subscales of the EORTC QLQ-C30 observed at week 24 were also maintained with long-term ruxolitinib therapy (Online Supplementary Figure S2). The Total Symptom Score was not collected beyond week 24.
Figure 2.
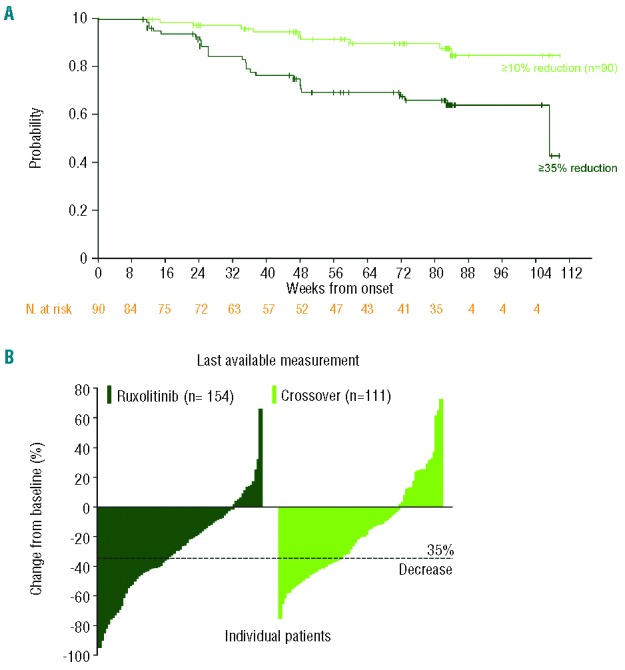
Durability of spleen volume reduction and individual percentage changes from baseline with ruxolitinib therapy. (A) Kaplan-Meier curve of durability of spleen volume reduction. In patients maintaining at least a 35% reduction in spleen volume (dark green line), duration of response was defined as the time from first 35% reduction to less than 35% reduction and 25% increase from nadir. Among patients achieving a 35% reduction in spleen volume, most patients maintained at least a 10% reduction from baseline (light green line), with duration defined as the time from first 35% reduction to less than 10% reduction from baseline. (B) Percentage change in spleen volume in individual patients from original baseline to last available spleen volume measurement in the ruxolitinib group (median follow-up, 24 months) and placebo group after crossover to ruxolitinib treatment (median follow-up after crossover, 14 months).
At the time of their last assessment during the follow-up period, most patients in the ruxolitinib group had some reduction in spleen volume from baseline (Figure 2B; mean reduction, 27.5%).7 Patients who crossed over from placebo to ruxolitinib (median follow-up on ruxolitinib after crossover, ~14 months) also experienced similar percentage reductions in spleen volume from the time of crossover to last assessment (mean, 30.0%); however, because patients in the crossover group experienced spleen growth prior to receipt of ruxolitinib, spleen volume changes relative to their original baseline values were not as robust (mean reduction, 18.0%).
Because the majority of patients had some adjustment of their ruxolitinib dose early in the course of therapy (mainly in the first 8–12 weeks), the changes in spleen volume and Total Symptom Score were evaluated by titrated dose at week 24. Changes in spleen volume by titrated dose were also assessed at week 48. Patients titrated to doses of 10 mg bid and higher experienced similar improvements in myelofibrosis-related symptoms, whereas those titrated to doses of ≥15 mg bid (or continued on their starting dose) had moderately greater improvements in spleen volume than those titrated to 10 mg bid (Figure 3).
Figure 3.
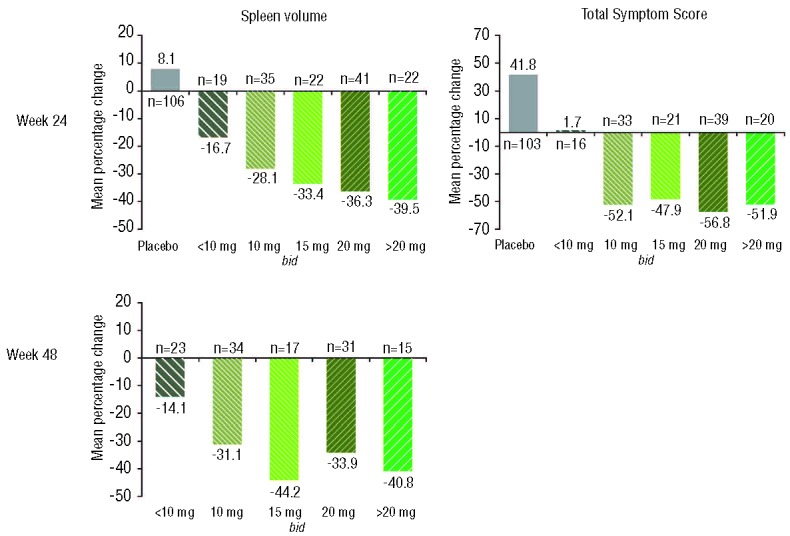
Mean percentage changes from baseline in spleen volume at weeks 24 and 48 and mean percentage changes in Total Symptom Score by titrated dose. Titrated dose was defined as the average dose patients received in the last 12 weeks before the time of assessment; bid: twice daily.
Survival
At the time of this analysis, 27 deaths were reported in the ruxolitinib group and 41 in the placebo group. The causes of death are listed in the Online Supplementary Table S1. Consistent with the overall survival analysis reported previously (median follow-up, 51 weeks),7 ruxolitinib treatment continued to be associated with an overall survival advantage relative to placebo with 1 additional year of follow-up (HR=0.58; 95% CI: 0.36, 0.95; P=0.03) (Figure 4). The 1- and 2-year survival probabilities for patients randomized to ruxolitinib were 92% (95% CI: 87%, 95%) and 82% (95% CI: 75%, 88%), respectively. In contrast, the 1- and 2-year survival probabilities for patients randomized to placebo (including those who crossed over to ruxolitinib) were 85% (95% CI: 79%, 90%) and 73% (95% CI: 65%, 80%), respectively. Because baseline age differed between the two treatment arms (median age: ruxolitinib arm, 66 years; placebo arm, 70 years), an age-adjusted survival analysis was also performed. The results (HR=0.61; 95% CI: 0.37, 0.99; P=0.04) were similar to those of the unadjusted analysis.
Figure 4.
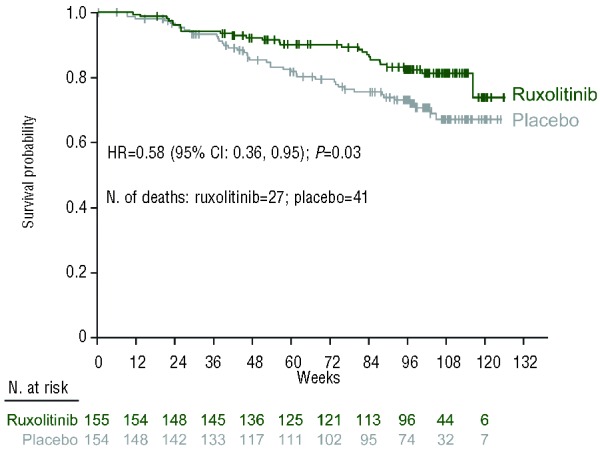
Overall survival by randomized treatment group (intent-to-treat population). CI: confidence interval; HR: hazard ratio.
Safety
Dose-dependent anemia and thrombocytopenia were the most common adverse events in the ruxolitinib group, but these events rarely led to discontinuation of the treatment. At the time of the primary analysis, there was one discontinuation for each event in the ruxolitinib group and in the placebo group;7 in the subsequent follow-up during ruxolitinib treatment, there was one discontinuation for anemia (median exposure, 107 weeks). The incidence of new-onset grade 3 or 4 anemia and thrombocytopenia decreased over time (Figure 5) to levels observed with placebo treatment (prior to crossover).
Figure 5.

Incidence of new-onset grade 3 and 4 anemia and thrombocytopenia over time. The incidence of anemia and thrombocytopenia after 6 months was summarized only for patients originally randomized to receive ruxolitinib because all patients receiving placebo had either crossed over to ruxolitinib or discontinued from the study after the primary analysis. Incidence was calculated using the life table method based on the time to first worsening grade 3 or 4 event censored at the time of discontinuation or data cutoff (earlier of the two); the effective sample size was used as the denominator.
Mean platelet counts decreased over the first 8–12 weeks of the study and remained relatively stable over the course of long-term therapy (Figure 6A). Mean hemoglobin values reached a nadir of 10–12% below baseline between weeks 8 and 12 and stabilized over time to a new steady state slightly below baseline by week 24, and then remained stable throughout the remaining follow-up (Figure 6B). This pattern of hemoglobin recovery to a new steady state over time was also observed in patients who did not receive RBC transfusions post-baseline (Online Supplementary Figure S3). Consistent with these changes in hemoglobin over time, the proportion of ruxolitinib-treated patients receiving RBC transfusions decreased to the level seen with placebo by week 36 and remained stable thereafter (Online Supplementary Figure S4).
Figure 6.
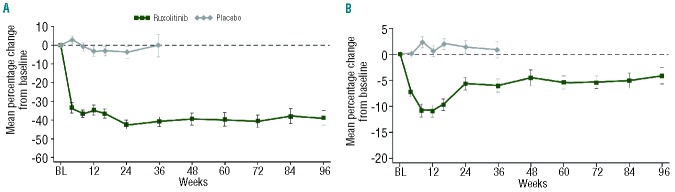
Mean percentage change (± standard error of the mean) in (A) platelet counts and (B) hemoglobin levels from baseline over time. BL: baseline.
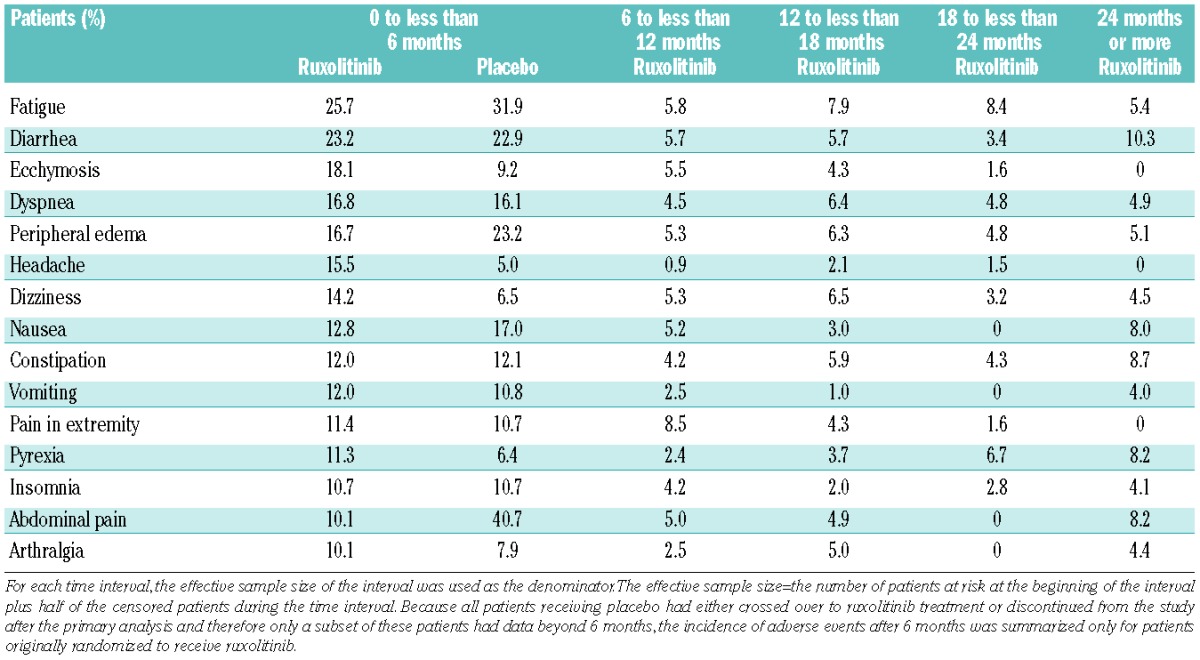
In the first 6 months of treatment, the most common non-hematologic adverse events that occurred more frequently in the ruxolitinib group than in the placebo group were ecchymosis, headache and dizziness (Table 1). In the ruxolitinib group, the incidence of new-onset non-hematologic adverse events in the subsequent 6-month intervals was lower than that observed in the initial 6-month interval. Most events were grade 1 or 2 (Online Supplementary Table S2).
Table 1.
Incidence of new-onset non-hematologic adverse events regardless of causality.
Two patients originally randomized to receive ruxolitinib developed acute myeloid leukemia (AML) at the time of the primary analysis, as described previously;7 no further cases of AML were reported in this group. Of the patients originally randomized to placebo, two developed AML. In one patient with a history of squamous cell carcinoma, bone marrow biopsy at the time of crossover showed 11% blasts and loss of chromosome 5 and deletion of 17p; this patient developed AML 21 days later. The second patient with a history of cervical cancer entered the study with abnormal cytogenetics and had a blast cell count of 4% prior to crossover; this patient developed AML 174 days after crossover.
As reported in the primary analysis, an evaluation of grade 3 and 4 adverse events and serious adverse events upon treatment discontinuation or interruption (including assessment of cardiac or respiratory events) showed no pattern of a withdrawal syndrome (Online Supplementary Tables S3 and S4).
Discussion
In this 2-year follow-up of COMFORT-I, ruxolitinib treatment was generally well tolerated in patients with myelofibrosis and provided durable and clinically meaningful reductions in spleen volume and improvements in quality of life. Furthermore, these data suggest a continued survival advantage for ruxolitinib over placebo. Similar findings were reported from a 2-year follow-up of patients in COMFORT-II, showing consistent reductions in spleen volume over time with ruxolitinib therapy and a survival benefit compared with best available therapy.10
Because all patients in the placebo group had discontinued or were receiving ruxolitinib at the time of this analysis, the COMFORT-I study provides insight into potential consequences of delayed ruxolitinib therapy. The analysis of overall survival evaluated patients by randomized treatment group regardless of crossover status. With a median time to crossover of 41 weeks, those originally randomized to receive placebo had shortened survival compared with those randomized to receive ruxolitinib (HR=0.58). Based on these data, patients with myelofibrosis who have symptoms or splenomegaly may benefit from earlier intervention with ruxolitinib.
Thrombopoietin and erythropoietin signal exclusively through JAK2 and, consequently, dose-dependent thrombocytopenia and anemia were expected with ruxolitinib treatment. These cytopenias were manageable with dose adjustments and RBC transfusions and rarely led to treatment discontinuation. Dose reductions were mandated for protocol-defined decreases in platelet counts, and most dose reductions occurred over the first 8–12 weeks of treatment. During this time frame, mean platelet counts declined and subsequently remained stable. Initial decreases in hemoglobin levels in the first 8–12 weeks recovered to near baseline levels, and RBC transfusion requirements followed a similar pattern. In a separate study of patients with myelofibrosis and baseline platelet counts 50×109/L – 100×109/L, a starting dose of 5 mg bid with subsequent titration to 10 mg bid resulted in stable platelet counts and mean hemoglobin values over time.11 It is, therefore, likely that dose reductions contributed to the recovery of hemoglobin values observed in COMFORT-I.
Mean titrated doses for patients in the 15 mg bid group (baseline platelet count 100×109/L – 200×109/L) and 20 mg bid group (baseline platelet count >200×109/L) were ~10 mg bid and 15–20 mg bid, respectively, by week 24 – a time at which most patients started to maintain a stable dose. At week 24, patients titrated to doses of 10 mg bid experienced improvements in symptoms similar to those receiving higher titrated doses, whereas spleen volume reductions were slightly less than those observed at higher doses. Titrated doses at <10 mg bid resulted in smaller improvements in spleen volume and symptoms but provided greater benefit than placebo.
Myelofibrosis is a progressive chronic myeloproliferative neoplasm that has a profound impact on the daily lives of patients. In the absence of a cure for myelofibrosis, some patients will require chronic therapy. The results of this long-term follow-up of patients in COMFORT-I underscore the importance of appropriate monitoring of patients and individualized dose adjustments, particularly early in the course of treatment, in order to achieve long-term benefits with ruxolitinib therapy. Consistent with earlier reports, these data reinforce the durable efficacy and tolerability of ruxolitinib in patients with myelofibrosis and suggest a continued survival advantage for patients treated with ruxolitinib over those given a placebo.
Acknowledgments
Medical writing assistance was provided by Stephanie Leinbach, PhD, whose work was funded by Incyte Corporation, and Kelly Reith of Incyte Corporation.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
COMFORT-I was supported by Incyte Corporation.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1): 14–22 [DOI] [PubMed] [Google Scholar]
- 2.Vannucchi AM. Management of myelofibrosis. Hematology Am Soc Hematol Educ Program. 2011;2011:222–30 [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13): 2895–901 [DOI] [PubMed] [Google Scholar]
- 4.Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1): 68–76 [DOI] [PubMed] [Google Scholar]
- 5.Vannucchi AM, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin. 2009;59(3): 171–91 [DOI] [PubMed] [Google Scholar]
- 6.Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10(2): 127–40 [DOI] [PubMed] [Google Scholar]
- 7.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366 (9): 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9): 787–98 [DOI] [PubMed] [Google Scholar]
- 9.Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AL, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31(10): 1285–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervantes F, Kiladjian JJ, Niederwieser D, Sirulnik A, Stalbovskaya V, McQuitty M, et al. Long-term safety, efficacy, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy (BAT) for the treatment of myelofibrosis (MF). Blood. 2012;120 Abstract 801 [Google Scholar]
- 11.Talpaz M, Paquette R, Afrin L, Hamburg SI, Prchal JT, Jamieson K, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol. 2013, 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]


