Abstract
Background
Minimally invasive mitral valve surgery has become a routine procedure at our institution. The present study analyzed the early and long-term outcomes of patients undergoing minimally invasive mitral valve surgery over the last decade, with special focus on mitral valve repairs (MVRp).
Methods
The preoperative variables, intraoperative data and postoperative outcomes of patients undergoing minimally invasive mitral valve surgery were prospectively collected in our database from May 1999 to December 2010. The survival and freedom from reoperation were evaluated with life tables and Kaplan-Meier analyses.
Results
A total of 3,438 patients underwent minimally invasive mitral valve surgery, of which 2,829 were MVRps and 609 were mitral valve replacements (MVR). Forty-five patients (1.6%) required MVR due to failure of repair. The mean age was 60.3±13 years. More than a third of patients underwent concomitant procedures like tricuspid valve surgery, atrial septal defect (ASD) closure and cryoablation. The rate of conversion to sternotomy was less than 1.4%. The 30-day mortality was 0.8%. The 5- and 10-year survival of all patients (MVR and MVRp) undergoing minimally invasive mitral valve surgery was 85.7±0.6% and 71.5±1.2%, respectively. For MVRp, the survival was 87.0±0.7% and 74.2±1.4% at 5 and 10 years, respectively. Freedom from reoperation was 96.6±0.4% and 92.9±0.9% at 5 and 10 years, respectively.
Conclusions
Minimally invasive MVRp can be performed safely and effectively with very few perioperative complications. The early and long-term outcomes in these patients are acceptable.
Keywords: Minimally invasive mitral valve surgery; short-, long-term outcomes; surgical technique; repair
Introduction
The enthusiasm to perform minimally invasive cardiac surgery emerged in the last decade of the twentieth century, due for the most part to the success of laparoscopic surgery and thoracoscopic procedures. The most remarkable innovation was the Port-access approach developed by the Stanford group, who proved that closed chest cardiopulmonary bypass (CPB) and cardioplegic arrest was safe and feasible and that mitral valve replacement (MVR) in a minimally invasive fashion was possible (1,2). Our group has expanded the use of this technique over the past fifteen years. Although the initial implementation of this technique was associated with a steep learning curve, resulting in a relatively higher complication rate than desired, repeated effective modifications in surgical techniques, equipment and instrumentation have enabled mitral valve surgery through a right small thoracotomy approach to become a routine procedure at our centre. Despite the fact that all conventional techniques of mitral valve repair (MVRp) can be performed with great precision through this small access, our group developed the so-called “loop technique” for correcting mitral valve prolapse in order to effectively simplify repair procedures, thus improving its reproducibility (3). Its results have proven to be comparable to those with the “gold standard leaflet-resection technique”. In fact, the loop technique results in a significantly longer line of leaflet coaptation and may therefore be more durable (4,5). Even complex repair procedures for severe bileaflet prolapse in patients with Barlow’s disease can be successfully performed through this approach (6). MVR using any of the commercially available prostheses can be performed with the same reliability in patients in whom the mitral valve is not amenable to repair. It is also a useful alternative for patients requiring a mitral valve procedure after a previous cardiac operation, particularly in patients with patent coronary bypass grafts or previous aortic valve replacement (7). At the present time this approach is being commonly used at many centres around the world with excellent short and long-term outcomes (8,9). It has also proven to be at least as good and safe as the standard sternotomy approach even in elderly patients (10). In the present series, the focus is chiefly on our experience with minimally invasive MVRp surgery through a right small thoracotomy approach over the last decade.
Methods
A total of 3,438 patients underwent minimally invasive mitral valve surgery through a right small thoracotomy approach at the Leipzig Heart Center between May 1999 and December 2010. Of these, 2,829 patients primarily underwent MVRp and 609 underwent MVR. Combined procedures performed in addition to mitral valve surgery included 390 tricuspid valve repairs (TVRp), 7 tricuspid valve replacements (TVR), 302 patent foramen ovale (PFO)/atrial septal defect (ASD) closures and 955 cryoablations for atrial fibrillation. The only patients who did not undergo surgery through a minimally invasive approach were those who had already received previous interventions through a right-sided thoracotomy, or required emergent surgery and the on-call-surgeon was not trained in minimally-invasive surgery, or had extensive mitral valve endocarditis requiring complex reconstructions. Clinical, operative and outcome data were prospectively collected in a computerized database. Our primary outcome was operative mortality, which was defined as death occurring within 30 days of the operation. The study was approved by our Institutional Ethics Committee. Being a retrospective study, individual patient informed consent was waived.
Surgical technique
The patient is intubated with a single lumen endotracheal tube. In patients weighing more than 75 kilograms and those requiring concomitant right-sided procedures like TVRp, TVR or ASD closures, an additional venous cannula is inserted percutaneously through the right internal jugular vein into the superior vena cava by the anaesthesiologist immediately after induction of anaesthesia. The patient is connected to CPB by cannulation of the femoral artery and vein (single venous cannula for isolated mitral valve procedures) through a 2 cm transverse incision in the groin. Transesophageal echocardiography (TEE) is mandatory to confirm the optimum location of the tip of the venous cannula in the right atrium. Body temperature is maintained around 34 °C and vacuum-assisted venous drainage is used throughout the procedure. A 5-6 cm right lateral mini-thoracotomy, just infero-lateral to the nipple in men and in the submammary crease in women, is used to enter the thorax through the fourth intercostal space (ICS). A dedicated instrument set designed for minimally invasive surgery is used to perform the operation (Geister Inc., Tuttlingen, Germany). A small thoracic and soft tissue retractor is utilized to spread the ribs. The pericardium is opened 3-4 cm anterior and parallel to the phrenic nerve from the distal ascending aorta to the diaphragm. A video camera and a transthoracic Chitwood aortic cross-clamp are inserted through 10 and 5 mm ports in the 2nd and 3rd right ICS, respectively. Two litres of antegrade crystalloid cardioplegia is delivered directly into the aortic root through a long cardioplegia needle and repeated after 90-120 minutes, if necessary. The mitral valve is accessed through a paraseptal incision and a left atrial retractor is used to expose the mitral valve. MVR is performed in a routine fashion using horizontal mattress pledgeted polyester sutures, with preservation of one or both leaflets. MVRp for degenerative mitral valve disease is most commonly performed utilising the Goretex neochordae by the “Loop technique”, the details of which have already been described previously (3). Assessment of the optimal length and precise fixation of neochordae to the papillary muscles and the free edge of the mitral leaflets are the fundamental aspects of this technique. A semi-rigid annuloplasty ring is implanted to support the repair. Mitral valve competency is restored in patients with Barlow’s disease, utilising a myriad of different techniques from leaflet resection to neochordae to Alfieri’s edge-to-edge repair. Ischemic MR is corrected utilising an undersized ring annuloplasty. Following the mitral valve procedure, the left atrium is de-aired by filling it with saline during closure. A direct closure of a PFO/ASD can be easily performed through the left atrial approach, however patch closure of the ASD, TVRp or TVR have to be accessed through the right atrium after establishing total CPB by clamping the superior and inferior vena cavae with large bull-dog clamps. TVRp or TVR can also be performed after releasing the aortic clamp. Following this, the patient is temporarily weaned from CPB to assess the quality of repair or replacement by TEE and to complete the de-airing procedure. Thereafter, CPB is resumed, the cardioplegia needle vent is removed, haemostasis is checked and the pericardium is closed. The patient is then finally weaned off CPB and decannulated.
Follow-up
Follow-up was obtained by personal contact, mailed questionnaires, or by phone contact with patients and family members, with supplemental information being supplied by family physicians and referring cardiologists. The mean follow-up interval was 5.4±3.1 years and was 100% complete.
Results
Of the 3,438 of patients undergoing minimally-invasive mitral valve surgery, 2,829 underwent MVRp and 609 underwent MVR, resulting in a repair rate of 81.2%. This also included patients with valve pathology that was not amenable to repair. Our database, however, does account for patients who undergo a formal repair attempt with annuloplasty, are weaned off CPB and then have to undergo MVR due to an unsatisfactory repair on TEE. A total of 45 patients (1.6%) required MVR due to failure of repair, either during the primary operation itself or at reoperation performed before discharge. This would result in a repair rate of 98.4% in patients whose mitral valves were considered highly reparable before the operation.
Demographic characteristics and intraoperative parameters
Demographic characteristics and intraoperative parameters of patients undergoing minimally invasive MVRp are depicted in Table 1. Almost two-thirds of patients were males. Most patients had good left ventricular function, a low preoperative risk profile and underwent elective surgery. Very few patients had active infective endocarditis requiring urgent or emergent surgery. The minimally invasive approach was avoided in patients with suspicion of paravalvular abscesses.
Table 1. Distribution of preoperative and intraoperative variables.
| Preoperative variables | |
| Age in years | 60.3±13 |
| Male | 1,733 (61.3) |
| Body-mass index (kg/m2) | 25.6±3.9 |
| Preoperative cerebrovascular accident | 90 (3.2) |
| Left ventricular ejection fraction (%) | 56.8±18.9 |
| Prior cardiac surgery | 152 (5.4) |
| Active endocarditis | 36 (1.3) |
| Timing of surgery | |
| Elective | 2,632 (93) |
| Urgent/emergent | 197 (7) |
| Log EuroSCORE (%) | 4.9±6 |
| Intraoperative parameters | |
| Mitral valve repair† | 2,829 (100) |
| Ring annuloplasty | 2,829 (100) |
| Complete ring | 2,440 (86.4) |
| Partial ring | 389 (13.6) |
| Ring size | 31.1±5.2 |
| Combined procedures | |
| Tricuspid valve repair | 303 (10.7) |
| Tricuspid valve replacement | 4 (0.1) |
| Atrial septal defect/patent foreamen ovale closure | 272 (9.6) |
| Cryoablation | 793 (28) |
| Excision of cardiac tumors | 3 (0.1) |
| Aortic cross-clamp time (minutes) | 76.4±35.1 |
| Cardiopulmonary bypass time (minutes) | 133.6±68.6 |
| Length of surgery (minutes) | 180.1±133.6 |
| Conversion to sternotomy | 39 (1.4) |
| Mitral valve repair failure | 45 (1.6) |
†, includes patients with failed mitral valve repair. Continuous variables expressed as mean ± standard deviation. Percentages are shown in parentheses.
It is the policy at our institution to use a ring annuloplasty for all repairs. The majority of patients received a complete ring. The right minithoracotomy approach also allows excellent access to the atrial septum, the tricuspid valve and the left and right atria for cryoablation. Less than 2% of patients required conversion to sternotomy.
Postoperative outcomes and follow-up
Overall 23 patients (0.8%) died within 30 days of surgery. The postoperative outcomes are presented in Table 2. All patients underwent transthoracic echocardiography before discharge. Of the 45 patients who required a MVR due to a failed repair, two patients (4.4%) died within 30 days and another five died within one year after surgery.
Table 2. Distribution of postoperative outcomes.
| Outcomes | n (%) |
|---|---|
| 30-day mortality | 23 (0.8) |
| Low output syndrome | 31 (1.1) |
| Failed mitral valve repair | 45 (1.6) |
| Re-exploration for bleeding | 198 (7) |
| Myocardial infarction | 18 (0.6) |
| Sepsis | 24 (0.8) |
| Stroke | 57 (2) |
| Postoperative new dialysis | 87 (3.1) |
| Postoperative symptomatic neuropsychotic syndrome | 71 (2.5) |
| Hospital stay, days | 12.2±9.4 |
Continuous variables expressed as standard ± mean deviation.
The survival of all patients (MVR and MVRp) undergoing minimally invasive mitral valve surgery and those undergoing MVRp is depicted in Figures 1 and 2, respectively. The 5- and 10-year survival of all patients (MVR and MVRp) undergoing minimally invasive mitral valve surgery was 85.7±0.6% and 71.5±1.2%, respectively. A total of 447 patients undergoing MVRp died during follow-up, resulting in a survival of 87.0±0.7% and 74.2±1.4% at five and ten years. One hundred and thirteen patients required a cardiac reoperation during follow-up, culminating in a freedom from reoperation of 96.6±0.4% and 92.9±0.9% at five and ten years (Figure 3).
Figure 1.
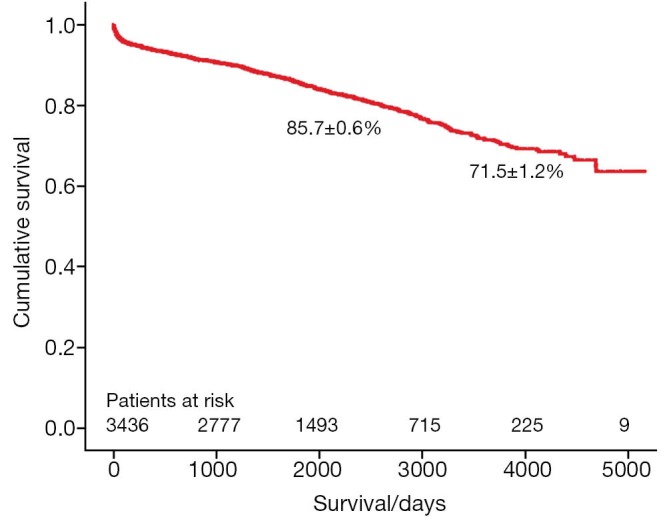
Survival of patients undergoing mitral valve repair and replacement.
Figure 2.
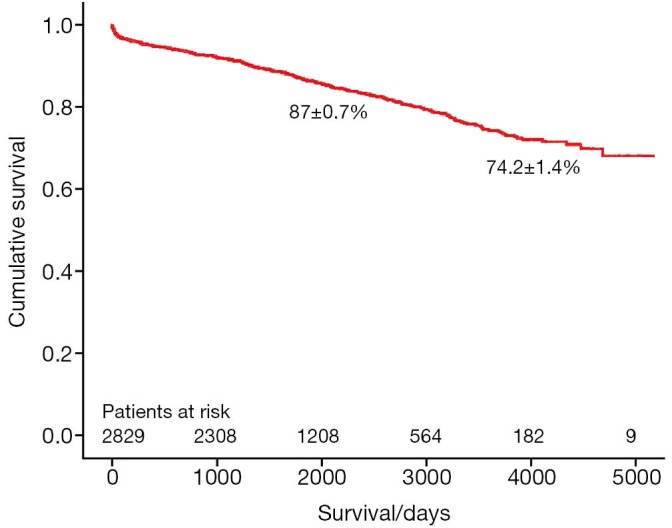
Survival of patients undergoing mitral valve repair.
Figure 3.
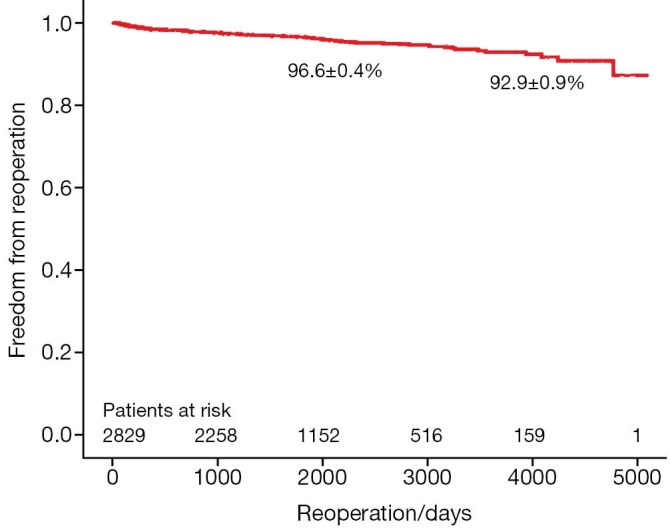
Freedom from reoperation in patients undergoing mitral valve repair.
Discussion
Ever since the description of the techniques of MVRp by Alain Carpentier in his famous publication “The French Correction” (11) MVRp has become the gold standard for patients with MR, especially due to degenerative and ischemic pathology.
The long-term outcomes after MVRp through a sternotomy approach have been excellent and have been reported by a multitude of publications in the literature (12-14). With the increasing use of laparoscopic and thoracoscopic surgery, minimally invasive access was extended to heart surgery as well. There has always been a lot of scepticism regarding the ability of a surgeon to perform the same quality of MVRp through this so-called “limited vision” access when compared to that with the sternotomy approach, thus presumably having a negative impact on the early and long-term outcomes. Our institution, which has contributed immensely to the development and progress of minimally invasive mitral valve surgery, performs all isolated mitral valve surgeries (except for active endocarditis with paravalvular abscess or severe mitral annular calcification) through a right anterolateral minithoracotomy, irrespective of the complexity of repair or left ventricular function. Having one of the largest experiences in minimally invasive MVRp, the present series includes patients undergoing minimally invasive mitral valve surgery, with particular focus on MVRp.
It is believed that the threshold for MVR as opposed to MVRp is much lower when performing minimally invasive mitral valve surgery. This was, however, not the case in our series. Although the overall repair rate was 81.2%, it included a large number of patients with dysfunctional mitral valve due to infective endocarditis, rheumatic heart disease, ischemic MR with severe restriction of leaflets, etc., which are not amenable for repair. Nevertheless, only 45 (1.6%) patients with an initial attempt at repair ultimately required replacement, resulting in a repair rate of 98.4% amongst the valves that were deemed to be highly reparable before surgery. This was similar to the repair rate of 97.5% observed by McClure et al. in 1,000 patients undergoing minimally-invasive MVRp predominantly through a lower hemi-sternotomy approach (15). Such a high repair rate is evidence enough that the minimally invasive approach was not a deterrent for the surgeons to perform MVRp and that the choice of procedure was influenced more by the valve pathology rather than the operative approach. In addition, the rate of conversion to sternotomy in this series was a meagre 1.4%. This was most commonly due to the presence of severe right-sided pleural adhesions or due to development of complications during surgery, which could not be controlled through the mini-thoracotomy incision.
Even though the overall risk profile of patients in this series appears to be relatively low, there were several patients who were obese with significantly reduced left ventricular function. We believe that patient selection should be considered by surgeons during their learning curve. Obesity, an excessively deep thoracic cavity, and chest wall deformities increase the level of difficulty of minimally invasive surgery. An unpublished analysis of 89 patients with body-mass index ≥35 kg/m2 (38.5±4.4 kg/m2) performed in our institution revealed that minimally invasive mitral valve surgery is safe and feasible in obese patients. The rate of conversion to sternotomy was, however, 5.7%, which was higher than that reported in this series. This can be lowered by obtaining a preoperative computed tomographic (CT) scan of the thorax to assess the distance between the mitral valve and right chest wall. It helps the surgeon determine if this approach is possible with regard to the length of the instruments available. Additionally, the skin incision is made slightly longer than usual and only experienced minimally invasive surgeons operate on obese patients in our institute.
Atluri and colleagues showed that patients with LV dysfunction were able to undergo minimally invasive mitral valve surgery with minimal mortality (2.1% vs. 1.7%, P=0.7) and morbidity, which was comparable to that of patients with normal ventricular function (16). Even reoperations in patients with previous aortic valve replacement or coronary artery bypass grafts can be performed with very good perioperative results (7). A preoperative CT scan of the thorax helps to rule out the presence of significant adhesions between the lung and chest wall. In this series, 5% of patients had undergone previous cardiac operations. The surgeries were performed either by clamping the aorta when possible or by inducing ventricular fibrillation under moderate hypothermia when it was not possible to clamp the aorta due to severe adhesions. Thus, with experience, almost every patient requiring isolated mitral valve surgery can be operated upon by this approach.
Annuloplasty bands or rings were used in all patients undergoing MVRp. A ring annuloplasty is necessary to achieve a durable repair (17). The mean operative, CPB 133.6±68.6 minutes and aortic clamp times 76.4±35.1 minutes (Table 2) are longer than one would usually encounter in patients undergoing conventional mitral valve surgery for several reasons. Firstly, all surgeons require more time especially during the initial period of their learning curves. We do have one or two surgeons training in this procedure every year. Secondly, many operative steps, which through a conventional approach would normally be performed by an assistant, have to be performed by the operating surgeon. Thirdly, the freedom of movement that one has when operating through a sternotomy is obviously restricted due to a small 5-7 cm area of access. Finally, all minimally invasive mitral valve surgeries in our institution are performed with a single-lumen endotracheal tube. Hence, CPB is established before thoracotomy and continues until pericardial closure and insertion of chest tubes. Despite this, the CPB and clamp times in this series were lower than those recently reported in an elegant meta-analysis on minimally-invasive versus conventional open mitral valve surgery by Cheng et al. The cross-clamp time 95±39 vs. 74±36 minutes and CPB time 144 vs. 111 minutes were significantly increased with mini- versus conventional mitral valve surgery, respectively (18).
In our series, the 30-day mortality for patients undergoing minimally invasive MVRp was low at 0.8%. The mortality is the same as that reported by McClure and colleagues in very low risk patients (15), but much better than 2.2% published by Galloway and co-workers in 1,601 patients undergoing minimally invasive MVRp for degenerative disease (19). It is also comparable to many contemporary series on MVRp through a sternotomy approach (20).
Excellent long-term outcomes can be achieved with minimally invasive mitral valve surgery. McClure et al. reported an overall survival of 79±3% for minimally invasive MVR and MVRp at 15 years. For MVRp, freedom from reoperation at 5, 10, and 15 years was 96±1%, 95±1% and 90±3%, respectively (15). Galloway and co-workers described an 8-year freedom from reoperation of 91±2% for sternotomy and 95±1% for minimally invasive P=0.24 isolated MVRp (19). In a 10-year follow-up of single-surgeon minimally invasive MVRp for degenerative disease, D’Alfonso and associates reported an overall survival of 98.7±1.2% and freedom from reoperation of 98.5±1.1% (21). These results are comparable with those of MVRp through a conventional sternotomy approach. In their study, Castillo et al. revealed a cumulative survival of 95.6±1.7% and 88.7±2.2% at 4 and 7 years, respectively. Seven-year freedom from reoperation was 94.1±0.5% (20). In a recent publication by David and associates, the freedom from all-cause mortality and reoperation on the mitral valve at 10 and 15 years was 85.8% and 72.5%, and 95.9% and 94.9%, respectively (14).
Although the long-term survival rates (Figures 1,2) in the present series are not as good as those in the above-mentioned studies, the freedom from reoperation (Figure 3) is excellent and comparable. Direct comparison of observational studies may be misleading, as patient populations are very variable across different. Our series includes not only patients operated on during the development phase of this technique, but also high risk patients with ischemic MR with poor left ventricular function, acute endocarditis, as well as technically challenging patients with Barlow’s disease operated in recent years. Secondly, one or two new surgeons are trained in this procedure every year, thus their learning curves should be taken into account as well. Holzhey et al. have elegantly shown that the complication rate reduces with increasing experience of the surgeon and the institution as a whole (22). Finally, the patients in our series were older than those included in some of the studies mentioned above (15,20).
One of the main limitations of this study is its retrospective nature and the resultant issues thereof. It is difficult to identify the exact “true repair rate” in a retrospective analysis, as cases in which the surgeons did attempt some amount of repair surgery, but then rapidly converted to replacement without further attempting to wean the patients off CPB, are sometimes not reported as such, either in the operative notes or in the database. However, those patients who undergo a formal repair attempt with annuloplasty, are weaned off CPB and then have to undergo MVR due to an unsatisfactory repair on TEE, are specifically coded in our database. Secondly, the patient population is a heterogenous group with varying causes of MR.
To conclude, minimally invasive MVRp can be safely performed with encouraging short and long-term outcomes. It is associated with very low rates of conversion to a conventional sternotomy. The failure rate of repairs is extremely low, especially in the hands of experienced surgeons.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Schwartz DS, Ribakove GH, Grossi EA, et al. Minimally invasive cardiopulmonary bypass with cardioplegic arrest: a closed chest technique with equivalent myocardial protection. J Thorac Cardiovasc Surg 1996;111:556-66 [DOI] [PubMed] [Google Scholar]
- 2.Pompili MF, Stevens JH, Burdon TA, et al. Port-access mitral valve replacement in dogs. J Thorac Cardiovasc Surg 1996;112:1268-74 [DOI] [PubMed] [Google Scholar]
- 3.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8 [DOI] [PubMed] [Google Scholar]
- 4.Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205; discussion 1205-6 [DOI] [PubMed] [Google Scholar]
- 5.Seeburger J, Falk V, Borger MA, et al. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: à ègalité. Ann Thorac Surg 2009;87:1715-20 [DOI] [PubMed] [Google Scholar]
- 6.Borger MA, Mohr FW. Repair of bileaflet prolapse in Barlow syndrome. Semin Thorac Cardiovasc Surg 2010;22:174-8 [DOI] [PubMed] [Google Scholar]
- 7.Seeburger J, Borger MA, Falk V, et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann Thorac Surg 2009;87:709-14 [DOI] [PubMed] [Google Scholar]
- 8.Modi P, Hassan A, Chitwood WR., Jr Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52 [DOI] [PubMed] [Google Scholar]
- 9.Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5 [DOI] [PubMed] [Google Scholar]
- 10.Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5 [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A.Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37 [PubMed] [Google Scholar]
- 12.David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9 [DOI] [PubMed] [Google Scholar]
- 13.Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998;116:734-43 [DOI] [PubMed] [Google Scholar]
- 14.David TE, Armstrong S, McCrindle BW, et al. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485-92 [DOI] [PubMed] [Google Scholar]
- 15.McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206 [DOI] [PubMed] [Google Scholar]
- 16.Atluri P, Woo YJ, Goldstone AB, et al. Minimally Invasive Mitral Valve Surgery Can Be Performed With Optimal Outcomes in the Presence of Left Ventricular Dysfunction. Ann Thorac Surg 2013;96:1596-602 [DOI] [PubMed] [Google Scholar]
- 17.Gillinov AM, Tantiwongkosri K, Blackstone EH, et al. Is prosthetic anuloplasty necessary for durable mitral valve repair? Ann Thorac Surg 2009;88:76-82 [DOI] [PubMed] [Google Scholar]
- 18.Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103 [DOI] [PubMed] [Google Scholar]
- 19.Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4 [DOI] [PubMed] [Google Scholar]
- 20.Castillo JG, Anyanwu AC, El-Eshmawi A, et al. All anterior and bileaflet mitral valve prolapses are repairable in the modern era of reconstructive surgery. Eur J Cardiothorac Surg. 2013 doi: 10.1093/ejcts/ezt196. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.D’Alfonso A, Capestro F, Zingaro C, et al. Ten years’ follow-up of single-surgeon minimally invasive reparative surgery for degenerative mitral valve disease. Innovations (Phila) 2012;7:270-3 [DOI] [PubMed] [Google Scholar]
- 22.Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91 [DOI] [PubMed] [Google Scholar]


