Abstract
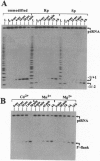
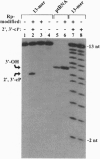
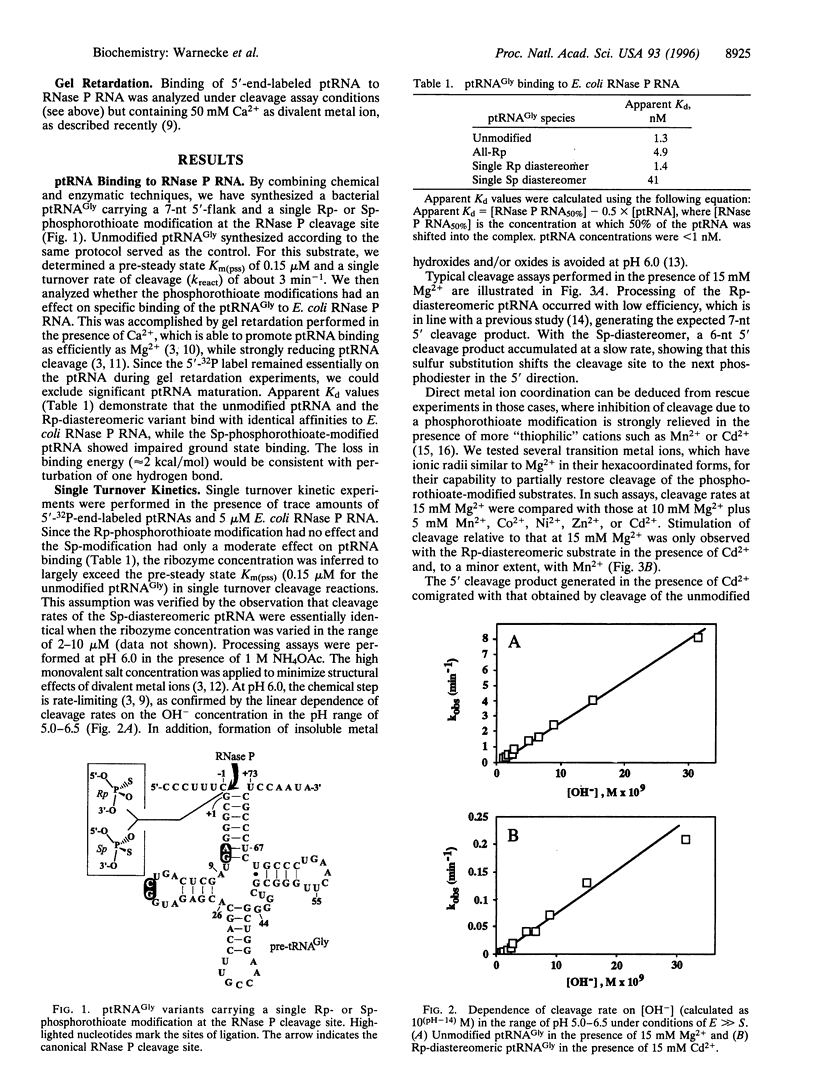
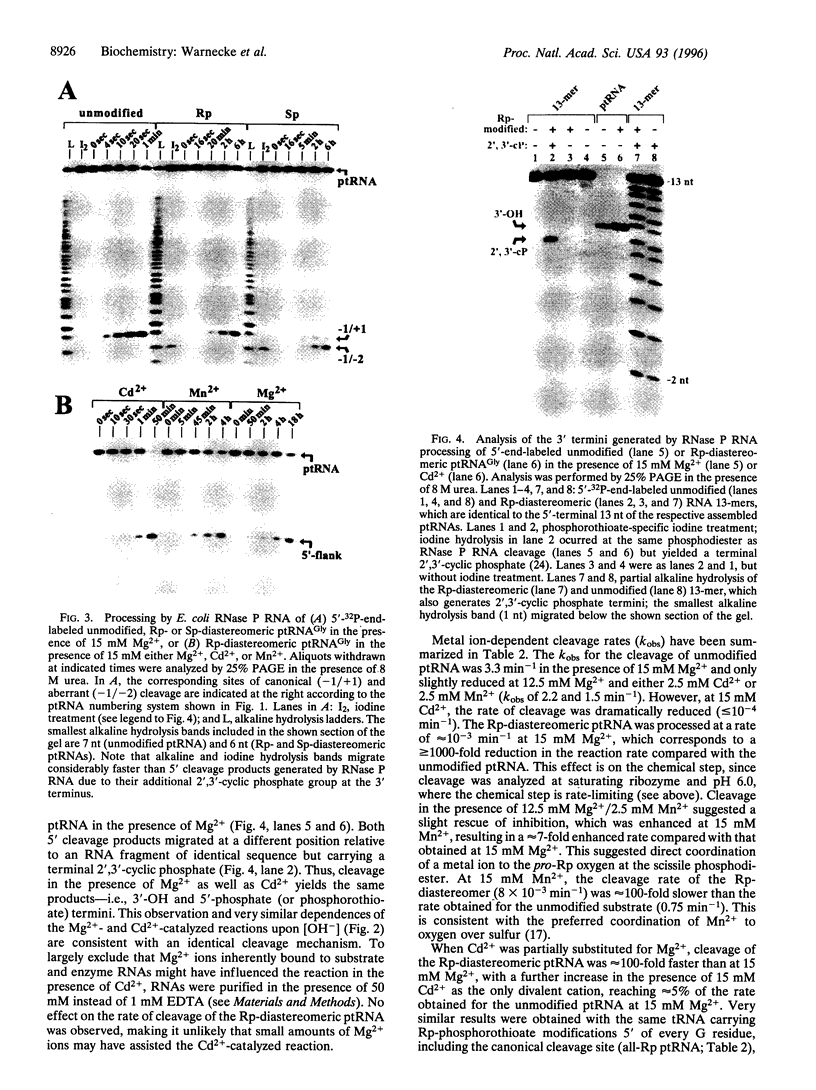
To study the cleavage mechanism of bacterial Nase P RNA, we have synthesized precursor tRNA substrates carrying a single Rp- or Sp-phosphorothioate modification at the RNase P cleavage site. Both the Sp- and the Rp-diastereomer reduced the rate of processing by Escherichia coli RNase P RNA at least 1000-fold under conditions where the chemical step is rate-limiting. The Rp-modification had no effect and the Sp-modification had a moderate effect on precursor tRNA ground state binding to RNase P RNA. Processing of the Rp-diastereomeric substrate was largely restored in the presence of the "thiophilic" Cd2+ as the only divalent metal ion, demonstrating direct metal ion coordination to the (pro)-Rp substituent at the cleavage site and arguing against a specific role for Mg(2+)-ions at the pro-Sp oxygen. For the Rp-diastereomeric substrate, Hill plot analysis revealed a cooperative dependence upon [Cd2+] of nH = 1.8, consistent with a two-metal ion mechanism. In the presence of the Sp-modification, neither Mn2+ nor Cd2+ was able to restore detectable cleavage at the canonical site. Instead, the ribozyme promotes cleavage at the neighboring unmodified phosphodiester with low efficiency. Dramatic inhibition of the chemical step by both the Rp- and Sp-phosphorothioate modification is unprecedented among known ribozymes and points to unique features of transition state geometry in the RNase P RNA-catalyzed reaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christian E. L., Yarus M. Analysis of the role of phosphate oxygens in the group I intron from Tetrahymena. J Mol Biol. 1992 Dec 5;228(3):743–758. doi: 10.1016/0022-2836(92)90861-d. [DOI] [PubMed] [Google Scholar]
- Dahm S. C., Derrick W. B., Uhlenbeck O. C. Evidence for the role of solvated metal hydroxide in the hammerhead cleavage mechanism. Biochemistry. 1993 Dec 7;32(48):13040–13045. doi: 10.1021/bi00211a013. [DOI] [PubMed] [Google Scholar]
- Frey P. A., Sammons R. D. Bond order and charge localization in nucleoside phosphorothioates. Science. 1985 May 3;228(4699):541–545. doi: 10.1126/science.2984773. [DOI] [PubMed] [Google Scholar]
- Gish G., Eckstein F. DNA and RNA sequence determination based on phosphorothioate chemistry. Science. 1988 Jun 10;240(4858):1520–1522. doi: 10.1126/science.2453926. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Haydock K., Allen L., Altman S. Metal ion requirements and other aspects of the reaction catalyzed by M1 RNA, the RNA subunit of ribonuclease P from Escherichia coli. Biochemistry. 1986 Apr 8;25(7):1509–1515. doi: 10.1021/bi00355a006. [DOI] [PubMed] [Google Scholar]
- Hardt W. D., Schlegl J., Erdmann V. A., Hartmann R. K. Gel retardation analysis of E. coli M1 RNA-tRNA complexes. Nucleic Acids Res. 1993 Jul 25;21(15):3521–3527. doi: 10.1093/nar/21.15.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt W. D., Schlegl J., Erdmann V. A., Hartmann R. K. Kinetics and thermodynamics of the RNase P RNA cleavage reaction: analysis of tRNA 3'-end variants. J Mol Biol. 1995 Mar 24;247(2):161–172. doi: 10.1006/jmbi.1994.0130. [DOI] [PubMed] [Google Scholar]
- Hardt W. D., Warnecke J. M., Erdmann V. A., Hartmann R. K. Rp-phosphorothioate modifications in RNase P RNA that interfere with tRNA binding. EMBO J. 1995 Jun 15;14(12):2935–2944. doi: 10.1002/j.1460-2075.1995.tb07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Piccirilli J. A., Cech T. R. Ribozyme-catalyzed and nonenzymatic reactions of phosphate diesters: rate effects upon substitution of sulfur for a nonbridging phosphoryl oxygen atom. Biochemistry. 1991 May 21;30(20):4844–4854. doi: 10.1021/bi00234a003. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Church G. M., Kim S. H. RNA-ligant interactions. (I) Magnesium binding sites in yeast tRNAPhe. Nucleic Acids Res. 1977 Aug;4(8):2811–2820. doi: 10.1093/nar/4.8.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. K., Cohn M. Diastereomers of the nucleoside phosphorothioates as probes of the structure of the metal nucleotide substrates and of the nucleotide binding site of yeast hexokinase. J Biol Chem. 1979 Nov 10;254(21):10839–10845. [PubMed] [Google Scholar]
- Kahle D., Küst B., Krupp G. Phosphorothioates in pre-tRNAs can change the specificities of RNAses P or reduce the cleavage efficiencies. Biochimie. 1993;75(11):955–962. doi: 10.1016/0300-9084(93)90145-i. [DOI] [PubMed] [Google Scholar]
- Moore M. J., Sharp P. A. Site-specific modification of pre-mRNA: the 2'-hydroxyl groups at the splice sites. Science. 1992 May 15;256(5059):992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- Nolte A., Klussman S., Lorenz S., Bald R., Betzel C., Dauter Z., Wilson K., Fürste J. P., Erdmann V. A. Crystallization and preliminary diffraction studies of the structural domain E of Thermus flavus 5S rRNA. FEBS Lett. 1995 Oct 30;374(2):292–294. doi: 10.1016/0014-5793(95)01136-3. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Podar M., Boulanger S. C., Perlman P. S. The stereochemical course of group II intron self-splicing. Science. 1994 Dec 9;266(5191):1685–1688. doi: 10.1126/science.7527587. [DOI] [PubMed] [Google Scholar]
- Pan T. Higher order folding and domain analysis of the ribozyme from Bacillus subtilis ribonuclease P. Biochemistry. 1995 Jan 24;34(3):902–909. doi: 10.1021/bi00003a024. [DOI] [PubMed] [Google Scholar]
- Pan T., Zhong K. Selection of circularly permuted ribozymes from Bacillus subtilis RNAse P by substrate binding. Biochemistry. 1994 Nov 29;33(47):14207–14212. doi: 10.1021/bi00251a032. [DOI] [PubMed] [Google Scholar]
- Pecoraro V. L., Hermes J. D., Cleland W. W. Stability constants of Mg2+ and Cd2+ complexes of adenine nucleotides and thionucleotides and rate constants for formation and dissociation of MgATP and MgADP. Biochemistry. 1984 Oct 23;23(22):5262–5271. doi: 10.1021/bi00317a026. [DOI] [PubMed] [Google Scholar]
- Slim G., Gait M. J. Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991 Mar 25;19(6):1183–1188. doi: 10.1093/nar/19.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Burgin A. B., Haas E. S., Pace N. R. Influence of metal ions on the ribonuclease P reaction. Distinguishing substrate binding from catalysis. J Biol Chem. 1992 Feb 5;267(4):2429–2436. [PubMed] [Google Scholar]
- Smith D., Pace N. R. Multiple magnesium ions in the ribonuclease P reaction mechanism. Biochemistry. 1993 May 25;32(20):5273–5281. doi: 10.1021/bi00071a001. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Steitz J. A. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]