Abstract
Background
Minimally invasive mitral valve surgery through a mini-thoracotomy approach was developed in the mid-1990s as an alternative to conventional sternotomy, but with reduced trauma and quicker recovery. However, technical demands and a paucity of comparative data have thus far limited the widespread adoption of minimally invasive mitral valve repair (MIMVR). Previous meta-analyses have grouped various surgical techniques and underlying valvular disease aetiologies together for comparison. The present study aimed to compare the clinical outcomes of MIMVR versus conventional mitral valve repair in patients with degenerative mitral valve disease.
Methods
A systematic review of the current literature was performed through nine electronic databases from January 1995 to July 2013 to identify all relevant studies with comparative data on MIMVR versus conventional mitral valve surgery. Measured endpoints included mortality, stroke, renal failure, wound infection, reoperation for bleeding, aortic dissection, myocardial infarction, atrial fibrillation, readmission within 30 days, cross clamp time, cardiopulmonary bypass time and durations of intensive care unit (ICU) stay and overall hospitalization. Echocardiographic outcomes were also assessed when possible.
Results
Seven relevant studies were identified according to the predefined study selection criteria, including one randomized controlled trial and six retrospective studies. Meta-analysis of clinical outcomes did not identify any statistically significant differences between MIMVR and conventional mitral valve repair. The duration of ICU stay was significantly shorter for patients who underwent MIMVR, but this did not translate to a shorter hospitalization period. Patients who underwent MIMVR required longer cross clamp time as well as cardiopulmonary bypass time. Both surgical techniques appeared to achieve satisfactory echocardiographic outcomes. Pain-related outcomes was assessed in one study and reported significantly less pain for patients who underwent MIMVR. However, this limited data was not suitable for meta-analysis.
Conclusions
The existing literature has limited data on comparative outcomes after MIMVR versus conventional mitral valve repair for patients with degenerative disease. From the available evidence, there are no significant differences between the two surgical techniques in regards to clinical outcomes. Patients who underwent MIMVR required longer cardiopulmonary bypass and cross clamp times, but the duration of stay in the ICU was significantly shorter than conventional mitral valve repair.
Keywords: Mini-mitral, meta-analysis, mitral valve repair
Introduction
Minimally invasive surgery has revolutionized many facets of surgical practice over the past few decades, including a range of procedures in cardiac surgery (1-3). Minimally invasive techniques aim to achieve similar or superior safety and efficacy to conventional surgery with the added advantages of reduced trauma, improved cosmesis and shorter hospitalization. Minimally invasive mitral valve surgery through a video-assisted thoracotomy approach was first introduced in the mid-1990s (4,5). Since then, a number of large studies have demonstrated the feasibility of performing minimally invasive mitral valve repair (MIMVR) for selected patients in specialized centres (6-8).
Despite encouraging institutional reports, broad adoption of the MIMVR technique has been limited. Although previous meta-analyses reported superior perioperative outcomes for minimally invasive mitral valve surgery compared to the conventional sternotomy approach, limited attempts were made to differentiate repair versus replacement procedures and account for the significant variations in the underlying valvular pathology (9,10). In addition, some surgeons remain concerned about the limited exposure of the mitral valve, arterial injuries and difficulties in deairing the heart that may result in an increased incidence of cerebrovascular accidents (11). To address some of these issues, the present study compares the clinical outcomes of MIMVR versus conventional mitral valve repair in patients with degenerative mitral valve disease.
Patients and methods
Literature search strategy
An electronic search was performed using nine electronic databases, including ACP Journal Club, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, Embase, Heath Technology Assessment, Ovid Medline and NHS Economic Evaluation Database from January 1995 to July 2013. To achieve the maximum sensitivity of the search strategy and identify all studies, we combined the terms “mini*” or “thoraco*” or “video*” or “robot*” or “laparoscop*” or “endoscop*” or “port-access” or “port access” or “partial sternotomy” or “keyhole” and “mitral*” or “Barlow*” as either keywords or MeSH terms. Reference lists of the selected articles were assessed for further identification of relevant studies.
Selection criteria
Selected comparative studies for the present meta-analysis included those that reported outcomes of patients with degenerative mitral valve disease who underwent mitral valve repair either through the conventional sternotomy or mini-thoracotomy approach. To focus on patients with similar underlying aetiology and surgical technique, studies that reported more than a third of patients who were diagnosed with non-degenerative mitral valve pathology or underwent valvular replacement in either treatment arm were excluded from analysis. In addition, studies that reported concomitant procedures other than patent foramen ovale closure or atrial fibrillation ablation in over a third of patients, or included less than 15 patients in either treatment cohort, were also excluded. When institutions reported duplicated trials, only the most complete studies were included where possible. Studies were limited to human subjects and in English language. Abstracts, case studies, editorials and letters were excluded.
Data extraction and critical appraisal
Data were extracted from article texts, tables and figures by three investigators, (D. C., T. A. N. and S. G.) who independently reviewed each retrieved article. Discrepancies between the investigators were resolved by discussion and consensus. The final results were reviewed by the senior investigators (C. C. and T. D. Y.).
Statistical analysis
Meta-analysis was performed by combining the results of reported incidences of mortality, stroke, reoperation for bleeding, renal failure, wound infection, aortic dissection, myocardial infarction, atrial fibrillation, and readmission within 30 days. In addition, the durations of cross-clamp time, cardiopulmonary bypass time, intensive care unit (ICU) stay and hospitalization were also meta-analysed when mean and standard deviation values were available. The relative risk (RR) was used as a summary statistic and the random effect model was tested, as these calculated ratios have a more conservative value (12). χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, due to heterogeneity rather than chance. An I2 value of greater than 50% was considered substantial heterogeneity. When there was substantial heterogeneity, the possible clinical and methodological reasons for this were investigated. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.1.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Quantity and quality of trials
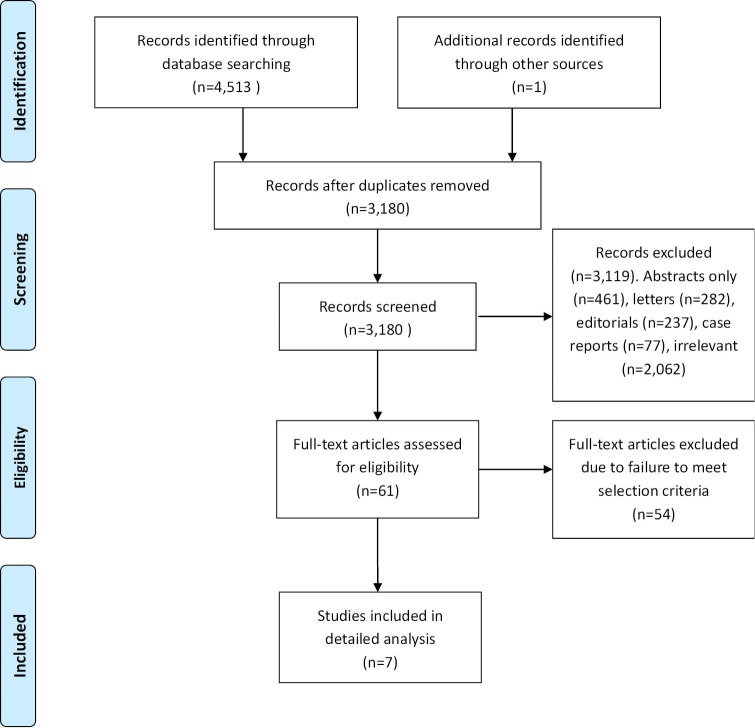
A systematic review of the nine electronic database searches identified 4,513 potentially relevant references. After exclusion of duplicate or irrelevant references, 61 potentially relevant articles were retrieved for more detailed evaluation. After applying the selection criteria, seven comparative studies remained eligible for quantitative assessment. A PRISMA chart summarizing the search strategy is presented in Figure 1 (13). The seven selected articles included one randomized-controlled trial and six retrospective studies, as summarized in Table 1 (14-20). In these seven studies, 1,964 patients who underwent mitral valve surgery were compared, including 953 patients who underwent the minimally invasive thoracotomy approach and 1,011 patients who underwent the conventional sternotomy approach. Three retrospective studies attempted to match patients according to important prognostic factors (14,16,18).
Figure 1.
Summary of search strategy performed to identify relevant comparative studies on mitral valve repair through minimally invasive thoracotomy versus conventional sternotomy approaches.
Table 1. Study characteristics of relevant articles identified for meta-analysis comparing mini-mitral versus conventional sternotomy approaches for patients undergoing mitral valve surgery.
| Author | Year | Institution | Study Period | MIMVR (n) | Sternotomy (n) | Follow-up period (months) |
|---|---|---|---|---|---|---|
| Goldstone | 2013 | University of Pennsylvaniva, USA | 2002-2011 | 153 | 153 | 50.4M |
| Speziale | 2011 | Villa Azzurra Hospital & Anthea Hospital, Italy | 2006-NR | 70 | 70 | 12.4 |
| Ryan | 2010 | Cardiopulmonary Research Science and Technology Institute, USA | 1996-2008 | 177 | 177 | 62.4±34.8 |
| Raanani | 2010 | Chaim Shebe Medical Centre, Israel | 2000-2009 | 61 | 82 | 41±24†; 28±22§ |
| Suri | 2009 | University of Pennsylvania (MIMVR) & Mayo Clinic (Sternotomy), USA | 1999-2006 | 350 | 365 | NR |
| Ruttman | 2006 | Medical University of Innsbruck, Austria | 2001-2005 | 42 | 64 | 43.8†; 41.8§ |
| Grossi | 2001 | New York University School of Medicine, USA | 1993-1999 | 100 | 100 | 33 |
MIMVR, minimally invasive mitral valve repair; NR, not reported; M, median; §, sternotomy; †, MIMVR.
Patient characteristics
Baseline patient characteristics such as age, gender, hypertension, diabetes mellitus and previous stroke were similar between the MIMVR and conventional sternotomy cohorts, as summarized in Table 2. In addition, preoperative New York Heart Association (NYHA) functional status was similar between the two treatment arms, as shown in Table 3. According to the study selection criteria, no studies included more than a third of patients with non-degenerative valvular disease. However, Grossi and colleagues included 18 patients with rheumatic (n=10), infective (n=7) or ischaemic (n=1) aetiology in their conventional sternotomy group (n=100). Sensitivity analysis excluding studies that reported non-degenerative valvular pathology did not alter our statistical findings (19,20).
Table 2. A summary of patient baseline characteristics in comparative studies on mini-mitral versus conventional sternotomy mitral valve repair.
| Author | Age (mean ± SD) |
Male gender (%) |
Diabetes mellitus (%) |
Hypertension, n (%) |
Previous stroke, n (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIMVR | Sternotomy | MIMVR | Sternotomy | MIMVR | Sternotomy | MIMVR | Sternotomy | MIMVR | Sternotomy | |||||
| Goldstone | 57±12 | 57±13 | 67 | 63 | NR | NR | 41 | 40 | 4§ | 5§ | ||||
| Speziale | 53±10 | 54±10 | 59 | 61 | NR | NR | NR | NR | NR | NR | ||||
| Ryan | 56±13 | 57±15 | 55 | 57 | 5 | 7 | 38 | 47 | 1 | 2 | ||||
| Raanani | 55±11 | 57±12 | 89 | 76 | NR | NR | NR | NR | NR | NR | ||||
| Suri | 58±13 | 55±15 | 58 | 67 | 5 | 2 | 33 | 31 | NR | NR | ||||
| Ruttman | 54±11 | 64±11 | 63 | 59 | NR | NR | NR | NR | 5† | 5† | ||||
| Grossi | 56±14 | 55±17 | 71 | 55 | 3 | 5 | NR | NR | NR | NR | ||||
SD, standard deviation; MIMVR, minimally invasive mitral valve repair; §, cerebrovascular disease; †, previous embolic event; NR, not reported.
Table 3. A summary of patient baseline functional status and underlying mitral valve pathology.
| Author | NYHA, n [%] |
Valvular pathology, n [%] |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIMVR |
Sternotomy |
MIMVR |
Sternotomy |
|||||||||||||||||
| I | II | III | IV | I | II | III | IV | Myxo. degen. | Isch. | Infec. | Rheum. | Funct. | Other | Myxo. degen. | Isch. | Infec. | Rheum. | Funct. | Other | |
| Goldstone | 53 [35] | 100 [65] | 47 [31] | 106 [69] | 153 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 153 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||
| Speziale | 49 [70] | 21 [30] | 51 [73] | 19 [27] | 70 [100]^ | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 70 [100]^ | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||
| Ryan | NR | NR | NR | 7 [4] | NR | NR | NR | 7 [4] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Raanani | 29 [48] | 15 [25] | 17 [28] | 0 [0] | 19 [23] | 33 [40] | 23 [28] | 7 [9] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Suri | 250 [71] | 100 [29] | 317 [87] | 48 [13] | 350 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 365 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||
| Ruttman | 2 [5] | 28 [68] | 11 [27] | 0 [0] | 10 [16] | 13 [20] | 30 [47] | 11 [17] | 28 [68] | 0 [0] | 1 [2] | 4 [10] | NR | NR | 58 [91] | 0 [0] | 3 [5] | 3 [5] | 0 [0] | 0 [0] |
| Grossi | NR | NR | NR | NR | NR | NR | NR | NR | 92 [92] | 1 [1] | 2 [2] | 2 [2] | 0 [0] | 3 [3] | 72 [72] | 1 [1] | 7 [7] | 10 [10] | 0 | 10 [10] |
NYHA, New York Heart Association functional classification; ^, Barlow’s disease; NR, not reported. MIMVR, minimally invasive mitral valve repair. Valvular pathologies are abbreviated as: myxo degen (myxomatous degeneration), isch (ischaemic), infec (infective), rheum (rheumatic) and funct (functional).
Surgical techniques
From the selected studies, MIMVR was performed through a 4-8 cm right thoracotomy with additional port access for instruments. Endoaortic occlusion was performed for selected patients in all retrospective studies, but direct aortic clamping was also described in four out of the six reports (14,17-19). The randomized controlled trial by Speziale was the only identified study that exclusively performed direct aortic clamping and aortic arterial cannulation for patients who underwent MIMVR (15). Concomitant procedures such as atrial fibrillation ablation and patent foramen ovale closures were reported in three studies (15,18,19). A summary of procedural details, including the cardioplegia strategy and repair techniques, are presented in Table 4.
Table 4. A summary of surgical techniques performed during mitral valve surgery through mini-mitral or sternotomy approaches.
| Author | Mini-mitral access approach | Mini-mitral clamp technique | Cardioplegia |
Mitral-valve repair details |
Concomitant surgery |
Conversion to sternotomy | |||
|---|---|---|---|---|---|---|---|---|---|
| Mini | Stern | Mini-mitral | Sternotomy | Mini-mitral | Sternotomy | ||||
| Goldstone | 4 cm right thoracotomy in infra-mammary grove | Aortic cross-clamp or endoballoon | AG & RG | Annuloplasty (5/153); leaflet resection (98/153); neochordae (29/153) | Annuloplasty (10/153); leaflet resection (121/153); neochordae (15/153) | NR | NR | NR | |
| Speziale | 2 ports & 4-5 cm right anterolateral mini-thoracotomy 3rd ICS & 5-7th ICS | Aortic cross-clamp | AG | NR | Annuloplasty & artificial chordal re-implantation | Annuloplasty & artificial chordal re-implantation | AF ablation (11/70) | AF ablation (12/70) | 1/70 (inadequate exposure) |
| Ryan | 4 cm incision 4th right ICS with port access | Endoballoon | AG & RG | Annuloplasty, chordal replacement and/or leaflet resection and sliding-plasty as required | Annuloplasty, chordal replacement and/or leaflet resection and sliding-plasty as required | NR | NR | 2/177 (repair of coronary sinus) |
|
| Raanani | 3 ports & 6-8 cm infra-mammary fold incision | Aortic cross-clamp or endoballoon | AG | AG & RG | Annuloplasty (61/61); leaflet resection (46/61); Alfieri edge-to-edge (6/61); artificial chordae (16/61) | Annuloplasty (82/82); leaflet resection (66/82); Alfieri edge-to-edge (1/82); artificial chordae (27/82) | NR | NR | 0/61 |
| Suri | 4 cm right infra-mammary incision with port access | Aortic cross-clamp or endoballoon | AG | NR | NR | NR | PFO closure | PFO closure | NR |
| Ruttman | 5-6 cm right anterolateral incision 4th ICS & port access ICS | Aortic cross-clamp OR endoballoon | AG & RG | NR | Ring annuloplasty (41/41); artificial chordae (7/41); rectangular resection p2 segment (22/41) | Ring annuloplasty (64/64); artificial chordae (7/64); rectangular resection p2 segment (41/64) | PFO closure (1/41); AF ablation (1/41) |
PFO closure (1/64); AF ablation (12/64) | 1/42 (repair bleed from aorta) |
| Grossi | Right thoracotomy & port access | Endoballoon | RG | NR | Ring annuloplasty | Ring annuloplasty | NR | NR | NR |
AG, antegrade; RG, retrograde; NR, not reported; PFO, patent foramen ovale; AF, atrial fibrillation; ICS, intercostal space.
Assessment of perioperative clinical outcomes
All comparable clinical outcomes reported by two or more studies were meta-analysed when data was available. Perioperative mortality was defined as all-cause death within 30 days or within the same hospital admission. Cerebral vascular accident included strokes (14,16,18) or a combination of strokes and transient ischaemic attacks (15,17,20). A summary of these and other clinical outcomes are summarized in Table 5. No clinical outcomes reached statistical significance between the two treatment arms and there was no significant heterogeneity between studies. Forest plots comparing perioperative mortality and cerebral vascular accidents between MIMVR and conventional mitral valve repair are presented in Figures 2,3, respectively.
Table 5. Perioperative clinical and time-related outcomes of patients who underwent mitral valve repair through a minimally invasive thoracotomy versus conventional sternotomy approach.
| Outcomes | Included studies | Overall statistics | ||||
|---|---|---|---|---|---|---|
| Clinical outcomes | No. of studies | MIMVR (n) | Sternotomy (n) | Relative risk (95% CI) | P-value | I2 (%) |
| Mortality | 7 | 952 | 1,011 | 1.23 (0.22-6.88) | 0.81 | 0 |
| Cerebrovascular accidents* | 6 | 906 | 929 | 1.43 (0.74-2.76) | 0.29 | 0 |
| Renal failure | 3 | 284 | 305 | 0.96 (0.31-3.00) | 0.95 | 0 |
| Wound infection | 4 | 634 | 670 | 2.97 (0.47-18.87) | 0.25 | 29 |
| Reoperation for bleeding | 6 | 848 | 896 | 1.25 (0.60-2.62) | 0.55 | 35 |
| Aortic dissection | 4 | 688 | 724 | 4.84 (0.55-42.43) | 0.15 | 0 |
| Myocardial infarction | 3 | 284 | 305 | 1.15 (0.24-5.64) | 0.86 | 0 |
| Readmission within 30 days | 2 | 308 | 315 | 0.61 (0.31-1.21) | 0.16 | 0 |
| Time-related outcomes | No. of studies | MIMVR (n) | Sternotomy (n) | Standard mean difference (95% CI) | P-value | I2 (%) |
| Cross-clamp time | 6 | 852 | 911 | 1.47 (0.52-2.42) | 0.003 | 99 |
| CPB time | 6 | 952 | 1,011 | 1.46 (0.40-2.51) | 0.007 | 99 |
| ICU stay | 2 | 247 | 247 | –0.77 (–1.36-0.17) | 0.01 | 88 |
| Length of hospitalization | 4 | 658 | 694 | –0.24 (–0.65-0.18) | 0.26 | 92 |
MIMVR, minimally invasive mitral valve repair; CI, confidence interval; *, includes stroke with or without transient ischaemic attack; CPB, cardiopulmonary bypass; ICU, intensive care unit.
Figure 2.
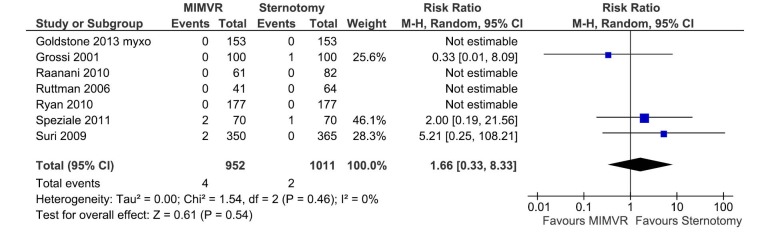
Forest plot of the relative risk (RR) of perioperative mortality after minimally invasive mitral valve repair (MIMVR) versus conventional sternotomy repair for degenerative mitral valve disease. The estimate of the RR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number treated are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary RR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics.
Figure 3.
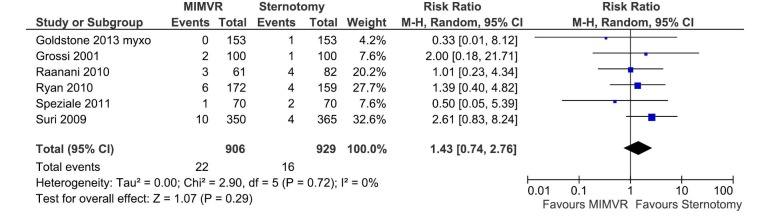
Forest plot of the relative risk (RR) of cerebrovascular accidents after minimally invasive mitral valve repair (MIMVR) versus conventional sternotomy repair for degenerative mitral valve disease. The estimate of the RR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number treated are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary RR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials within a subgroup is given below the summary statistics.
Assessment of time-related outcomes
Meta-analysis was performed when mean and standard deviation values were available for cross-clamp time, cardiopulmonary bypass time, duration of ICU stay and overall duration of hospitalization, as summarized in Table 5. The length of ICU stay was significantly shorter for patients who underwent MIMVR. However, there was no statistical difference in regards to the entire duration of hospitalization between the two treatment arms. Patients who underwent MIMVR required significantly longer periods of cross-clamp time and cardiopulmonary bypass time.
Echocardiography outcomes
When available echocardiographic findings from individual studies were summarized and categorized into predefined severities of none/trivial/mild mitral regurgitation (MR) and moderate/severe MR, patients who underwent MIMVR were reported to have moderate/severe MR in 98.7% of cases preoperatively, compared to 98.4% of patients who underwent conventional sternotomy. Postoperatively, patients who underwent MIMVR had persistent moderate/severe MR in 0.1% of cases compared to 0.3% of patients who underwent conventional sternotomy. A summary of these echocardiographic findings before and after surgery is presented in Figure 4A,B.
Figure 4.
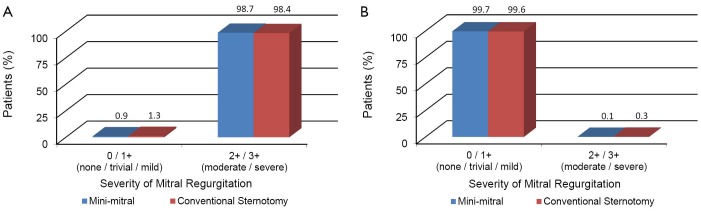
A summary of severity of mitral regurgitation before (A) and after (B) mitral valve repair through the minimally invasive (blue) or conventional sternotomy (red) approach.
Discussion
To achieve minimal surgical access and reduced trauma, a number of novel approaches to mitral valve surgery were developed in the mid-1990s, including right parasternal incisions (21), upper hemisternotomy and lower hemisternotomy (22). Minimally invasive mitral valve surgery through a thoracotomy approach was initially developed with the aim of performing similar surgical techniques as through a conventional sternotomy, but with reduced perioperative morbidities and quicker recovery. The first video-assisted MIMVR through a mini-thoracotomy was reported by Carpentier in 1996 (5), followed shortly by the first mitral replacement by Chitwood (23). Different aortic occlusion strategies have been explored, including a direct transaortic clamping technique using a specialized clamp that can be passed through the chest wall via a small incision (24). Alternatively, an endovascular aortic clamp can be placed through the femoral artery and guided to the ascending aorta using transesophageal echocardiography, as described by Mohr (11). An early series of 51 patients who underwent the port-access technique involving endoaortic clamping reported relatively high mortality and morbidity, including technical complications related to the misplacement of the intraaortic balloon clamp causing migration into the left ventricle, rupture of the aorta, or transient hemiparesis (11). In addition, retrograde aortic dissections and a high incidence of strokes were described, possibly related to intimal tears at the site of the common iliac artery from balloon insertion and inadequate de-airing, respectively. Vascular injuries at the site of femoral cannulation and interference with atherosclerotic plaques in the aorta posed additional potential adverse outcomes. Advocates of the direct transaortic clamping technique suggested that this technique was safer than the intraaortic occlusive approach, and also at a lower cost, resulting in a change in practice at some institutions (24,25). However, concerns have been raised regarding clamping injuries to the pulmonary artery and atrial appendage (11,16), and one study involving 36 patients using transcranial Doppler reported fewer embolic signals with endoclamp usage compared to transaortic clamping (26).
Regardless of the specific surgical technique, proponents of the minimally invasive approach highlight findings of decreased hospitalization duration (16), reduced bleeding (27) and improved cosmetic outcomes (28) compared to conventional sternotomy. Indeed, two previous comprehensive meta-analyses reported minimally invasive mitral surgery to be associated with reduced need for reoperation for bleeding, decreased bleeding, need for transfusions, atrial fibrillation, sternal wound infection, scar dissatisfaction, ventilation time, ICU stay, hospitalization, and reduced time to return to normal activity (9,10). However, these meta-analyses included a number of different surgical procedures deemed to be ‘minimally invasive’, including hemisternotomies and parasternal incisions that are no longer performed currently (10). In addition, comparative studies that included patients with significant variations in valvular disease aetiology and surgical procedures between the ‘minimally invasive’ versus standard sternotomy groups were categorized together for analysis. This may have falsely reported superior outcomes for patients who underwent MIMVR, as this cohort usually consisted of more favourable surgical candidates with better functional status and less aggressive valvular pathology compared to patients who underwent conventional sternotomy. Certainly, patients diagnosed with infective endocarditis who had previous cardiac surgery and subsequently requiring mitral valve replacement can be expected to have significantly different clinical outcomes compared to patients with Barlow’s disease who undergo first-time mitral valve repair.
In contrast to previous reports, our meta-analysis focused on a specific selection of comparative studies that involved patients with degenerative mitral valve disease who underwent mitral valve repair. Our findings suggest there were no statistically significant differences between MIMVR and conventional mitral valve repair in regards to mortality, stroke, renal failure, wound infection, reoperation for bleeding, aortic dissection, myocardial infarction, atrial fibrillation, or readmission within 30 days. The duration of ICU stay was shorter for patients who underwent MIMVR, but there was no significant difference between the two approaches in the duration of hospitalization. Patients who underwent MIMVR required longer cross clamp time as well as cardiopulmonary bypass time. No significant heterogeneity was detected between studies in regards to clinical outcomes. Both MIMVR and conventional sternotomy groups demonstrated satisfactory echocardiographic outcomes, with the incidence of moderate/severe MR dropping from 98.7% and 98.4% preoperatively to 0.1% and 0.3% postoperatively, respectively. Systematic evaluation of pain-related outcomes was only measured in the RCT by Speziale et al., which reported significantly lower pain scores measured by visual analogue scale at second, fourth and sixth days postoperatively. Anecdotal reporting of pain-related outcomes from earlier series on MIMVR was relatively disappointing (11).
A number of limitations to our study should be acknowledged and our results should be interpreted with caution. Firstly, our systematic review of the current literature has demonstrated that the quantity of the existing evidence for MIMVR versus conventional surgery in patients with degenerative mitral valve disease who undergo surgical repair is relatively limited. Although propensity score matching and other attempts have been made to balance the two treatment arms, all but one study were retrospective studies that may be liable to patient selection bias. Follow-up periods of the selected studies were generally shorter than five years, and long-term echocardiographic data was scarce. It should be acknowledged that the definitions of certain endpoints varied between studies, such as the inclusion of stroke and transient ischaemic attack (TIA) in some reports and only stroke in others. Finally, there was significant heterogeneity in time-related endpoints, which may reflect the varying degrees of complexity involved in mitral valve repair techniques between individual institutions and the divergent discharge patterns in different countries.
In conclusion, the present meta-analysis comparing MIMVR with the conventional sternotomy approach for patients with degenerative mitral valve disease requiring repair did not identify any statistically significant difference in regards to perioperative clinical outcomes. Patients who underwent MIMVR required significantly longer periods of cross-clamping and cardiopulmonary bypass. However, patients who underwent the minimally invasive approach had a significantly shorter ICU stay period, although this was not translated into a shorter hospitalization duration. Although previous studies claim MIMVR results in reduced pain and quicker recovery (29), there appears to be a relative paucity of evidence to support these claims. Only one study reported improved pain outcomes for patients who underwent MIMVR within the first week postoperatively (15). In view of the learning curve and multi-disciplinary training required to develop and maintain a successful MIMVR program, these procedures should currently be limited to specialist centres until more robust evidence supports broader adoption of this surgical technique. Future studies should aim to attain longer clinical and echocardiographic follow-up in a randomized setting.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Cosgrove DM, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7 [PubMed] [Google Scholar]
- 2.Calafiore AM, Angelini GD, Bergsland J, et al. Minimally invasive coronary artery bypass grafting. Ann Thorac Surg 1996;62:1545-8 [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013;2:10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitwood WR, Jr, Wixon CL, Elbeery JR, et al. Video-assisted minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg 1997;114:773-80; discussion 780-2 [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23 [PubMed] [Google Scholar]
- 6.McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206 [DOI] [PubMed] [Google Scholar]
- 7.Arcidi JM, Jr, Rodriguez E, Elbeery JR, et al. Fifteen-year experience with minimally invasive approach for reoperations involving the mitral valve. J Thorac Cardiovasc Surg 2012;143:1062-8 [DOI] [PubMed] [Google Scholar]
- 8.Modi P, Rodriguez E, Hargrove WC, 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7 [DOI] [PubMed] [Google Scholar]
- 9.Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103 [DOI] [PubMed] [Google Scholar]
- 10.Modi P, Hassan A, Chitwood WR., Jr Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52 [DOI] [PubMed] [Google Scholar]
- 11.Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6 [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg 2011;142:77-83 [DOI] [PubMed] [Google Scholar]
- 16.Ryan WH, Brinkman WT, Dewey TM, et al. Mitral valve surgery: comparison of outcomes in matched sternotomy and port access groups. J Heart Valve Dis 2010;19:51-8; discussion 59 [PubMed] [Google Scholar]
- 17.Raanani E, Spiegelstein D, Sternik L, et al. Quality of mitral valve repair: median sternotomy versus port-access approach. J Thorac Cardiovasc Surg 2010;140:86-90 [DOI] [PubMed] [Google Scholar]
- 18.Suri RM, Schaff HV, Meyer SR, et al. Thoracoscopic versus open mitral valve repair: a propensity score analysis of early outcomes. Ann Thorac Surg 2009;88:1185-90 [DOI] [PubMed] [Google Scholar]
- 19.Ruttmann E, Laufer G, Muller LC. Development of a minimally invasive mitral valve surgery program – The Innsbruck experience. Eur Surg 2006;38:320-5 [Google Scholar]
- 20.Grossi EA, LaPietra A, Ribakove GH, et al. Minimally invasive versus sternotomy approaches for mitral reconstruction: comparison of intermediate-term results. J Thorac Cardiovasc Surg 2001;121:708-13 [DOI] [PubMed] [Google Scholar]
- 21.de Vaumas C, Philip I, Daccache G, et al. Comparison of minithoracotomy and conventional sternotomy approaches for valve surgery. J Cardiothorac Vasc Anesth 2003;17:325-8 [DOI] [PubMed] [Google Scholar]
- 22.Gaudiani VA, Grunkemeier GL, Castro LJ, et al. Mitral valve operations through standard and smaller incisions. Heart Surg Forum 2004;7:E337-42 [DOI] [PubMed] [Google Scholar]
- 23.Chitwood WR, Jr, Elbeery JR, Chapman WH, et al. Video-assisted minimally invasive mitral valve surgery: the “micro-mitral” operation. J Thorac Cardiovasc Surg 1997;113:413-4 [DOI] [PubMed] [Google Scholar]
- 24.Chitwood WR, Jr, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg 1997;63:1477-9 [DOI] [PubMed] [Google Scholar]
- 25.Dogan S, Aybek T, Risteski PS, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg 2005;79:492-8 [DOI] [PubMed] [Google Scholar]
- 26.Maselli D, Pizio R, Borelli G, et al. Endovascular balloon versus transthoracic aortic clamping for minimally invasive mitral valve surgery: impact on cerebral microemboli. Interact Cardiovasc Thorac Surg 2006;5:183-6 [DOI] [PubMed] [Google Scholar]
- 27.Grossi EA, Galloway AC, Ribakove GH, et al. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg Forum 1999;2:212-5 [PubMed] [Google Scholar]
- 28.Wang D, Wang Q, Yang X, et al. Mitral valve replacement through a minimal right vertical infra-axillary thoracotomy versus standard median sternotomy. Ann Thorac Surg 2009;87:704-8 [DOI] [PubMed] [Google Scholar]
- 29.Walther T, Falk V, Metz S, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg 1999;67:1643-7 [DOI] [PubMed] [Google Scholar]