Natural killer (NK) cells provide an early defense against intracellular pathogens, such as parasites and viruses, and protect against spontaneous tumors. The NK cell response is not only cytotoxicity, which is mediated through polarized release of cytolytic granules towards target cells, but also secretion of cytokines and chemokines. In many cases, the potent release of cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, is sufficient for the effector function of NK cells, in the absence of perforin-dependent killing of other cells (Strowig et al., 2008). Removal of tumor cells by NK cells occurs not only through a perforin-dependent pathway, but also by expression of the Fas ligand and ‘tumour necrosis factor (TNF)-related apoptosis-inducing ligand’ (TRAIL) (Smyth et al., 2002). NK cells belong to the innate immune system, but they share some features of adaptive immunity, and are integrated in the immune system as a whole, through reciprocal regulation with dendritic cells, macrophages, and other cell types (Moretta et al., 2008). For example, NK cells can promote a T helper type 1 (Th1) response through their ability to prime dendritic cells and to produce interferon (IFN)-γ. Priming in vivo is required for NK cells to become responsive and this step involves specific interactions with other cells types. There are four distinct phenotypic subsets of NK cells: CD16+ CD56dim NK cells in peripheral blood, CD16− CD56bright NK cells that are preferentially recruited into tissues through expression of L-selectin (CD62L) (Cooper et al., 2001), mucosal NK cells that produce IL-22 (Vivier et al., 2009), and uterine NK cells that may promote proper vascularization during early pregnancy (Ashkar et al., 2000; Hanna et al., 2006; Rajagopalan et al., 2006).
In the absence of a receptor repertoire generated by somatic DNA recombination and mutation, NK cells must rely on innate, germ-line encoded receptors to distinguish healthy cells from those that should be disposed of. To achieve this task, NK cells use a large number of receptors, each one with unique specificity for ligands and signaling properties. Some of the ligands are expressed broadly on many cell types, while others are expressed predominantly by hemapoietic cells. The expression of several ligands of NK cell activation receptors is induced as a result of infection, stress, or cell transformation. Some of the NK cell receptors have inhibitory functions and serve to protect healthy cells by recognizing major histocompatibility complex (MHC) class I molecules.
The focus of this Unit is on signaling by receptors that activate NK cell effector function, and those that inhibit NK cell responses. How NK cells respond to soluble stimulators, such as cytokines and chemokines, is fairly well understood and will not be reviewed here. However, it should be noted that the response of NK cells to cytokines and chemokines during viral infections is highly regulated by positive and negative signaling through different signal transducers and activators of transcription (STAT) molecules (Lee et al., 2007; Nguyen et al., 2002). To a great extent, it is generally assumed that signaling components in NK cells participate in signal transduction in a way similar to pathways established in other cells. However, it is important to keep in mind that it is not always the case. As much as possible, work done with NK cells will be highlighted here.
NK cell activation
NK cell cytotoxicity is a highly regulated process. It involves NK cell adhesion to target cells and the interaction between activating NK cell receptors and their respective ligands on the target cell surface. This induces intracellular signaling pathways resulting in polarization and release of cytotoxic granules towards the attached target. Various experimental systems have been used to study signaling after receptor stimulation. In this respect, it is worth noting that crosslinking with Abs is no substitute for the receptor–ligand interactions that occur upon contact of NK cells with other cells. Likewise, NK cell lines may not faithfully reproduce the signal transduction that occurs in primary NK cells.
Activation Receptors in Natural Cytotoxicity
In contrast to T or B cells, NK cells do not possess a single activating receptor that dominates their regulation. Instead, they possess a large array of receptors, which can act in synergy to regulate NK cell effector functions (Bryceson et al., 2006a; Lanier, 2005). These receptors recognize ligands on infected or transformed cells (Bottino et al., 2005; Gasser and Raulet, 2006) and thereby enable NK cells to detect and eliminate these cells. Interestingly, freshly isolated resting human NK cells can only be activated by triggering two or more activating receptors in combination (Bryceson et al., 2006b). The only exception seems to be the low affinity Fc receptor Fc γRIIIa (CD16), which is sufficient by itself to induce activation of resting NK cells. Therefore, all stimulatory receptors for natural cytotoxicity should be considered as co-activating. In the following we will discuss the receptor proximal signaling pathways of the different co-activating NK cell receptors.
Natural Cytotoxicity Receptors (NCR)
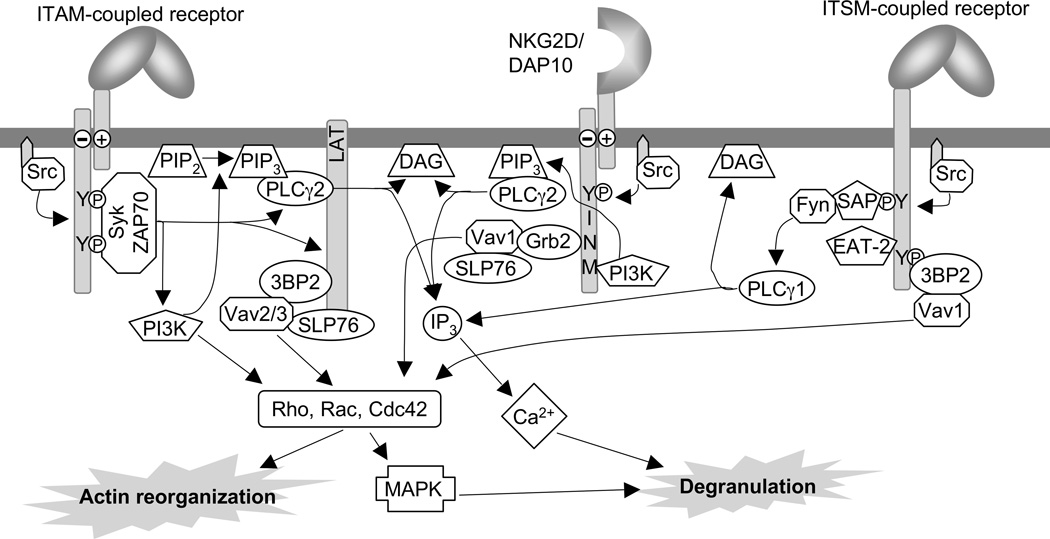
The NCR NKp30, NKp44, and NKp46 were among the first identified activating receptors in NK cells (Cantoni et al., 1999; Pende et al., 1999;). While NKp46 expression is specific for NK cells, NKp30 and NKp44 can also be found on subsets of T cells. NCR are immunoglobulin-like type I transmembrane glycoproteins that are expressed as monomers and contain a positively charged amino acid in their transmembrane domain. This charge facilitates the association of the NCR with signaling partner chains containing a negative charge in their transmembrane region. NKp46 and NKp30 associate with disulfide-linked homodimers or heterodimers of the TCR ζ and/or the FcR γ chains, whereas NKp44 associates with the disulfide-linked homodimer DAP12. All these signaling partners contain an immunoreceptor-based activation motif (ITAM) in their cytoplasmic tail. While several viral ligands for the NCR have been identified (Arnon et al., 2006), the cellular ligands for these receptors remain poorly defined (Byrd et al., 2007). The intracellular protein BAT3 has been described as an NKp30 ligand (Pogge von Strandmann et al., 2007). How BAT3 is exposed on the surface of target cells is unknown. NCR-mediated NK cell activation is critically dependent on the ITAM motif of the associated partner chains and is very similar to the extensively studied signal transduction of the T cell receptor or B cell receptor. Briefly, the most important signaling steps are as follows (Figure 1). NCR engagement induces Src-family kinase-mediated tyrosine phosphorylation of the ITAM sequence in the associated partner chains. This induces binding of tyrosine kinases Syk and ZAP70 to the ITAM. These kinases phosphorylate transmembrane adapter molecules such as LAT and NTAL leading to the association, phosphorylation and activation of several signaling complexes, including phosphatidyl-inositol-3-OH kinase (PI3K), phospholipase C (PLC-γ1, PLC-γ2) and Vav-1, 2, 3. There are differences between human and mouse NK cells and between different ITAM-coupled receptors as to the importance of the different PLC-γ and Vav isoforms (Billadeau et al., 1998; Cella et al., 2004; Tassi et al., 2005; Ting et al., 1992; Upshaw et al., 2005).
Figure 11.9B.1.
Complexity in Activation Pathways. The redundant signaling pathways emanating from ITAM-, DAP10-, and ITSM-coupled receptors are shown. NKG2D/DAP10 and the ITSM-coupled receptor 2B4 synergize for activation of primary resting NK cells.
NKG2D
The C-type lectin-like receptor NKG2D is a type II transmembrane glycoprotein which is expressed as a disulfide-linked homodimer on the surface of NK cells, γδ T cells and CD8+ T cells (Bauer et al., 1999). NKG2D carries an arginine residue in the central portion of its transmembrane region, which mediates the association with the signaling partner chain DAP10 (Wu et al., 1999). DAP10 is also a disulfide-linked homodimer. One homodimer of NKG2D associates with two homodimers of DAP10, thereby forming a hexameric complex (Garrity et al., 2005). NKG2D can bind several ligands such as the human MICA/B and ULBP1, 2, 3, or 4, and the mouse Mult1, H60, and the Rae-1 family. The expression of these ligands is up-regulated on infected, stressed, or transformed cells (Gasser et al., 2005; Joncker and Raulet, 2008). This makes NKG2D an important co-activating receptor for the recognition of tumors (Guerra et al., 2008). Signaling of human NKG2D is critically dependent of DAP10, while a mouse-specific splice variant of NKG2D can also associate with DAP12 (Diefenbach et al., 2002). The short cytoplasmic tail of DAP10 contains a tyrosine based signaling motif (YINM), which can be phosphorylated by Src-family kinases and bind either the p85 subunit of PI3K or the adapter molecule Grb2 (Figure 1). Binding of both molecules is essential for NKG2D-mediated Ca2+ flux and cytotoxicity (Upshaw et al., 2006). PI3K activity is important for the production of phosphatidyl-inositol-3,4,5-trisphosphate (PIP3), facilitating the plasma membrane recruitment of pleckstrin homology domain containing proteins such as PLC-γ1, PLC-γ2, Vav1 and Tec-family kinases. Grb2 recruits Vav1 and is important for the phosphorylation and activation of SLP76 and PLC-γ2. Vav1 is important for the induction of actin reorganization and the polarization of the microtubule organizing center (MTOC) toward target cells (Cella et al., 2004; Graham et al., 2006). NKG2D signaling does not require Syk or ZAP70 kinases, and is independent of the transmembrane adapters LAT and NTAL (Billadeau et al., 2003; Chiesa et al., 2006; Zompi et al., 2003).
2B4, NTB-A, and CRACC
2B4, NTB-A and CRACC are members of the SLAM-related receptors (Claus et al., 2008). The expression of these monomeric immunoglobulin-like type I transmembrane glycoproteins is not limited to NK cells and can be found on many other immune cells. While 2B4 binds to CD48, which is widely expressed on cells of hematopoietic origin, NTB-A and CRACC are homophilic. The intracellular domain of these receptors contains immunoreceptor tyrosine-based switch motifs (ITSM), which can be phosphorylated by Src-family kinases upon receptor cross-linking (Figure 1). The phosphorylated ITSM then recruits small SH2-domain containing adapters such as SAP (SH2D1A) (Sayos et al., 1998), EAT-2 and ERT (which is only expressed in mice) (Eissmann et al., 2005; Eissmann and Watzl, 2006; Roncagalli et al., 2005). SAP then recruits the Src-family kinase Fyn by an untypical SH2-SH3 domain interaction (Chan et al., 2003; Latour et al., 2003). This interaction is crucial for 2B4-mediated NK cell activation. Mutations in SAP are the cause for a genetic disorder called X-linked lymphoproliferative disease (XLP). These patients show an uncontrollable and fatal lymphoproliferation in response to EBV infection (Nichols et al., 2005). 2B4 and NTB-A-mediated NK cell activation is defective in XLP patients (Benoit et al., 2000; Bottino et al., 2001; Nakajima et al., 2000; Parolini et al., 2000; Tangye et al., 2000), demonstrating the importance of SAP for the function of these receptors. Similarly, 2B4-mediated NK cell activation is also defective in Fyn-deficient mice (Bloch-Queyrat et al., 2005). CRACC signaling is independent of SAP and seems to rely on the adapter EAT-2 (Bouchon et al., 2001; Cruz-Munoz et al., 2009). Another adapter protein that binds to phosphorylated 2B4 is 3BP2 (Saborit-Villarroya et al., 2005). Phosphorylated 3BP2 interacts with the signaling molecules Vav1, LAT, and PLC-γ. Stimulation of 2B4 leads to phosphorylation of LAT, Vav1, PLC-γ1, c-Cbl, Grb2 and SHIP (Bottino et al., 2000; Chen et al., 2004; Watzl et al., 2000).
Under certain circumstances 2B4, NTB-A and CRACC can also inhibit NK cell functions (Veillette, 2006). In humans inhibitory signaling of these receptors has been reported in rare cases of SAP deficiency, such as XLP patients (Parolini et al., 2000; Bottino et al., 2001), or in immature NK cells, which express low levels of SAP (Sivori et al., 2002). However, inhibitory signaling of 2B4 in mice has been reported. A recent report has suggested that the amount of 2B4 expression, the strength of cross-linking, and the expression level of SAP determine if 2B4 is activating or inhibitory (Chlewicki et al., 2008). High levels of 2B4 expression, heavy cross-linking, and low SAP expression would favor inhibitory signaling of the receptor. The basis for this inhibitory signaling is not completely understood. Phosphorylated 2B4 can recruit the phosphatases SHP-1, SHP-2, and SHIP and the inhibitory kinase Csk (Eissmann et al., 2005). SAP competes with these inhibitory molecules for binding to the receptor, which could explain the negative signaling in the absence of SAP. EAT-2 and ERT can mediate inhibitory signaling by 2B4 when bound to the receptor (Roncagalli et al., 2005). However, as EAT-2 can induce positive signals when bound to NTB-A (Eissmann and Watzl, 2006) or CRACC (Cruz-Munoz et al., 2009), the basis for its inhibitory signaling when bound to 2B4 is controversial.
Other Receptors
Other co-activating NK cell receptors include DNAM-1, NKp80, and CD2. Not much is known about the signal transduction of these molecules. DNAM-1 (CD226, also known as PTA-1) is an immunoglobulin-like type I transmembrane glycoprotein that is expressed on NK cells, T cells and monocytes (Shibuya et al., 1996). The ligands for DNAM-1 are poliovirus receptor (CD155) and Nectin-2 (CD112), both of which are expressed on epithelial and endothelial cells and are over-expressed on certain tumors (Bottino et al., 2003; Tahara-Hanaoka et al., 2004). Tumor surveillance is therefore impaired in DNAM-1 deficient mice (Gilfillan et al., 2008; Iguchi-Manaka et al., 2008). The cytoplasmic tail of DNAM-1 contains three tyrosine residues that can be phosphorylated by Src-family kinases upon receptor engagement and recruit actin-binding proteins (Ralston et al., 2004; Shibuya et al., 2003). Interestingly, DNAM-1-mediated NK cell activation is dependent on its association with lymphocytes function-associated antigen 1 (LFA-1) and DNAM-1 signaling is therefore involved in promoting cellular adhesion (Shibuya et al., 2003).
The C-type lectin-like receptor NKp80 (also known as KLRF1) is a type II transmembrane glycoprotein that is expressed as a disulfide-linked homodimer on the surface of NK cells and a subset of CD8+ T cells (Kuttruff et al., 2009; Vitale et al., 2001). The ligand for NKp80 is the activation-induced C-type lectin (AICL) (Welte et al., 2006), which is selectively expressed on myeloid cells, such as monocytes and macrophages, and the NKp80-AICL interaction seems to contribute to reciprocal activation of NK and myeloid cells at sites of inflammation. NKp80 carries two tyrosine-based signaling motifs in its cytoplasmic tail, but nothing is known so far about the signal transduction of this receptor.
CD2 is a member of the immunoglobulin super-family and is expressed as a type I transmembrane glycoprotein on T cells and NK cells. The ligands for CD2 are CD58 (LFA-3) and, to a lower extent, CD48 in humans, but CD48 is the only ligand in mice (Davis et al., 1998). The cytoplasmic domain of CD2 contains a proline-rich sequence that interacts with the Src-family kinase Lck (Bell et al., 1996) and several adapter molecules (Li et al., 1998). However, the exact signaling mechanism of CD2 is still unclear. Signaling through CD2 has been shown to enhance cellular adhesion and CD2 is linked to the cytoskeleton.
Several members of the killer cell immunoglobulin-like receptors (KIR) in humans, some of the Ly49 receptors in mice, and the CD94/NKG2C receptor complex carry a negative charge in their transmembrane region through which they associate with the ITAM-containing partner chain DAP12 (Lanier et al., 1998; Lanier et al., 1998; Smith et al., 1998). Except for Ly49H and Ly49P in mice, which confer resistance to mouse cytomegalovirus (Arase et al., 2002; Kielczewska et al., 2009; Smith et al., 2002), the function of activating isoforms in the KIR, Ly49, and CD94 receptor families remains unknown.
Fcγ Receptor Signaling
The CD56dim subset of NK cells expresses the Fcγ receptor CD16, through which NK cells mount antibody-dependent cellular cytotoxicity (ADCC). CD16 associates with disulfide-linked homodimers or heterodimers of the TCR ζ and/or the FcR γ chains (Lanier et al., 1991). The signaling of CD16 follows therefore the classical ITAM pathway of Src-family kinase-mediated tyrosine phosphorylation, Syk association leading to PI3K, Vav1 and PLC-γ1 and PLC-γ2 activation (Billadeau et al., 1998; Ting et al., 1992). Signaling for ADCC involves a PI3K-dependent activation of the ADP-ribosylation factor (Arf)-6, which couples CD16 to the production of phosphatidylinositol-4,5-bisphosphate (PIP2) by a phosphatidylinositol-4-phosphate 5-kinase (PI5K) and the activation of phospholipase D (Galandrini et al., 2005). PI5K activity in primary human NK cells is required for degranulation but not for granule polarization (Micucci et al., 2008). In contrast to other ITAM coupled NK cell receptors, triggering of CD16 is by itself sufficient to activate cytotoxicity and cytokine production in resting human NK cells (Bryceson et al., 2006b). So far it is unknown which part of the CD16 signaling cascade is responsible for this unique ability of this receptor.
Signaling by Integrins
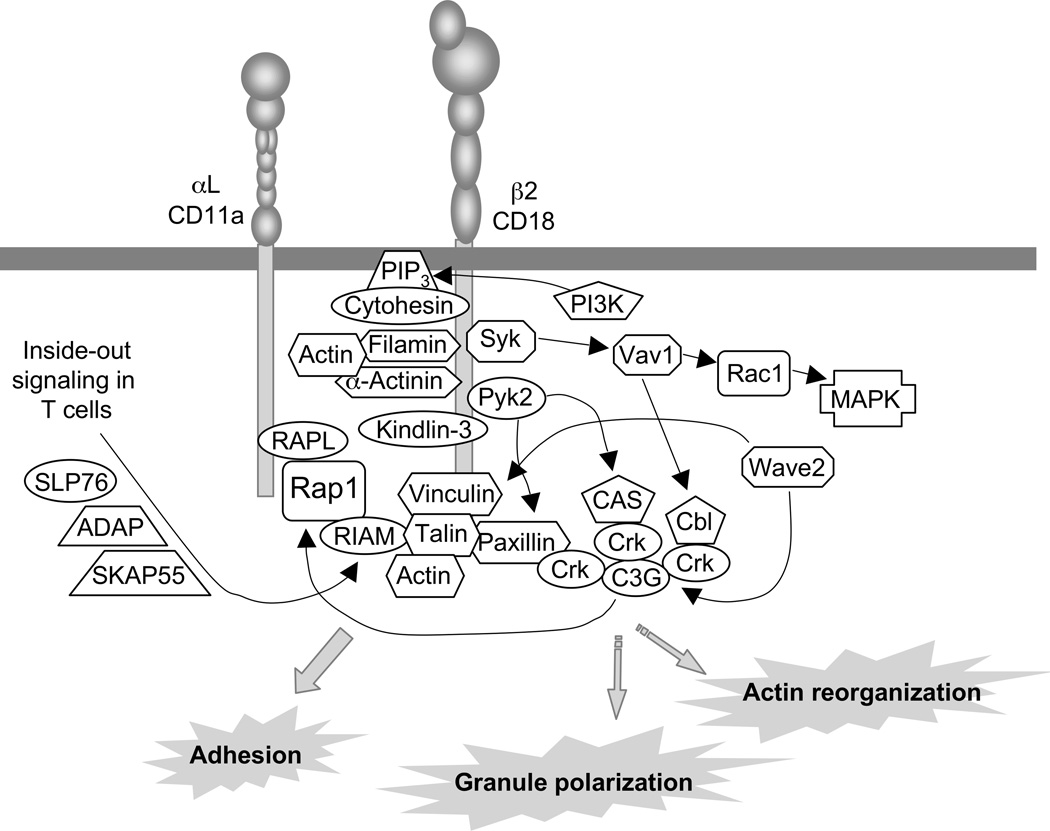
NK cells express several types of integrins, including LFA-1 (αLβ2, CD11a/CD18) and VLA-4 (α4β1, CD49d/CD29). Integrin α4β1 binds to the vascular cell adhesion molecule (VCAM)-1 (CD106), an Ig-like molecule expressed on activated endothelial cells, and to the extracellular matrix component fibronectin. LFA-1 binds to ICAM-1, ICAM-2, and ICAM-3, which are expressed on endothelium and upregulated during inflammation. Interaction of integrins with their ligand on other cells is a complex process, as it requires an ‘inside-out’ signal from other receptors that promotes a change in the integrin from a bent, low affinity conformation to extended forms, which are of intermediate or high affinity. The strength of adhesion is determined by the integrin affinity and avidity for the ligand, and by the interaction of the integrin cytoplasmic domains with the cytoskeleton and signaling molecules. The β1 and β2 integrin cytoplasmic tails bind to talin, a protein that links them to the actin cytoskeleton and is essential for ‘inside-out’ activation, even when such signals originate from different receptors and signaling pathways (Cantor et al., 2008). Inside-out signaling, which has been studied mainly following stimulation of the T-cell receptor (TCR), requires the scaffolding proteins ‘adhesion and degranulation-promoting adapter protein’ (ADAP, formerly known as SLAP-130 or Fyb) and ‘Src kinase-associated phosphoprotein of 55kDa’ (SKAP55) (Mor et al., 2007; Wang and Rudd, 2008). ADAP is required, independently of LFA-1, for polarization of the MTOC and recruitment of dynein in T cells (Combs et al., 2006). The molecule cytohesin is a guanine-nucleotide exchange factor for Arf-6, and promotes inside-out signaling through binding to the membrane-proximal part of the β2 cytoplasmic tail (Geiger et al., 2000). The small GTPase Rap1 and its effector ‘regulator for cell adhesion and polarization enriched in lymphoid tissues’ (RAPL) are key elements of inside-out signaling. RAPL binds directly to the αL subunit of LFA-1. Furthermore, WAVE2 regulates inside-out signaling by recruiting vinculin and talin to the immunological synapse and by promoting Rap1 activation through a CrkL–C3G pathway (Nolz et al., 2007; Nolz et al., 2008). The current model (Figure 2) proposes that a complex RAPL–Rap1GTP–RIAM interacts with talin, and that RIAM provides a link to the ADAP/SKAP-55 complex (Cantor et al., 2008; Menasche et al., 2007; Mor et al., 2007; Wang and Rudd, 2008). C3G and RIAM are guanine exchange factors for Rap1. However, ADAP is dispensable for NK cell function, including adhesion, cytotoxicity, and cytokine production (Fostel et al., 2006). This could either reflect the fact that an inside-out signal is not essential for LFA-1 signaling in NK cells, or be due to a redundancy of ADAP with another signaling component.
Figure 11.9B.2.
LFA-1 Signaling. For the sake of clarity, Src-family kinases, which are involved in many of the signaling steps, have not been included in the diagram. Most of the information shown is derived from data obtained with T cells. LFA-1 signaling in NK cells may be different in some respects. For example, inside-out signals for LFA-1 are not a stringent requirement in NK cells, and LFA-1 signaling in NK cells is sufficient to induce granule polarization. The detailed pathways leading to actin reorganization and to granule polarization are not known.
The importance of β2 (CD18) integrins is underscored by the severe infections suffered by individuals with homozygous mutations in CD18, a disease known as leukocyte adhesion deficiency type I (LAD-I). Recently, the genetic basis for the even more severe LAD-III disease has been mapped to the gene encoding Kindlin-3 (Hidalgo and Frenette, 2009). Kindlin-3, which is expressed in hematopoietic cells, binds to the cytoplasmic tail of β1, β2, and β3 integrins and promotes inside-out signaling. Other proteins that link β1 and β2 integrin directly to the cytoskeleton include filamin and α-actinin (Cantor et al., 2008). Conformational changes in integrins, which are required to achieve high affinity binding to ligands, are facilitated by force, as it occurs during adhesion under flow (Alon and Dustin, 2007). Linkage of integrins to the underlying cellular cytoskeleton is required for their response to shear forces and the ensuing switch to higher affinity states.
β1 Integrins
Protein kinase D1 (PKD1) promotes clustering and activation of integrin β1 in T cells through membrane recruitment of Rap1 (Burbach et al., 2007). The analysis of ‘outside-in’ signaling by β1 integrins is complicated due to a number of cis interactions with tetraspannin proteins, such as CD9, CD63, and CD81, which recruit the signaling enzymes PI4K, PKCα and PKCβII (Berditchevski, 2001). The cytoplasmic tail of β1 integrin binds to integrin-linked kinase (ILK), which phosphorylates myosin light chain and Akt (Wu and Dedhar, 2001). Through a direct association of α4 with paxillin, α4β1 integrin provides an ‘inside-out’ signal to the β2 integrin LFA-1 (Cantor et al., 2008).
Engagement of β1 integrin specifically on NK cells results in phosphorylation of tyrosine kinase Pyk2 and the cytoskeleton protein paxillin, and formation of a phospho-Pyk2–paxillin complex (Gismondi et al., 1997). β1 integrin crosslinking induces formation of a Shc-Pyk2-Grb2 complex and leads to Erk-dependent secretion of interferon-γ (Mainiero et al., 1998). Furthermore, NK cells secrete IL-8 in response to β1 integrin binding to fibronectin through a signaling pathway that involves activation of the small GTPase Rac1 by Vav, and of the Rac1 effector Pak1, which leads to activation of the p38 MAPK (Mainiero et al., 2000).
β2 Integrins
Some of the known interactions of LFA-1 with signaling components are depicted in Figure 2. Most of them are derived from experiments performed with T cells. It is therefore important to keep in mind that LFA-1 has unique signaling properties in NK cells, which are not shared with LFA-1 in T cells. First, in resting NK cells, inside-out signaling is not strictly required for binding of LFA-1 to ICAM-1 (Barber and Long, 2003). Slow and signaling-dependent binding of resting NK cells to ICAM-1 implies that LFA-1 can transmit its own inside-out signal to other LFA-1 molecules. However, co-engagement of NK cell activation receptors 2B4 and CD2 results in stronger inside-out signals (Barber and Long, 2003). Second, binding of LFA-1 to ICAM-1, either attached to beads or expressed on insect cells, is sufficient to promote polarization of cytolytic granules (Barber et al., 2004; Bryceson et al., 2005). This contrasts with signaling in T cells, where LFA-1 cannot signal on its own. Nevertheless, LFA-1 engagement in T cells does enhance TCR-dependent polarization of cytolytic granules (Anikeeva et al., 2005). In addition, Mg2+-induced binding of the high affinity form of LFA-1 on T cells to ICAM-1 stimulates F-actin reorganization independently of TCR signals (Porter et al., 2002).
As first described in platelets with β3 integrin, which activates MAP kinase signaling through Vav, integrins can signal independently of actin polymerization (Miranti et al., 1998). In NK cells, engagement of LFA-1 by ICAM-1 on insect cells was sufficient to induce actin-independent activation of the Vav1-Rac1 pathway (Riteau et al., 2003). Therefore, LFA-1 has the unique ability to provide early activation signals to NK cells. These early LFA-1-dependent signals are sensitive to inhibition by MHC class I-specific inhibitory receptors (Barber et al., 2004). Consistent with its ability to signal independently of actin polymerization, LFA-1 engagement on NK cells induces phosphorylation of the Wiskott-Aldrich syndrome protein (WASp), a known regulator of the actin cytoskeleton (Gismondi et al., 2004), and phosphorylation of Pyk2 and Pyk2 association with paxillin (Gismondi et al., 2003).
The tyrosine kinase Syk binds directly to integrin β chains through its SH2 domain but in a phospho-tyrosine independent way (Woodside et al., 2002). Signaling by β2 and β3 integrins in neutrophils, platelets, and myeloid cells requires Syk and an ITAM-containing transmembrane adapter molecule, which can be either DAP12 or the FcR γ chain (Jakus et al., 2007). Similarly, calcium signaling through the β2 cytoplasmic tail of LFA-1 in T cells required the TCR, a requirement that could be fulfilled by the TCR ζ chain alone (Sirim et al., 2001). It remains to be seen whether these unusual signaling properties contribute to the unique ability of LFA-1 to trigger granule polarization in primary NK cells.
Key Events in NK cell Activation
NK cell activation uses highly redundant signaling pathways originating from many different surface receptors (Figure 1). Additionally, while several critical and related signaling molecules are expressed selectively in B-cells (e.g. Syk and SLP-65) and T-cells (e.g. ZAP70 and SLP-76), NK cells express all of these proteins. Therefore, it has been difficult to identify critical signaling pathways using genetic approaches (Tassi et al., 2006). Furthermore, even the genetic deletion of both Syk and ZAP70 does not significantly impair NK cell development and cytotoxic function. NK cell activity is also largely intact in mice lacking all three ITAM-containing signaling subunits DAP12, TCR ζ and FcR γ (Chiesa et al., 2006; Colucci et al., 2002). However, a few molecules and signaling pathways have emerged as essential for NK cell activation.
Src-family Kinases
Basically, all activating NK cell receptors depend for their phosphorylation and function on the activity of Src-family kinases. This makes this family of kinases essential for NK cell functions. NK cells express many different members of this family, including Lck, Fyn, Src, Lyn, Yes and Fgr, and the activity of these kinases is highly redundant. Therefore, no single Src-family kinase is essential for NK cell function (Mason et al., 2006; van Oers et al., 1996). However, chemical inhibition of Src-family kinases efficiently blocks NK cell activation mediated by ITAM and non-ITAM coupled receptors. Several of the Src-family kinases are enriched in membrane microdomains, also referred to as lipid rafts, through the posttranslational addition of a lipid anchor (Cherukuri et al., 2001).
The initial step in co-activating NK cell receptor signaling seems to be receptor clustering induced by ligand binding. Receptor cross-linking either by ligands, some of which are pre-clustered on target cells (Eleme et al., 2004), or plate-bound antibodies is sufficient to induce signal transduction by all co-activating NK cell receptors. Whether ligand-induced conformational change occurs in these receptors is unknown. Receptor clustering induces a concentration of co-activating receptors such as 2B4 or NKG2D in membrane microdomains that are enriched in Src-family kinases, and where receptor phosphorylation is facilitated (Endt et al., 2007; Watzl and Long, 2003). In line with this, the 2B4 receptor is phosphorylated exclusively in membrane microdomains (Watzl and Long, 2003).
PLC-γ2
Exocytosis of lytic granules in NK cells is strongly dependent on PLC-γ phosphorylation and subsequent mobilization of Ca2+ from the endoplasmic reticulum. While the two isoforms of PLC-γ (PLC-γ1 and PLC-γ2) may be redundant in ITAM signaling pathways, signaling by the NKG2D/DAP10 receptor has an exclusive requirement for PLC-γ2 (Upshaw et al., 2005). PLC-γ2 is essential for NK cell cytotoxicity (Caraux et al., 2006; Tassi et al., 2005) and over-expression of PLC-γ1 can only partially rescue the cytotoxicity defect in PLC-γ2 deficient NK cells (Regunathan et al., 2006).
Vav
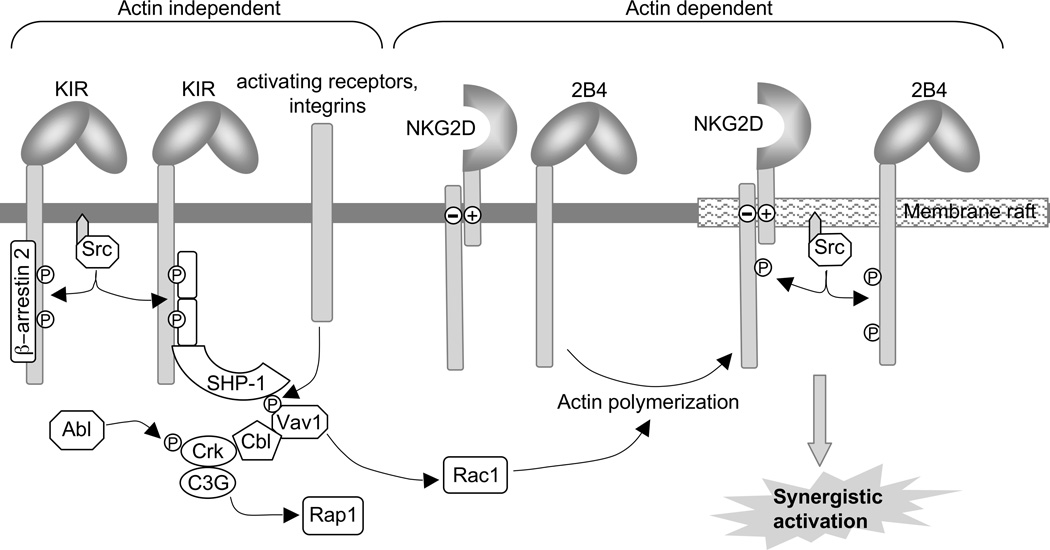
Vav proteins are essential components for NK cell activation pathways. NK cells express all three Vav isoforms (Vav1, Vav2 and Vav3), all of which have guanine nucleotide exchange factor (GEF) activity and thereby activate small GTPase proteins of the Rho family, primarily Rac1. These GTPases are important mediators of actin cytoskeleton reorganization, which is essential for the clustering of co-activating receptors and the polarization of the lytic granules toward the target cell (Figure 3). Rac1 also activates a PAK1–MEK–Erk pathway, which is important for granule polarization and release (Jiang et al., 2000). Vav1 is required for DAP10-mediated signaling pathways, whereas Vav2 and Vav3 are essential for the function of ITAM-coupled receptors (Cella et al., 2004; Chan et al., 2001; Colucci et al., 2001). Absence of all three Vav isoforms severely compromises NK cell cytotoxicity (Cella et al., 2004). The central role of Vav in the activation of NK cell responses is highlighted by the fact that it is selectively inactivated during inhibition by MHC class I-specific receptors as a preferred substrate of the ITIM-bound tyrosine phosphatase SHP-1 (Figure 3).
Figure 11.9B.3.
Model of Inhibitory Signaling in NK Cells. Binding of β-arrestin 2 to phosphorylated ITIM enhances the recruitment of SHP-1 and SHP-2 to phosphorylated ITIMs. The dephosphorylation of Vav1 by SHP-1 blocks its GEF activity and activation of Rac1. Phosphorylation of the adapter Crk by Abl, which is induced by inhibitory receptor engagement, results in disassembly of the Cbl–Crk–C3G complex. Inhibition is dominant over activation because it occurs upstream of the actin-dependent clustering and phosphorylation of co-activation receptors.
Granule Polarization and Degranulation
Killing of target cells by cytotoxic lymphocytes is a complex process (Fischer et al., 2007; Orange, 2008; Stinchcombe and Griffiths, 2007). The effector cell has to reorient its cytoskeleton, including the MTOC, and move cytolytic granules towards the target cell. Granules have to make contact with the plasma membrane where they will eventually fuse and release their content into the synaptic cleft. Studies of NK cells from patients with genetically defined immuno-deficiencies have identified the Wiskott-Aldrich syndrome protein (WASp) as an essential component of actin-dependent formation of the NK cell immune synapse (Gismondi et al., 2004; Orange et al., 2002a).
Several signals can induce granule polarization in NK cells. Granule polarization in a human NK cell line during contact with sensitive target cells required activation of the MAPK Erk through a PI3K–Rac1–MEK pathway (Jiang et al., 2000). Pharmacological inhibition of another MAP kinase, JNK, suggested that it is required for polarization (Li et al., 2008). A role for tyrosine kinase Pyk2 in polarization is supported by experiments with a dominant-negative mutant of Pyk2, which blocked MTOC and paxillin movement to the synapse, and NK cell cytotoxicity (Sancho et al., 2000). There may be a direct link between cytolytic granules and the actin cytoskeleton during polarization. Granule polarization in NK cells is dependent on the WASp-interacting protein (WIP) (Krzewski et al., 2008). A key connection between microtubules and actin during granule polarization may be provided by the Cdc42-interacting protein-4 (CIP4), a molecule known to bind directly to tubulin. CIP4 is associated with WASp at the MTOC and is required for MTOC polarization to the NK cell immune synapse (Banerjee et al., 2007). Myosin IIA, which is known as a regulator of actin dynamics, is required for a late step in the degranulation pathway, downstream of granule polarization in the cell line NKL (Andzelm et al., 2007). A feature unique to human NK cells is that LFA-1 binding to ICAM-1 is sufficient to induce granule polarization (Barber et al., 2004; Bryceson et al., 2005), which is not the case in T cells. Furthermore, in mouse NK cells that were derived from embryonic stem cells polarization required co-engagement of LFA-1 and NKG2D, and was dependent on talin (Mace et al., 2009).
As shown in T cells, the Rab27a GTPase effector protein Munc13-4, which is defective in patients with familial hemophagocytic lymphohistiocytosis 3 (FHL3) (Fischer et al., 2007), is required for NK cell degranulation (Marcenaro et al., 2006). FHL4 is a disease caused by mutations in the ‘soluble N-ethylmaleimide-sensitive factor’ ‘(SNF) attachment protein receptor’ (SNARE) syntaxin 11. The defect could be traced to a lack of degranulation by NK cells (Arneson et al., 2007; Bryceson et al., 2007). Expressed mutations of the ‘NF-κB essential modifier’ (NEMO) result in a syndrome known as hypohidrotic ectodermal dysplasia with immune deficiency (HED-ID). NK cells from HED-ID patients were defective in natural cytotoxicity but not ADCC (Orange et al., 2002b). How activation of the transcription factor NF-κB by NEMO contributes to NK cell cytotoxicity is still unknown.
INHIBITION
It was appreciated long ago that the activity of NK cells was under stringent negative regulation. Specifically, normal expression of MHC class I molecules protects cells from lysis by NK cells (Ljunggren and Kärre, 1985). These early findings were given a mechanistic basis when NK cell inhibitory receptors specific for MHC class I were identified in mouse and man (Ciccone et al., 1992; Karlhofer et al., 1992). As knowledge about these inhibitory receptors advanced, it became clear that they were the prototypes of a very large group of receptors with similar signaling properties (Long, 2008). The definition of a sequence motif, now known as the ‘immunoreceptor tyrosine-based inhibition motif’ (ITIM), for binding of the tyrosine phosphatase SHP-1 to human inhibitory ‘killer cell Ig-like receptors’ (KIR) (Burshtyn et al., 1996) led to the identification of many other ITIM-containing receptors with inhibitory properties in various types of cells (Long, 2008). Although the ITIM is evolutionary ancient, conserved from bony fish to primates, some of the ITIM-containing receptor families have evolved rapidly. For example, the inhibitory receptors for classical MHC class I molecules in mice, the Ly49 family, are completely different from their counterparts in humans, which are members of the KIR family. Despite the structural differences between KIR and the lectin-like Ly49 and CD94-NKG2A, signaling through the conserved ITIM is remarkably similar.
Inhibitory Receptors
There are many ITIM-containing receptors on different cell types and they bind to a large variety of ligands (Long, 2008). NK cells alone express several of these ITIM-containing receptors, some of which bind to non-MHC ligands. The human KIR family (also known as CD158) includes inhibitory receptors for HLA-B and HLA-C. In the mouse, members of the Ly49 family bind to H-2 class I molecules with varying degrees of specificity. The ITIM-containing CD94-NKG2A (also known as KLRC1 and CD159) heterodimeric receptor is conserved in both species but the ligands are not. Human CD94-NKG2A binds to the non-classical MHC molecule HLA-E, whereas the ligand in the mouse is Qa1. LILR (also known as ILT, LIR, and CD85) is the third family of ITIM-containing receptors on human NK cells that bind to MHC class I, and they display permissive binding to several different MHC class I molecules. In addition to these MHC-specific receptors, NK cells express several other ITIM-containing receptors (Long, 2008): NKR-P1 (CD161) binds to human LLT1 and to mouse Ocil/Clrb molecules; Siglec-7 (CD328) binds to sialic acids; LAIR-1 (CD305) binds to collagen; KLRG1 binds to cadherins; CEACAM-1 (also known as BGP and CD66a) binds to itself; PILRα binds to CD99; and finally CD300a (also known as IRp60), which has no known ligand. Considering how many cells express ligands for any one of these inhibitory receptors, one wonders how much inhibition has to be overcome during NK cell activation by contact with target cells.
Individual receptors within the KIR, Ly49, and CD94-NKG2 families are expressed randomly among NK cells. Thus, each individual NK cell expresses its own small repertoire of inhibitory receptors. Furthermore, some of these receptors may have a self MHC class I ligand, but others may not. Therefore, within most individuals, there are NK cells that have no inhibitory receptor for self, and others that express several. To ensure proper balance between activation and inhibition for every NK cell, NK cells undergo a form of education, which is determined by the MHC class I repertoire of the host (Anfossi et al., 2006; Gasser and Raulet, 2006; Yokoyama and Kim, 2006). The more MHC class I detected by inhibitory receptors on any given NK cell, the more intrinsically responsive that NK cell will be. Conversely, NK cells with no inhibitory receptor for self MHC will be hyporesponsive. The education mechanism is not known yet. It could be a form of anergy provoked by continuous stimulation due to weak inhibition (Gasser and Raulet, 2006). Alternatively, inhibitory receptors may deliver an instructive signal that permits acquisition of responsiveness (Yokoyama and Kim, 2006).
Inhibitory Signals
The first step in the inhibitory signaling pathway is phosphorylation of ITIM sequences in the inhibitory receptor. How this is achieved is still unknown. The model proposing that ITIMs become phosphorylated in trans by tyrosine kinases that are involved in activation pathways is difficult to reconcile with evidence that inhibition occurs at a very early step, independently of actin polymerization (Figure 3). Once phosphorylated, ITIMs recruit mainly the tyrosine phosphatases SHP-1 and SHP-2. Relative selectivity for SHP-1 or SHP-2 among different ITIM-containing receptors has been observed (Long, 2008). Furthermore, some of the ITIM-containing receptors bind additional molecules through their phosphorylated ITIMs: LAIR-1 and human LILR bind the inhibitory ‘C-terminal Src kinase’ Csk; Siglecs bind SOCS3; KLRG1 binds SHIP; CD300 binds PI3K; and KIR binds β-arrestin 2 (Yu et al., 2008). Deficiency in β-arrestin 2 results in diminished inhibition through KIR. By an unknown mechanism, which involves binding to phosphorylated ITIM, β-arrestin 2 facilitates SHP-1 and SHP-2 recruitment to inhibitory KIR (Figure 3).
During inhibition of NK cells by an ITIM-containing receptor, most of the tyrosine-phosphorylated proteins detectable during activation have greatly diminished phosphorylation. Rather than being a consequence of fairly ‘permissive’ dephosphorylation of many signaling components by the ITIM-bound SHP, it is due to inhibition of a very proximal signaling step, which is independent of actin polymerization, and occurs through dephosphorylation of Vav1 by SHP-1 (Figure 3). Given the central role of Vav1 in promoting Rac1-dependent actin cytoskeleton rearrangement, synapse formation, and clustering of receptors, this inhibitory mechanism prevents tyrosine phosphorylation of activation receptors even before it occurs (Endt et al., 2007; Watzl and Long, 2003). The important concept here is not so much that Vav1 per se is selectively targeted for dephosphorylation, but rather that inhibition does rely on selection of a key component in a signaling pathway. This opens the possibility of multiple, finely tuned inhibitory mechanisms, which could be controlled by the engagement of different inhibitory receptors in different cellular contexts. It would be interesting to identify SHP substrates during inhibition by various ITIM-containing receptors in different cells.
The early, proximal, and actin-independent signals that lead to Vav1 activation during NK cell contact with other cells are not well characterized. However, engagement of integrins alone is sufficient to induce Vav1 phosphorylation (Gismondi et al., 2003; Riteau et al., 2003). This would be consistent with the model (Figure 3), since LFA-1-dependent NK cell activation by ICAM-1 is blocked by co-engagement of KIR with its cognate ligand HLA-C (Barber et al., 2004).
Inhibition of natural cytotoxicity by KIR and by CD94-NKG2A involves a second component in the negative signaling pathway besides Vav1 dephosphorylation by SHP-1 (Figure 3). During incubation of NK cells with target cells that express an MHC class I ligand for the inhibitory receptor, the small adapter Crk becomes phosphorylated and forms a complex with the tyrosine kinase Abl (Peterson and Long, 2008). Crk phosphorylation by Abl, and Abl binding to Crk, are steps known to dissociate Crk from the scaffold protein Cbl and from the Rap1-specific GEF C3G. The Cbl–Crk–C3G complex, as well as the CAS–Crk–C3G and the paxillin–Crk–C3G complexes, that form during NK cell activation may contribute to the strength of LFA-1-mediated adhesion through activation of Rap1 (Figure 2). Phosphorylated Vav1 is also bound to phosphorylated Cbl during activation (Peterson and Long, 2008). During inhibition, the Cbl–Crk–C3G complex is not simply absent, due to a position downstream of the point of inhibition, but it is actively disassembled through recruitment of a tyrosine kinase and specific phosphorylation of an adapter protein (Peterson and Long, 2008). The ability of ITIM-containing receptors to activate a specific tyrosine phosphorylation event suggests additional signaling functions of this large receptor family.
This Unit has described many positive and negative signaling pathways that act in parallel and in synergy to regulate NK cell responses. To achieve some understanding of these different signaling pathways it is necessary to study each one in isolation, and a few in controlled combinations, using reductionist approaches. However, a challenge for the future is to study and to understand the complex integration and interaction of these different signals in NK cells.
References
- Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–3401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–269. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, Orange JS. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DF, Long EO. Coexpression of CD58 or CD48 with Intercellular Adhesion Molecule 1 on Target Cells Enhances Adhesion of Resting NK Cells. J Immunol. 2003;170:294–299. doi: 10.4049/jimmunol.170.1.294. [DOI] [PubMed] [Google Scholar]
- Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- Bloch-Queyrat C, Fondaneche MC, Chen R, Yin L, Relouzat F, Veillette A, Fischer A, Latour S. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J Exp Med. 2005;202:181–192. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006a;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006b;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjold M, Henter JI, Long EO, Ljunggren HG. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP (SHP-1) by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A, Hoffmann SC, Jarahian M, Momburg F, Watzl C. Expression analysis of the ligands for the Natural Killer cell receptors NKp30 and NKp44. PLoS ONE. 2007;2:e1339. doi: 10.1371/journal.pone.0001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JM, Ginsberg MH, Rose DM. Integrin-associated proteins as potential therapeutic targets. Immunol Rev. 2008;223:236–251. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- Cherukuri A, Dykstra M, Pierce SK. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity. 2001;14:657–660. doi: 10.1016/s1074-7613(01)00156-x. [DOI] [PubMed] [Google Scholar]
- Ciccone E, Pende D, Viale O, Than A, Di Donato C, Orengo AM, Biassoni R, Verdiani S, Amoroso A, Moretta A, Moretta L. Involvement of HLA class I alleles in natural killer (NK) cell-specific functions: expression of HLA-Cw3 confers selective protection from lysis by alloreactive NK clones displaying a defined specificity (specificity 2) J Exp Med. 1992;176:963–971. doi: 10.1084/jem.176.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus M, Meinke S, Bhat R, Watzl C. Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front Biosci. 2008;13:956–965. doi: 10.2741/2735. [DOI] [PubMed] [Google Scholar]
- Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10:297–305. doi: 10.1038/ni.1693. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Ikemizu S, Wild MK, van der Merwe PA. CD2 and the nature of protein interactions mediating cell-cell recognition. Immunol Rev. 1998;163:217–236. doi: 10.1111/j.1600-065x.1998.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- Eissmann P, Watzl C. Molecular Analysis of NTB-A Signaling: A Role for EAT-2 in NTB-A-Mediated Activation of Human NK Cells. J Immunol. 2006;177:3170–3177. doi: 10.4049/jimmunol.177.5.3170. [DOI] [PubMed] [Google Scholar]
- Endt J, McCann FE, Almeida CR, Urlaub D, Leung R, Pende D, Davis DM, Watzl C. Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol. 2007;178:5606–5611. doi: 10.4049/jimmunol.178.9.5606. [DOI] [PubMed] [Google Scholar]
- Fischer A, Latour S, de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr Opin Immunol. 2007;19:348–353. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Fostel LV, Dluzniewska J, Shimizu Y, Burbach BJ, Peterson EJ. ADAP is dispensable for NK cell development and function. Int Immunol. 2006;18:1305–1314. doi: 10.1093/intimm/dxl063. [DOI] [PubMed] [Google Scholar]
- Galandrini R, Micucci F, Tassi I, Cifone MG, Cinque B, Piccoli M, Frati L, Santoni A. Arf6: a new player in FcgammaRIIIA lymphocyte-mediated cytotoxicity. Blood. 2005;106:577–583. doi: 10.1182/blood-2004-10-4100. [DOI] [PubMed] [Google Scholar]
- Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci U S A. 2005;102:7641–7646. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- Geiger C, Nagel W, Boehm T, van Kooyk Y, Figdor CG, Kremmer E, Hogg N, Zeitlmann L, Dierks H, Weber KS, Kolanus W. Cytohesin-1 regulates beta-2 integrin-mediated adhesion through both ARF-GEF function and interaction with LFA-1. EMBO J. 2000;19:2525–2536. doi: 10.1093/emboj/19.11.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase-2 activation by b1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol. 1997;159:4729–4736. [PubMed] [Google Scholar]
- Gismondi A, Jacobelli J, Strippoli R, Mainiero F, Soriani A, Cifaldi L, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration. J Immunol. 2003;170:3065–3073. doi: 10.4049/jimmunol.170.6.3065. [DOI] [PubMed] [Google Scholar]
- Gismondi A, Cifaldi L, Mazza C, Giliani S, Parolini S, Morrone S, Jacobelli J, Bandiera E, Notarangelo L, Santoni A. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104:436–443. doi: 10.1182/blood-2003-07-2621. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Frenette PS. When integrins fail to integrate. Nat Med. 2009;15:249–250. doi: 10.1038/nm0309-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z, Fodor S, Abram CL, Lowell CA, Mocsai A. Immunoreceptor-like signaling by beta 2 and beta 3 integrins. Trends Cell Biol. 2007;17:493–501. doi: 10.1016/j.tcb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Kielczewska A, Pyzik M, Sun T, Krmpotic A, Lodoen MB, Munks MW, Babic M, Hill AB, Koszinowski UH, Jonjic S, Lanier LL, Vidal SM. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Chen X, Strominger JL. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:2568–2573. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Yu G, Phillips JH. Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation. J Immunol. 1991;146:1571–1576. [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Li C, Ge B, Nicotra M, Stern JN, Kopcow HD, Chen X, Strominger JL. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace EM, Monkley SJ, Critchley DR, Takei F. A dual role for talin in NK cell cytotoxicity: activation of LFA-1-mediated cell adhesion and polarization of NK cells. J Immunol. 2009;182:948–956. doi: 10.4049/jimmunol.182.2.948. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Gismondi A, Soriani A, Cippitelli M, Palmieri G, Jacobelli J, Piccoli M, Frati L, Santoni A. Integrin-mediated Ras-extracellular regulated kinase (ERK) signaling regulates interferon gamma production in human natural killer cells. J Exp Med. 1998;188:1267–1275. doi: 10.1084/jem.188.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Soriani A, Strippoli R, Jacobelli J, Gismondi A, Piccoli M, Frati L, Santoni A. RAC1/P38 MAPK signaling pathway controls β1 integrin-induced interleukin-8 production in human natural killer cells. Immunity. 2000;12:7–16. doi: 10.1016/s1074-7613(00)80154-5. [DOI] [PubMed] [Google Scholar]
- Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–2323. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol. 2007;27:4070–4081. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micucci F, Capuano C, Marchetti E, Piccoli M, Frati L, Santoni A, Galandrini R. PI5KI-dependent signals are critical regulators of the cytolytic secretory pathway. Blood. 2008;111:4165–4172. doi: 10.1182/blood-2007-08-108886. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Leng L, Maschberger P, Brugge JS, Shattil SJ. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- Mor A, Dustin ML, Philips MR. Small GTPases and LFA-1 reciprocally modulate adhesion and signaling. Immunol Rev. 2007;218:114–125. doi: 10.1111/j.1600-065X.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O'Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, Billadeau DD. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Ramesh N, Remold-O'Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, Rosen FS, Geha RS, Strominger JL. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002a;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, Nurko S, Rasmussen WL, Köhler JR, Gellis SE, Ferguson BM, Strominger JL, Zonana J, Ramesh N, Ballas ZK, Geha RS. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002b;109:1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JC, Bracke M, Smith A, Davies D, Hogg N. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J Immunol. 2002;168:6330–6335. doi: 10.4049/jimmunol.168.12.6330. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncagalli R, Taylor JE, Zhang S, Shi X, Chen R, Cruz-Munoz ME, Yin L, Latour S, Veillette A. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat Immunol. 2005;6:1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- Saborit-Villarroya I, Del Valle JM, Romero X, Esplugues E, Lauzurica P, Engel P, Martin M. The Adaptor Protein 3BP2 Binds Human CD244 and Links this Receptor to Vav Signaling, ERK Activation, and NK Cell Killing. J Immunol. 2005;175:4226–4235. doi: 10.4049/jimmunol.175.7.4226. [DOI] [PubMed] [Google Scholar]
- Sancho D, Nieto M, Llano M, Rodríguez-Fernández JL, Tejedor R, Avraham S, Cabañas C, López-Botet M, Sánchez-Madrid F. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249–1261. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirim P, Zeitlmann L, Kellersch B, Falk CS, Schendel DJ, Kolanus W. Calcium signaling through the beta2-cytoplasmic domain of LFA-1 requires intracellular elements of the T cell receptor complex. J Biol Chem. 2001;276:42945–42956. doi: 10.1074/jbc.M103224200. [DOI] [PubMed] [Google Scholar]
- Sivori S, Falco M, Marcenaro E, Parolini S, Biassoni R, Bottino C, Moretta L, Moretta A. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc Natl Acad Sci USA. 2002;99:4526–4531. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. doi: 10.1111/j.1600-065X.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- Veillette A. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol Rev. 2006;214:22–34. doi: 10.1111/j.1600-065X.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med. 2003;197:77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside DG, Obergfell A, Talapatra A, Calderwood DA, Shattil SJ, Ginsberg MH. The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J Biol Chem. 2002;277:39401–39408. doi: 10.1074/jbc.M207657200. [DOI] [PubMed] [Google Scholar]
- Wu CY, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- Yu MC, Su LL, Zou L, Liu Y, Wu N, Kong L, Zhuang ZH, Sun L, Liu HP, Hu JH, Li D, Strominger JL, Zang JW, Pei G, Ge BX. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]