Abstract

Leishmaniasis is a disease caused by the intracellular protozoan, Leishmania. A current treatment for cutaneous leishmaniasis involves the delivery of imidazoquinolines via a topical cream. However, there are no parenteral formulations of imidazoquinolines for the most deadly version of the disease, visceral leishmaniasis. This work investigates the use of electrospray to encapsulate the imidazoquinoline adjuvant resiquimod in acid sensitive microparticles composed of acetalated dextran (Ac-DEX) or Ac-DEX/Tween blends. The particles were characterized and tested both in vitro and in vivo. Solutions of Ac-DEX and resiquimod in ethanol were electrosprayed to generate approximately 2 µm Ac-DEX particles containing resiquimod with an encapsulation efficiency of 85%. To prevent particle aggregation, blends of Ac-DEX with Tween 20 and Tween 80 were investigated. Tween 80 was then blended with the Ac-DEX at ~10% (w/w) of total polymer and particles containing resiquimod were formed via electrospray with encapsulation efficiencies between 40% and 60%. In vitro release profiles of resiquimod from Ac-DEX/Tween 80 particles exhibited the acid-sensitive nature of Ac-DEX, with 100% drug release after 8 h at pH 5 (phagosomal pH) and after 48 h at pH 7.4 (physiological pH). Treatment with Ac-DEX/Tween 80 particles elicited significantly greater immune response in RAW macrophages over free drug. When injected intravenously into mice inoculated with Leishmania, parasite load reduced significantly in the bone marrow compared to blank particles and phosphate-buffered saline controls. Overall, electrospray appears to offer an elegant, scalable way to encapsulate adjuvant into an acid sensitive delivery vehicle for use in treating visceral leishmaniasis.
Keywords: electrospray, drug delivery, immune adjuvant, subunit vaccine, Leishmania
INTRODUCTION
Nearly 10% of the world’s population is at risk of acquiring a form of leishmaniasis, a parasitic based disease. Among parasitic infections, leishmaniasis is responsible for the highest number of DALYs (Disability Adjusted Life Years—a measure of health burden) after malaria. Currently around 12 million people are infected with leishmaniasis worldwide, and the disease can be attributed to nearly 50 000 deaths per year.1 One major concern is the contraction of the disease by U.S military service members. In 2004, 1178 U.S military personnel were reported to have contracted the cutaneous form of the disease, but the actual number of cases is estimated to be 3000–5000,2 with up to 30% of those cases leading to deadly visceral leishmaniasis.3 The military has attempted to halt transmission of the disease through insecticide spraying of bases, bed netting, and uniforms containing insect repellent. Service people, however, rarely wore protective clothing in the hot climates where the disease is prevalent, and spraying insecticides was shown to be ineffective.2
Leishmaniasis is caused by Leishmania, an intracellular protozoan parasite genus that is transmitted to humans via sandflies. Infection with the Leishmania parasite can cause either cutaneous leishmaniasis or systemic/visceral leishmaniasis. Lesions on the skin are indicative of cutaneous leishmaniasis, but when not properly contained, cutaneous may become visceral leishmaniasis, which usually manifests itself in the spleen, liver, bone marrow, and/or lymph nodes. According to the Centers for Disease Control (CDC), visceral leishmaniasis has a 90% mortality rate without treatment.
Leishmania is an obligate intracellular parasite which has several mechanisms4–6 to evade the immune system once inside the host macrophage. The migration of infected macrophages from the site of inoculation to the bone marrow, liver, and spleen may be a major contributing factor in the transition from cutaneous to visceral leishmaniasis, although the exact mechanism of transition is still an active area of research.7 Imidazoquinolines, such as resiquimod and imiquimod, have been used topically to treat cutaneous leishmaniasis,8 but due to their poor water solubility, they have not been used inside the body to treat visceral leishmaniasis. The poor water solubility of imidazoquinolines like resiquimod restricts the delivery of these compounds to primarily topical formulation. Imidazoquinolines act on cutaneous leishmaniasis by modulating the helper T cell response from a Th2 response to a Th1 response through upregulating pro-inflammatory response such as nitric oxide production and cytokines like IL-6 and TNF-α.
One potential method to deliver poorly soluble imidazoquinolines parenterally is to incorporate the adjuvant in a micro/ nano particle, thereby facilitating the passive targeting of macrophages by size exclusion.9,10 Additional passive targeting to parasitic rich regions can be also achieved with intravenous injection of polymeric particles. Numerous studies have concluded that hydrophobic polymeric particles will primarily accumulate in areas with discontinuous epithelium (e.g., liver, spleen, bone marrow, lymph nodes) and areas of high vasculature (e.g., lungs).11–13 We have previously encapsulated the imidazoquinoline imiquimod in a microparticle made from the polymer acetalated dextran (Ac-DEX) via emulsion chemistry.9 Ac-DEX is unique among biopolymers because it is both acid-sensitive for triggered release inside the macrophages’ phagosome where pH is ~5, and it has tunable degradation rates that can range from hours to months.9,14–16 In the emulsion approach solvent–polymer–imidazoquinoline droplets are dispersed in an aqueous phase, an approach that works well for the encapsulation of hydrophobic imiquimod9 since this molecule does not readily partition into the aqueous phase. Another imidazoquinoline, resiquimod, is, however, preferred because it has a more potent effect on cytokine expression.17 Since resiquimod is less hydrophobic than imiquimod, it is more easily lost to the aqueous phase, severely limiting the maximum amount of imidazoquinoline that can be encapsulated via emulsion chemistry. In addition to the particle formulation limits of emulsion techniques that are specific to resiquimod, there are other disadvantages to using emulsion chemistry. In particular, emulsion methods involve harsh solvents that can remain in residual quantities after manufacturing, and the method is a batch operation that is not easily scaled due to the fact that power requirements also scale up as batch mixer size is increased. The challenges inherent in emulsion chemistry thus limit the applications of Ac-DEX microparticles and the encapsulation of resiquimod.
Aerosol methods are an alternate way to produce micrometer-sized polymer particles that can address some of the limitations of emulsion technology. As droplets containing the solvent, drug, polymer, and surfactants move through the air, the solvent rapidly evaporates, while the nonvolatile components remain in the particle. Since the drug is not lost by diffusion to an aqueous phase, aerosol methods allow for a greater range of and control over drug loadings than emulsion chemistry. Furthermore, a broader range of solvents can be used since immiscibility in water is not required. Finally, aerosol processes operate continuously and lend themselves to industrial scale production.18
Although there are a number of well-established ways to produce aerosols, including spray drying,19 ultrasonic atomization, 20 and impact-jet atomization,21 in this study we chose to use electrohydrodynamic spraying22,23 (electrospray) to produce the Ac-DEX microparticles. While our work investigates electrospray on the laboratory scale, there have been multiple studies performed showing the scale up of such an apparatus by operating an array of electrospray nozzles in parallel, which is referred to as multiplexed electrospray.18,24,25 We used a single capillary electrospray device (Figure S.1 of the Supporting Information) to encapsulate resiquimod in polymer microparticles comprised of either Ac-DEX or Ac-DEX blended with surfactant (either Tween 20 or Tween 80). These two surfactants were evaluated, without drug, with regard to their ability to improve particle dispersion in buffer. In initial formulation studies we investigated the effect of Peclet Number (Pe) on the morphology of pure Ac-DEX particles. The Peclet Number is a dimensionless group, calculated from the properties of the solvent and the polymer used, which quantifies the ratio of solvent evaporation to polymer diffusion for an aerosol droplet. When this number is high, a polymer shell forms around the droplet leading to a collapsed morphology. When it is low, the polymer concentration can equilibrate as the solvent evaporates, yielding more spherical droplets. The effect of the Peclet Number was studied by systematically changing the solvent while maintaining a fixed polymer concentration. Although Tween 20 and 80 were both blended with Ac-DEX for improving particle dispersion in aqueous medium, only Tween 80 was blended with Ac-DEX for the particles used in the in vitro and in vivo experiments, because using this surfactant resulted in more spherical particles (Figure S.3) which were also more easily dispersed in buffer. Resiquimod loaded Ac-DEX/Tween 80 particles were then fabricated and evaluated in vitro for encapsulation efficiency, drug release at physiological and phagosomal pH, and the ability of the particles to stimulate an immune response in macrophages. In contrast to most work with electrospray particles where only in vitro studies were performed,26 we also evaluated our system in vivo by infecting mice with L. donovani and treating them with intravenous delivery of resiquimod loaded Ac-DEX particles.
EXPERIMENTAL SECTION
Materials
All chemicals used in these experiments were purchased from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise indicated. Resiquimod was purchased from Alexis Biochemicals, Enzo Life Sciences (Farmingdale, NY). Ac-DEX was prepared from dextran with a molecular weight of 71 kDa using the procedure described by Kauffman et al.27 The cyclic:acyclic ratio of acetal substitution and degrees of substitution per 100 glucose were determined by evaluating the NMR spectra peaks as described in an earlier publication.15
Emulsion Particle Fabrication
Resiquimod (1.5 mg/mL) and Ac-DEX (50 mg/mL) were dissolved in dichloromethane (DCM) and added to an aqueous solution containing 3% (w/ v) poly(vinyl alcohol) (PVA). The mixture was vortexed for 30 min and sonicated for 30 s (Misonix Ultrasonic Liquid Processor, 60 W, duty cycle 50%), and the emulsion was immediately pipetted into the dispersed phase (0.3% PVA). This solution was then centrifuged (12 min, 17 500 rpm, 4 °C), the supernatant was discarded, and the particle sediment was resuspended and washed with basic water (2 × 12 min, 17 500 rpm, 4 °C). The microparticles were then suspended in basic water and freeze-dried. Blank microparticles were made following the same procedure but without adding adjuvant.
Electrospray Particle Fabrication
Ac-DEX microparticles were fabricated using the electrospray apparatus illustrated in Figure S.1a. Solutions were delivered to a 20 gauge stainless steel capillary (908 µm OD, 603 µm ID) using a programmable syringe pump (KD Scientific, KDS100) at flow rates between 0.1 and 0.3 mL/h. The needle tip was 7 cm above a grounded stainless steel plate (7.6 × 7.6 cm2) and a high voltage power supply provided the positive high voltage (~4.5 kV) to the stainless steel capillary. Particle collection began once a stable Taylor cone was observed. The collected particles were removed from the plate using a microspatula and stored in a freezer at −20 °C.
Ac-DEX/Tween composite particles (using either Tween 20 or Tween 80) were all electrosprayed using a flow rate of 0.3 mL/h and an applied voltage of 4.5 kV. To prepare the electrospray solutions for Ac-DEX/Tween particles, 0.03 mL of Tween was added to 5.97 mL ethanol to make a 0.5% v/v Tween solution. Ac-DEX was then added to produce a solution containing 50 mg/mL Ac-DEX and 5 mg/mL Tween.
Resiquimod loaded Ac-DEX/Tween 80 blended particles were prepared with two drug loadings for drug release and macrophage studies. They were prepared by dissolving resiquimod at levels of 0.5, and 0.05 mg/mL in the Ac-DEX/ Tween 80 solutions described above. This yielded solutions where the mass of the drug was 0.9 wt % and 0.09 wt %, respectively, of the total dissolved solute (Ac-DEX and Tween 80). Each solution was electrosprayed for 6 h and ~50 mg of white powder was recovered. About 50 mg of blank Ac-DEX/ Tween 80 particles were also produced.
Scanning Electron Microscopy
The morphology of microparticles was observed by scanning electron microscopy (SEM), using either a Hitachi s-4300 Cold Field Emission SEM or an FEI NOVA NanoSEM 400. Electrosprayed particles were collected on aluminum foil and either imaged directly or suspended in basic water and a small amount was placed on an SEM stub and allowed to air-dry. Particle size distributions were measured from SEM images using ImageJ.28 For a given particle diameter (Dp), the primary droplet size (Dd) was determined using:
| (1) |
where χ is the particle shape factor (see eq S.2 in Supporting Information), ρp is the polymer density, and Cp is the polymer concentration in the original solution.
Dynamic Light Scattering
Dynamic light scattering (DLS) measurements (Brookhaven Instruments Corporation, BI 200SM) were made using a laser wavelength of 633 nm, a pinhole setting of 200 µm, and a detector angle of 90°. For DLS analysis the particles were collected directly in basic water, and samples were diluted with additional basic water to reduce the average count rate to ~110 kcps.
Morphology Analysis
Six short chain alcohols were tested for use as solvents to systematically study the effect of Peclect number (Pe) on particle morphology by changing the solvent evaporation rate. We studied all 1-alcohols from methanol to pentanol as well as trifluouroethanol. These solvents were chosen since they aptly dissolve Ac-DEX, and their volatility changes with chain length and halogenation The morphology was observed via SEM, and Pe was calculated to compare our observations with theory. The Okuyama Peclet number, described by eq 2, is defined as the ratio of the rate of solvent evaporation from the droplet surface to the rate of polymer diffusion within the solvent droplet.29,30
| (2) |
In eq 2, Dsolvent,air and Dpolymer,solvent are the diffusion coefficients for solvent vapor in air (gas phase) and for polymer in solvent (liquid phase), respectively, ρgas and ρsolvent are the densities of air and solvent, respectively, while mvap,0 and mvap,∞ are the vapor phase solvent mass fractions at the droplet surface and in the bulk air, respectively. The details of this calculation are discussed in the Supporting Information (eq S.3).
Encapsulation Efficiency and Drug Loading
The encapsulation efficiency (EE) of resiquimod is defined as the mass ratio of resiquimod to polymer in the particles divided by the mass ratio of resiquimod to polymer in the initial electrospray solution, given as a percent. Resiquimod fluorescence (Ex: 260 nm/Em: 360 nm) was quantified using a Spectra Max Gemini XS microplate reader (Molecular Devices, Sunnyvale, CA). Three separate samples of particles containing resiquimod and three blank particle samples were dissolved in DMSO at 1 mg/mL. Each sample was pipetted in triplicate into a 96 well plate and read using the microplate reader. The concentration of resiquimod was determined by using a calibration curve.
Polymer Microparticle Degradation
The degradation of Ac-DEX/Tween polymer microparticles (using either Tween 20 and 80) was examined qualitatively by dispersing 2 mg particles in 2 mL of either sodium acetate buffer (pH 5) or phosphate buffered saline (PBS) (pH 7.4). The samples were examined visually and photographed at various time points. For these micrometer-sized particles, a decrease in the turbidity of the samples indicates particle degradation. Experiments were continued until the sample became clear (Figure S.4). Quantitative particle degradation was observed for Ac-DEX/ Tween 80 blends using a method similar to that of Broaders et al.15 Particles were suspended in both sodium acetate buffer at pH 5 and PBS at pH 7.4, and samples were taken at various time points. The samples were centrifuged and the supernatants stored in a 96 well plate at −20 °C. After all of the samples were collected, a bicinchoninic acid based assay (Micro BCA Protein Assay, Pierce) was used to detect the presence of reducing saccharides and calculate the quantitative degradation of the Ac-DEX microparticles.
Resiquimod Release Profile
Release profiles were measured in sodium acetate buffer at pH 5 and PBS at pH 7.4. Both resiquimod loaded and blank particles were weighed, placed in microcentrifuge tubes, and suspended in buffer at a concentration of 1 mg/mL. The particle suspensions were incubated at 37 °C. At different time points, the suspensions were mixed by briefly vortexing, and then 100 µL of supernatant was isolated after centrifugation. Sample supernatant was stored in a 96 well plate at −20 °C. After all samples were collected they were brought up to room temperature and the fluorescence of the supernatants was measured using a microplate reader. The fluorescence of the blank particle samples was subtracted as a background, and the remaining intensities were scaled linearly relative to the highest value.
In Vitro Cytotoxicity
The bioactivity of the encapsulated resiquimod was evaluated using murine RAW 264.7 macrophages (ATCCC, Manassas, VA). Cells were plated in a culture treated 96 well plate at 5 × 104 cells per well and incubated for 24 h in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone Logan, UT) with high glucose (4.5 g glucose/L), l-glutamine, sodium pyruvate, 10 % (v/v) fetal bovine serum (FBS; characterized; Hyclone Logan, UT), and 1 % (v/v) penicillin–streptomycin. Twenty-four hours after seeding, the medium in each well was removed and replaced with 200 µL of treatment medium containing blank Ac-DEX particles at the same concentration as that of the highest dose of loaded particles (0.35 mg/mL), serial dilutions of resiquimod loaded particles, or serial dilutions of free (unencapsulated) resiquimod. Controls included three wells without cells, three wells with untreated cells, and three wells containing cells treated with 0.1 µg/mL lipopolysaccharide (LPS) as a positive control. After another 24 h of treatment, medium was removed and analyzed for nitric oxide and inflammatory cytokine production using the methods described below. The remaining cells were analyzed for viability. To the cells still adhered to the culture plate, 180 µL of fresh medium and 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dissolved in medium (5 mg/mL) were added. The plate was incubated for ~2 h, until the MTT metabolic product formazan was visible as dark purple crystals. The incubation time for all MTT analysis was constant for a given series of experimental conditions and controls. The medium was then aspirated off; the formazan was resuspended in 200 µL of isopropyl alcohol and placed on a shaker plate for 5 min, and absorbance was read using a microplate reader. The absorbance at 670 nm (background) was subtracted from the absorbance at 560 nm. The net absorbance scaled by the net absorbance of the untreated cells (×100%) corresponds to the cell viability.
Nitrite Analysis
The supernatants that were removed 24 h after cell treatment were placed in microcentrifuge tubes and centrifuged to separate out the particles. A 50 µL aliquot of supernatant from each sample was pipetted into a new 96 well plate for nitrite analysis. The Griess assay (Promega, Madison, WI) was performed following the manufacturer’s directions.
Inflammatory Cytokine Analysis
A 100 µL aliquot of supernatant from each sample was pipetted into a new 96 well plate for cytokine analysis by Affymetrix (Santa Clara, CA) Procarta assay service. Six cytokines (IL-1β, IL-6, IL-12p70, RANTES, TNF-α, and MIP-1α) were assayed using a mouse multiplex assay system.
Parasite and Infection Protocol
Leishmania donovani (1 Sudanese strain) were grown and maintained in Syrian Golden Hamsters. BALB/c mice were infected with 1 × 107 amastigotes by tail vein injection.
Treatment Procedure
L. donovani infected BALB/c mice were treated intravenously via a single 100 µL tail-vein injection with either phosphate-buffered saline, empty microparticles (1 mg MPs), or resiquimod microparticles (1 mg MPs, 55 µg resiquimod) 14 days after infection. These mice were sacrificed two weeks post treatment.
Spleen and Liver LDU
Parasite loads in the spleen and liver were quantified using a previously described method.31 Briefly, the liver and spleen impression smears were stained using Giemsa, and parasite loads were quantified microscopically. LDU (L. donovani units) was determined by the number of amastigotes per 1000 nuclei multiplied by the weight (mg) of the liver or spleen.
Bone Marrow Counts
Bone marrow counts were performed as previously described.32 Briefly, the parasite load in the bone marrow was examined microscopically. Femurs of mice were removed, gently broken, and the bone marrow extracted. Bone marrow smears were made, stained with Giemsa, and bone marrow counts were determined by the number of amastigotes per 200 nuclei.
Statistics
Error bars in all graphs show the standard deviation of three replicates unless otherwise stated. A two-tailed t test was used to compare means of data sets using an F-test to test the assumption of equal variance for each case. A p-value of less than 0.05 was considered significantly different.
RESULTS
Electrospray of Encapsulation of Resiquimod in Ac-DEX Particles
To evaluate the first use of electrospraying for the fabrication of Ac-DEX microparticles, a screening experiment was performed with concentrations of 5 and 50 mg Ac-DEX/mL in ethanol at flow rates of 0.1 and 0.3 mL/h. Ethanol was chosen because this solvent is more benign than dichloromethane and chloroform, and residual solvent could exist during the manufacturing of these particles. Thus, Ac-DEX has an advantage over poly(lactic-co-glycolic acid) (PLGA) and other polyester polymers since its high solubility in ethanol makes it possible to use this solvent in electrospraying. SEM images showed that particle sizes were between 1 and 5 µm, and all particles exhibited a collapsed morphology. There was no significant effect of polymer concentration or flow rate on particle size or morphology in the ranges explored. All further experiments used particles produced from solutions with an Ac-DEX concentration of 50 mg/mL at a flow rate of 0.3 mL/h.
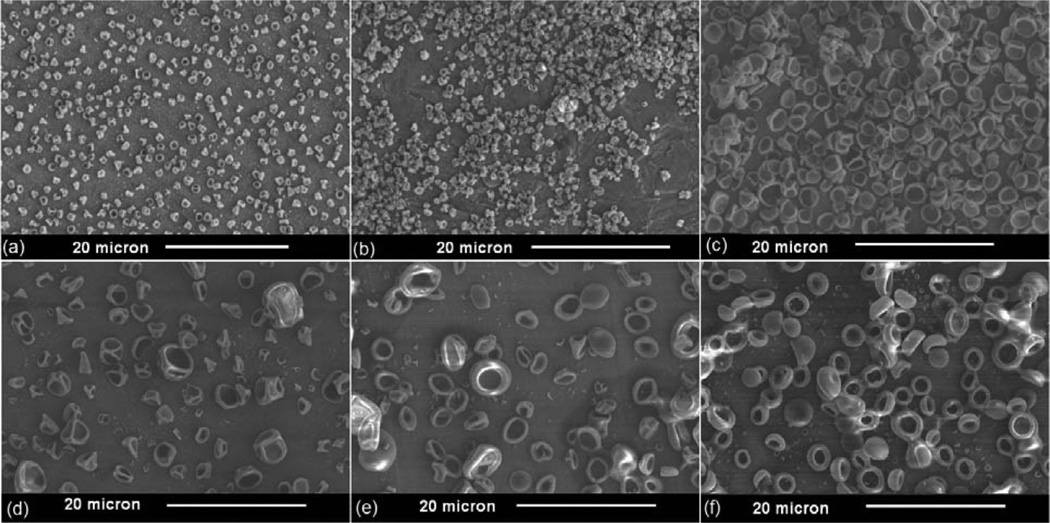
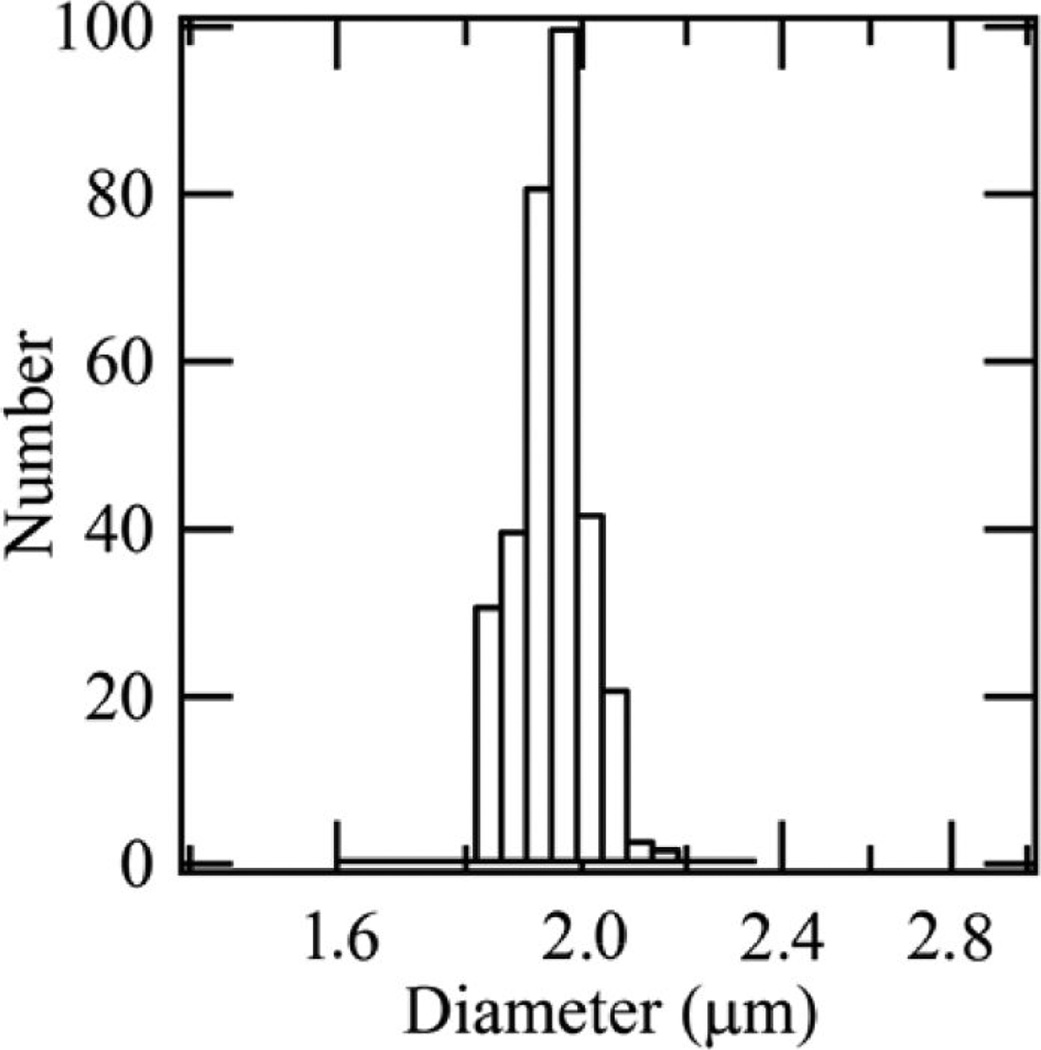
Five further solvents were also investigated, and Table 1 summarizes the key properties of the six solvent–polymer systems including the calculated Pe. SEM images of the particles are presented in Figure 1. The number weighted size distribution of particles electrosprayed from an ethanol-Ac-DEX solution and measured by dynamic light scattering is shown in Figure 2. The mean particle diameter by number, 1.9 µm, is in good agreement with the particle size observed using SEM (Figure 1c), and the sample is quite monodisperse with a polydispersity index of 0.005. Table 2 displays the encapsulation efficiencies achieved in resiquimod loaded Ac-DEX particles fabricated using both electrospray and emulsion methods.
Table 1.
Parameters of the Solvent Ac-DEX Mixtures Including the Polymer Diffusivity in Solvent (Dpolymer,solvent), the Solvent Diffusivity in Air (Dsolvent,air), the Vapor Pressure (Psat) of the Pure Solvent at 20 °C, and the Corresponding Pe Numbera
| solvent | Dpolymer,solvent (cm2/s) × 107 | Dsolvent,air (cm2/s) | Psat (mmHg) | Pe | Dp,mean (µm) | Dp,mode (µm) |
|---|---|---|---|---|---|---|
| trifluoroethanol | 3.8 | 0.1096 | 70 | 77 | 1.3 ± 0.2 | 1.3 |
| methanol | 11.3 | 0.1469 | 96 | 30 | 1.0 ± 0.3 | 1.2 |
| ethanol | 5.8 | 0.1140 | 49 | 31 | 2.5 ± 0.5 | 2.7 |
| 1-propanol | 3.0 | 0.0958 | 18 | 24 | 2.3 ± 1.2 | 3.1 |
| 1-butanol | 2.3 | 0.0830 | 4 | 8 | 2.6 ± 1.5 | 3.7 |
| 1-pentanol | 1.7 | 0.0688 | 3 | 8 | 2.2 ± 1.4 | 3.6 |
The number average diameter (Dp,mean) and the mode (Dp,mode) of the particles was estimated from the SEM images (Figure S.2). The solvents investigated include trifluoroethanol, methanol, ethanol, 1-propanol, 1-butanol, and 1-pentanol.
Figure 1.
SEM images of Ac-DEX particles generated using single capillary electrospray and the following solvents: (a) trifluoroethanol; (b) methanol; (c) ethanol; (d) 1-propanol; (e) 1-butanol; and (f) 1-pentanol.
Figure 2.
Number weighted particle size distribution for empty Ac-DEX particles generated by electrospray with ethanol, measured using DLS.
Table 2.
Drug Loading of Resiquimod Loaded Ac-DEX Particles Fabricated Using Electrospray and Emulsion Methods
| method | initial loading (wt %) |
weight loading (wt %) |
encapsulation efficiency (%) |
|---|---|---|---|
| electrospray | 3.0 | 2.5 | 83 |
| emulsion | 3.0 | 0.2 | 6 |
Ac-DEX/Tween Blends
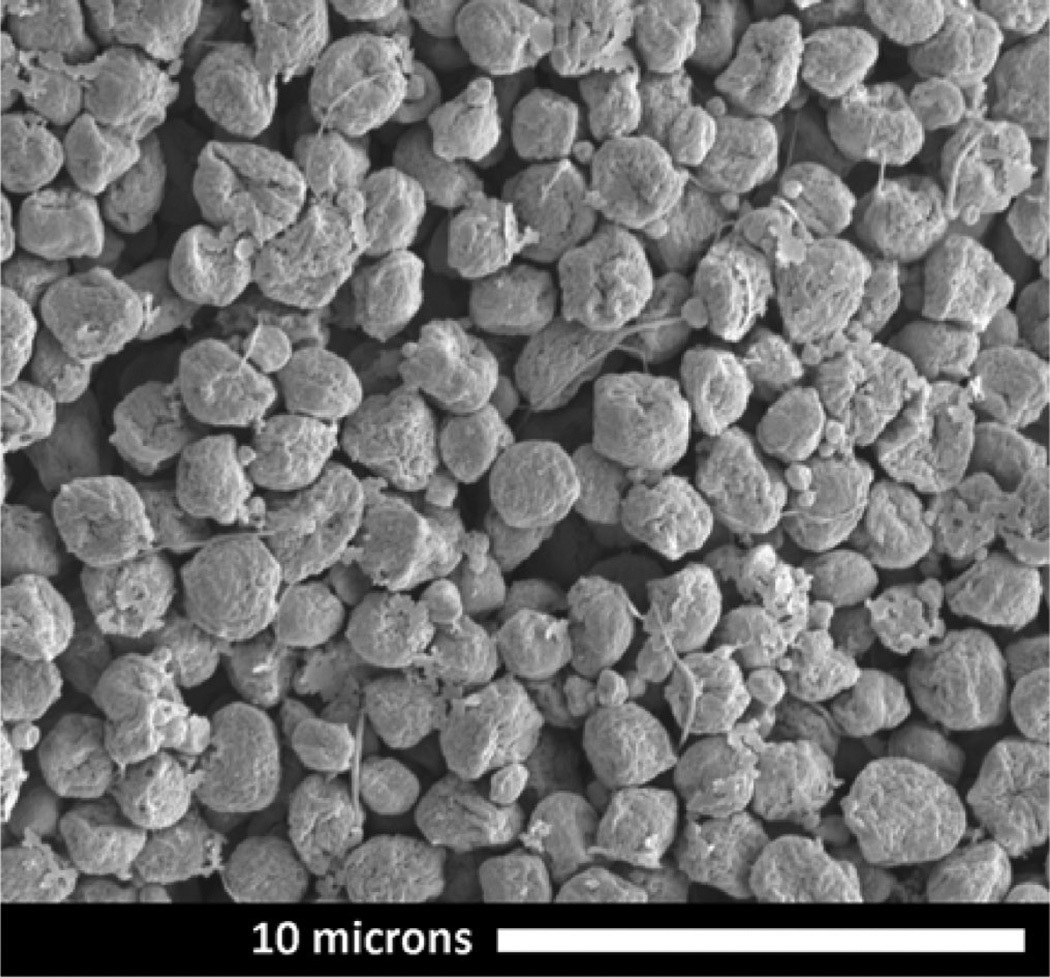
The particles made from Ac-DEX blends with Tween 80 were more spherical and compact and were easier to suspend in buffer than those made with Tween 20. SEM images of Ac-DEX/Tween 20 and Ac-DEX/Tween 80 blended particles are illustrated in the Supporting Information, Figure S.3. Suspensions of particles in buffer made with either surfactant/polymer formulation were completely clear within approximately 4 h at pH 5 and still turbid after 96 h at pH 7.4 (Figure S.4). The Ac-DEX/Tween 80 particles appeared to be less collapsed than the Ac-DEX/Tween 20 particles, which display the same collapsed morphology observed for the pure Ac-DEX particles. Table 3 shows the encapsulation efficiencies measured for two loading levels of resiquimod encapsulated in Ac-DEX/Tween 80 blended particles. The encapsulation efficiencies are lower than for the pure Ac-DEX particles, and the encapsulation efficiency decreases with a decrease in the initial drug loading. Figure 3 is an SEM image of the Ac-DEX/ Tween 80 particles with the higher resiquimod loading. The morphology was not affected by the presence of resiquimod at the concentrations investigated.
Table 3.
Encapsulation Efficiencies of Resiquimod Loaded into Ac-DEX/Tween 80 Blended Particles with Both High and Low Drug Loading
| sample | initial loading (wt %) |
weight loading (wt %) |
encapsulation efficiency (%) |
|---|---|---|---|
| high loaded particles | 0.90 | 0.540 | 60 |
| low loaded particles | 0.09 | 0.035 | 39 |
Figure 3.
Scanning electron microscope image of Ac-DEX/Tween 80 composite electrosprayed microparticles containing 0.54 wt % resiquimod. These particles were used for both the release profile experiments and in vitro experiments. Nanofiber strands and the presences of a subpopulation of smaller particles indicate that a small fraction of the electrosprayed droplets have undergone coulomb fissioning before the polymer hardened.24
The average size of the high resiquimod loaded particles (Figure 3) used in the cell treatment experiments is 1.3 ± 0.5 µm, as measured from SEM images. The low drug loaded particles (not shown) were essentially the same with an average size of 1.5 ± 0.5 µm. The particles in Figure 3 also exhibit some fibers extending outward (called “tails”) and a subpopulation of smaller particles that are evidence for Coulomb fissioning.24,33 Coulomb fissioning occurs as the solvent evaporates, and the charge density on the droplet surface builds up to levels that induce instability of the surface. Under these conditions, the droplet breaks up to reduce the charge on the parent droplet. The ejection of progeny droplets from the droplet surface may be a problem for the purpose of encapsulating a surface active payload, which can include some proteins, and may have to be addressed in future work. However, in our study, Coulomb fissioning did not prevent the demonstration of improved encapsulation efficiency through electrospray or the in vitro or in vivo efficacy of the electrospray encapsulated drug.
In Vitro Bioactivity
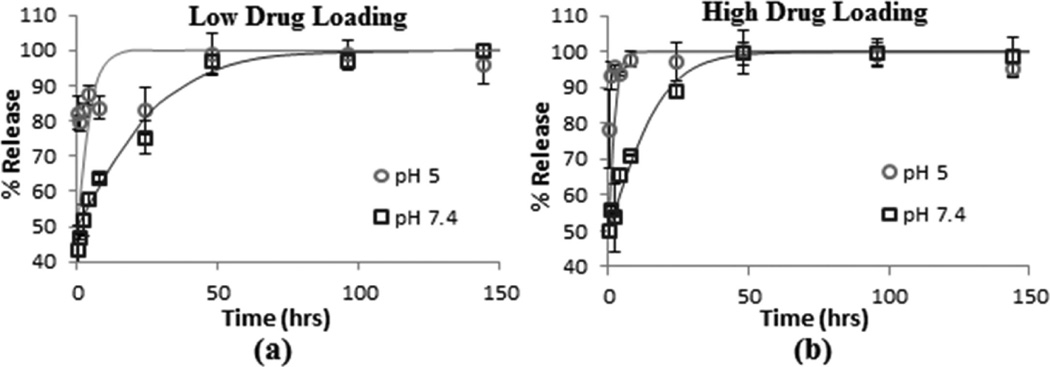
Figure 4a and b shows the resiquimod release profiles from Ac-DEX/Tween 80 particles with low and high loadings of resiquimod, respectively. Each graph shows a comparison between the release profile at pH 5 and pH 7.4. In the pH 5 buffer with either adjuvant loading, close to 80% of the resiquimod was released from the particles in the first 0.5 h and at the high loading resiquimod release was ~100% within 8 h. In the pH 7.4 buffer, the initial drug burst release was 40–50% with 100% of the drug released within 50 h.
Figure 4.
Resiquimod release profiles measured for electrosprayed Ac-DEX/Tween 80 particles containing (a) low drug loading and (b) high drug loading. Error bars show the standard deviation for three independent particle suspensions. The solid lines, added as a visual guide, are sigmoidal functions of the form % Release = (100) × (1/(1 + e−t/τ)), where t is time and τ is the characteristic time for drug release.
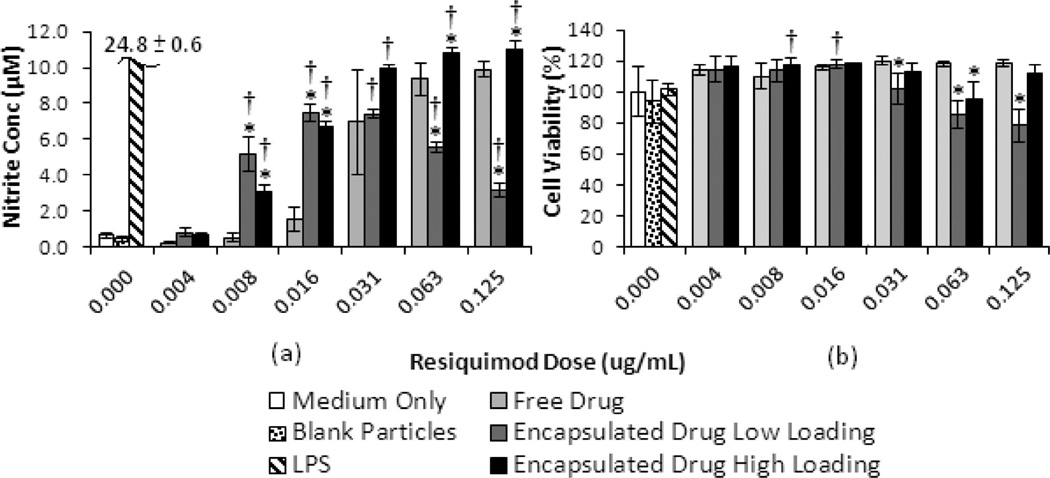
Figure 5a and b shows the observed nitric oxide (NO) production and cell viability (respectively) in macrophages treated with increasing doses of free and encapsulated resiquimod. Figure 5a shows that for encapsulated drug at the low loading, the NO production initially increases with resiquimod dose, reaching a maximum at doses of 0.016 and 0.031 µg/mL. All doses of encapsulated drug (except for the lowest dose) showed significantly higher NO production than the blank particle control. For the high drug loaded particles, the NO production was significantly greater than that for free resiquimod at every dose level, with the exception of 0.031 µg/ mL (where there was a large variance in replicates) and 0.004 µg/mL (the lowest dose).
Figure 5.
(a) Nitrite production and (b) cell viability of RAW macrophages treated with free and encapsulated resiquimod. Both graphs show results from one experiment run in triplicate (n = 3). Error bars represent one standard deviation. An * indicates significant difference with respect to free drug at the respective dose (p < 0.05). A † indicates significant difference with respect to blank particles (p < 0.05).
For the low loaded particles, the NO production was significantly higher than that for free drug at lower doses of 0.008 and 0.016 µg/mL, while the opposite is observed at higher doses. Figure 5b shows that relative to the media only control, there was no significant effect of free resiquimod, compared to medium only, on the cell viability over the concentration range investigated. Moreover, the presence of blank particles does not show a significant effect on cell viability compared to media only. However, when compared to free drug at the same resiquimod dose, cell viability was significantly lower when cells are exposed to particles containing resiquimod at low drug loading for each of the three highest doses.
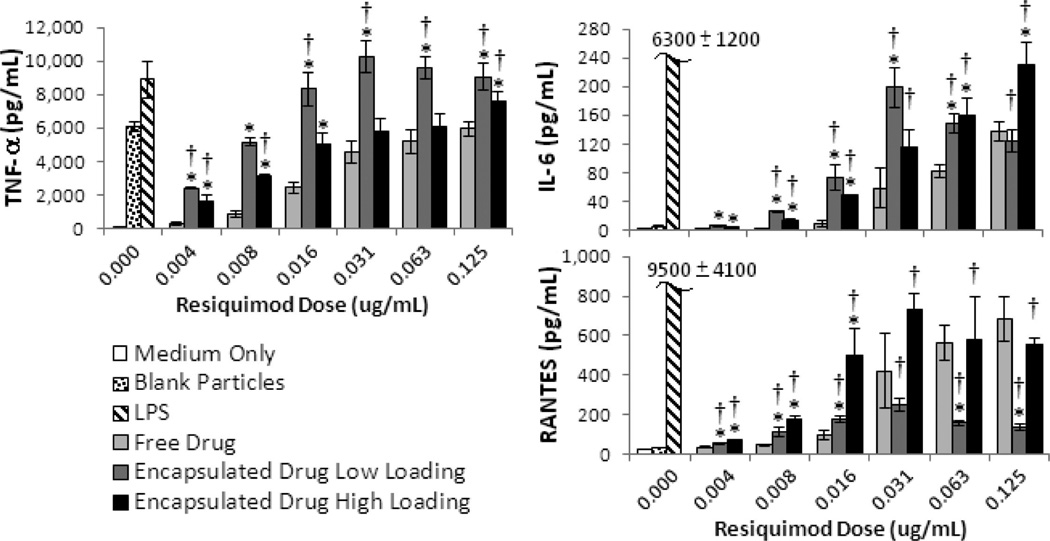
The production of three inflammatory cytokines (TNF-α, IL-6, and RANTES) is shown in Figure 6. Although we also measured the production of IL-1β, MIP-1α, and IL-12p70, these results are shown in the Supporting Information (Figure S.6) due to the fact that they are either too low to be considered practically significant (IL-1 β and IL-12p70), or they lack statistical significance compared to free drug or blank particle controls (MIP-1α).
Figure 6.
Inflammatory cytokine production in RAW macrophages treated with free and encapsulated resiquimod. All graphs show cytokine results from triplicate wells. Error bars represent one standard deviation. An * indicates significant difference with respect to free drug at the same dose (p < 0.05). A † indicates a significant difference relative to the blank particle control (p < 0.05).
In Vivo Treatment of Leishmaniasis
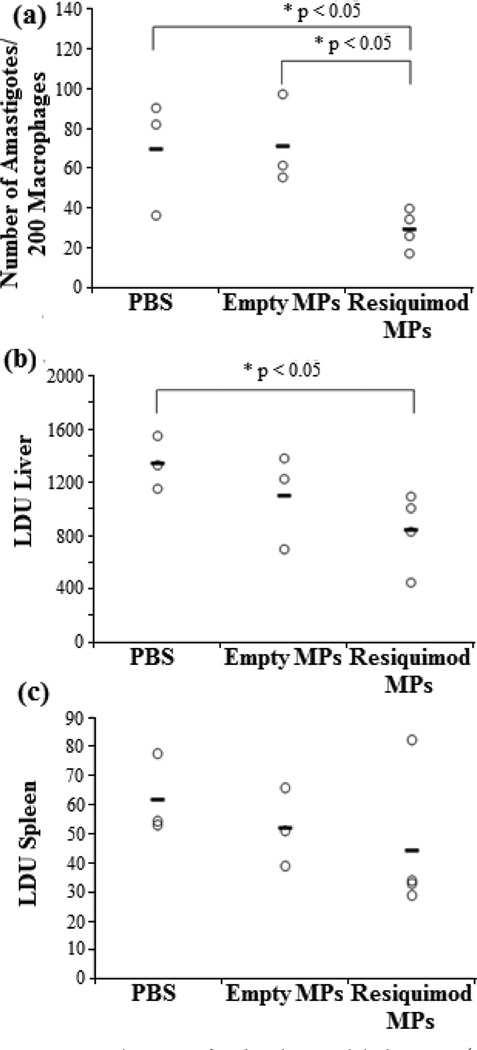
There is a statistically significant decrease in the level of L. donovani amastigotes in the bone marrow and liver, Figure 7a and b, respectively, of mice treated with resiquimod loaded micro-particles compared to the PBS control. In the spleen, Figure 7c, there is no statistical difference between the groups, but on average the parasite titers are lower compared to the other two groups.
Figure 7.
BALB/C mice infected with visceral leishmaniasis (L. donovani) and two weeks later treated with either PBS, empty Ac-DEX/Tween 80 particles, or Ac-DEX/Tween 80 encapsulated resiquimod via intravenous injection. Data shows L. donovani concentration in (a) bone marrow, (b) liver, and (c) spleen.
DISCUSSION
Controlled Morphology and Drug Loading through Electrospray
The morphology of a particle formed from an evaporating aerosol droplet reflects the competition between solvent evaporation and solute diffusion. If the solvent evaporates more quickly than the polymer can diffuse toward the center of the shrinking droplet, a higher polymer concentration develops near the droplet surface forming a polymer shell. As the volume of the liquid core decreases, the polymer shell collapses if it cannot withstand the internal change in volume,29,34–36 and the residual particle adopts the collapsed shell morphology observed in Figure 1. The Peclet number (Pe) has been used to correlate changes in the morphology of polymer particles produced by aerosol methods.29,36 In our experiments, the solvents corresponding to the highest Pe (trifluoroethanol (TFE) and methanol) were more spherical than those with the lowest Pe (1-propanol, 1-butanol, and 1-pentanol), an inverse relationship compared to the observations of Yao et al., where high Pe (~100) yielded a collapsed morphology, and low Pe (~10) resulted in smooth, spherical particles.29 One reason for this difference is that in our experiments the primary droplet size was not constant. Recent work by Iskandar et al.35 demonstrated that final particle morphology also depends on the initial droplet size with larger droplets resulting in collapsed residual particles due to greater mechanical instability. The electrospray scaling law developed by Ganan-Calvo,37 predicts that for a fixed liquid flow rate the primary droplet size varies as (ρsol/γK)1/6 where ρsol, γ, and K are the density, surface tension, and conductivity of the solution. The experimentally estimated primary droplet sizes appear to follow this scaling law reasonably well (Figure S.7). Thus, our results illustrate that tuning the primary droplet size can be more important than adjusting Pe for controlling the morphology of electrosprayed particles and may aid in controlling the morphology of future polymeric particles. All solvents investigated showed potential for generating particles through electrospray, but ethanol was chosen as the best for our application since it is a relatively benign solvent compared to longer chain or halogentated alcohols and the particles made using ethanol were a good size for phagocytosis.10
In addition to altering particle morphology with electrospray, we were also able to significantly increase the resiquimod encapsulation efficiencies achievable for Ac-DEX by approximately 10 times over the emulsion formulation. In addition to reducing the loss of drug during the encapsulation process, the electrospray approach makes it possible to achieve higher drug loadings, and therefore, a larger range of drug loadings is possible than through current emulsion technology. However, pure Ac-DEX microparticles made by electrospray were difficult to disperse in aqueous medium while particles prepared by the emulsion method dispersed readily.
Electrospray Allows Blending with Surfactants for Improved Particle Dispersion in Water
The current electrospray process does not need a surfactant, such as the poly(vinyl alcohol) (PVA) required in the emulsion method for droplet dispersion and stabilization, to make particles. Nevertheless, to improve the dispersibility of the final Ac-DEX microparticles in water, two types of Tween (20 and 80) were added to the electrospray solution. Tween was chosen because it is already used as a pharmaceutical excipient,38 and it is soluble in ethanol.
Ac-DEX and Tween are not the first polymer–surfactant combinations to be electrosprayed. Xie et al.39 electrosprayed poly(lactic-co-glycolic acid) (PLGA)/pluronic solutions where the pluronic comprised 2–16% by weight of the total solute. The concentration of surfactant (10% w/w) used in our work lies within this concentration range. For PLGA, the addition of pluronic was reported to reduce agglomeration but did not affect the final particle morphology relative to the pure PLGA particles. In our work, we found that Tween 80, but not Tween 20, has an impact on particle morphology (Figure S.3). Both surfactants increase the solution conductivities by the same order of magnitude, which could contribute to changes in particle morphology by reducing the primary droplet size, but this does not explain the discrepancy observed between the effects of the two polymers on shape. Further characterization of the particles’ physical properties is required to better understand the difference in morphologies observed when using these two surfactants.
By adding Tween 80, we were able to disperse the particles in aqueous medium without altering the acid sensitivity of the Ac-DEX (Figure S.4). Furthermore, the Ac-DEX/Tween 80 particles showed quantitative acid sensitive degradation profiles (Figure S.8) with time scales similar to those measured for particles made by emulsion methods.15 The pH sensitivity of the drug release observed in vitro provides a basis for intracellular delivery of the adjuvant, which can then result in a dose dependent immune response in cells as shown in previous work.9 This result is significant since it provides a way to modulate the dispersion of the drug delivery vehicle in aqueous medium and address solubility issues for drugs such as resiquimod. Tween 80 was therefore incorporated in particle formulations used in the drug encapsulation, release profile, and macrophage studies.
The encapsulation efficiencies for the Ac-DEX/Tween 80 blended particles (Table 3) are lower than that measured for electrosprayed Ac-DEX particles without Tween 80 (Table 2). The reason for this difference may be a concentration effect since encapsulation efficiency appears to decrease as initial loading decreases. Nevertheless, even the lowest encapsulation efficiencies are still more than 6 times higher than those found for the emulsion based method.
Electrospray Encapsulation Improves in Vitro Bioactivity of Resiquimod
Particles in the 1–2 µm size range are reported to be taken up by phagocytic cells such as macrophages through macropinocytosis.10 Particle shape is also reported to effect uptake by cells,40 and Champion and Mitragoti41 report that, for particles with very high aspect ratios, phagocytosis can decrease. Electrospray fabricated particles used to treat macrophages in our work show morphologies with aspect ratios close to 1 (Figure 3) and should, therefore, be readily taken up by macrophages.
Nitric oxide has been shown to be essential in the clearance of Leishmania infection.42,43 Imidazoquinolines have been shown to increase NO activity by interacting with TLR 7/8.8 Previously, we showed that the encapsulation of imiquimod results in a higher level of activity in both macrophages and dendritic cells.9 Similarly, with the encapsulation of resiquimod, we were able to generally increase the activity of the encapsulated drug (NO production and cytokine activity). Initially, the low loaded particles had the highest level of NO production, but above 0.031 µg/mL, these particles resulted in a lower activity compared to free resiquimod. This is probably due to the fact that at higher concentrations the low loaded particles decrease the viability of the macrophages. The toxicity is due to the encapsulated drug, since treatment with empty particles at the same concentration does not affect the viability. Compared to the high loaded particles, a greater number of low loaded particles are required to achieve the same drug dose, resulting in a greater particle-to-cell ratio. Because a greater number of particles are available in culture for phagocytosis, it is likely that a greater drug concentration is available intracellularly with the low loaded particles than the high loaded particles, thus increasing the toxicity. We hypothesize that this leads to an enhanced resiquimod activity. This hypothesis is supported by the work of Jhunjhunwala et al.44 who report that increasing the ratio of rapamycin loaded particles to cells enhanced the drug’s activity. The decrease in cell viability at high particle to cell ratios of drug loaded particles is consistent with findings that high concentrations of imidazoquinolines can result in apoptosis.45–47 The particle-to-cell ratio estimations are summarized in the Supporting Information (Figure S.5).
The two cytokines that are the strongest indicators of a cells’ response to resiquimod, IL-6 and TNF-α, have in vitro profiles (Figure 6) that reflect those observed in the NO experiments. The production of IL-1β, IL-12p70, and MIP-1α lacked either statistical or practical significance and are included in the Supporting Information (Figure S.6).
The drug loaded particles induced a significantly higher level of production of IL-6, TNF-α, and RANTES when compared to free drug. The production of TNF-α and RANTES has been shown to be important in the clearance of Leishmania.48–50 In addition, the production of these cytokines suggests that the phagocytosis of resiquimod loaded Ac-DEX particles can stimulate an immune response related to these pro-inflammatory TH1 cytokines. The concentration of IL-6 and TNF-α produced versus drug dose was similar to that observed in the NO production profile, in which a maximum production level was observed at 0.0313 µg/mL of encapsulated drug with low loading. This further supports the hypothesis that the amount of drug delivered is dependent on the dose as well as the drug loading. Based on these results, the electrosprayed particles could also be used for the development of vaccines. By tuning the electrospray parameters, we can synthesize particles in the optimal size range for phagocytosis by cells such as macrophages and dendritic cells.51,52 By incorporating an adjuvant and a protein, multiplexed electrospray could be used produce microparticles on a large scale for vaccine applications.
In Vivo Treatment of Leishmaniasis Is Demonstrated for Electrospray Encapsulated Drug
The in vivo results support the in vitro bioactivity results. Our result is important because currently there are very few in vivo investigations of electrosprayed particles.26 Furthermore, the few in vivo studies that have been conducted indicate that in vitro data may not always be consistent with in vivo behavior.53–55 The fact that the in vivo results are consistent with the in vitro data in our work strengthens the chance for the eventual application of electrosprayed particles in the clinical setting. To the best of our knowledge, this is the first time that an imidazoquinoline has been used for the parenteral treatment of visceral leishmaniasis. In the treatment of visceral leishmaniasis, the bone marrow is a persistent site of infection that must be cleared for successful treatment.56 The first line drug, antimony does not effectively clear this site of infection.57 Additionally, there has been a significant increase in antimony resistant Leishmania strains that require the development of new drugs for treatment.58 Since resiquimod and imiquimod activate the host and do not directly interact with the parasite, there is less opportunity to develop drug resistant strains,59 and the drug is already FDA approved. Additionally, the encapsulated resiquimod lowered the L. donovani concentration in both the spleen and liver, but not statistically. In future experiments we will study the biodistribution of the electrosprayed particles as well as optimize the dose. By changing the size and the surfactant used we could potentially alter the distribution of these particles in vivo.60
CONCLUSIONS
This work has demonstrated methods to improve the delivery of resiquimod as a treatment for visceral leishmaniasis and has opened a new field for electrospray research in drug delivery. To the best of our knowledge, this is the first time electrospraying has been used to produce particles for the treatment of a parasitic disease, and these particles were shown to clear leishmania from the bone marrow, a previously difficult task. Furthermore, the in vivo data are consistent with the in vitro results. Electrospray was shown to greatly improve the encapsulation efficiency of resiquimod over that of the emulsion method, while the morphology of these particles could be tuned by adding surfactants or by changing the solvent. In particular Tween 80, initially added to increase the ability to disperse the particles in aqueous media, also yielded more spherical particles. Our work provides a path forward to improve future treatment of diseases with resiquimod. The increased flexibility in drug loading will make it possible to design therapies for parasitic diseases by encapsulating multiple agents. Additionally this flexibility can also be applied to vaccines, since we can increase adjuvant loads, making it possible to tune vaccines and obtain the desired immune response. Future work will involve the fine-tuning of the formulation to increase the uptake of the particles in the liver and spleen.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under Grant No. EEC-0914790, National Institutes of Health, and DARPA W911NF-10-1-0264.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supplemental figures as described in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Kobets T, Grekov I, Lipoldova M. Leishmaniasis: Prevention, Parasite Detection and Treatment. Curr. Med. Chem. 2012;19:1443–1474. doi: 10.2174/092986712799828300. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Hochberg LP, Swanson KI, Lee JS, McAvin JC, Moulton JK, Eddington DO, Groebner JL, O’Guinn ML, Putnam JL. Impact of Phlebotomine Sand Flies on US Military Operations at Tallil Air Base, Iraq: 4. Detection and Identification of Leishmania Parasites in Sand Flies. J. Med. Entomol. 2009;46(3):649–663. doi: 10.1603/033.046.0333. [DOI] [PubMed] [Google Scholar]
- 3.Furlow B. US Army reports fewer cases of leishmaniasis, but a complex threat persists. Military Disease Surveillance, EPI News. 2007 Jun 3; [Google Scholar]
- 4.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 5.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 1994;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, Recker TJ, Miller MA, Wilson ME. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 2003;170(5):2613–2620. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- 7.Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20(11):524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J. Infect. Dis. 1999;179(6):1485–1494. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 9.Bachelder EM, Beaudette TT, Broaders KE, Frechet JM, Albrecht MT, Mateczun AJ, Ainslie KM, Pesce JT, Keane-Myers AM. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharmaceutics. 2010;7(3):826–835. doi: 10.1021/mp900311x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16(5):645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado A, Soriano I, Sanchez E, Oliva M, Evora C. Radiolabelled biodegradable microspheres for lung imaging. Eur. J. Pharm. Biopharm. 2000;50(2):227–236. doi: 10.1016/s0939-6411(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 13.Gratton SEA, Pohhaus PD, Lee J, Guo I, Cho MJ, DeSimone JM. Nanofabricated particles for engineered drug therapies: A preliminary Biodistribution study of PRINT (TM) nanoparticles. J. Controlled Release. 2007;121(1–2):10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Frechet JM. Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 2008;130(32):10494–10495. doi: 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JM. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc. Natl. Acad. Sci U.S.A. 2009;106(14):5497–5502. doi: 10.1073/pnas.0901592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meenach SA, Kim YJ, Kauffman KJ, Kanthamneni N, Bachelder EM, Ainslie KM. Synthesis, optimization, and characterization of camptothecin-loaded acetalated dextran porous microparticles for pulmonary delivery. Mol. Pharmaceutics. 2012;9(2):290–298. doi: 10.1021/mp2003785. [DOI] [PubMed] [Google Scholar]
- 17.Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 2001;48(6):751–755. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 18.Deng WW, Waits CM, Morgan B, Gomez A. Compact multiplexing of monodisperse electrosprays. J. Aerosol. Sci. 2009;40(10):907–918. [Google Scholar]
- 19.Jain R, Shah NH, Malick AW, Rhodes CT. Controlled Drug Delivery by Biodegradable Poly(Ester) Devices: Different Preparative Approaches. Drug Dev. Ind. Pharm. 1998;24(8):703–727. doi: 10.3109/03639049809082719. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly TD, Hogan J, Mugler A, Schubmehl M, Schommer N, Bernoff AJ, Dasnurkar S, Ditmire T. Using ultrasonic atomization to produce an aerosol of micron-scale particles. Rev. Sci. Instrum. 2005;76(11):10. [Google Scholar]
- 21.Boysen DA, Peters TM. Impactor designed to increase mass output rate of nanoparticles from a pneumatic nebulizer. J. Aerosol. Sci. 2010;41(2):170–179. [Google Scholar]
- 22.Wu Y, Chalmers JJ, Wyslouzil BE. The Use of Electrohydrodynamic Spraying to Disperse Hydrophobic Compounds in Aqueous Media. Aerosol Sci. Technol. 2009;43(9):902–910. [Google Scholar]
- 23.Jaworek A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007;176(1):18–35. [Google Scholar]
- 24.Almeria B, Deng WW, Fahmy TM, Gomez A. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J. Colloid Interface Sci. 2010;343(1):125–133. doi: 10.1016/j.jcis.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Almeria B, Fahmy TM, Gomez A. A multiplexed electrospray process for single-step synthesis of stabilized polymer particles for drug delivery. J. Controlled Release. 2011;154(2):203–210. doi: 10.1016/j.jconrel.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Bock N, Dargaville TR, Woodruff MA. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012;37:1510–1551. [Google Scholar]
- 27.Kauffman K, Kanthamneni N, Meenach S, Pierson B, Bachelder E, Ainslie K. Optimization of Rapamycin-Loaded Acetalated Dextran Microparticles for Immunosuppression. Int. J. Pharmaceutics. 2012;422:356–363. doi: 10.1016/j.ijpharm.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with Image. J. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 29.Yao J, Lim LK, Xie JW, Hua JS, Wang CH. Characterization of electrospraying process for polymeric particle fabrication. J. Aerosol. Sci. 2008;39(11):987–1002. [Google Scholar]
- 30.Spalding D. B. Combustion and mass transfer: a textbook with multiple-choice exercises for engineering students. Oxford: Pergamon Press; 1979. [Google Scholar]
- 31.Murray HW. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect. Immun. 2000;68(11):6294–6299. doi: 10.1128/iai.68.11.6294-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob. Agents Chemotherapy. 1992;36(8):1630–1634. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.delaMora JF. On the outcome of the coulombic fission of a charged isolated drop. J. Colloid Interface Sci. 1996;178(1):209–218. [Google Scholar]
- 34.Raula J, Eerikainen H, Kauppinen EI. Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles. Int. J. Pharmaceutics. 2004;284(1–2):13–21. doi: 10.1016/j.ijpharm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Iskandar F, Gradon L, Okuyama K. Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. J. Colloid Interface Sci. 2003;265(2):296–303. doi: 10.1016/s0021-9797(03)00519-8. [DOI] [PubMed] [Google Scholar]
- 36.Fiegel J, Garcai-Contreras L, Elbert K, Hickey A, Edwards D. AICHE Annual Meeting. Cincinnati, OH: 2005. Dry Powder Aerosols for Multi-Drug Resistant Tuberculosis (Mdr-Tb) Treatment. [Google Scholar]
- 37.Ganan-Calvo AM. The surface charge in electrospraying: Its nature and its universal scaling laws. J. Aerosol Sci. 1999;30(7):863–872. [Google Scholar]
- 38.Gonzalez RCB, Huwyler J, Boess F, Walter I, Bittner B. In vitro investigation on the impact of the surface-active excipients Cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm. Drug Dispos. 2004;25(1):37–49. doi: 10.1002/bdd.383. [DOI] [PubMed] [Google Scholar]
- 39.Xie J, Lim LK, Phua Y, Hua J, Wang C-H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Colloid Interface Sci. 2006;302(1):103–112. doi: 10.1016/j.jcis.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 40.Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Controlled Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champion JA, Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009;26(1):244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar A, Saha P, Mandal G, Mukhopadhyay D, Roy S, Singh SK, Das S, Goswami RP, Saha B, Kumar D, Das P, Chatterjee M. Monitoring of intracellular nitric oxide in leishmaniasis: its applicability in patients with visceral leishmaniasis. Cytometry A. 2011;79(1):35–45. doi: 10.1002/cyto.a.21001. [DOI] [PubMed] [Google Scholar]
- 43.von Stebut E, Belkaid Y, Nguyen B, Wilson M, Sacks DL, Udey MC. Skin-derived macrophages from Leishmania majorsusceptible mice exhibit interleukin-12- and interferon-gammaindependent nitric oxide production and parasite killing after treatment with immunostimulatory DNA. J. Invest. Dermatol. 2002;119(3):621–628. doi: 10.1046/j.1523-1747.2002.01850.x. [DOI] [PubMed] [Google Scholar]
- 44.Jhunjhunwala S, Raimondi G, Thomson AW, Little SR. Delivery of rapamycin to dendritic cells using degradable microparticles. J. Controlled Release. 2009;133(3):191–197. doi: 10.1016/j.jconrel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang SW, Liu KT, Chang CC, Chen YJ, Wu CY, Tsai JJ, Lu WC, Wang YT, Liu CM, Shieh JJ. Imiquimod simultaneously induces autophagy and apoptosis in human basal cell carcinoma cells. Brit. J. Dermatol. 2010;163(2):310–320. doi: 10.1111/j.1365-2133.2010.09827.x. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J. Immunol. 2007;178(10):6495–6503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- 47.Kim SO, Ono K, Han J. Apoptosis by pan-caspase inhibitors in lipopolysaccharide-activated macrophages. Am. J. Physiol. 2001;281(5):L1095–L1105. doi: 10.1152/ajplung.2001.281.5.L1095. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca SG, Romao PR, Figueiredo F, Morais RH, Lima HC, Ferreira SH, Cunha FQ. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur‥ Immunol. 2003;33(8):2297–2306. doi: 10.1002/eji.200320335. [DOI] [PubMed] [Google Scholar]
- 49.Liew FY, Parkinson C, Millott S, Severn A, Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- 50.Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect. Immun. 2004;72(8):4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008;25(8):1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 53.Naraharisetti PK, Ong BYS, Xie JW, Lee TKY, Wang C-H, Sahinidis NV. In vivo performance of implantable biodegradable preparations delivering Paclitaxel and Etanidazole for the treatment of glioma. Biomaterials. 2007;28(5):886–894. doi: 10.1016/j.biomaterials.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 54.Ranganath SH, Kee I, Krantz WB, Chow PK-H, Wang C-H. Hydrogel Matrix Entrapping PLGA-Paclitaxel Microspheres: Drug Delivery with Near Zero-Order Release and Implantability Advantages for Malignant Brain Tumour Chemotherapy. Pharm. Res. 2009;26(9):2101–2114. doi: 10.1007/s11095-009-9922-2. [DOI] [PubMed] [Google Scholar]
- 55.Nie H, Fu Y, Wang C-H. Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. Biomaterials. 2010;31(33):8732–8740. doi: 10.1016/j.biomaterials.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 56.Cotterell SE, Engwerda CR, Kaye PM. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect. Immun. 2000;68(4):1840–1848. doi: 10.1128/iai.68.4.1840-1848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter KC, Dolan TF, Alexander J, Baillie AJ, McColgan C. Visceral leishmaniasis: drug carrier system characteristics and the ability to clear parasites from the liver, spleen and bone marrow in Leishmania donovani infected BALB/c mice. J. Pharm. Pharmacol. 1989;41(2):87–91. doi: 10.1111/j.2042-7158.1989.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 58.Ait-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D. Leishmania antimony resistance: what we know what we can learn from the field. Parasitol. Res. 2011;109(5):1225–1232. doi: 10.1007/s00436-011-2555-5. [DOI] [PubMed] [Google Scholar]
- 59.Wu JJ, Huang DB, Tyring SK. Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses. Antiviral Res. 2004;64(2):79–83. doi: 10.1016/j.antiviral.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Dunn SE, Coombes AGA, Garnett MC, Davis SS, Davies MC, Illum L. In vitro cell interaction and in vivo biodistribution of poly(lactide-co-glycolide) nanospheres surface modified by poloxamer and poloxamine copolymers. J. Controlled Release. 1997;44(1):65–76. [Google Scholar]
- 61.Mohr PJ, Taylor BN, Newell DB. CODATA recommended values of the fundamental physical constants: 2006. Rev. Mod. Phys. 2008;80(2):633–730. doi: 10.1103/RevModPhys.93.025010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaws CL. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds. New York: Knovel; 2003. [Google Scholar]
- 63.Yaws CL. Yaws’ Thermophysical Properties of Chemicals and Hydrocarbons. New York: Knovel; 2009. , electronic ed. [Google Scholar]
- 64.Smallwood IM. Handbook of Organic Solvent Properties. New York: Elsevier; 1996. [Google Scholar]
- 65.Yaws CL. Yaws’ Transport Properties of Chemicals and Hydrocarbons. New York: Knovel; 2009. electronic ed.; [Google Scholar]
- 66.Yaws CL, Narasimhan PK, Gabbula C. Yaws’ Handbook of Antoine Coefficients for Vapor Pressure. Knovel; New York: 2007. 2nd electronic ed.; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.