Abstract
Hepatic glucose production is normally activated at birth, but has been observed in response to experimental hypoglycemia in fetal sheep. The cellular basis for this process remains unknown. We determined the impact of 2 weeks of fetal hypoglycemia during late gestation on enzymes responsible for hepatic gluconeogenesis, focusing on the insulin signaling pathway, transcription factors, and coactivators which regulate gluconeogenesis. Hepatic PEPCK and glucose-6-phosphatase mRNA increased 12-fold and 7-fold respectively following chronic hypoglycemia with no change in hepatic glycogen. Chronic hypoglycemia decreased fetal plasma insulin with no change in glucagon, but increased plasma cortisol 3.5-fold. PGC1 mRNA and phosphorylation of CREB at serine 133 were both increased, with no change in Akt, FOXO1, HNF4α, or C/EBPβ. These results demonstrate that chronic fetal hypoglycemia triggers signals which can activate gluconeogenesis in the fetal liver.
Keywords: glucose, gluconeogenesis, cortisol, CREB, PGC1
INTRODUCTION
Intrauterine growth restriction (IUGR) affects 4-8% of newborns and is commonly associated with placental insufficiency and decreased fetal nutrient delivery (12; 45; 52). In addition to a wide variety of perinatal morbidities, IUGR increases the risk of developing several adult onset metabolic diseases, including type II diabetes mellitus, a disease characterized by peripheral insulin resistance and insufficient insulin secretion (24; 49). One of the hallmarks of type II diabetes is reduced ability of insulin to suppress hepatic glucose production (22). It is significant, therefore, that in several animal models of IUGR there is an early and persistent increase in fetal and neonatal hepatic phosphoenolpyruvate carboxykinase (PEPCK) expression, the enzyme that catalyzes the first committed step of gluconeogensis (9; 21; 32; 42; 47; 65). The various nutrient and secondary metabolic abnormalities in IUGR fetuses that might cause such changes in liver enzyme function and glucose production is uncertain. The most common metabolic condition in all IUGR fetuses that would have direct bearing on hepatic glucose production is decreased placental glucose supply to the fetus and relative fetal hypoglycemia. Studies of experimental fetal hypoglycemia without placental insufficiency or global nutrient restriction, however, have shown variable results for induction of hepatic PEPCK (11; 20; 23; 40; 43), which may reflect species, timing/duration, and other methodological differences.
Among the complex network of transcription factors and cofactors that regulate PEPCK gene expression, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) and cAMP response element binding protein (CREB) are particularly important effectors of the cAMP pathway. PGC-1α does not bind directly to the PEPCK promoter. Instead it facilitates the transcriptional activity of hepatocyte nuclear factor (HNF)-4α, the glucocorticoid receptor, and forkhead transcription factor FOXO1 to increase PEPCK gene transcription (7; 37). The CCAAT enhancer binding protein (C/EBP) α and C/EBPβ bind to the CRE of the PEPCK promoter and play an important role in cAMP induction (7; 55). The prevailing model is that induction by cAMP is mediated by phosphorylation of CREB, which must interact with C/EBP and other factors bound to an upstream accessory enhancer to stimulate gene transcription (25; 38). Furthermore, CREB induces PGC1α mRNA expression (37). FOXO1, which is negatively regulated by insulin signaling through Akt via nuclear exclusion, also facilitates PEPCK gene expression (7).
To evaluate the impact of experimental hypoglycemia on fetal glucose metabolism, we previously used late gestation hypoglycemic fetal sheep produced by a continuous maternal insulin infusion (10). This renders the fetus chronically hypoglycemic and these fetal sheep increase endogenous glucose production but the cellular basis for this response is unknown. Given the propensity for increased glucose production and its contribution to the risk for Type II diabetes among IUGR offspring, it is important to understand the cellular mechanisms responsible for increased hepatic glucose production in response to fetal hypoglycemia.
MATERIALS AND METHODS
Animal Model and Organ Isolation
Studies were conducted in pregnant Columbia-Rambouillet ewes (singleton) during the final 20% of gestation (term of 147 days). Indwelling catheters were surgically placed into the ewe and fetus as previously described (34; 56). All animal procedures were in compliance with guidelines of the United States Department of Agriculture, the National Institutes of Health, and the American Association for the Accreditation of Laboratory Animal Care. The animal care and use protocols were approved by the University of Colorado Health Sciences Center Institutional Animal Care and Use Committee. Data for many of the animals used in this study have been reported, as described in the results section (34; 56). As previously described animals were randomly placed into one of two groups: euglycemic control (C) animals (n=15) or hypoglycemic (H) animals (n=16). The H ewes received a two week intravenous insulin infusion (30-60 pmol min-1 kg-1; Humulin R, Eli Lilly and Co., Indianapolis, IN) in 0.5% bovine serum albumin (BSA; Sigma Chemicals, St. Louis, MO) in 0.9% NaCl adjusted on average twice daily to produce a 50% reduction in maternal plasma glucose (from 60-70 to 30-35 mg/dl) which also decreased fetal glucose concentrations by 50%. The insulin infusion was started on day 122.5±0.6. Gestational ages at necropsy are in table 1.
Table 1.
Fetal Characteristics
| Control | Hypoglycemic | p value | |
|---|---|---|---|
| Gestational Age, days | 138.6 ± 0.3 | 138.8 ± 0.6 | NS |
| Weight, kg | 4.380 ± 0.116 | 3.370 ± 0.137* | <0.0001 |
| Liver Weight, g | 121.96 ± 5.55 | 93.19 ± 5.54* | <0.005 |
| Liver/Body Weight, % | 2.78 ± 0.09 | 2.80 ± 0.12 | NS |
| Glucose, mmol/L | 1.12 ± 0.03 | 0.58 ± 0.02* | <0.0001 |
| Lactate, mmol/L | 1.98 ± 0.16 | 1.33 ± 0.10* | <0.005 |
| Oxygen, mmol/L | 3.20 ± 0.17 | 4.15 ± 0.18* | <0.001 |
| Insulin, ng/ml | 0.32 ± 0.04 | 0.12 ± 0.02* | <0.0001 |
| Cortisol, ng/ml | 5.3 ± 0.7 | 19.0 ± 4.2* | <0.0005 |
Values are means ± SE.
The refers to a significant difference between Hypoglycemic and Control fetuses by Student's t test (parametric) or the Mann-Whitney test (nonparametric).
Necropsies were performed as follows: the ewe and fetus were anesthetized with maternally administered intravenous ketamine (4.4 mg/kg) and diazepam (0.11 mg/kg). After a hysterectomy, the fetus was removed, weighed, and dissected for organ weights. Sections of the right hepatic lobe were snap frozen in liquid nitrogen and then transferred to -80°C. The ewe was then euthanized by administering intravenous concentrated sodium pentobarbital (10 ml; Sleepaway, Fort Dodge Animal Health, IA). The fetus died under anesthesia following an intracardiac injection of sodium pentobarbital (1ml).
Biochemical Analysis
Whole blood was collected in EDTA-coated syringes and centrifuged (14,000g) for 3 min at 4°C. Plasma was removed and the glucose and lactate concentrations were determined using the YSI model 2700 select biochemistry analyzer (Yellow Springs Instruments, Yellow Springs, OH). The remainder of the plasma was stored at -70°C for hormone measurements. Plasma insulin concentrations were measured by an ovine insulin ELISA (Alpco, Windham, NH; inter- and intra-assay coefficients of variation: 2.9 and 5.6%, respectively) and plasma cortisol concentrations were measured by a salivary cortisol ELISA (Alpco; inter- and intra-assay coefficients of variation: 5.7 and 4.4%, respectively). Blood oxygen content was determined using an ABL 520 blood gas analyzer (Radiometer, Copenhagen, Denmark) (36).
Glycogen Content
Hepatic glycogen content was determined as previously described and results are expressed as mg glycogen/g liver (wet weight) (2).
Cloning and Real Time PCR for Relative Gene Expression
Total RNA was extracted from pulverized hepatic tissue (100 mg) and reverse transcribed into complimentary DNA (cDNA) as previously described (35). Cloning and real time quantitative PCR (qPCR) for ovine ribosomal protein s15, PEPCK, Glucose-6-Phosphatase (G6Pase), and PGC1α (GenBank accession nos.: AY949774, EF062862, EF062861, and AY957611, respectively) has been previously described (35; 57). cDNA samples were run in triplicate and the qPCR was performed as previously described (57) with the standard curve method of relative quantification used to compare results (66). s15 was used as a housekeeping gene and was not different between groups.
Protein Extraction and Western Blot Analysis
Protein was extracted from pulverized hepatic tissue (200 mg) by the addition of 600 μl of ice cold lysis buffer (NaCl 150 mmol/L, Tris, pH 7.4, 20 mmol/L, Nonidet P-40 1% v/v, EDTA 2 mmol/L, Na4P2O7 2.5 mmol/L, Glycerol 10% v/v. β-Glycerophosphate 20 mmol/L, phenylmethylsulfonyl fluoride 0.575 mmol/L, Sigma Mammalian Protease Inhibitor Cocktail 2% v/v, Sigma Phosphatase Inhibitor 0.5% v/v) followed by 30 min on an orbital rocker at 4°C. The samples were then sonicated for 30 sec, agitated, and placed on an orbital rocker for another 30 min at 4°C. The protein was separated from cellular debris by centrifugation at 21,000g for 20 min at 4°C. The supernatant was removed and the protein concentration was quantified with the BioRad DC Protein Assay (BioRad, Hercules, CA).
Equal amounts of protein were separated by polyacrylamide gel electrophoresis under reduced conditions (5% β-mercaptoethanol). Proteins were then transferred to a polyvinylidene difluoride membrane (BioRad). Unless otherwise noted, all Western blot membranes were blocked for one hour in phosphate buffered saline with 0.1% tween 20 (PBST; BioRad) and 5% nonfat dried milk w/v (NFDM) for one hour at room temperature. The following primary antibodies were diluted in PBST with 5% NFDM: C/EBPβ (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), CREB (1:1000, Santa Cruz Biotechnology), HNF4α (1:750, Santa Cruz Biotechnology), and β-Actin (1:40,000, Medimmune, Inc., Gaithersburg, MD). Other primary antibodies were diluted in PBST with 5% BSA: ser133 phosphorylated CREB (1:500, Cell Signaling Technology Inc., Danvers, MA), Akt (1:500, Cell Signaling Technology), ser473 phosphorylated Akt (1:500, Cell Signaling Technology), FOXO1 (1:250, Cell Signaling Technology), and ser256 phosphorylated FOXO1 (1:500, Cell Signaling Technology). Membranes probed for insulin receptor β (IRβ) were blocked for one hour at room temperature in PBST with 5% NFDM with 1% BSA and the primary antibody (Santa Cruz Biotechnology) was diluted 1:1250 in the same buffer. Horseradish peroxidase conjugated secondary antibodies were diluted in PBST with 5% NFDM and applied to membranes for one hour at room temperature. Immunocomplexes were detected with enhanced chemiluminescence (Amersham ECL Plus, Piscataway, NJ). Densitometry was performed using Scion Image software (Scion Corporation, Frederick, MD). All results were normalized to β-Actin to control for loading differences, and a reference sample was analyzed on every membrane to control for differences in transfer efficiency. Ser473 phosphorylated Akt and ser256 phosphorylated FOXO1 also were normalized to the total amount of each protein. Antibodies were stripped from the membranes with Restore Western Stripping Buffer (Pierce, Rockford, IL).
Statistical Analysis
Statistical analysis was performed using SAS version 9.1 (58). All results are presented as mean ± standard error and groups were compared using either the Student's t test (parametric) or the Mann-Whitney test (nonparametric), both two tailed, and a level of 0.05 or less was considered significant.
RESULTS
Fetal Characteristics
Information on the experimental conditions and necropsy measurements have been previously reported for many of the fetuses used in these experiments (34; 56). Characteristics for the group of fetuses used in this study are summarized in table 1. We previously reported no difference in fetal arterial plasma glucagon, epinephrine or norepinephrine concentrations between the groups (34). Reported here for the first time (table 1), fetal arterial plasma cortisol concentrations are significantly greater (3.5-fold increase, p<0.0005) in the H group than in the C group. The percent of male fetuses was not statistically different (60% C, 40% H) and there was no distinguishable effect of fetal sex on any measurements.
G6Pase and PEPCK mRNA Expression and Glycogen Content
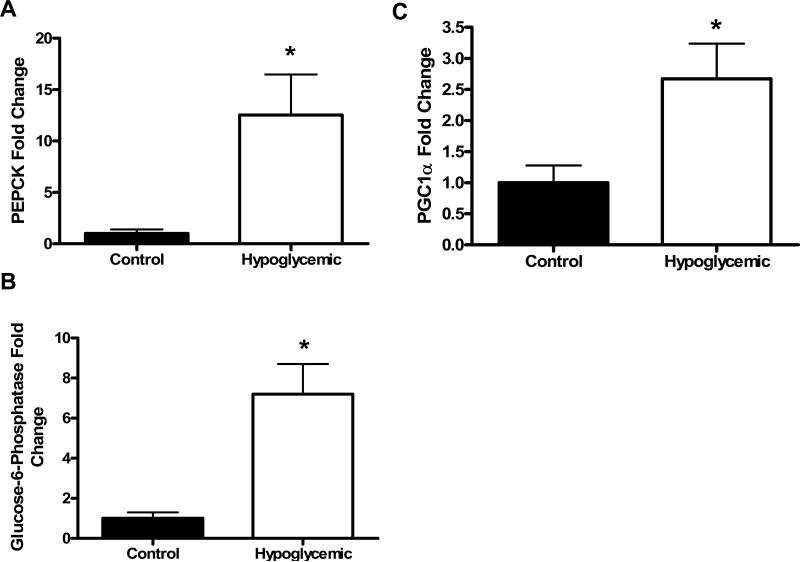
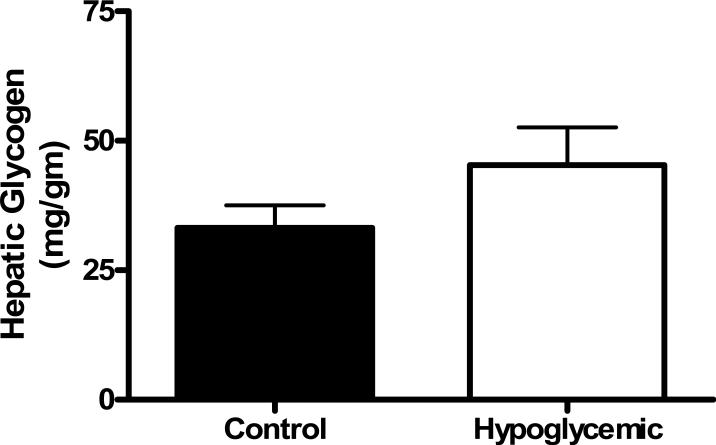
PEPCK mRNA was significantly greater (12-fold, p<0.05) in H fetal livers compared to C fetuses (figure 1a). The same expression pattern was found for G6Pase mRNA (7-fold increase, p<0.0005, figure 1b). Chronic fetal hypoglycemia did not affect hepatic glycogen content (figure 2).
Figure 1. Hepatic mRNA concentrations.
PEPCK (A), G6Pase (B), and PGC1α (C) mRNA concentrations were determined in livers from Control and Hypoglycemic fetuses by real-time quantitative PCR. Data was normalized to ribosomal protein s15 and is presented as fold change relative to control fetuses with SEM bars. Treatment groups are listed on the x-axis. The * denotes a higher amount of PEPCK (p<0.05) and G6Pase (p<0.0005) and PGC1α (p<0.05) in Hypoglycemic livers compared to Control livers. All statistics are from the Mann-Whitney test for nonparametric analysis.
Figure 2. Hepatic Glycogen.
Glycogen content (milligram per gram of tissue) with SEM bars was determined for Control and Hypoglycemic fetuses. No differences were found between treatment groups, which are listed on the x-axis.
Insulin Receptor and Akt
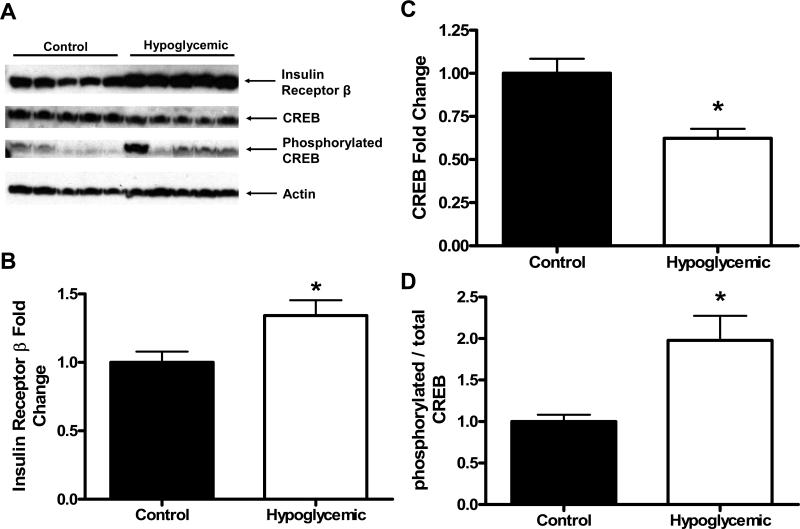
Hepatic content of the β-subunit of the insulin receptor was significantly higher (34% increase) in H fetuses compared to C fetal livers (p<0.05, figure 3a,b). There were no differences in the hepatic content of Akt (1.00±0.13 C, 0.87±0.09 H, arbitrary units relative to C) or in the ratio of phosphorylated Akt at the serine 473 position to total Akt (1.00±0.23 C, 1.17±0.29 H, arbitrary units relative to C).
Figure 3. Hepatic Protein Concentrations.
(A) Representative Western Blots for Insulin Receptor β, CREB, ser133 phosphorylated CREB, and β-actin. (B-D) Results of Western Blot analysis for Insulin Receptor β normalized to β-actin (B), CREB normalized to β-actin (C), and ser133 phosphorylated CREB normalized to total CREB (D). Treatment groups are listed on the x-axis. The * denotes a higher amount of Insulin Receptor β (p<0.05) and a higher ratio of ser133 phosphorylated CREB to total CREB (p<0.01) in Hypoglycemic livers compared to Control livers, and a lower amount of CREB in Hypoglycemic livers compared to Control livers (p<0.001). Statistics are from either Student's t test (parametric) or the Mann-Whitney test (nonparametric).
Transcription Factors and Transcription Co-Activators (CREB, C/EBPβ, HNF4α, FOXO1, and PGC1)
There was a significantly lower amount of total CREB (38% decrease) present in H livers compared to C fetal livers (p<0.001, figure 3a,c). The ratio of phosphorylated (active) CREB on serine 133 to total CREB was 2-fold higher in H fetal livers compared to C (p<0.01, figure 3a,d). C/EBPβ (1.00±0.08 C, 1.12±0.12 H, arbitrary units relative to C), HNF4α (1.00±0.16 C, 0.88±0.17 H, arbitrary units relative to C), FOXO1 (1.00±0.10 C, 0.94±0.11 H, arbitrary units relative to C), and the ratio of FOXO1 phosphorylated at the serine 256 position to total FOXO1 (1.00±0.08 C, 0.77±0.09 H, arbitrary units relative to C) were not different between groups. PGC-1 mRNA was 2.5-fold greater in H compared to C fetal livers (p<0.05, figure 1c).
DISCUSSION
The major finding in the present study is that fetal glucose deprivation activates hepatic PEPCK and G6Pase mRNA expression. Fetal hypoglycemia does not affect hepatic glycogen content. This demonstrates that fetal glucose production following chronic hypoglycemia is due to sustained gluconeogenesis as previously postulated (10) and not to persistent glycogenolysis. Normally, hepatic gluconeogenesis in fetal sheep does not occur until very late in gestation when it develops in response to a surge in fetal cortisol secretion, which occurs at gestational ages beyond the time point used in this study (18; 19). The central role of fetal cortisol secretion in activating glucose production has been determined by studies showing that hypophysectomy in fetal sheep renders them incapable of increasing plasma cortisol concentrations. Such fetuses have significantly decreased hepatic activities of gluconeogenic enzymes. Furthermore, fetal cortisol infusions increase these enzyme activities (17; 18). Our data suggest that fetal hypoglycemia increases fetal cortisol production and plasma concentrations and induces both PGC1α mRNA and phosphorylated CREB, all of which are important regulatory components in the gluconeogenic response.
In our model glucagon and epinephrine concentrations are not elevated, although the insulin to glucagon ratio is decreased (34). Plasma cortisol is higher, and excess glucocorticoids increase PEPCK gene expression directly and act permissively to augment induction by other stimuli (6). Glucagon, a decrease in the insulin to glucagon ratio, or epinephrine, activate CREB by stimulating phosphorylation at position 133, which in turn increases expression of the nuclear co-activator PGC1α as well as directly increases PEPCK and G6Pase expression (27; 54; 60). Insulin in contrast suppresses hepatic PGC1α transcriptional activity in part via Akt-mediated phosphorylation and nuclear export of the forkhead family activator FOXO1 (50). In addition insulin has recently been shown to stimulate phosphorylation of PGC1α directly to inhibit its ability to activate PEPCK gene transcription (33). Given that we found no changes in the distal insulin signaling targets, either phosphorylated FOXO1 or Akt, our results suggest that the up-regulation of PEPCK during hypoglycemia was more likely due to increased activation by cortisol and a decrease in the insulin to glucagon ratio through either CREB or PGC1α, rather than a reduction in insulin signaling.
The increase in PGC1α mRNA by chronic fetal hypoglycemia is similar to the findings in the bilateral uterine artery ligation model of IUGR in the rat in which both PGC1α and PEPCK mRNA are increased (32). In addition to PGC1α we also measured other factors that are known to increase PEPCK and G6ase expression and activity, including C/EBPβ and HNF4α (5; 8; 53). However, neither of these factors was increased by chronic fetal hypoglycemia. An interesting negative result was no change in HNF4α because it differs from fetal rats exposed to exogenous glucocorticoids. These fetuses have increased hepatic concentrations of PEPCK and HNF4α mRNA but normal hepatic concentrations of PGC1α (41). In our model of fetal hypoglycemia, with increased endogenous fetal glucocorticoids, hepatic PEPCK and PGC1α mRNA are increased but HNF4α protein is not different. These differences suggest that the surge in fetal cortisol may not be the sole mechanism up-regulating PEPCK in the hypoglycemic fetal sheep.
The maintenance of hepatic glycogen content in the hypoglycemic group, despite a lower insulin concentration and decreased glycogenic precursors (glucose and lactate), confirms the results of some earlier fetal experiments, but is in conflict with others. Several experimental models of IUGR and nutrient deprivation have demonstrated decreased hepatic glycogen (4; 39; 43; 44; 46). In each of these models, when reported, fetal oxygen values (partial pressure, hemoglobin-oxygen saturation, or blood oxygen content) either are normal or decreased and fetal plasma glucagon concentration is increased. It is possible that the increased fetal oxygenation in our hypoglycemic group allows for maintenance of hepatic glycogen. When tested in late gestation fetal sheep, hypoxemia without hypoglycemia decreases fetal hepatic glycogen content (63). Another difference between this model and the models in which hepatic glycogen decreases is that fetal glucagon is not elevated in the hypoglycemic group (34). In a different late gestation fetal sheep model of nutrient deprivation, fetuses subjected to a five day maternal fast had significantly lower fetal weight and maternal hepatic glycogen content, but did not have different fetal glucagon concentrations or hepatic glycogen contents (29; 59). In addition, piglets did not have lower liver glycogen contents following a maternal fast for the final seven or 21 days of gestation (15) and unilateral ligation of the uterine artery in guinea pigs produced IUGR fetuses that had increased hepatic glycogen content (31). Our results are consistent with the studies that demonstrate no decrease in hepatic glycogen following fetal nutrient deprivation, but there clearly are variations among studies.
Cortisol is important for hepatic glycogen accumulation and at gestational ages beyond 135 days, fetal sheep plasma cortisol is almost entirely of fetal origin (26). Increased cortisol concentrations in response to hypoglycemia have been described before in a variety of late gestation and neonatal mammals (13; 28; 62). In the sheep, like many mammalian species, liver glycogen content increases during the later part of gestation (61). The increase in hepatic glycogen during the last part of gestation is dependent on cortisol (1; 51; 64) and in fact exogenous cortisol can augment and accelerate late gestation hepatic glycogen synthesis and deposition (3; 16; 30; 64). These results have been confirmed with in vitro studies using fetal liver explants and primary fetal hepatocytes which show glucocorticoids are necessary for allowing insulin stimulated glycogen synthesis and deposition (14; 48; 64).
In conclusion, two weeks of experimental hypoglycemia in late gestation fetal sheep increases hepatic PEPCK and G6Pase mRNA and stimulates hepatic glucose output (10). This is associated with increased fetal plasma cortisol concentrations, increased hepatic PGC1α mRNA, and activation of hepatic CREB. In addition, fetal hepatic glycogen content is maintained despite decreased insulin and glycogen precursors. However, hepatic glucose production was not enough to restore fetal glucose concentrations to normal indicating that maternal glucose supply to the fetus is a critical factor regulating fetal glucose concentrations.
Grant Support and Acknowledgements
Supported by NIH grants HD42815 (WWH, PI), DK52138 (WWH, PI), HD28794 (WWH, PI), HD07186 (WWH, PI), RR00069 (WWH, Associate Director), NIH-CNRU Pilot and Feasibility Project 2 P30 DK048520-11 (PR, PI), and The Children's Hospital Research Institute Research Scholar Award (PR, PI). Sean W. Limesand was supported by NIH DK067393 (SWL, PI). DNA sequencing core services were provided by the Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, which is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-57516 (J.C. Hutton, PI).
Reference List
- 1.Barnes RJ, Comline RS, Silver M. Effect of cortisol on liver glycogen concentrations in hypophysectomized, adrenalectomized and normal foetal lambs during late or prolonged gestation. J Physiol. 1978;275:567–579. doi: 10.1113/jphysiol.1978.sp012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry JS, Davidsen ML, Limesand SW, Galan HL, Friedman JE, Regnault TRH, Hay WW., Jr. Developmental Changes in Ovine Myocardial Glucose Transporters and Insulin Signaling Following Hyperthermia-Induced Intrauterine Fetal Growth Restriction. Experimental Biology and Medicine. 2006;231:566–575. doi: 10.1177/153537020623100511. [DOI] [PubMed] [Google Scholar]
- 3.Benito M, Lorenzo M, Medina JM. Relationship between lipogenesis and glycogen synthesis in maternal and foetal tissues during late gestation in the rat. Effect of dexamethasone. Biochem J. 1982;204:865–868. doi: 10.1042/bj2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossi E, Greenberg RE. Sources of blood glucose in the rat fetus. Pediatr Res. 1972;6:765–772. doi: 10.1203/00006450-197210000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Boustead JN, Stadelmaier BT, Eeds AM, Wiebe PO, Svitek CA, Oeser JK, O'Brien RM. Hepatocyte nuclear factor-4 alpha mediates the stimulatory effect of peroxisome proliferator-activated receptor gamma co-activator-1 alpha (PGC-1 alpha) on glucose-6-phosphatase catalytic subunit gene transcription in H4IIE cells. Biochem J. 2003;369:17–22. doi: 10.1042/BJ20021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassuto H, Kochan K, Chakravarty K, Cohen H, Blum B, Olswang Y, Hakimi P, Xu C, Massillon D, Hanson RW, Reshef L. Glucocorticoids Regulate Transcription of the Gene for Phosphoenolpyruvate Carboxykinase in the Liver via an Extended Glucocorticoid Regulatory Unit. J Biol Chem. 2005;280:33873–33884. doi: 10.1074/jbc.M504119200. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- 8.Croniger CM, Millward C, Yang J, Kawai Y, Arinze IJ, Liu S, Harada-Shiba M, Chakravarty K, Friedman JE, Poli V, Hanson RW. Mice with a Deletion in the Gene for CCAAT/Enhancer-binding Protein beta Have an Attenuated Response to cAMP and Impaired Carbohydrate Metabolism. J Biol Chem. 2001;276:629–638. doi: 10.1074/jbc.M007576200. [DOI] [PubMed] [Google Scholar]
- 9.Desai M, Byrne CD, Zhang J, Petry CJ, Lucas A, Hales CN. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1083–G1090. doi: 10.1152/ajpgi.1997.272.5.G1083. [DOI] [PubMed] [Google Scholar]
- 10.DiGiacomo JE, Hay WW., Jr. Fetal glucose metabolism and oxygen consumption during sustained hypoglycemia. Metabolism. 1990;39:193–202. doi: 10.1016/0026-0495(90)90075-n. [DOI] [PubMed] [Google Scholar]
- 11.Domenech M, Gruppuso PA, Susa JB, Schwartz R. Induction in utero of hepatic glucose-6-phosphatase by fetal hypoinsulinemia. Biol Neonate. 1985;47:92–98. doi: 10.1159/000242096. [DOI] [PubMed] [Google Scholar]
- 12.Economides DL, Nicolaides KH. Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;160:385–389. doi: 10.1016/0002-9378(89)90453-5. [DOI] [PubMed] [Google Scholar]
- 13.Edwards LJ, Symonds ME, Warnes KE, Owens JA, Butler TG, Jurisevic A, McMillen IC. Responses of the fetal pituitary-adrenal axis to acute and chronic hypoglycemia during late gestation in the sheep. Endocrinology. 2001;142:1778–1785. doi: 10.1210/endo.142.5.8143. [DOI] [PubMed] [Google Scholar]
- 14.Eisen HJ, Goldfine ID, Glinsmann WH. Regulation of Hepatic Glycogen Synthesis during Fetal Development: Roles of Hydrocortisone, Insulin, and Insulin Receptors. PNAS. 1973;70:3454–3457. doi: 10.1073/pnas.70.12.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezekwe MO. Effects of maternal starvation on some blood metabolites, liver glycogen, birth weight and survival of piglets. J Anim Sci. 1981;53:1504–1510. doi: 10.2527/jas1982.5361504x. [DOI] [PubMed] [Google Scholar]
- 16.Fowden AL, Comline RS, Silver M. The effects of cortisol on the concentration of glycogen in different tissues in the chronically catheterized fetal pig. Q J Exp Physiol. 1985;70:23–35. doi: 10.1113/expphysiol.1985.sp002894. [DOI] [PubMed] [Google Scholar]
- 17.Fowden AL, Coulson RL, Silver M. Endocrine regulation of tissue glucose-6-phosphatase activity in the fetal sheep during late gestation. Endocrinology. 1990;126:2823–2830. doi: 10.1210/endo-126-6-2823. [DOI] [PubMed] [Google Scholar]
- 18.Fowden AL, Mijovic J, Silver M. The effects of cortisol on hepatic and renal gluconeogenic enzyme activities in the sheep fetus during late gestation. J Endocrinol. 1993;137:213–222. doi: 10.1677/joe.0.1370213. [DOI] [PubMed] [Google Scholar]
- 19.Fowden AL, Mundy L, Silver tlM. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J Physiol (Lond) 1998;508:937–947. doi: 10.1111/j.1469-7793.1998.937bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund N, Kervran A, Assan R, Geloso JP, Girard J. Fetal metabolic response to phloridzin-induced hypoglycemia in pregnant rats. Biol Neonate. 1980;38:321–327. doi: 10.1159/000241382. [DOI] [PubMed] [Google Scholar]
- 21.Girard J, Ferre P, Gilbert M, Kervran A, Assan R, Marliss EB. Fetal metabolic response to maternal fasting in the rat. Am J Physiol Endocrinol Metab. 1977;232:E456–E463. doi: 10.1152/ajpendo.1977.232.5.E456. [DOI] [PubMed] [Google Scholar]
- 22.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, Defronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruppuso PA, Migliori R, Susa JB, Schwartz R. Chronic maternal hyperinsulinemia and hypoglycemia. A model for experimental intrauterine growth retardation. Biol Neonate. 1981;40:113–120. doi: 10.1159/000241479. [DOI] [PubMed] [Google Scholar]
- 24.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 26.Hennessy DP, Coghlan JP, Hardy KJ, Scoggins BA, Wintour EM. The origin of cortisol in the blood of fetal sheep. J Endocrinol. 1982;95:71–79. doi: 10.1677/joe.0.0950071. [DOI] [PubMed] [Google Scholar]
- 27.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 28.Jackson L, Williams FL, Burchell A, Coughtrie MW, Hume R. Plasma catecholamines and the counterregulatory responses to hypoglycemia in infants: a critical role for epinephrine and cortisol. J Clin Endocrinol Metab. 2004;89:6251–6256. doi: 10.1210/jc.2004-0550. [DOI] [PubMed] [Google Scholar]
- 29.Kaneta M, Liechty EA, Moorehead HC, Lemons JA. Ovine fetal and maternal glycogen during fasting. Biol Neonate. 1991;60:215–220. doi: 10.1159/000243411. [DOI] [PubMed] [Google Scholar]
- 30.Klepac R. Effect of dexamethasone on glycogen deposition in pregnant rats and their fetuses. Exp Clin Endocrinol. 1985;86:305–309. doi: 10.1055/s-0029-1210502. [DOI] [PubMed] [Google Scholar]
- 31.Lafeber HN, Rolph TP, Jones CT. Studies on the growth of the fetal guinea pig. The effects of ligation of the uterine artery on organ growth and development. J Dev Physiol. 1984;6:441–459. [PubMed] [Google Scholar]
- 32.Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased Hepatic Peroxisome Proliferator-Activated Receptor-{gamma} Coactivator-1 Gene Expression in a Rat Model of Intrauterine Growth Retardation and Subsequent Insulin Resistance. Endocrinology. 2002;143:2486–2490. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1[agr] transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 34.Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol (Lond) 2003;547:95–105. doi: 10.1113/jphysiol.2002.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limesand SW, Rozance PJ, Smith D, Hay J. Increased Insulin Sensitivity and Maintenance of Glucose Utilization Rates in Fetal Sheep with Placental Insufficiency and Intrauterine Growth Restriction. Am J Physiol Endocrinol Metab. 2007;293:E1716–E1725. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 36.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr. Attenuated Insulin Release and Storage in Fetal Sheep Pancreatic Islets with Intrauterine Growth Restriction. Endocrinology. 2006;147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen EL, Kliegman RM. Fetal and neonatal responses to extended maternal canine starvation. II. Fetal and neonatal liver metabolism. Pediatr Res. 1983;17:639–644. doi: 10.1203/00006450-198308000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Narkewicz MR, Carver TD, Hay WW., Jr. Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res. 1993;33:493–496. doi: 10.1203/00006450-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Nyirenda MJ, Dean S, Lyons V, Chapman KE, Seckl JR. Prenatal programming of hepatocyte nuclear factor 4alpha in the rat: A key mechanism in the ‘foetal origins of hyperglycaemia’? Diabetologia. 2006;49:1412–1420. doi: 10.1007/s00125-006-0188-5. [DOI] [PubMed] [Google Scholar]
- 42.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogata ES, Paul RI, Finley SL. Limited maternal fuel availability due to hyperinsulinemia retards fetal growth and development in the rat. Pediatr Res. 1987;22:432–437. doi: 10.1203/00006450-198710000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35:970–977. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 45.Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental Transport of Leucine, Phenylalanine, Glycine, and Proline in Intrauterine Growth-Restricted Pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- 46.Parimi PS, Croniger CM, Leahy P, Hanson RW, Kalhan SC. Effect of reduced maternal inspired oxygen on hepatic glucose metabolism in the rat fetus. Pediatr Res. 2003;53:325–332. doi: 10.1203/01.PDR.0000047643.26484.48. [DOI] [PubMed] [Google Scholar]
- 47.Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E1258–E1266. doi: 10.1152/ajpendo.00437.2002. [DOI] [PubMed] [Google Scholar]
- 48.Plas C, Duval D. Dexamethasone binding sites and steroid-dependent stimulation of glycogenesis by insulin in cultured fetal hepatocytes. Endocrinology. 1986;118:587–594. doi: 10.1210/endo-118-2-587. [DOI] [PubMed] [Google Scholar]
- 49.Polonsky KS, Sturis J, Bell GI. Non-Insulin-Dependent Diabetes Mellitus --A Genetically Programmed Failure of the Beta Cell to Compensate for Insulin Resistance. N Engl J Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 50.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 51.Randall GC. Tissue glycogen concentrations in hypophysectomized pig fetuses following infusion with cortisol. J Dev Physiol. 1988;10:77–83. [PubMed] [Google Scholar]
- 52.Resnik R, Creasy R. Intrauterine Growth Restriction. In: Creasy R, Resnik R, Iams J, editors. Maternal-Fetal Medicine. Saunders; Philadelphia: 2004. pp. 495–512. [Google Scholar]
- 53.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): Requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. PNAS. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roesler WJ. What is a cAMP response unit? Mol Cell Endocrinol. 2000;162:1–7. doi: 10.1016/s0303-7207(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 55.Roesler WJ. The role of C/EBP in nutrient and hormonal regulation of gene expression. Annu Rev Nutr. 2001;21:141–165. doi: 10.1146/annurev.nutr.21.1.141. [DOI] [PubMed] [Google Scholar]
- 56.Rozance PJ, Limesand SW, Hay WW., Jr. Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab. 2006;291:E404–E411. doi: 10.1152/ajpendo.00643.2005. [DOI] [PubMed] [Google Scholar]
- 57.Rozance PJ, Limesand SW, Zerbe GO, Hay WW., Jr. Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2007;292:E1256–E1264. doi: 10.1152/ajpendo.00265.2006. [DOI] [PubMed] [Google Scholar]
- 58.SAS Institute Inc SAS/STAT® 9.1 User's Guide. 2004. Ref Type: Serial (Book,Monograph)
- 59.Schreiner RL, Nolen PA, Bonderman PW, Moorehead HC, Gresham EL, Lemons JA, Escobedo MB. Fetal and maternal hormonal response to starvation in the ewe. Pediatr Res. 1980;14:103–108. doi: 10.1203/00006450-198002000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res. 2002;275:143–154. doi: 10.1006/excr.2002.5491. [DOI] [PubMed] [Google Scholar]
- 61.Shelley HJ. Glycogen Reserves and Their Changes at Birth and in Anoxia. British Medical Bulletin. 1961;17:137–143. [Google Scholar]
- 62.Silver M, Fowden AL. Sympathoadrenal and other endocrine and metabolic responses to hypoglycaemia in the fetal foal during late gestation. Exp Physiol. 1995;80:651–662. doi: 10.1113/expphysiol.1995.sp003875. [DOI] [PubMed] [Google Scholar]
- 63.Stratford LL, Hooper SB. Effect of hypoxemia on tissue glycogen content and glycolytic enzyme activities in fetal sheep. Am J Physiol. 1997;272:R103–R110. doi: 10.1152/ajpregu.1997.272.1.R103. [DOI] [PubMed] [Google Scholar]
- 64.Tye LM, Burton AF. Glycogen deposition in fetal mouse tissues and the effect of dexamethasone. Biol Neonate. 1980;38:265–269. doi: 10.1159/000241375. [DOI] [PubMed] [Google Scholar]
- 65.Vuguin P, Raab E, Liu B, Barzilai N, Simmons R. Hepatic Insulin Resistance Precedes the Development of Diabetes in a Model of Intrauterine Growth Retardation. Diabetes. 2004;53:2617–2622. doi: 10.2337/diabetes.53.10.2617. [DOI] [PubMed] [Google Scholar]
- 66.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]