Abstract
Epidemiological data suggest robust associations of high vegetable intake with decreased risks of bladder cancer incidence and mortality, but translational prevention studies have yet to be performed. We designed and tested a novel intervention to increase vegetable intake in patients with non-invasive bladder cancer. We randomized 48 patients aged 50 to 80 years with biopsy-proven non-invasive (Ta, T1, or carcinoma in situ) urothelial cell carcinoma to telephone- and Skype-based dietary counseling or a control condition that provided print materials only. The intervention behavioral goals promoted 7 daily vegetable servings, with at least 2 of these as cruciferous vegetables. Outcome variables were self-reported diet and plasma carotenoid and 24-hour urinary isothiocyanate (ITC) concentrations. We used 2-sample t-tests to assess between-group differences at 6-month follow-up. After 6 months, intervention patients had higher daily intakes of vegetable juice (p=0.02), total vegetables (p=0.02), and cruciferous vegetables (p=0.07); lower daily intakes of energy (p=0.007), (p=0.002) and energy from fat (p=0.06); and higher plasma alpha-carotene concentrations (p=0.03). Self-reported cruciferous vegetable intake correlated with urinary ITC concentrations at baseline (p<0.001) and at 6 months (p=0.03). Although urinary ITC concentrations increased in the intervention group and decreased in the control group, these changes did not attain between-group significance (p=0.32). In patients with non-invasive bladder cancer, our novel intervention induced diet changes associated with protective effects against bladder cancer. These data demonstrate the feasibility of implementing therapeutic dietary modifications to prevent recurrent and progressive bladder cancer.
Introduction
In the U.S. in 2012, there were an estimated 73,510 new cases of and 14,480 deaths from bladder cancer.(1) The U.S. population prevalence is approximately 600,000 persons and continues to increase annually.(2) Bladder cancer is the fourth most frequently diagnosed cancer among men and—due to the high costs of diagnosis, treatment, and post-treatment surveillance—the single most expensive cancer to treat.(3) Collectively, these observations underscore the considerable challenges bladder cancer poses to the public health and highlight an important need to develop innovative, novel therapies for bladder cancer prevention and control.
A potential means of decreasing the morbidity and mortality of bladder cancer is through lifestyle change. Modifiable risk factors present novel, practical targets for primary and tertiary bladder cancer chemoprevention because modulations of these factors potentially exert beneficial, disease-specific health effects. For example, smoking is strongly associated with an increased risk of incident bladder cancer, and a recent cohort analysis of patients with non-invasive bladder cancer observed that longer-term smoking cessation was associated with reduced risks of disease recurrence and progression of 34% and 58%, respectively.(4)
Robust epidemiological data indicate beneficial associations of increased vegetable intake, particularly cruciferous vegetables, with decreased risks of incident and progressive bladder cancer.(5) In the Health Professional’s Follow-Up Study, those in the highest quartile of cruciferous vegetable intake had a 50% reduced risk of urothelial cancer compared to those in the lowest quartile.(6) In a cohort of bladder cancer patients, increased consumption of raw broccoli was associated with a 43% decreased risk of death from bladder cancer.(7) Other population-based studies have observed similar patterns.(8, 9)
Translational studies of lifestyle modifications and bladder cancer, however, have yet to be performed. In a randomized clinical trial, we tested a novel intervention to increase vegetable intake in patients with non-invasive bladder cancer.
Materials and Methods
Study population
We recruited 48 patients aged 50 to 80 years at 4 study sites (Moores Comprehensive Cancer Center, University of California San Diego and San Diego Veterans Affairs Medical Center, La Jolla, CA; Roswell Park Cancer Institute, Buffalo, NY; and Waikato Hospital, Hamilton, New Zealand) with biopsy-proven non-invasive (Ta, T1, or carcinoma-in-situ) urothelial cell carcinoma with at least a three-year life expectancy and a willingness to be randomized to receive information about diet or to participate in dietary intervention. Institutional Review Board approval was obtained at all sites.
Exclusion criteria included psychiatric illness precluding compliance with the intervention and/or obtainment of informed consent; medical conditions which in the opinion of the treating physician made the protocol unreasonably hazardous, including infection, chronic diseases (such as diabetes mellitus, cardiac disease, ulcerative colitis, and Crohn’s disease); intolerance of cruciferous vegetables; bladder cancer with distant metastases; prior cystectomy or radiotherapy; current oral anticoagulation therapy with coumadin; and unwillingness to adopt a vegetable-rich diet.
Intervention: telephone- and Skype-based dietary counseling
Patients were randomized to 6 months of telephone- or Skype-based (for New Zealand patients, n=1) dietary counseling or a control condition that provided print materials only.
The principle strategy to promote dietary change in the intervention arm was a counseling protocol with individualized, one-on-one assistance tailored to each participant. The counseling protocol followed a step-wise, phased approach employing social cognitive theory.(10, 11) Motivational interviewing techniques were utilized to help participants assume and maintain responsibility for their own behavior change.(12) Similar to a prior study we conducted in prostate cancer patients,(13) we included 12 telephone calls over the 6-month intervention, with more frequent calls occurring during the early phase of the intervention when participants required more support in making dietary change. The protocol specified 5 calls during month one and 3 calls during month two, followed by monthly maintenance calls during months three through six.
The primary intervention behavioral goal was 7 daily vegetable servings, with at least 2 of these as cruciferous vegetables. We defined a serving size as ½ cup cut-up, chopped, or shredded vegetables; ½ cup vegetable sauce or puree; 1 cup of raw, leafy vegetables; or ¾ cup (6 fl. oz.) vegetable juice. Within the context of these overall targets, participants were guided to obtain an adequate intake of all essential nutrients. To enhance quality control, all counseling was performed centrally from the Moores UCSD Cancer Center. Prior to beginning counseling, counselors completed an intensive 80-hour training program; moreover, counselor performance was monitored throughout the study to ensure counselor consistency and quality.
The control group was provided the Dietary Guidelines for Americans, 2005, which recommended 5 daily servings of vegetables daily and did not emphasize cruciferous vegetables.(14)
Outcome variables
Diets were evaluated at baseline and again at 6-month follow-up by a series of 3 separate 24-hour dietary recalls collected interactively via telephone interview. Each set of recalls included two weekdays and 1 weekend day during a 2-week period to provide data on average intake over that time period. To reduce the potential for reporting bias, the assessors were blinded to the randomization allocation. Dietary data was collected and analyzed utilizing Minnesota Nutrition Data System (NDS) software (Nutrition Coordinating Center, University of Minnesota).
Plasma carotenoids are established biomarkers of vegetable intake.(13, 15) Fasting blood samples were collected at baseline and at 6-month follow-up and, using high performance liquid chromatography (HPLC), analyzed for lutein, cryptoxanthin, lycopene, alpha carotene, and beta carotene concentrations, which account for > 90% of carotenoids in the circulation.(15)
Urinary isothiocyanate (ITC) concentrations are indicators of cruciferous vegetable intake.(16) Twenty-four hour urine samples were collected at baseline and at 6-month follow-up and analyzed for cumulative urinary ITC concentrations with HPLC methodology using the cyclocondensation reaction.(16–18)
All laboratory analyses were performed in the Moores UCSD Comprehensive Cancer Center Nutrition Shared Resource Laboratory, which participates in the National Institute of Standards and Technology (NIST), U.S. Department of Commerce, Micronutrients Measurement Quality Assurance (QA) Programs and College of American Pathologists QA Program. Blood and urine samples collected at the San Diego VA, Roswell Park Cancer Institute, and Waikato Hospital were stored at −80 degrees until shipment to UCSD.
Statistical analysis
The study was designed to have > 80% power to detect a moderate to large 0.7 effect-size (i.e. mean difference in diet change between arms divided by the SD of change) with 50 total participants, based on a 1-sided t-test with alpha=0.05. The primary outcomes were changes in objective dietary biomarkers, namely alpha-carotene and urinary isothyocyanates, since vegetables, especially cruciferous vegetables, were a primary target of the intervention. Secondary outcomes were self-reported dietary intake of vegetables, fruits, grains, and fat. For this pilot feasibility study, no multiple comparisons adjustment for sample-size were made for the multiple outcomes.
We randomized participants in a 3:2 fashion to the dietary intervention (N=30) or control arm (N=18) using a block design. We randomized a larger proportion to the intervention arm in order to specifically and intensively investigate the feasibility of administering the behavior intervention in this pilot trial and to obtain estimates of effect-size and precision for planning a larger trial.
To test if randomization achieved comparable groups at baseline, we compared diet groups on baseline participant characteristics using 2-sample tests. To examine dietary changes, we used 2-sample nonparametric Wilcoxon tests to assess between-group differences from baseline to 6-month follow-up. We evaluated self-reported dietary values and biomarkers.
We conducted a sensitivity analysis to examine the robustness of results to missing data by fitting a linear mixed-effects model with dietary intake at baseline and 6 months as repeated outcome measures.(19) We included a person-specific random effect to model person-person variability in intake and diet group, time (0, 6months), and the group*time interaction as fixed effects in the models. A significant diet*group interaction would indicate that dietary changes differed significantly between study arms. We transformed biomarker values to better approximate a Gaussian distribution in these models. We used a 5% significance level for all analyses.
Results
Baseline characteristics
The groups did not differ significantly in age, BMI, gender, ethnicity, education, time from diagnosis to randomization, or tumor stage (Table 1).
Table 1.
Participant characteristics at baseline, stratified by study arm, in the Dietary Intervention in Bladder Cancer Study (DIBS).
| Overall N = 48 |
Control N = 18 |
Intervention N = 30 |
p-value† | |
|---|---|---|---|---|
| Mean age, years (SE) | 66 (9) | 65 (8) | 66 (9) | 0.93 |
| Mean Body Mass Index, kg/m2 (SD) | 29 (7) | 29 (7) | 30 (8) | 0.38 |
| Gender, % | 0.74 | |||
| Male | 75 | 72 | 77 | |
| Female | 25 | 28 | 23 | |
| Ethnicity, % | 0.53 | |||
| Non-Hispanic white | 88 | 89 | 87 | |
| Hispanic | 4 | 0 | 7 | |
| Black | 6 | 11 | 3 | |
| Other | 2 | 0 | 3 | |
| Education, % | 0.61 | |||
| High school graduate | 19 | 22 | 17 | |
| Some college education | 31 | 22 | 37 | |
| College graduate | 29 | 28 | 30 | |
| Post-college graduate | 19 | 28 | 13 | |
| Unknown | 2 | 0 | 3 | |
| Mean time from diagnosis to randomization, years (SE) | 1.5 (1.3) | 1.2 (1.2) | 1.7 (1.3) | 0.19 |
| Tumor stage, % | 0.50 | |||
| Ta | 63 | 56 | 67 | |
| T1 | 27 | 28 | 26.5 | |
| Carcinoma in situ | 10 | 17 | 6.5 |
Comparing control and intervention
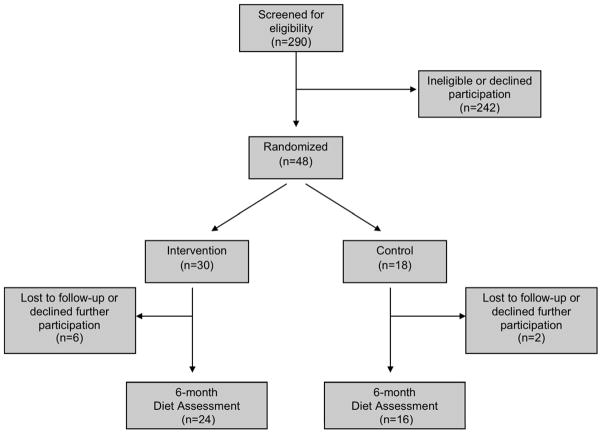
At 6-month follow-up, 83% of the participants completed the diet recall assessments (Table 2) and 81% (Table 3) provided blood and urine samples to complete the biomarker assays. Six (20%) participants in the intervention arm and 2 (11%) in the control arm did not complete the study (Figure 1). The reasons for participant discontinuation were as follows: in the intervention group, 1 could not continue due to bladder cancer progression, 1 withdrew due to other medical reasons, 2 could not be contacted by phone, and 2 voluntarily withdrew for unspecified reasons; in the control group, 1 could not continue due to bladder cancer progression and 1 could not be contacted by phone.
Table 2.
Dietary composition comparing intervention to control at baseline and 6-month follow-up in the Dietary Intervention in Bladder Cancer Study (DIBS).
| Baseline | 6-month Follow-up | p-valuec | |||
|---|---|---|---|---|---|
| Control Mean (SE) | Intervention Mean (SE) | Control Mean (SE) | Intervention Mean (SE) | ||
|
| |||||
| N = 18 | N = 30 | N = 16 | N = 24 | ||
|
| |||||
| Energy (kcal/day) | 1927.2 (178.3) | 1946.5 (78.3)* | 2012.6 (148.5) | 1773.3 (89.8)* | 0.007 |
| Fat (g/day) | 67.6 (7.9)† | 80.521 (4.8)†** | 77.4 (6.7) | 66.1 (4.8)** | 0.002 |
| Vegetable juice (servings/day) | 0.07 (0.07) | 0.16 (0.07)** | 0.06 (0.06) | 0.74 (0.2)** | 0.02 |
| Total vegetables (servings/day) | 2.3 (0.2) | 2.4 (0.23)** | 2.1 (0.3) | 3.9 (0.4)** | 0.02 |
| Cruciferous vegetables (servings/day) | 0.40 (0.12) | 0.49 (0.14)** | 0.35 (0.1) | 1.2 (0.2)** | 0.07 |
| Total fruit (servings/day) | 3.74 (0.69) | 3.27 (0.45) | 2.46 (0.40) | 2.12 (0.37) | 0.97 |
| Refined grain products (servings/day) | 1.79 (0.29) | 1.90 (0.15) | 1.7 (0.3) | 1.4 (0.3) | 0.06 |
| Whole grain products (servings/day) | 1.08 (0.22) | 0.86 (0.15) | 1.378 (0.2) | 1.35 (0.2) | 0.95 |
| Legumes, total (servings/day) | 0.28 (0.21) | 0.21 (0.06)** | 0.132 (0.049) | 0.47 (0.1)** | 0.06 |
| Legumes, soy (servings/day) | 0.13 (0.08) | 0.02 (0.007) | 0.035 (0.023) | 0.17 (0.09) | 0.15 |
| Energy from fat (%) | 31.2 (2.0)‡ | 37.1 (1.5)‡ | 34.5 (2.3) | 33.4 (1.8) | 0.06 |
p-value based on Wilcoxon rank sum test comparing changes in each marker between groups.
between group difference at baseline Wilcoxon test p-value < 0.1
between group difference at baseline Wilcoxon test p-value < 0.05
within group change p-value < 0.1
within group change p-value < 0.05
Table 3.
Dietary biomarkers comparing intervention to control at baseline and 6-month follow-up in the Dietary Intervention in Bladder Cancer Study (DIBS).
| Baseline | 6-month Follow-up | p-valuec | |||
|---|---|---|---|---|---|
| Control Mean (SE) | Intervention Mean (SE) | Control Mean (SE) | Intervention Mean (SE) | ||
|
| |||||
| N = 18 | N = 30 | N = 15 | N = 24 | ||
|
| |||||
| Lutein plus zeaxanthin (umol/L) a | 0.38 (0.04)‡ | 0.33 (0.07)‡ | 0.37 (0.03) | 0.32 (0.05) | 0.97 |
| Cryptoxanthin (umol/L) a | 0.19 (0.04) | 0.19 (0.06)* | 0.18 (0.04) | 0.13 (0.03)* | 0.42 |
| Lycopene (umol/L) a | 0.78 (0.06) | 0.65 (0.06) | 0.76 (0.11) | 0.68 (0.08) | 0.38 |
| Alpha-carotene (umol/L) a | 0.20 (0.03)‡ | 0.14 (0.04)‡** | 0.17 (0.03) | 0.16 (0.03)** | 0.03 |
| Beta-carotene (umol/L) a | 0.62 (0.09)‡ | 0.47 (0.09)‡ | 0.69 (0.16) | 0.55 (0.08) | 0.40 |
| Total carotenoids (umol/L) a | 2.2 (0.18)‡ | 1.70 (0.19)‡ | 2.2 (0.29) | 1.80 (0.20) | 0.60 |
| Isothiocyanate (umol/L) b | 95.5 (42.9) | 40.0 (14.4) | 56.9 (23.0) | 59.9 (14.8) | 0.32 |
Plasma
Urine
p-value based on Wilcoxon rank sum test comparing changes in each marker between groups.
between group difference at baseline Wilcoxon test p-value < 0.1
between group difference at baseline Wilcoxon test p-value < 0.05
within group change p-value < 0.1
within group change p-value < 0.05
Figure 1.
Composition of the study population in the Dietary Intervention in Bladder Cancer Study (DIBS).
Self-reported dietary intake
At 6-month follow-up, intervention patients reported significant increases in daily intakes of vegetable juice and total vegetables and lower daily intakes of total energy (kcal/d) and fat (g/d) compared to control patients. Although not significant at the 5% level, daily intakes of cruciferous vegetables and legumes increased and energy from fat decreased in the intervention compared to the control groups (Table 2).
Dietary biomarkers
At 6-month follow-up, intervention patients demonstrated significant increases from baseline in plasma alpha-carotene concentrations compared to controls. Although intervention patients also had increases from baseline in plasma lycopene, beta-carotene, and total carotenoids compared to controls, these differences did not attain significance (Table 3).
Self-reported cruciferous vegetable intake correlated significantly with 24-hour urinary ITC concentrations at both baseline (Spearman r=0.63, p=0.005) and 6-month follow-up (Spearman r=0.45, p=0.03). Although urinary ITC concentrations increased in the intervention arm and decreased in the control arm, these changes did not attain significance among the patients for whom 6-month comparison data were available (Table 3). Sensitivity analysis using mixed model yielded concordant results (Appendix).
Discussion
In this randomized pilot trial in patients with non-invasive bladder cancer, our novel dietary counseling intervention significantly increased vegetable intake and plasma alpha-carotene concentrations, significantly decreased fat and total energy intake, and marginally significantly increased cruciferous vegetable intake. In the intervention group, self-reported intake of cruciferous vegetables more than doubled (p<0.05 for within group change and p=0.07 for between group change, Table 2) and for total vegetable intake increased by more than 60% (p<0.05 for within group change and p=0.02 for between group change, Table 2). This trial is the first clinical study of a dietary intervention for bladder cancer and demonstrates the feasibility of implementing therapeutic, chemopreventive lifestyle modifications in patients with bladder cancer.
The aim of this pilot study was to develop a feasible clinical intervention that produces changes in the diets of bladder cancer patients consistent with the putative benefits of prior epidemiological and pre-clinical data. Although the majority of prior evidence supports a role for dietary modification in primary prevention, (6, 8, 9) at least one study observed a survival benefit for higher crucifer intake among bladder cancer survivors.(7) Moreover, given the relatively high incidence of recurrence and progression, a compelling argument can be made in favor of the clinical relevance of tertiary prevention applications. Approximately 75% to 85% of patients with bladder cancer initially present with non-invasive disease; of these, 50% to 90% will recur or progress to invasive disease within 3 to 5 years despite aggressive local therapy. (20, 21)
An additional finding was that self-reported cruciferous vegetable intake correlated with 24-hour urinary ITC concentrations. This finding is consistent with prior feeding studies in cohorts of hospitalized and non-hospitalized study participants (16, 22–26) and confirms that urinary ITC concentration is a robust biomarker for cruciferous vegetable intake in the setting of an outpatient clinical trial for cancer.
Unlike the increases in reported total and cruciferous vegetable intakes, changes in total carotenoids, lycopene, beta-carotene and in the urinary ITC concentrations did not attain significance, most likely because of the relatively small sample sizes (Figure 1). Although the changes were non-significant, total carotenoid and isothiocyanate concentrations in the intervention group increased while those in the control group decreased or remained stable, consistent with the intervention emphasis on cruciferous vegetables. Larger sample sizes would be needed to definitively demonstrate biomarker changes.
In addition, although objective biomarkers are useful metrics, they should not necessarily be regarded as a gold standard for measuring relevant intakes of nutrients in clinical trials, nor should they substitute for diet recall data. Individual variations in variables including, but not limited to, recent macronutrient intake (especially fat), BMI, and ethnicity may potentially influence serum and urinary concentrations of these biomarkers and introduce systematic biases.(27) Biomarkers and dietary recall measures should thus be utilized as complementary metrics.
We believe these results will inform the design of a Phase III trial of dietary modification to prevent recurrence and progression among patients with non-invasive bladder cancer. Specific data we will consider for trial design include the following: first, the carotenoid and ITC biomarker results, which suggest that power calculations for a Phase 3 trial will require consideration of a larger sample size than originally anticipated; second, the adherence data, which suggest an anticipated dropout rate of up to 20% (Figure 1); and, finally, the observation that 24-hour urinary ITC concentrations correlated with self-reported cruciferous vegetable intake, which potentially allows for greater reliance on patient self report, which would free patients of burdensome 24-hour urine collections and possibly increase adherence to follow-up.
Our intervention is practicable, demands few resource commitments on the part of the patient, and is low-cost to implement among relatively large and geographically diverse study populations. The intervention employs straightforward, rational strategies adopted from social cognitive theory (10, 11) using the techniques of motivational interviewing.(12) Similar interventions have produced marked changes in the diet and increased plasma carotenoids in both breast cancer (28, 29) and prostate cancer (13, 30) patients.
Notably, since obesity and poor diet disproportionately affect African Americans and Hispanics, (31) this intervention may be particularly relevant for addressing health care disparities for bladder cancer among underserved minorities. Compared to non-Hispanic whites, African Americans with bladder cancer experience diminished cancer-specific and overall survival, are more likely to present with higher grade and higher stage disease, and have increased risks of adverse outcomes after cystectomy, including prolonged length of stay and death.(32) Bladder cancer incidence and mortality are also higher among Hispanics compared to non-Hispanic whites.(33, 34)
Carotenoids, which occur in high concentrations in tomatoes, carrots, and other deeply pigmented vegetables, are putative anti-carcinogenic agents. Prior studies have observed strong associations of higher plasma carotenoid concentrations with decreased risks of incident bladder cancer. In one analysis comparing the highest quartiles to the lowest, plasma total carotenoids (RR 0.64; 95% CI: 0.44, 0.93; p-trend = 0.04) and beta-carotene (RR: 0.51; 95% CI: 0.30, 0.88; P-trend = 0.02) were inversely associated with aggressive and plasma lutein (RR: 0.56; 95% CI: 0.32, 0.98; P-trend = 0.05) with nonaggressive cancers, respectively.(35) In another study, higher concentrations of alpha-carotene (0.22, 95% CI 0.05 to 0.92), lutein (0.42, 95% CI 0.18 to 1.00), zeaxanthin (0.16, 95% CI 0.02 to 1.06) lycopene (0.94, 95% CI 0.89 to 0.99) and beta-cryptoxanthin (0.90, 95% CI 0.81 to 1.00) were associated with decreased risks of incident disease.(36)
Cruciferous vegetables—such as broccoli, kale, and radishes—are rich in ITCs. ITCs are potent inducers of phase 2 cytoprotective enzymes, including glutathione S-transferase (GST) and NAD(P)H:quinolonone oxidoreductase 1 (NQ01). Cruciferous vegetables are believed to exert anti-carcinogenic effects through induction of these enzymes. Several studies have observed an increased risk of urothelial carcinoma in individuals with GST and NQ01 genotypes associated with null or sub-optimal phenotypes.(37–40) Similarly, a case control study reported that increased consumption of ITCs was associated with a 29% decreased risk of incident bladder cancer,(41) and ingestion of ITC-rich broccoli sprout extract significantly inhibited bladder carcinogenesis in a rat model.(42)
Strengths of this pilot study include its novel yet practical therapeutic strategy for bladder cancer primary or tertiary chemoprevention, randomized trial design that produced balanced study arms, 85% participant retention rate, and comprehensive dietary assessments. One potential limitation was the limited sample size, which could have reduced the study power to detect significant differences in most of the biomarkers. Another potential limitation is that it is unclear whether the observed changes in diet and plasma alpha-carotene will persist beyond 6 months. However, a prior study of breast cancer suggests that these changes could continue for at least four years after the initial application of the intervention.(28) Finally, it is possible that recall bias occurred in that the intervention participants may have differentially reported consuming the recommended foods in order to please the interviewer. However, a prior analysis of diet change in breast cancer patients concluded that, while there is a potential increase in systematic error for diet assessment in an intervention group, the validity (i.e., correlation with “true” intake) of self-report is significantly higher during follow-up for intervention versus nonintervention participants.(43) Thus, although the potential for error existed among self-reported diet in the intervention group, so did the potential for increased validity.
Conclusions
In patients with non-invasive bladder cancer, our novel intervention induced dietary changes associated with protective effects against bladder cancer. These data demonstrate the feasibility of implementing therapeutic, chemopreventive dietary modifications in bladder cancer patients and support the performance of Phase 3 clinical trials focused on preventing incident, recurrent, and progressive disease.
Supplementary Material
Acknowledgments
Funding: NCI grant CA037447-25
Footnotes
The authors have no relevant conflicts of interest.
References
- 1.American Cancer Society. Bladder cancer. 2013 Jun 7; Available from: http://www.cancer.org.
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Busby JE, Kamat AM. Chemoprevention for bladder cancer. J Urol. 2006;176:1914–20. doi: 10.1016/j.juro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, et al. Impact of Smoking and Smoking Cessation on Oncologic Outcomes in Primary Non-muscle-invasive Bladder Cancer. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silberstein JL, Parsons JK. Evidence-based principles of bladder cancer and diet. Urology. 2010;75:340–6. doi: 10.1016/j.urology.2009.07.1260. [DOI] [PubMed] [Google Scholar]
- 6.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–13. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 7.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1806–11. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:938–44. doi: 10.1158/1055-9965.EPI-07-2502. [DOI] [PubMed] [Google Scholar]
- 9.Zeegers MP, Goldbohm RA, van den Brandt PA. Consumption of vegetables and fruits and urothelial cancer incidence: a prospective study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:1121–8. [PubMed] [Google Scholar]
- 10.Kahneman D, Tversky A, editors. Choices, Values, and Frames. New York: Cambridge University Press and Russell Sage Foundation; 2000. [Google Scholar]
- 11.Bandura A. Self-Efficacy: The Exercise of Self-Control. Vol. 1997. New York, New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 12.WRM, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 2002. [Google Scholar]
- 13.Parsons JK, Newman V, Mohler JL, Pierce JP, Paskett E, Marshall J. The Men’s Eating and Living (MEAL) study: a Cancer and Leukemia Group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology. 2008;72:633–7. doi: 10.1016/j.urology.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2005. 2013 Jun 7; Available from: http://www.health.gov/dietaryguidelines/dga2005/document/
- 15.Muller H, Bub A, Watzl B, Rechkemmer G. Plasma concentrations of carotenoids in healthy volunteers after intervention with carotenoid-rich foods. Eur J Nutr. 1999;38:35–44. doi: 10.1007/s003940050044. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen M, Krogholm KS, Frederiksen H, Bugel SH, Rasmussen SE. Urinary excretion of total isothiocyanates from cruciferous vegetables shows high dose-response relationship and may be a useful biomarker for isothiocyanate exposure. Eur J Nutr. 2007;46:377–82. doi: 10.1007/s00394-007-0676-5. [DOI] [PubMed] [Google Scholar]
- 17.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clinica chimica acta; international journal of clinical chemistry. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Analytical biochemistry. 1996;239:160–7. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 19.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxofrd University Press; 1994. pp. 1–254. [Google Scholar]
- 20.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Alkhateeb SS, Van Rhijn BW, Finelli A, van der Kwast T, Evans A, Hanna S, et al. Nonprimary pT1 nonmuscle invasive bladder cancer treated with bacillus Calmette-Guerin is associated with higher risk of progression compared to primary T1 tumors. J Urol. 2010;184:81–6. doi: 10.1016/j.juro.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Fowke JH, Fahey JW, Stephenson KK, Hebert JR. Using isothiocyanate excretion as a biological marker of Brassica vegetable consumption in epidemiological studies: evaluating the sources of variability. Public Health Nutr. 2001;4:837–46. doi: 10.1079/phn2000113. [DOI] [PubMed] [Google Scholar]
- 23.Fowke JH, Hebert JR, Fahey JW. Urinary excretion of dithiocarbamates and self-reported Cruciferous vegetable intake: application of the ‘method of triads’ to a food-specific biomarker. Public Health Nutr. 2002;5:791–9. doi: 10.1079/PHN2002345. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutrition and cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7:1091–100. [PubMed] [Google Scholar]
- 26.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:501–8. [PubMed] [Google Scholar]
- 27.Rock CL. Carotenoids: biology and treatment. Pharmacological Therapy. 1997;75:185–97. doi: 10.1016/s0163-7258(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 28.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold EB, Pierce JP, Natarajan L, Stefanick ML, Laughlin GA, Caan BJ, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women’s healthy eating and living trial. J Clin Oncol. 2009;27:352–9. doi: 10.1200/JCO.2008.16.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101:1227–31. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 31.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs BL, Montgomery JS, Zhang Y, Skolarus TA, Weizer AZ, Hollenbeck BK. Disparities in bladder cancer. Urol Oncol. 2012;30:81–8. doi: 10.1016/j.urolonc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic differences in bladder cancer survival. Urology. 2011;78:544–9. doi: 10.1016/j.urology.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieder AM, Mackinnon JA, Huang Y, Fleming LE, Koniaris LG, Lee DJ. Florida bladder cancer trends 1981 to 2004: minimal progress in decreasing advanced disease. J Urol. 2008;179:491–5. doi: 10.1016/j.juro.2007.09.082. discussion 5. [DOI] [PubMed] [Google Scholar]
- 35.Ros MM, Bueno-de-Mesquita HB, Kampman E, Aben KK, Buchner FL, Jansen EH, et al. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2012;96:902–10. doi: 10.3945/ajcn.111.032920. [DOI] [PubMed] [Google Scholar]
- 36.Hung RJ, Zhang ZF, Rao JY, Pantuck A, Reuter VE, Heber D, et al. Protective effects of plasma carotenoids on the risk of bladder cancer. J Urol. 2006;176:1192–7. doi: 10.1016/j.juro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Park SJ, Zhao H, Spitz MR, Grossman HB, Wu X. An association between NQO1 genetic polymorphism and risk of bladder cancer. Mutat Res. 2003;536:131–7. doi: 10.1016/s1383-5718(03)00041-x. [DOI] [PubMed] [Google Scholar]
- 38.Salagovic J, Kalina I, Habalova V, Hrivnak M, Valansky L, Biros E. The role of human glutathione S-transferases M1 and T1 in individual susceptibility to bladder cancer. Physiol Res. 1999;48:465–71. [PubMed] [Google Scholar]
- 39.Schulz WA, Krummeck A, Rosinger I, Eickelmann P, Neuhaus C, Ebert T, et al. Increased frequency of a null-allele for NAD(P)H: quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics. 1997;7:235–9. doi: 10.1097/00008571-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Toruner GA, Akyerli C, Ucar A, Aki T, Atsu N, Ozen H, et al. Polymorphisms of glutathione S-transferase genes (GSTM1, GSTP1 and GSTT1) and bladder cancer susceptibility in the Turkish population. Arch Toxicol. 2001;75:459–64. doi: 10.1007/s002040100268. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Lin J, Grossman HB, Hernandez LM, Dinney CP, Wu X. Dietary isothiocyanates, GSTM1, GSTT1, NAT2 polymorphisms and bladder cancer risk. Int J Cancer. 2007;120:2208–13. doi: 10.1002/ijc.22549. [DOI] [PubMed] [Google Scholar]
- 42.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 43.Natarajan L, Pu M, Fan J, Levine RA, Patterson RE, Thomson CA, et al. Measurement error of dietary self-report in intervention trials. American journal of epidemiology. 2010;172:819–27. doi: 10.1093/aje/kwq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.