Abstract
Background and aims
Thiopurines, including 6-mercaptopurine (6-MP) and azathioprine (AZA), are the mainstay of maintenance therapy for Crohn’s disease (CD). However, studies examining their effectiveness in routine practice among diverse patient populations are lacking. Among a cohort of new users of 6MP/AZA, we described treatment patterns and changes in subsequent therapy.
Methods
Using the Truven Health Analytics databases, we identified all individuals diagnosed with CD and initiating 6-MP/AZA monotherapy from 2001–2008 (n=3,657). We estimated the proportion of CD patients remaining on 6-MP/AZA monotherapy, using Kaplan–Meier methods, and identified predictors of treatment noncontinuation, using multivariable Cox regression. Among the “noncontinuers,” we described subsequent patterns of maintenance therapy and summarized the diagnosis and procedure codes and prescription drug claims preceding treatment discontinuation.
Results
The 1-year 6-MP/AZA treatment continuation rate was 42%. Children (age ≤18 years) and individuals with no prior anti-tumor necrosis factor (TNF) use were more likely to continue 6-MP/AZA, while those dispensed more (>4) outpatient prescriptions for any drug before initiation of 6-MP/AZA were less likely to continue maintenance treatment. Overall, 1,128 (39%) and 105 (4%) individuals experienced a clinical event potentially indicating active disease or 6-MP/AZA-intolerance prior to discontinuation, respectively. Most patients discontinued therapy; among the remaining patients who failed to continue 6-MP/AZA, most augmented with an anti-TNF.
Conclusion
Most patients initiating 6-MP/AZA monotherapy did not continue beyond 1 year. In contrast to trial evidence showing 1-year remission rates of 40%–80%, this study observed a lower effectiveness of 6-MP/AZA treatment, possibly due to differences in disease severity, patient demographics, comorbidity, adherence, and health care utilization.
Keywords: immunomodulators, outcomes research/measurement, inflammatory bowel disease, patterns of care
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) affecting close to 500,000 Americans.1,2 CD is characterized by flares of abdominal pain, diarrhea, rectal bleeding, and extraintestinal manifestations, followed by periods of remission. Because the disease is typically relapsing and remitting, the two goals of medical therapy are to treat disease flares and prolong remission.
Since the initial trial conducted by Present et al in 1980 describing the efficacy of 6-mercaptopurine in (6-MP) in the treatment of CD, thiopurines, including 6-MP and azathioprine (AZA), have become mainstays in the IBD therapeutic arsenal.3–5 Subsequent randomized controlled trials (RCTs) have reported 1-year remission rates for 6-MP/AZA in adult CD populations of 40%–70%;6–9 and a single RCT in pediatric CD demonstrated a >80% remission rate at 12 and 18 months.10 Consequently, a recent meta-analysis from the Cochrane Collaboration concluded that thiopurines are effective for inducing and maintaining remission among adult CD patients.4
In addition to RCTs, which are the gold standard for evaluating treatment efficacy, clinical effectiveness research involves the study of the benefits and harms of medications when used in “real-world” settings, in which patients tend to be older, have more comorbidity,11 are not as carefully monitored or adherent to their medications, and remain on treatment longer than subjects in RCTs.12 An early study by Fraser et al13 using 30 years of data from an IBD clinic in Oxford, England reported overall rates of remission in CD patients initiating AZA of 45% (123/272), consistent with, though on the low end of, RCT findings. However, data from more recent studies examining the real world use of 6-MP/AZA have suggested that the clinical effectiveness of thiopurines in practice may be less than the efficacy demonstrated in clinical trials. A study by Goodhand et al14 reported that 6-month steroid-free remission was achieved in only 30% (15/50) of children and 38% (19/50) of adults treated with thiopurines. Another study, by Riello et al,15 found similar rates of steroid-free remission in a small, single-center observational pediatric cohort of CD patients. Most recently, an observational study by Hyams et al16 reported that children who initiated immunomodulator treatment had similar rates of remission at 1 year when compared with children who did not initiate immunomodulator or anti-tumor necrosis factor (TNF) treatment, indicating a lack of effectiveness.
To further evaluate the clinical effectiveness of thiopurines in a large and diverse population, we used health insurance claims data in the USA to undertake a retrospective cohort study of individuals diagnosed with CD who initiated 6-MP/AZA monotherapy. Specifically, we estimated the proportion of CD patients that remained on this maintenance regimen over time and identified independent patient-level predictors of 6-MP/AZA monotherapy noncontinuation. We also described clinical events occurring before discontinuation, and examined the subsequent treatment strategies utilized.
Materials and methods
Data source
The data for this study were drawn from Truven Health Analytics databases (Ann Arbor, MI, USA), including the Commercial Claims and Encounters database (January 1, 2000 – December 31, 2009) and the Medicare Supplemental and Coordination of Benefits database (January 1, 2006 – December 31, 2009), collectively referred to as “the databases.” The databases capture person-specific clinical utilization, expenditures, and enrollment information across inpatient, outpatient, prescription drug, and carve-out services from a selection of large employers, health plans, and government and public organizations in the USA. The paid claims and encounter data for the study period were linked to detailed patient information across sites and types of providers, and over time. The annual medical databases include commercial health data from approximately 100 payers.
Inpatient and outpatient diagnoses and procedures were recorded using International Classification of Diseases, ninth revision (ICD-9 CM) codes, Common Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes. Drug information, including administration and the dispensing of specific treatments, were identified using HCPCS codes and National Drug Codes (NDCs).17 Patient demographic data, and start and stop dates for plan enrollments were recorded. As all records used in the analysis were previously collected and deidentified, this study did not constitute human subjects research.
Study sample
All individuals with a first prescription claim for 6-MP/AZA between January 1, 2001 and December 31, 2008 were eligible for study inclusion. The date of the first 6-MP/AZA prescription claim was defined as the “index prescription date.” Each individual was required to have plan enrollment for at least 12 - months prior to (“preindex period”) and following (“postindex period”) the index prescription date, creating a cohort of incident users of 6-MP/AZA with sufficient health care utilization information to assess the important predictors and outcomes of interest.18 To ensure that the study population included only CD patients, individuals were only included if they had at least two claims for CD (ICD-9: 555) on different days and no claims for ulcerative colitis (UC) (ICD-9: 556) during the preindex period. To ensure a study population in which 6-MP/AZA monotherapy was the primary maintenance strategy, all individuals who used other CD treatment (ie, methotrexate, an anti-TNF agent, or natalizumab) during the 90-day “washout period” prior to the index prescription date were excluded.
Outcome definitions
Treatment continuation
Because administrative data lack the detail necessary to identify all clinically relevant outcomes, including disease exacerbations, changes in disease activity, and medication-related toxicity, the primary outcome for this study, determined a priori, was the continuation of 6-MP/AZA as the primary treatment strategy. This is an appropriate proxy outcome for clinical effectiveness because patients who discontinue 6-MP/AZA, who augment their treatment with another agent, or who switch agents are likely either intolerant to or “failures of 6-MP/AZA.” Treatment continuation was defined as continuous 6-MP/AZA monotherapy (without concurrent use of other CD maintenance treatment, such as methotrexate, anti-TNF agents, or natalizumab). The period of use was determined by the date of 6-MP/AZA dispensing, plus days supplied, plus an additional 90-day “grace” period to account for delays in prescription dispensing or poor adherence. If the number of days supplied was missing, it was assumed to be 30 days.
Treatment augmentation, switch, and discontinuation
Three mutually exclusive secondary outcomes were defined, which together accounted for the failure of treatment continuation (Figure 1). The first category was a “treatment augmentation,” defined as the presence of a claim for methotrexate, an anti-TNF agent, or natalizumab (a “new maintenance treatment”) occurring alongside continuous 6-MP/AZA treatment, allowing for a 90-day grace period. The date of augmentation was defined as the date of the first new maintenance treatment claim. The second category was a “treatment switch,” defined as the presence of a claim for a new maintenance treatment in addition to the lack of a claim for 6-MP/AZA during the period from the date of the new maintenance treatment plus the number of days supplied and a 90-day grace period. The date of switch was the date of the first new maintenance treatment claim. The third outcome was a “treatment discontinuation,” defined as the lack of a claim for any maintenance treatment (ie, 6-MP/AZA or a new maintenance treatment) during the period from the date of last prescription claim for 6-MP/AZA plus the days supplied and a 90-day grace period. The date of discontinuation was the date of the last 6-MP/AZA claim plus the days supplied. All remaining individuals were considered to be censored either at disenrollment from the health plan or at the end of the study period (December 31, 2009).
Figure 1.
Illustration of the cohort of CD patients who initiated 6-mercaptopurine/azathioprine, and the three secondary outcomes of treatment, discontinuation, augmentation, and switch.
Abbreviations: 6-MP, 6-mercaptopurine; AZA, azathioprine; CD, Crohn’s disease; TNF, tumor necrosis factor.
Patient-level predictors
Data on patient characteristics that were hypothesized to be independently associated with failure to continue 6-MP/AZA treatment were obtained from the inpatient, outpatient, and pharmacy claims during the 12-month preindex period. The demographic information included age (at the index prescription date), sex, and region of residence (Northeast, North Central, South, and West). Treatment-related variables included the year of 6-MP/AZA initiation and prior corticosteroid, 5-aminosalicylate (5-ASA), and antibiotic use during the preindex period. Prior methotrexate, anti-TNF, and natalizumab use was assessed during the 9 months prior to the 90-day washout period. Other measures, of comorbid illness and health care utilization, measured during the preindex period included the Charlson comorbidity score (0, 1, ≥2),19 a prior diagnosis of rheumatologic disease or other autoimmune disease (including rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, juvenile idiopathic arthritis, and multiple sclerosis), the number of unique outpatient visits, the number of unique outpatient gastrointestinal visits, the number of unique generic drug prescriptions (a marker of health care utilization), the number of unique gastrointestinal procedures, and the number of unique hospitalizations (occurring at least 2 days apart) with a CD discharge diagnosis.
Clinical events occurring prior to study outcomes
In an exploratory analysis, we summarized the diagnosis codes and procedure and outpatient pharmaceutical claims occurring prior to the study outcomes. We defined two broad categories of events that might indicate intolerance to the initial 6-MP/AZA treatment or active disease. “Possible indicators of intolerance” included a diagnosis of pancreatitis, liver toxicity, or low white blood cell count during the 60-day period prior to reaching any of the above study endpoints (augmentation, switch, or discontinuation). “Possible indicators of active disease” included a prescription for an oral steroid, a gastrointestinal surgical procedure, or a hospitalization with a primary discharge diagnosis of CD within the 60 days prior to any of the above endpoints. The period of 60 days was selected based on clinical input. All the ICD-9 diagnosis and procedure codes, NDCs, and HCPCS codes used to define the study outcomes are listed online (Tables S1 and S2).
Statistical analysis
We utilized nonparametric Kaplan–Meier survival analysis to estimate the time-to-event (failure to continue 6-MP/AZA monotherapy) among the cohort of CD patients initiating 6-MP/AZA between January 1, 2001 and December 31, 2008. Descriptive statistics were generated to report the mean and interquartile range of the time-to-treatment failure. We estimated the 1-year event-free rate, defined as the proportion of the cohort remaining on their initial continuous 6-MP/AZA therapy at 1-year postinitiation and reported this for the cohort overall and stratified by children (≤18 years) and adult (>18 years) populations. We performed multivariable Cox proportional hazards regression to identify patient-level predictors that were independently associated with failure to continue 6-MP/AZA. Our models estimated unadjusted and adjusted hazard ratios (HRs) (controlling for all other predictors) and 95% confidence intervals (CIs) for all the predictors of interest. As 8–16 weeks of 6-MP/AZA treatment are required in order to obtain pharmacokinetic effects, we conducted a subanalysis examining the effectiveness of initial 6-MP/AZA treatment among the CD patients remaining on monotherapy for at least 3 months.
For individuals failing to continue 6-MP/AZA mono-therapy, we reported the number and proportion of individuals who started on alternative treatment strategies. Finally, we summarized the number and proportion of diagnosis and procedure codes and outpatient prescription drug claims for the clinical events occurring 60 days prior to the failure of treatment continuation that possibly indicated either 6-MP/AZA treatment intolerance or active CD. All the statistical analyses were conducted using SAS version 9.2 (Cary, NC, USA).
Results
Study population
The study sample comprised 3,657 individuals with a diagnosis of CD initiating 6-MP/AZA therapy from January 1, 2001–December 31, 2008, with a median follow up of 2.5 years (interquartile range 1.6, 4.0). Table 1 reports the demographic and clinical characteristics of the cohort. Children (aged 0–18 years) accounted for 16% of the study sample, while those aged 60 years and older made up approximately 11%. The majority of the individuals in the cohort were women (54%) and most frequently lived in the South (40%). Prior to 6-MP/AZA initiation, the use of methotrexate (<1%) and anti-TNFs (2%) was rare; however, the majority of individuals in the cohort used corticosteroids (59%), 5-ASAs (72%), and antibiotics (64%).
Table 1.
Characteristics of 3,657 Crohn’s disease patients initiating 6-MP/AZA from 2001–2008
| Characteristic | Number of patients | Percentage of patients |
|---|---|---|
| Female | 1,986 | 54.3% |
| Male | 1,671 | 45.7% |
| Age (mean, SD) | 42 (16.77) | |
| 0 to 18 | 575 | 15.7% |
| 19 to 29 | 537 | 14.7% |
| 30 to 39 | 618 | 16.9% |
| 40 to 49 | 719 | 19.7% |
| 50 to 59 | 809 | 22.1% |
| ≥60 | 399 | 10.9% |
| Regiona | ||
| Northeast | 443 | 12.1% |
| North Central | 1,167 | 31.9% |
| South | 1,463 | 40.0% |
| West | 574 | 15.7% |
| Prior drug useb | ||
| Methotrexate | 17 | 0.5% |
| Anti-TNFs | 68 | 1.9% |
| Concomitant drug usec | ||
| Corticosteroids | 2,149 | 58.8% |
| 5-ASAs | 2,637 | 72.1% |
| Antibiotics | 2,333 | 63.8% |
| Measures of comorbidity | ||
| Charlson score, 0 | 2,903 | 79.4% |
| Charlson score, 1 | 484 | 13.2% |
| Charlson score, ≥2 | 270 | 7.4% |
| Prior rheumatologic condition | 120 | 3.3% |
| Number of GI outpatient visits | ||
| 0 visits | 1,128 | 30.8% |
| 1 to 2 visits | 871 | 23.8% |
| ≥3 visits | 1,658 | 45.3% |
| Number of distinct generic drug prescriptions | ||
| 1 to 4 | 523 | 14.3% |
| 5 to 8 | 1,153 | 31.5% |
| 9 to 12 | 973 | 26.6% |
| ≥13 | 1,008 | 27.6% |
Notes:
Region was missing for ten individuals
prior use was defined as occurring during the 3–12 months prior to 6-MP/AZA initiation
concomitant use was defined as occurring during the 12 months prior to 6-MP/AZA initiation.
Abbreviations: 5-ASA, 5-aminosalicylate; 6-MP, 6-mercaptopurine; AZA, azathioprine; GI, gastrointestinal; SD, standard deviation; TNF, tumor necrosis factor.
Rates and predictors of treatment noncontinuation
Figure 2 illustrates the nonparametric Kaplan–Meier curve estimating the time-to-noncontinuation among the cohort of CD initiators of 6-MP/AZA over time (measured in months from initiation). Nearly one-quarter of individuals in the cohort did not continue 6-MP/AZA monotherapy longer than 2 months. Similarly, more than 50% and 75% of individuals in the cohort failed to continue 6-MP/AZA treatment for 8 months and 28 months, respectively. The overall 1-year continuation rate of 6-MP/AZA monotherapy was 42%. Stratified by age group, the 1-year continuation rate was 54% for children (≤18 years) and 41% for adults (>18 years). For individuals with prior anti-TNF use, the 1-year continuation rate was 22%. Among the individuals who continued on 6-MP/AZA treatment for 3 or more months, the continuation rate at 12, 18, and 24 months was 63%, 50%, and 42%, respectively. The median follow up among CD patients after failure to continue 6-MP/AZA was 2.03 years (interquartile range 1.20–3.42).
Figure 2.
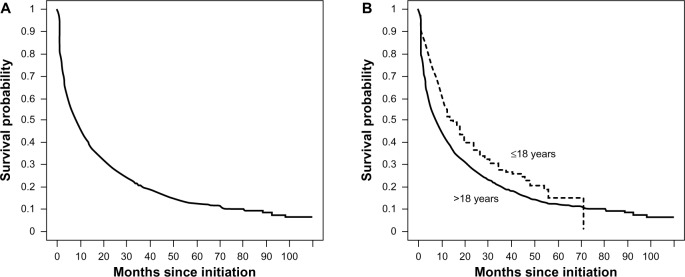
Time-to-treatment noncontinuation of initial 6-MP/AZA maintenance therapy.
Notes: Nonparametric Kaplan–Meier survival curves estimate the time-to-noncontinuation of initial 6-MP/AZA maintenance therapy among a cohort of CD patients (n=3,657) initiating 6-MP/AZA maintenance therapy between January 1, 2001 and December 31, 2008, overall (A) and stratified by age (≤18 vs >18 years), (B). At 12, 24, and 36 months, the numbers of CD patients at risk were 1,544, 730, and 352. Stratified by age, the same numbers of patients at risk were 170, 67, and 32 and 1,374, 663, and 320 among those ≤18 years and >18, respectively.
Abbreviations: 6-MP, 6-mercaptopurine; AZA, azathioprine; CD, Crohn’s disease.
We estimated unadjusted and adjusted HRs (aHRs) and 95% CIs for the association between each patient-level predictor of interest and initial 6-MP/AZA treatment failure (Table 2), using multivariable Cox proportional hazards regression. The individuals who used anti-TNFs prior to their initial 6-MP/AZA prescription were more likely to fail to continue on treatment compared with those who did not (aHR =1.64, 95% CI: 1.26, 2.13). Age appeared to have a nonlinear association with noncontinuation, where individuals aged 19–29, 30–39, and 40–49 years all had an elevated hazard compared with individuals aged 0–18 years, while older adults aged 50–59 and ≥60 years did not. When age was dichotomized (age ≤18 vs >18 years), adults were more likely than children to fail to continue on 6-MP/AZA (aHR =0.85, 95% CI: 0.76, 0.95). Having a diagnosis of a rheumatologic or other autoimmune disease with an indication for an anti-TNF prior to 6-MP initiation increased the hazard of treatment noncontinuation (aHR =1.23, 95% CI: 1.00, 1.51). Lastly, individuals receiving a higher number of unique generic prescription drug fills in the year prior to their 6-MP/AZA initiation (5–8, 9–12, and ≥13 vs 1–4 unique generic prescriptions) were more likely to fail to continue 6-MP/AZA monotherapy.
Table 2.
Patient-level predictors of failure to continue initial 6-MP/AZA among 3,657 Crohn’s disease patients
| Independent variable | Unadjusted HR (95% CI) | Adjusted HRc (95% CI) |
|---|---|---|
| Female vs male | 0.87 (0.81–0.93) | 0.92 (0.85–0.99) |
| 19–29 years vs 0–18 years | 1.47 (1.28–1.68) | 1.39 (1.21–1.59) |
| 30–39 years vs 0–18 years | 1.35 (1.19–1.54) | 1.25 (1.09–1.43) |
| 40–49 years vs 0–18 years | 1.27 (1.12–1.43) | 1.16 (1.02–1.32) |
| 50–59 years vs 0–18 years | 1.17 (1.03–1.32) | 1.04 (0.91–1.19) |
| ≥60 years vs 0–18 years | 1.20 (1.04–1.40) | 1.07 (0.91–1.25) |
| Northeast vs South | 0.81 (0.72–0.92) | 0.86 (0.76–0.97) |
| North Central vs South | 0.90 (0.82–0.98) | 0.91 (0.84–1.00) |
| West vs South | 0.93 (0.83–1.03) | 0.95 (0.85–1.07) |
| Anti-TNF prescription prior to initiationa (yes vs no) | 1.81 (1.40–2.33) | 1.64 (1.26–2.13) |
| Methotrexate prescription prior to initiationa (yes vs no) | 1.35 (0.82–2.25) | 1.21 (0.73–2.03) |
| Salicylate prescription prior to initiationb (yes vs no) | 0.96 (0.88–1.04) | 0.94 (0.86–1.02) |
| Steroid prescription prior to initiationb (yes vs no) | 1.00 (0.93–1.08) | 0.95 (0.88–1.03) |
| Antibiotic prescription prior to initiation (yes vs no) | 1.19 (1.10–1.28) | 1.06 (0.96–1.16) |
| History of rheumatologic diseaseb (yes vs no) | 1.27 (1.04–1.55) | 1.23 (1.00–1.51) |
| 5–8 vs 1–4 generic prescriptionsb | 1.16 (1.03–1.31) | 1.20 (1.05–1.37) |
| 9–12 vs 1–4 generic prescriptionsb | 1.24 (1.09–1.40) | 1.28 (1.10–1.49) |
| ≥13 vs 1–4 generic prescriptionsb | 1.43 (1.27–1.62) | 1.46 (1.23–1.73) |
Notes:
Prior use was defined as occurring during the 3–12 months prior to 6-MP/AZA initiation
all variables were assessed during the 12 months prior to 6-MP/AZA initiation
all estimates were adjusted for all the variables above in addition to year of initiation, Charlson score, number of office visits, number of gastrointestinal procedures, and number of hospitalizations.
Abbreviations: 6-MP, 6-mercaptopurine; AZA, azathioprine; CI, confidence interval; HR, hazard ratio; TNF, tumor necrosis factor; vs, versus.
Subsequent maintenance therapy strategy changes
Table 3 reports the number and proportion of the 2,845 individuals who did not continue on 6-MP/AZA monotherapy and who changed to another maintenance therapy strategy. A total of 484 individuals (17%) augmented their 6-MP/AZA treatment with an anti-TNF agent. Almost 9% discontinued 6-MP/AZA and switched to another CD strategy, such as methotrexate alone (1.6%), an anti-TNF alone (7%), or an anti-TNF in combination with methotrexate (<1%). Most CD patients (n=2,108 or 74%) completely discontinued 6-MP/AZA without augmenting or switching.
Table 3.
Maintenance therapy changes among 2,845 patients diagnosed with Crohn’s disease who failed to continue initial 6-MP/AZA therapy
| Treatment strategy | Number of patients (%) |
|---|---|
| Discontinued all therapy | 2,108 (74.02) |
| Switched to another therapy | |
| Natalizumab | 0 (0.00) |
| Methotrexate | 45 (1.58) |
| Anti-TNF | 203 (7.13) |
| Anti-TNF + methotrexate | 5 (0.18) |
| Augmented with another therapy | |
| 6-MP/AZA + anti-TNF | 484 (16.99) |
Abbreviations: 6-MP, 6-mercaptopurine; AZA, azathioprine; TNF, tumor necrosis factor.
Possible indicators of 6-MP/AZA intolerance or active disease
Among individuals who did not continue on 6-MP/AZA monotherapy (n=2,845), Table 4 reports the number and proportion of patients who received a diagnosis, procedure, or prescription for clinical events, which could indicate a possible intolerance to 6-MP/AZA or active disease flare. Overall, 105 individuals (4%) received diagnoses that could indicate a possible intolerance, including a diagnosis for pancreatitis (2%), liver toxicity (1%), and low white blood cell count (1%). Similarly, 1,128 individuals received diagnoses, procedures, or prescriptions for clinical events that could indicate an active disease flare (39%), including a prescription for an oral steroid (27%), a gastrointestinal-related procedure (16%), and hospitalization with a CD-related discharge diagnosis (8%). Almost 2% of the cohort experienced both indicators during the study period.
Table 4.
Clinical events occurring among 2,845 CD patients during the 60 days prior to failure of initial 6-MP/AZA treatment continuation
| Event of interest | Number of patients (%a) |
|---|---|
| Possible indicator of intolerance | 105 (3.6) |
| Pancreatitis diagnosis | 71 (2.4) |
| Liver toxicity diagnosis | 17 (0.6) |
| Low white blood cell count diagnosis | 18 (0.6) |
| Possible indicator of active disease | 1,128 (38.6) |
| Oral steroid prescription | 799 (27.4) |
| GI surgery procedure | 480 (16.4) |
| Hospitalization related to CD | 246 (8.4) |
| Both | 56 (1.9) |
Notes:
Individuals can experience multiple events in each category, therefore the column percentages do not sum to 100%.
Abbreviations: 6-MP, 6-mercaptopurine; AZA, azathioprine; CD, Crohn’s disease; GI, gastrointestinal.
Discussion
An examination of the patterns and effectiveness of 6-MP/AZA treatment for maintenance of CD remission is important because patients treated in the routine setting are different to those enrolled in RCTs. In this retrospective cohort of 3,657 individuals with CD initiating on 6-MP/AZA (98% of whom were immunomodulator or biologic naïve), we found that approximately 42% of the CD patients who initiated 6-MP/AZA monotherapy remained on this treatment strategy after 1 year, and only 28% remained at 2 years. When restricted to patients who remained on therapy for at least 3 months (an adequate amount of time to achieve pharmacokinetic effects), 63% and 42% remained on their 6-MP/AZA without augmenting or switching at 1 and 2 years, respectively. Taken together, these data suggest that most individuals benefit from 6-MP/AZA monotherapy at 1 year; however, the longer-term benefits remain uncertain, and our data do not allow us to comment directly on these benefits. Despite this uncertainty, many clinical algorithms recommend this approach, and it has become a mainstay of medical therapy for moderate to severe CD.20,21
Because the primary outcome of our study, treatment continuation, is different than the clinical outcomes of disease remission or steroid-free remission used in clinical trials and retrospective chart reviews, direct comparisons with the existing literature are not possible. However, as treatment continuation may either over- or underestimate clinical remission, we believe that, on balance, it is a reasonable proxy indicator. Treatment continuation will overestimate remission in instances where patients remain on 6-MP/AZA despite active disease, or may underestimate clinical remission in instances where discontinuation occurs due to poor adherence or other reasons aside from treatment failures.
Our findings are in line with emerging evidence suggesting that the real-world clinical effectiveness of 6-MP/AZA may be somewhat lower than the efficacy initially observed in randomized trials. The Cochrane Collaboration conducted a meta-analysis of eight published randomized, double-blind, placebo-controlled trials of AZA or 6-MP therapy in adult patients (≥19 years) with CD4 and concluded that both treatments were effective in maintaining the remission of CD, with a summary odds ratio (OR) in the AZA trials of 2.32 (95% CI: 1.55, 3.49) and in the 6-MP trials of 3.32 (95% CI: 1.40, 7.87). The 1-year remission rates reported in these trials ranged from 40%–70%.6–9 The Markowitz et al pediatric study10 showed similar, if not higher, efficacy, with >80% of children in remission at 1 year. However, the success of 6-MP/AZA when used in clinical practice has not been consistently documented in the literature. The 30-year retrospective study by Fraser et al13 investigating 272 patients from an IBD referral center in Oxford, England reported the rate of overall remission (defined as being steroid-free for at least 3 months) was 45%, which increased to 64% among CD patients who completed 6 or more months of treatment. Two small observational studies have shown relatively low clinical effectiveness of 6-MP/AZA in pediatric CD in clinical settings. Riello et al15 conducted a retrospective observational cohort study of AZA effectiveness in 105 pediatric CD patients from a single center in Paris, France. Remission (as measured by a pediatric CD activity index ≤10 without any steroid medication) at 12 months of follow up occurred in only 40% of patients. More recently, Hyams et al16 drew upon a multicenter, inception cohort to compare 1-year remission outcomes for initiators of anti-TNFs, initiators of immunomodulators, and children who did not initiate immunomodulator therapy. After propensity score matching, the authors found no comparative benefit of initiating immunomodulators versus not receiving treatment (OR: 1.3, 95% CI: 0.6, 2.8). Therefore, our findings in a larger and more diverse sample are consistent with this emerging literature regarding real-world clinical effectiveness.
One particularly notable finding from our study was the effects of age on length of treatment continuation. Children had longer periods of treatment continuation than did adults, which may indicate a higher response to 6-MP/AZA in the pediatric versus adult populations. Increased efficacy of 6-MP/AZA in the pediatric population was suggested by the Markowitz study,10 where it was hypothesized that treatment earlier in the course of the disease may be a potential disease-modifying strategy.22 Another possible explanation for the longer treatment continuation among children observed here is that children may experience less toxicity than adults, as has been suggested by a small, single-center retrospective study by Goodhand et al,14 which found that 6-MP/AZA was better tolerated in children than adults (albeit with no difference in efficacy). Finally, nonmechanistic reasons may also explain the elevated continuation in children, where parents’ involvement in the management of their child’s disease may improve adherence. We also noted that individuals initiating 6-MP/AZA in older age (≥50 years) were at a reduced hazard of treatment noncontinuation compared with other adults (19–49 years), consistent with the findings by Fraser et al.13 This may indicate that 6-MP/AZA is more effective in older individuals, or may reflect differences in disease course, adherence, and/or insurance coverage in the elderly in the USA.
An additional finding worth highlighting is that although prior anti-TNF users were less likely to continue on long-term 6-MP/AZA monotherapy than were anti-TNF naïve patients, approximately 22% continued on thiopurines for longer than 1 year. This suggests that the long-term benefits of sequential therapy with anti-TNF inhibitors followed by thiopurines in “anti-TNF failures” may equal the benefits of the combination of anti-TNF and thiopurines initiated simultaneously, as has recently been suggested by Lewis.23
We were surprised that 2,108 (74%) of all treatment failures were considered discontinuations of treatment. Accordingly, we performed a series of analyses to further examine the discontinuers and exclude the possibility of misclassification of either CD itself or medication discontinuation. Approximately, 57% of those that discontinued had claims with an ICD-9 diagnosis code for CD within 90 days of 6-MP/AZA discontinuation, and 86% had CD claims at any point following 6-MP/AZA discontinuation, suggesting that these patients did, in fact, have CD and received ongoing care for their condition. In the 90 days following all maintenance treatment discontinuation, we found that 12% (n=259) had a claim for hospital admission, 5% (n=96) had a claim for a gastrointestinal-related surgery, and 0.3% (n=6) had a claim for cyclosporine, tacrolimus, or mycophenolate, which could be indicative of a maintenance treatment failure. However, in the same time period, we identified 674 individuals (32%) who had a prescription for 5-ASA or sulfasalazine, which would be consistent with a “step down” strategy due to improvements in disease course. As such, these data suggest that discontinuers comprise a heterogeneous group of patients. Indeed, our findings of medication nonuse are consistent with the findings of a recent population-based study by Melesse et al,24 which used the University of Manitoba IBD Epidemiology Database linked to an outpatient prescription drug database. Researchers found that by the end of 5 years, only 46% of CD patients continued to use IBD medications.
Because our study relied upon administrative data, there are several limitations to consider when interpreting the results. One key issue discussed previously is the use of the outcome variable, continuation of 6-MP/AZA monotherapy, as a proxy variable for clinical effectiveness. Similarly, information on drug dosage was unavailable, and therefore, detection of underdosing, which may impact clinical effectiveness, could not be measured. Misclassification of the primary or secondary outcomes (augmentation, switch, and discontinuation) may have also occurred; however, medication dispensings are generally reliable administrative data because they require reimbursement by the health plans. We also recognize that our exploratory analysis of events suggestive of treatment intolerance and failure was limited by our reliance on algorithms using diagnosis and procedure codes and outpatient prescription drug claims submitted to insurance companies that have not been validated. As additional clinically relevant events not contained within administrative data may have occurred, it is likely that we have underestimated such events but may have also overestimated events, such as colonoscopy, that may have been used for routine surveillance. Results from a large Spanish prospective cohort study of IBD patients by Chaparro et al25 demonstrated that 25% of patients using thiopurines experience an adverse event during treatment, with nausea being the most common event (and one which is difficult to capture in claims data). Fraser et al13 reported similar findings of 28% of patients stopping AZA due to side effects, with nausea and vomiting as the main reason. Finally, our analysis relied upon information from commercially insured individuals and as such, patients who frequently change health plans would not have been captured, and the overall findings may not be generalizable to uninsured patients.
The primary strengths of this study were the incident user design and the broad diversity and large size of the study population. The CD cohort included initiators of 6-MP/AZA instead of prevalent users (“survivors of early 6-MP/AZA treatment”) so that we could identify early noncontinuation events and patient-level characteristics associated with failure to continue 6-MP/AZA monotherapy. As shown in Figure 2, the hazard of failure to continue treatment is highest in the early months following initiation and therefore, the incident user design is appropriate and necessary in this setting.18 Additionally, the databases utilized for this study included diverse, commercially insured individuals diagnosed with CD, ranging in age from 3 to 100 years and residing in all regions of the USA. Furthermore, the study cohort had a wide distribution of comorbidity and health care utilization, which is often lacking in RCTs. Most of the prior observational studies examining the effectiveness of 6-MP/AZA in a routine care setting were small, single-center experiences. With 3,657 individuals included in this cohort and 2,845 experiencing a noncontinuation event, the sample size was sufficiently large to examine a wide range of patient-level predictors, while allowing for broad generalizability to the commercially insured CD population in the USA.
In summary, most patients who initiate on 6-MP/AZA monotherapy for CD do not continue on this treatment beyond 1 year, suggesting the limited long-term impact of this therapeutic strategy. In contrast to some adult and the pediatric RCTs, the lower real-world clinical effectiveness of 6-MP/AZA monotherapy may be due to differences in a variety of factors, such as disease severity, patient demographics, comorbidity, adherence, and health care utilization.
Supporting information
Administrative codes used to identify an initial 6-MP/AZA treatment discontinuation, switch, and augmentation
| Source | Description | Codes |
|---|---|---|
| NDC, HCPCS | Azathioprine | 49452078301 51552077902 51552077904 51927225800 00173059871 55390060020 60976059871 65483055101 65649024141 66591024141 00054408425 00054808425 00081059756 00173059755 00378100501 00406200301 00781105901 00781507501 23490511009 52959007900 54868092101 54868092104 54868531000 54868531001 54868531002 54868531004 57866902101 60976059755 65483059010 66479030110 66591022141 68084022901 68084022911 68382000301 68382000305 68462050201 65649023141 66591023141 38779031203 38779031206 49452078201 49452078203 49452078302 |
| C9436 J7500 J7501 K0119 K0120 | ||
| NDC, HCPCS | 6-MP | 00054458111 00054458127 00081080725 00081080765 00093551006 00173080725 00173080765 00378354725 00378354752 38779142703 38779142704 38779142706 49884092202 49884092204 51927200000 54868528200 57844052206 57844052207 57844052252 68084032521 68258910301 |
| S0108 | ||
| NDC, HCPCS | Methotrexate | 49452460002 49452460003 49452460102 49452460104 51552105401 51552105409 51927156500 00205532618 63323012140 58406068318 61703040804 63323012104 66479013613 00555092901 51285036801 00555094501 51285036901 55390014301 58406067105 63323012250 66479013929 00005450704 00005450705 00005450707 00005450709 00005450723 00005450791 00054455015 00054455025 00054855003 00054855005 00054855006 00054855007 00054855010 00054855025 00182153901 00182153995 00364249901 00364249936 00378001401 00378001450 00405464301 00405464336 00536399801 00536399836 00555057202 00555057235 00555057245 00555057246 00555057247 00555057248 00555057249 00603449921 00677161001 00781107601 00781107636 00904174960 00904174973 00904601260 23490588900 49999038024 51079067001 51079067005 51079067086 51079067087 51285050902 51432052203 52959024400 54569181800 54868382600 54868382601 54868382602 54868382603 54868382604 54868382605 54868382606 54868382607 55289092430 59911587401 62701094036 62701094099 67253032010 67253032036 67253058042 67253058043 67253058044 67253058045 67253058046 68115063200 58406068312 63323012108 58406067301 66479013721 54868471600 58406068316 63323012110 63323012310 66479013619 00186142013 00205455626 00469288030 10019094001 10019094002 10019094101 10139006202 10139006210 10139006240 53905003110 53905003210 53905003410 55390003110 55390003210 55390003310 55390003410 58406068114 58406068117 61703035038 61703040707 61703040732 61703040813 61703040822 61703040832 61703040841 61703040858 66479013501 66479013509 66758004002 66758004008 66758004101 00205933792 54569452500 54868017301 54868479600 58406068315 61703040807 63323012102 63323012302 66479013611 00555092701 51285036601 00555092801 51285036701 38779003503 38779003504 38779003506 38779003511 62991120001 62991120002 63370015410 63370015415 63370015425 |
| J8610 J9250 J9260 | ||
| NDC, HCPCS | Natalizumab | 59075073015, C9126, J2323, Q4079 |
| NDC, HCPCS | Anti-TNFs | 50474070062 50474071079 00074379902 00074433902 00074433906 00074433907 00074937402 54569552400 54868482200 57894003001 |
| C9249 J0135 J0718 J1745 S9359 |
Abbreviations: NDC, National Drug Code; HCPCS, Healthcare Common Procedural Coding System; TNF, tumor necrosis factor.
Administrative codes used to identify possible indicators of intolerance or active disease
| Source | Description | Codes |
|---|---|---|
| ICD-9 CM | Acute pancreatitis | 577.0 |
| ICD-9 CM | Liver toxicity | 570, 790.4 |
| ICD-9 CM | Low white blood cell count | 288.5, 288.0 |
| ICD-9 CM | GI procedures | 43200–43273, 44360–44397, 45300–45392, 46600–46615 |
| NDC | Oral steroid prescription | Codes |
| ICD-9 CM | Crohn’s disease-related hospitalization | 555.0, 555.1, 555.2, 555.9 |
Abbreviations: NDC, National Drug Code; ICD-9 CM, International Classification of Diseases, ninth revision codes; GI, gastrointestinal.
Author contributions
SFC, MDK, and JLL conceived and designed the study. CFC and JKA acquired and analyzed the data. JLL, MDK and SFC interpreted the data. JLL, MDK, CFC, JKA, and SFC drafted the article or revised it critically for important intellectual content. All authors approved the final version of the manuscript.
Disclosure
Results of this study were presented at the 28th International Conference on Pharmacoepidemiology and Therapeutic Risk Management in Barcelona, Spain on August 23–26, 2012. SFC, JKA, and CFC are employees of GlaxoSmithKline. JLL and MDK are consultants to GlaxoSmithKline. As such, the study sponsor had involvement in all aspects of the study including, study design, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication; the study sponsor did not have a role in data collection. The authors report no other conflicts of interest.
References
- 1.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13(4):451–461. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease [review] Cochrane Database Syst Rev. 2010;6:CD000545. doi: 10.1002/14651858.CD000545.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease [review] Cochrane Database Syst Rev. 2009;1:CD000067. doi: 10.1002/14651858.CD000067.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. New Engl J Med. 1980;302(18):981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- 6.Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37(5):674–678. doi: 10.1136/gut.37.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derijks LJ, Gilissen LP, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(5):715–729. doi: 10.1111/j.1365-2036.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 8.Mantzaris GJ, Christidou A, Sfakianakis M, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15(3):375–382. doi: 10.1002/ibd.20777. [DOI] [PubMed] [Google Scholar]
- 9.Reinisch W, Panés J, Lémann M, et al. A multicenter, randomized, double-blind trial of everolimus versus azathioprine and placebo to maintain steroid-induced remission in patients with moderate-to-severe active Crohn’s disease. Am J Gastroenterol. 2008;103(9):2284–2292. doi: 10.1111/j.1572-0241.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119(4):895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 11.Ha CY, Ullman TA, Siegel CA, Kornbluth A. Are patients enrolled in randomized controlled trials (RCTs) representative of the general inflammatory bowel disease (IBD) patient population? Gastroenterology. 2011;140(5 Suppl 1):S113. doi: 10.1016/j.cgh.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Strom BL, editor. Textbook of Pharmacoepidemiology. 4 ed. Chichester: John Wiley and Sons Ltd; 2005. [Google Scholar]
- 13.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50(4):485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodhand JR, Tshuma N, Rao A, et al. Do children with IBD really respond better than adults to thiopurines? J Pediatr Gastroenterol Nutr. 2011;52(6):702–707. doi: 10.1097/MPG.0b013e31820ba46c. [DOI] [PubMed] [Google Scholar]
- 15.Riello L, Talbotec C, Garnier-Lengliné H, et al. Tolerance and efficacy of azathioprine in pediatric Crohn’s disease. Inflamm Bowel Dis. 2011;17(10):2138–2143. doi: 10.1002/ibd.21612. [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Kim MO, Denson L, et al. Early anti-TNFα therapy is superior to early immunomodulator therapy in newly diagnosed children with Crohn’s disease. Gastroenterology. 2013;144(5):S146. [Google Scholar]
- 17.US Food and Drug Administration . National Drug Code Database Background Information. Silver Spring, MD: US Food and Drug Administration; 2012. [Accessed November 6, 2013]. [June]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/UCM070829. [Google Scholar]
- 18.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Herrinton LJ, Liu L, Fireman B, et al. Time trends in therapies and outcomes for adult inflammatory bowel disease, Northern California, 1998–2005. Gastroenterology. 2009;137(2):502–511. doi: 10.1053/j.gastro.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 21.Punati J, Markowitz J, Lerer T, et al. Pediatric IBD Collaborative Research Group Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm Bowel Dis. 2008;14(7):949–954. doi: 10.1002/ibd.20412. [DOI] [PubMed] [Google Scholar]
- 22.Hyams JS, Markowitz JF. Can we alter the natural history of Crohn disease in children? J Pediatr Gastroenterol Nutr. 2005;40(3):262–272. doi: 10.1097/01.mpg.0000154660.62359.fe. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JD. Is there still a rationale for monotherapy after SONIC and SUCCESS? [Accessed October 22, 2012];AGA Perspectives. 2011 7(6):5–7. Available from: http://www.gastro.org/mobiletools/news-item/1297. [Google Scholar]
- 24.Melesse DY, Targownik LE, Singh H, et al. Patterns of drug avoidance in patients with inflammatory bowel disease. Gastroenterology. 2013;144(5):S48–S49. [Google Scholar]
- 25.Chaparro M, Ordás I, Cabré E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19(7):1404–1410. doi: 10.1097/MIB.0b013e318281f28f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Administrative codes used to identify an initial 6-MP/AZA treatment discontinuation, switch, and augmentation
| Source | Description | Codes |
|---|---|---|
| NDC, HCPCS | Azathioprine | 49452078301 51552077902 51552077904 51927225800 00173059871 55390060020 60976059871 65483055101 65649024141 66591024141 00054408425 00054808425 00081059756 00173059755 00378100501 00406200301 00781105901 00781507501 23490511009 52959007900 54868092101 54868092104 54868531000 54868531001 54868531002 54868531004 57866902101 60976059755 65483059010 66479030110 66591022141 68084022901 68084022911 68382000301 68382000305 68462050201 65649023141 66591023141 38779031203 38779031206 49452078201 49452078203 49452078302 |
| C9436 J7500 J7501 K0119 K0120 | ||
| NDC, HCPCS | 6-MP | 00054458111 00054458127 00081080725 00081080765 00093551006 00173080725 00173080765 00378354725 00378354752 38779142703 38779142704 38779142706 49884092202 49884092204 51927200000 54868528200 57844052206 57844052207 57844052252 68084032521 68258910301 |
| S0108 | ||
| NDC, HCPCS | Methotrexate | 49452460002 49452460003 49452460102 49452460104 51552105401 51552105409 51927156500 00205532618 63323012140 58406068318 61703040804 63323012104 66479013613 00555092901 51285036801 00555094501 51285036901 55390014301 58406067105 63323012250 66479013929 00005450704 00005450705 00005450707 00005450709 00005450723 00005450791 00054455015 00054455025 00054855003 00054855005 00054855006 00054855007 00054855010 00054855025 00182153901 00182153995 00364249901 00364249936 00378001401 00378001450 00405464301 00405464336 00536399801 00536399836 00555057202 00555057235 00555057245 00555057246 00555057247 00555057248 00555057249 00603449921 00677161001 00781107601 00781107636 00904174960 00904174973 00904601260 23490588900 49999038024 51079067001 51079067005 51079067086 51079067087 51285050902 51432052203 52959024400 54569181800 54868382600 54868382601 54868382602 54868382603 54868382604 54868382605 54868382606 54868382607 55289092430 59911587401 62701094036 62701094099 67253032010 67253032036 67253058042 67253058043 67253058044 67253058045 67253058046 68115063200 58406068312 63323012108 58406067301 66479013721 54868471600 58406068316 63323012110 63323012310 66479013619 00186142013 00205455626 00469288030 10019094001 10019094002 10019094101 10139006202 10139006210 10139006240 53905003110 53905003210 53905003410 55390003110 55390003210 55390003310 55390003410 58406068114 58406068117 61703035038 61703040707 61703040732 61703040813 61703040822 61703040832 61703040841 61703040858 66479013501 66479013509 66758004002 66758004008 66758004101 00205933792 54569452500 54868017301 54868479600 58406068315 61703040807 63323012102 63323012302 66479013611 00555092701 51285036601 00555092801 51285036701 38779003503 38779003504 38779003506 38779003511 62991120001 62991120002 63370015410 63370015415 63370015425 |
| J8610 J9250 J9260 | ||
| NDC, HCPCS | Natalizumab | 59075073015, C9126, J2323, Q4079 |
| NDC, HCPCS | Anti-TNFs | 50474070062 50474071079 00074379902 00074433902 00074433906 00074433907 00074937402 54569552400 54868482200 57894003001 |
| C9249 J0135 J0718 J1745 S9359 |
Abbreviations: NDC, National Drug Code; HCPCS, Healthcare Common Procedural Coding System; TNF, tumor necrosis factor.
Administrative codes used to identify possible indicators of intolerance or active disease
| Source | Description | Codes |
|---|---|---|
| ICD-9 CM | Acute pancreatitis | 577.0 |
| ICD-9 CM | Liver toxicity | 570, 790.4 |
| ICD-9 CM | Low white blood cell count | 288.5, 288.0 |
| ICD-9 CM | GI procedures | 43200–43273, 44360–44397, 45300–45392, 46600–46615 |
| NDC | Oral steroid prescription | Codes |
| ICD-9 CM | Crohn’s disease-related hospitalization | 555.0, 555.1, 555.2, 555.9 |
Abbreviations: NDC, National Drug Code; ICD-9 CM, International Classification of Diseases, ninth revision codes; GI, gastrointestinal.



