Abstract
Objective
Genetic contribution to left ventricular (LV) structure is generally recognized, but whether and how this influence varies by ethnicity or with age is unknown.
Design and methods
Participants were 517 European American (EA) and African American (AA) twin pairs (mean age: 14.6 ± 3.0) at visit 1 and 422 EA and AA at follow-up 4.1 years later. Echocardiograms were obtained on both visits. Data were analyzed using structural equation modeling software Mx.
Results
Body mass index (BMI) was a strong predictor for all LV measures at both visit 1 and 2, accounting for 3.5-24.2% of the total variance. Hemodynamics explained up to 4.5% additional LV measures variance. After adjusting for BMI, LV measures showed substantial heritability (range: 21%-71%). Best-fitting longitudinal models revealed considerable novel genetic effects on the interventricular septum, posterior wall and relative wall thickness (but not LV inner diameter), accounting for 32-41% of the phenotypic variance at visit 2, with no significant gender and ethnic effects. There was a gender difference for LV mass index in AA (P < 0.01), with a significant influence of novel genetic effects in males (47%), but not in females. No gender difference was seen in EA, with 34% of the phenotypic variance at visit 2 attributable to novel genetic effects.
Conclusions
The heritability of cardiac structure and geometry was equally substantial in both AA and EA. Significant novel genetic influences were detected for all measures but LV inner diameter and LV mass index in AA females. Further developmental genetic studies are warranted to elucidate the nature of the emerging gene effects during the transition from adolescence into adulthood.
Keywords: heritability, left ventricular mass, ethnicity
INTRODUCTION
The strong relationship between increased left ventricular mass (LVM) and increased cardiovascular morbidity and mortality is well established[Casale, 1986 #2006; Koren, 1991 #2914; Brown, 2000 #8912]. Mild to moderate genetic contributions and possible ethnic differences in the heritability of LVM have been reported independent of covariates [Post, 1997 #4644; Juo, 2005 #22591; Verhaaren, 1991 #1280; Busjahn, 1997 #19585; Arnett, 2001 #27798; Sharma, 2006 #27810]. However, earlier studies have predominantly been performed in European Americans (EA) and, with one exception [Arnett, 2001 #27798], did not directly compare and test differences in heritabilities between different ethnic groups precluding a conclusive answer as to the importance of the genetic influence on left ventricular structure in African Americans in which an increased LVM is highly prevalent.
Previous studies have shown that the contribution of various LVM determinants change from childhood to adulthood [Dekkers, 2002 #8910; Treiber, 1999 #5017; Li, 2004 #18438; Daniels, 1995 #8794]. For instance, body size is the most important contributor to cardiac growth in childhood while hemodynamics play a greater role in adulthood. Although, a genetic contribution to LV structure is generally recognized, little is known about the variation of genetic influence over time. We hypothesized that genetic control of cardiac structure fluctuates with age and new gene expression would emerge during the transition period from adolescence to adulthood as we have recently observed for hemodynamics [Kupper, 2006 #23091].
Therefore, the present study was conducted to longitudinally assess the influence of genetic factors on constituents of LV structure in a large cohort of adolescent AA and EA twins with average ages between 14 and 18 years, while taking into account potential ethnic and gender differences in the influence of these genetic factors.
METHODS
Subjects
Subjects visited the laboratory twice, with an intervening time period of 4.1 (± 0.49) years (range 2.4 – 6.3 yrs). Data from a total of 296 EA twin pairs [153 monozygotic (MZ), 143 dizygotic (DZ)] and 221 AA twin pairs (106 MZ, 115 DZ) and 1 singleton (DZ) was available from the first measurement. Data of 69.9% of these EA twins and 74.7% of these AA twins was available from the second visit (i.e., 372 pairs and 10 singletons in total). There was no significant difference in drop-out rate between EAs and AAs (P=0.27) or between MZ and DZ twins (P=0.25). During the second visit, an additional 25 EA pairs and 18 AA pairs and 1 AA singleton took part for the first time. The group of DZ twins included both same-sex and opposite sex twins. The age limit for these additional subjects to be included in the present analysis was 14 (lowest age at time 1 plus inter-test time interval). Recruitment and zygosity determination of twin pairs into the Georgia Cardiovascular Twin Study have been described previously as have been the criteria to classify subjects as AA or EA [Snieder, 2002 #10656]. The study was approved by the institutional review board and all subjects (and parents if subjects were <18 years) provided written informed consent.
On each visit subject’s height (Ht) in centimeters (cm) and weight (Wt) in kilograms (kg) were measured without shoes using a Health-O-Meter medical scale (Bridgeview, USA), which was calibrated daily. Body mass index (BMI) was calculated as Wt/Ht2.
Hemodynamic Evaluations
The subject was placed in a supine position and given standardized instructions to relax as completely as possible for 15 min. During minutes 11, 13 and 15 of this rest period, blood pressure and heart rate measurements were obtained using a Dinamap 1864 SX (Criticon Incorporated, Tampa, FL, USA). Diastolic blood pressure was subtracted from systolic blood pressure to obtain pulse pressure (PP).
Echocardiographic Studies
2D directed M- mode echocardiograms were performed using a Hewlett-Packard Sonos 1500 echocardiograph (Andover, MA). LV posterior wall thickness in diastole (LVPWD), interventricular septal thickness in diastole (IVSD), and LV internal dimension in diastole (LVIDD) were measured according to the American Society of Echocardiography convention [Schiller, 1989 #5074]. LVM was derived using the formula of Devereux et al. [Devereux, 1986 #969] which has been validated for use in individuals with normal hearts. Based upon the recommendation of de Simone et al [de Simone, 1992 #1400], LVM was indexed by Ht2.7 (i.e., LVMI) to adjust for normal growth without removing the contribution of obesity. Relative wall thickness (RWT) was calculated as: RWT = (LVPWD + IVD)/LVIDD. Intra and interrater coefficients of variation for all cardiac structure were <10%[Cook, 2001 #6378].
Analytical Approach
The analytical strategy of our study was as follows. First, we investigated the contribution of demographic, anthropometric and hemodynamic determinants of LV structure using hierarchic multiple regression. Second, we used bivariate model fitting analyses including both visit 1 and visit 2 data to estimate: 1) the relative influence of genetic and environmental factors on individual differences in LV structure variables at both measurement occasions, 2) the extent to which genetic and environmental effects on LV structure at age 18 are the same or different from those at age 14, and 3) to what extent tracking correlations between visit 1 and visit 2 can be explained by common genes and/or common environment. In these bivariate models, tests for ethnicity and gender differences were conducted as described in detail elsewhere for the univariate case[Wang, 2005 #27806].
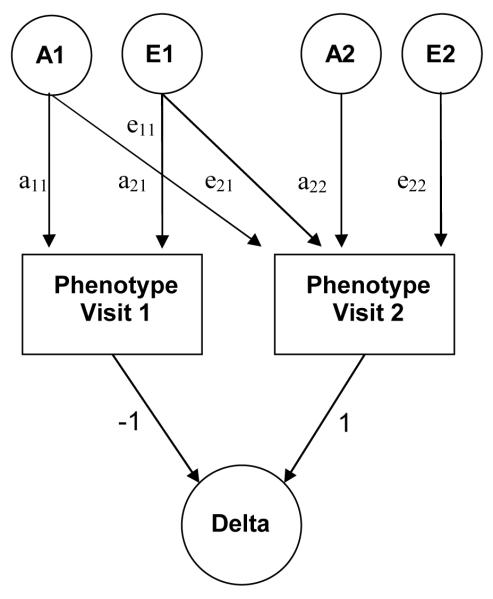
We used a bivariate Cholesky decomposition, in which there is a main factor that loads on all variables (visit 1 & visit 2), followed by a second factor that loads only on the second variable (visit 2). Figure 1 shows such a bivariate model containing only additive genetic (A) and unique environmental (E) factors. We first determined whether a model without ethnic or gender differences showed a better statistical fit than a model in which variance components were estimated separately for ethnicity and gender. The variance components for which significant ethnic or gender differences were found were estimated separately for the ethnic and/or gender groups in subsequent variance components analyses. Then, the most parsimonious variance components model (ADE, ACE, AE, CE, or E) was determined for each of the variables. By constraining certain path coefficients to zero (see Figure 1) we subsequently tested for the significance of 1) the emergence of novel genetic effects at visit 2 (a22 = 0), 2) the genetic correlation between visit 1 and 2 (a21 = 0), and 3) the unique environmental correlation between visit 1 and 2 (e21 = 0). Within the best fitting bivariate model of LV structure variables we also calculated the heritability of the change over time in these phenotypes (between visit 1 and 2). It can be shown that this heritability of the difference score (e.g., LVMI visit2 – LVMI visit1) can simply be derived from the parameter estimates of the phenotype levels in the bivariate model (see Figure 1).
Figure 1.
Path diagram for a bivariate model.
For clarity only one twin is depicted. A1, A2 = Genetic variance components, E1, E2 = Non-shared environmental variance components, a11 through a22 = genetic path coefficients (or factor loadings) of which a22 represents novel genetic influences on visit 2, e11 through e22 = non-shared environmental path coefficients. (or factor loadings) of which e22 represents novel environmental influences on visit 2. Delta = difference in levels of measured phenotype between visit 2 and 1. Subtracting the level of the measured phenotype at time 2 from the level of the measured phenotype at time 1 is established by setting the path coefficients originating from the two measured phenotypes (and pointing towards delta) to -1 (time 1) and 1 (time 2).
- h2 total (visit1) = a112 / (a112 + e112)
- h2 total (visit2) = (a212 + a222) / (a212 + a222 + e212 + e222)
- h2 specific (visit2) = a222 / (a212 + a222 + e212 + e222)
- h2 delta (visit2 – visit1) = ((a21- a11)2+ a222) / ((a21 - a11)2+ a222 + (e21- e11)2+ e222)
Models were fitted to the raw data using normal theory maximum likelihood allowing the use of information provided by unpaired twin and/or single visit observations [Neale, 1992 #27802]. The significance of components A, C and D was assessed by testing deterioration in model fit after each component was dropped from the full model (ACE or ADE), leading to the most parsimonious model in which the pattern of variance/covariance are explained by as few parameters as possible. Standard hierarchic χ2 tests were used to select the best fitting model in combination with Akaike’s Information Criterion (AIC= χ2 - 2df) [Kendler, 1992 #2875]. The model with the lowest AIC reflects the best balance of goodness-of-fit and parsimony.
Tracking correlations
Phenotypic tracking correlations (and the underlying genetic and environmental tracking correlations) reflecting the stability over time of the individual cardiac structure variables from early adolescence into young adulthood (i.e., between visit 1 and 2) were determined from the best fitting models in Mx.
Statistical analysis and software
LV structure variables were logarithmically transformed before analysis to obtain approximately normal distributions. Multiple regression models were performed using Generalized Estimating Equations (GEE) to allow for the dependency between twins[Tregouet, 1997 #27804]. Data handling, preliminary analyses, calculation of twin correlations, and GEE were done with STATA (StataCorp, College Station, Texas). Quantitative genetic modeling was performed with Mx software [Neale, 1992 #27802].
RESULTS
Descriptive statistics
Table 1 shows means and standard deviations of age, anthropometric, hemodynamic and LV structure variables for both visits, grouped by ethnicity and gender. Age was similar across the subgroups. All anthropometric and hemodynamic variables significantly differed by gender. All means were significantly higher at visit 2 than at visit 1 except for heart rate, which was significantly lower at the 2nd visit. In general there were significant main effects of gender, ethnicity and visit for LV structure phenotypes. AAs and males had greater LVMI compared to EAs and females.
Table 1.
General characteristics and echocardiography data of European- and African-American males and females during visit 1 and visit 2
| European Americans |
African Americans |
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 |
Visit 2 |
Visit 1 |
Visit 2 |
Ethnicity Gender Visit |
|||||||
| Males | Females | Males | Females | Males | Females | Males | Females | P | P | P | |
| n | 266 | 295 | 224 | 221 | 191 | 237 | 164 | 190 | … | … | … |
| Age, y | 14.7±2.9 | 14.6±3.0 | 18.3±3.1 | 18.3±2.9 | 14.2±2.7 | 14.8±3.3 | 17.9±2.7 | 17.9±3.0 | NS | NS | <.001 |
| Height, m | 1.64±0.14 | 1.58±0.10 | 1.76±0.07 | 1.63±0.07 | 1.63±0.14 | 1.59±0.08 | 1.75±0.08 | 1.63±0.06 | NS | <.001 | <.001 |
| Weight, kg | 57.9±18.3 | 53.4±14.8 | 72.6±16.6 | 61.1±14.2 | 58.5±20.0 | 58.2±16.8 | 74.1±17.6 | 67.3±19.5 | NS | .04 | <.001 |
| BMI, kg/m2 | 21.1±4.3 | 21.1±4.7 | 23.4±4.6 | 22.8±4.7 | 21.6±5.0 | 22.9±5.7 | 24.0±4.8 | 25.3±6.7 | <.001 | <.001 | .004 |
| Heart rate, bpm | 67.3±11.1 | 72.4±12.0 | 61.0±9.5 | 66.6±9.9 | 65.7±10.0 | 71.9±10.9 | 59.6±10.5 | 67.5±10.3 | NS | <.001 | <.001 |
| SBP, mmHg | 109.8±9.0 | 105.8±8.3 | 114.5±9.9 | 106.7±8.3 | 112.5±10.5 | 110.1±10.2 | 118.0±10.2 | 111.6±11.1 | <.001 | <.001 | <.001 |
| DBP, mmHg | 56.2±5.7 | 57.5±5.5 | 56.8±6.3 | 59.4±6.4 | 59.4±5.9 | 60.9±7.0 | 59.2±6.5 | 62.7±7.8 | <.001 | .001 | <.001 |
| PP, mmHg | 53.6±9.0 | 48.3±7.2 | 57.7±9.5 | 47.3±7.3 | 53.0±9.1 | 49.3±8.1 | 58.8±9.7 | 48.9±9.03 | NS | <.001 | <.001 |
| IVSD, mm | 7.3±1.0 | 6.8±0.8 | 8.6±1.2 | 7.6±0.9 | 7.7±1.2 | 7.3±0.9 | 8.8±1.2 | 7.9±1.1 | <.001 | <.001 | <.001 |
| LVPWD, mm | 7.5±1.0 | 6.9±0.8 | 8.5±1.1 | 7.5±0.9 | 7.9±1.2 | 7.4±0.9 | 8.7±1.1 | 7.8±1.0 | <.001 | <.001 | <.001 |
| LVIDD, mm | 47.7±4.6 | 44.7±3.9 | 50.3±3.7 | 46.2±3.6 | 45.7±4.4 | 44.1±3.6 | 49.5±4.2 | 45.3±3.2 | <.001 | <.001 | <.001 |
| RWT, % | 31.3±3.9 | 31.0±3.8 | 34.0±4.7 | 32.7±4.2 | 34.3±4.8 | 33.5±4.3 | 35.6±5.1 | 34.8±4.9 | <.001 | <.001 | <.001 |
| LVM, g | 116.3±33.9 | 93.9±22.1 | 151.1±35.2 | 110.4±23.3 | 115.9±34.6 | 99.3±21.3 | 151.9±34.7 | 113.8±24.5 | NS | <.001 | <.001 |
| LVMI, (g/m2.7) | 30.2±5.4 | 27.2±5.7 | 33.0±7.2 | 29.3±6.1 | 30.8±6.4 | 28.5±5.3 | 33.3±7.0 | 30.5±6.2 | .03 | <.001 | <.001 |
Data are mean ± SD unless stated otherwise. BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; IVSD, interventricular septum in diastole; LVPWD, left ventricular posterior wall in diastole; LVIDD, left ventricular internal diameter in diastole; RWT, relative wall thickness; LVM, left ventricular mass; LVMI, left ventricular mass index (LVM/height 2.7).
There were significant gender-visit interactions (P values <.001) for height, weight, SBP, DBP, IVSD, LVPWD, and LVM, indicating that the gender difference (male > female except for DBP for which female > male) became larger over the transition period from adolescence to young adulthood for these measures. There were also significant ethnicity-visit interactions for IVSD (P=.02) and RWT (P=.003), with the ethnic difference (AA>EA) becoming smaller at visit 2 because EAs showed a faster increase than AAs for these two measures.
Cardiac structure prediction
Multivariate analysis of determinants of LV structure variables for visits 1 and 2 are presented in Table 2. The first column shows the proportion of variance (R2) explained by the base model of age, gender, ethnicity, and their interactions. Subsequent columns show the R2 values for the models that in addition to the base model include BMI (base + anthropometric) or both BMI and hemodynamic predictors (base + anthropometric + hemodynamic). BMI was a strong predictor for all LV structure measures at both visit 1 and 2, accounting for 3.5-24.2% of additional variance after allowing for the effects of age, gender, ethnicity and their interactions. Substituting waist circumference for BMI gave very similar results, but explained slightly less variance (not shown). Inclusion of hemodynamic variables resulted in a more modest increase in R2 at both visits, with up to 4.5% of variance of LV measures explained in addition to the effects of demographic and anthropometric variables.
Table 2.
Proportion of explained variance of left ventricular structure phenotypes by different explanatory models.
| Visit 1 |
Visit 2 |
|||||
|---|---|---|---|---|---|---|
| Variable | Base model* |
Base +Anthropometric † |
Base +Anthropometric +Hemodynamic ‡ |
Base model* |
Base +Anthropometric † |
Base +Anthropometric +Hemodynamic ‡ |
| IVSD | 22.1% | 38.4% | 39.7% | 19.1% | 33.5% | 34.4% |
| LVPWD | 25.2% | 40.6% | 43.3% | 22.1% | 35.2% | 36.8% |
| LVIDD | 26.6% | 36.1% | 39.8% | 25.3% | 33.1% | 34.1% |
| RWT | 11.7% | 15.2% | 18.6% | 6.8% | 11.0% | 13.0% |
| LVM | 33.6% | 53.1% | 56.9% | 32.4% | 49.5% | 53.9% |
| LVMI | 6.5% | 30.7% | 30.7% | 6.5% | 28.3% | 30.8% |
Composed of age, gender, ethnicity, and their interactions;
Composed of base model and BMI;
Tested measures included: systolic and diastolic blood pressure, pulse pressure, and heart rate.
IVSD, interventricular septum in diastole; LVPWD, left ventricular posterior wall in diastole; LVIDD, left ventricular internal diameter in diastole; RWT, relative wall thickness; LVMI, left ventricular mass index (LVM/height 27).
Genetic Model Fitting
Based on hierarchic multiple regression results, all LV structure variables were adjusted for age, gender, ethnicity, their interactions, and BMI prior to calculation of twin correlations and genetic model fitting.
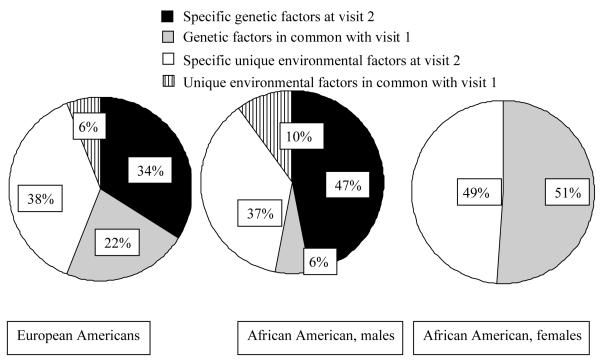
In general, both AA and EA showed MZ correlations that were substantially higher than DZ correlations indicating the importance of genetic factors in the two ethnic groups. Subsequent model fitting of the data confirmed this pattern. LV structure measures showed substantial heritability for both visits (range: 21%-71%, Table 3). The best-fitting longitudinal model revealed considerable influence of novel genetic effects on IVSD, LVPWD and RWT (but not for LVIDD), accounting for 33-41% of the phenotypic variance at visit 2, with no significant gender and ethnic effects. A gender difference for LVM and LVMI in AAs could be detected (P < 0.01), with a significant influence of novel genetic effects in males (46% and 47%, respectively), but not in females. There was no detectable gender difference in EAs, with 40% (LVM) and 35% (LVMI) of the phenotypic variance at visit 2 attributable to novel genetic effects. Figure 2 shows a decomposition of the sources of phenotypic variance of LVMI at visit 2 based on the best-fitting bivariate model. It is clearly shown which part of the genetic and environmental LVMI variance is common to both visits and which part is specific to visit 2. In AA females, the same set of genes that influence LVMI at visit 1 is entirely responsible for the genetic influence at visit 2.
Table 3.
Total (Visit 1 and 2) and Specific Heritability (Visit 2) Estimates and Heritability of the Change Score between Visits (95%CI) of Best Fitting Multivariate Models.
| Visit 1 |
Visit 2 |
|||
|---|---|---|---|---|
| Measures | h2 tot(95%CI) | h2tot(95%CI) | h2spec(95%CI) | h2delta(95%CI) |
| IVSD | 0.35(0.24-0.45) | 0.47(0.36-0.57) | 0.41(0.29-0.51) | 0.39(0.25-0.50) |
| LVPWD | 0.43(0.33-0.51) | 0.42(0.30-0.53) | 0.35(0.22-0.46) | 0.35(0.21-0.47) |
| LVIDD | 0.21(0.05-0.41) | 0.55(0.35-0.64) | * | * |
| RWT | 0.40(0.30-0.49) | 0.41(0.29-0.52) | 0.32(0.19-0.43) | 0.33(0.19-0.46) |
| LVM | ||||
| EA | 0.56(0.45-0.65) | 0.62(0.50-0.71) | 0.40(0.27-0.52) | 0.48(0.32-0.61) |
| AA, male | 0.71(0.57-0.81) | 0.58(0.37-0.72) | 0.46(0.28-0.62) | 0.65(0.43-0.79) |
| AA, female | 0.51(0.36-0.64) | 0.56(0.39-0.70) | * | * |
| LVMI | ||||
| EA | 0.42(0.30-0.53) | 0.56(0.44-0.66) | 0.34(0.19-0.47) | 0.39(0.22-0.54) |
| AA, male | 0.66(0.49-0.78) | 0.53(0.31-0.69) | 0.47(0.27-0.63) | 0.65(0.41-0.79) |
| AA, female | 0.44(0.29-0.58) | 0.51(0.34-0.66) | * | * |
h2 tot = total h2 ; h2 spec = specific h2 for visit 2; h2 delta = h2 for change score between the two visits; * = dismissed from the final model.
All models were adjusted for age, gender, ethnicity, their interactions, and BMI.
IVSD; interventricular septum in diastole; LVPWD; left ventricular posterior wall in diastole; LVIDD, left ventricular internal diameter in diastole; RWT, relative wall thickness; LVMI, left ventricular mass index (LVM/height 27).
Figure 2.
Sources of variance of LVMI at visit 2 shared with visit 1, based on the best-fitting bivariate model.
As shown in Table 4, tracking correlations over the 4.1 year period between visit 1 and visit 2 were moderate for IVSD, LVPWD and RWT (0.29 - 0.35), and moderate to high for LVIDD, LVM and LVMI (0.39 - 0.60). For IVSD, LVIDD, LVPWD and RWT 49% to 62% of the tracking correlation could be attributed to genetic effects that were common to both visits (%G in Table 5). For LVM and LVMI, depending on ethnicity and gender, 52% to 100% of the phenotypic tracking correlations were due to genetic effects with the remainder (if any) explained by stable environmental influences.
Table 4.
Tracking (rTR), genetic (rG), and environmental (rE) correlations between visit 1 and 2
| Measures | rTR (%G) | rG (95% CI) | rE (95% CI) |
|---|---|---|---|
| IVSD | 0.31 (49%) | 0.37(0.13-0.59) | 0.26(0.12-0.40) |
| LVPWD | 0.29 (62%) | 0.42(0.21-0.63) | 0.19(0.06-0.33) |
| LVIDD | 0.60 (58%) | 1.00 | 0.38(0.25-0.49) |
| RWT | 0.35 (54%) | 0.48(0.25-0.69) | 0.27(0.13-0.40) |
| LVM | |||
| EA | 0.49 (70%) | 0.59(0.43-0.73) | 0.35(0.18-0.50) |
| AA, male | 0.45 (64%) | 0.44(0.17-0.66) | 0.47(0.17-0.68) |
| AA, female | 0.53 (100%) | 1.00 | * |
| LVMI | |||
| EA | 0.50 (62%) | 0.63(0.43-0.81) | 0.37(0.21-0.52) |
| AA, male | 0.39 (52%) | 0.34(0.02-0.61) | 0.47(0.17-0.68) |
| AA, female | 0.48 (100%) | 1.00 | * |
The correlations have been adjusted for the effects of age, gender, ethnicity, their interactions, and BMI.
%G, percentage of genetic contribution to the phenotypic tracking correlation. * = dismissed from the final model.
IVSD; interventricular septum in diastole; LVPWD; left ventricular posterior wall in diastole; LVIDD, left ventricular internal diameter in diastole; RWT, relative wall thickness; LVMI, left ventricular mass index (LVM/height 27)
DISCUSSION
The present study longitudinally estimated the relative influence of genetic and environmental factors on constituents of LV structure in a large sample of AA and EA adolescent twins between 14 and 18 years of age on average, while adjusting for demographic factors and BMI. Genetic contributions to the individual differences at both measurement occasions were considerable for all variables. Results showed moderate to high tracking correlations between the two measurement occasions for all variables with genes contributing more than environmental factors to the stability of the LV structure phenotypes over time. At the same time, a substantial portion of the total genetic influence at visit 2 was specific to this measurement occasion.
A possible explanation for this emergence of novel genetic effects between 14 and 18 years of age is that hormonal changes during and after puberty affect the activation and deactivation of genes influencing individual differences in cardiac structure. Such switching on and off of genes may (partly) be caused by epigenetic changes such as methylation affecting the expression of genes and their influence on the phenotype.
Hierarchic multiple regression findings of our study confirm previous studies showing that adiposity explains a large portion of the variance in LVM [Urbina, 1995 #3980; Daniels, 1995 #8794; Li, 2004 #18438; Janz, 2000 #18439]. BMI explained up to 24% additional variance after allowing for the effects of age, gender and ethnicity. In keeping with previous studies in childhood, the contribution of hemodynamics was significant but of lesser magnitude than body composition [Urbina, 1995 #3980; Daniels, 1995 #8794]. Contributions of demographic, anthropometric and hemodynamic factors were similar for LVIDD, IVSD and LVPWD at both visits suggesting these determinants may equally explain changes occurring to inner cavity (e.g., LVIDD) and the myocardium as measured by parietal thickness. We previously reported in a normotensive sample of singleton youth that adiposity, sex, resting vascular tone and BP reactivity to a challenging behavioral stressor were independent predictors of future LVM [Kapuku, 1999 #5018]. Herein we extend our observation to the role of heredity.
Our study has several unique strengths such as the inclusion of a large group of AA twins, which allowed us to directly compare and test differences in relative influence of genetic and environmental factors on cardiac structure in AAs and EAs. We are aware of only one other twin study that included AA twins. This early study by Harshfield et al [Harshfield, 1990 #702] did not include any EA twins and only a small number of AA twins and observed the genetic effect on cardiac mass only after controlling for body surface area, gender and systolic blood pressure. We are aware of only one family study studied a population-based sample of European American (EA) and African-American (AA) hypertensives. [Arnett, 2001 #27798] Results indicated higher sibling correlations for LVM among AA ranging from 0.22 (brother-sister) to 0.44 (brother-brother) compared to EA siblings (ranging from to 0.05 (brother-sister) to 0.22 (sister-sister). Correlations for relative wall thickness, a measure of LV concentricity, were lower in AA siblings (0.04-0.12) than their EA counterparts (0.19-0.28) suggesting ethnic heterogeneity among genes influencing LV structure and geometry. This conclusion was not supported by the results of our study that including both ethnic groups. The Hypertension Genetic Epidemiology Network (HyperGEN) found equally substantial contributions of genetic factors (i.e., total heritabilities) in both ethnic groups by 18 years of age.
We observed higher mean values for most LV structure variables in AAs and males compared to EAs and females for both measurement occasions, thereby confirming observations from our other longitudinal studies [Dekkers, 2002 #8910; Kapuku, 1999 #5018] and those of others [Schieken, 1998 #4652; Malcolm, 1993 #3083] that ethnic and gender differences in cardiac structure are already present in childhood. The classic twin study is established as the ideal study design to estimate the relative importance of genetic and environmental factors to the variance of traits and diseases in human populations [Snieder, 2003 #10654], but without actual measurement of specific genes or environments it cannot attribute the ethnic difference in mean values to either of these factors. However, our study does show that apart from the gender difference in LVM and LVMI for AAs, the observed difference in mean values did not translate in major differences in relative importance of genetic and environmental factors between ethnic and gender groups.
Another strength of our study lies in its longitudinal design, which allowed investigation of genetic and environmental sources of stability and change of LV structure phenotypes between 14 and 18 years of age. To the best of our knowledge this is the first study to show the emergence of genetic influences on cardiac structure at Visit 2 that were not present at Visit 1 in late adolescence. Previous studies have shown cardiac structure components to track over time [Janz, 2000 #18439; Schieken, 1998 #4652; Mahoney, 1988 #185]. Here, we observed mild to moderate tracking, which could be mostly attributed to genetic factors after adjusting for age, gender, ethnicity, and BMI. Cardiac development includes changes in myocardium and chamber size. Cardiac size is mainly determined by load conditions (i.e., preload, afterload). After birth, the heart cell loses the ability to divide and increases in size to achieve heart growth. It is evident that the gene-environment interaction modulates heart enlargement. From animal and human studies [Innes, 1998 #22329; Baessler, 2006 #18441; Poch, 2000 #18442; Kupari, 1998 #18444; Schunkert, 1994 #4388] candidates genes including GHSR, G-protein beta (3) subunit, human aldosterone synthase (CYP11B2) and ACE have been shown to variably affect cardiac size and LVM. Genes have been described as switching on and off during the normal growth process. Our results suggest that some genes were shared between visit 1 and 2, others were specific to visit 2, implying that these genes were turned on during the 4 year follow-up period. However, at visit 2, LVIDD was still controlled by the same set of genes also influencing the phenotype 4 years earlier, suggesting a differential gene control of myocardium and chamber size.
In AA females, no novel genetic effects on LVM and LVMI emerged during the study follow-up. The reason and significance of this unexpected genetic stability is unknown. Whether this phenomenon is linked to earlier maturation and age at menarche in AA females [Kaplowitz, 2001 #7153; Herman-Giddens, 2006 #27758; Daniels, 1996 #8822] deserves attention. Continued follow up of our twin sample [Ge, 2006 #27803] will enable us to examine whether the genetic component stabilizes in early adulthood for the other gender by ethnicity groups as well.
In conclusion, this longitudinal twin study demonstrates moderate tracking and moderate to high heritability of LV structure and geometry after taking in account BMI, age, gender, and ethnicity. A great deal of LVM variability was linked to aging-related changes in genetic expression as there was a significant amount of new genes expressed between 14 and 18 years of age in all but AA females. Further work is required to provide the clinical implications of this observation. At the same time it means that one should exercise caution pooling adolescent and adult subjects in large studies aimed at evaluating the contribution of the gene/environment interaction on cardiac growth.
Acknowledgments
Source of support: This study was supported by grants HL56622 and HL69999 from the National Heart Lung and Blood Institute.
REFERENCE
- Casale PN, Devereux RB, Milner M. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105:173–178. doi: 10.7326/0003-4819-105-2-173. al. e. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Devereaux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Int Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of Left Ventricular Mass The Framingham Heart Study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- Juo SH, Di Tullio MR, Lin HF, Rundek T, Boden-Albala B, Homma S, Sacco RL. Heritability of left ventricular mass and other morphologic variables in Caribbean Hispanic subjects: the Northern Manhattan Family Study. J Am Coll Cardiol. 2005;46:735–737. doi: 10.1016/j.jacc.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaaren HA, Schieken RM, Mosteller M, Hewitt JK, Eaves LJ, Nance WE. Bivariate genetic analysis of left ventricular mass and weight in pubertal twins (The Medical College of Virginia Twin Study) American Journal of Cardiology. 1991;68:661–668. doi: 10.1016/0002-9149(91)90361-n. [DOI] [PubMed] [Google Scholar]
- Busjahn A, Knoblauch H, Knoblauch M, Bohlender J, Menz M, Faulhaber HD, Becker A, Schuster H, Luft FC. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms, plasma levels, cardiac dimensions. A twin study. Hypertension. 1997;29:165–170. doi: 10.1161/01.hyp.29.1.165. [DOI] [PubMed] [Google Scholar]
- Arnett DK, Hong Y, Bella JN, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Devereux RB. Sibling correlation of left ventricular mass and geometry in hypertensive African Americans and whites: the HyperGEN study. Hypertension Genetic Epidemiology Network. Am J Hypertens. 2001;14:1226–1230. doi: 10.1016/s0895-7061(01)02200-2. [DOI] [PubMed] [Google Scholar]
- Sharma P, Middelberg RP, Andrew T, Johnson MR, Christley H, Brown MJ. Heritability of left ventricular mass in a large cohort of twins. J Hypertens. 2006;24:321–324. doi: 10.1097/01.hjh.0000202815.18083.03. [DOI] [PubMed] [Google Scholar]
- Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–951. doi: 10.1161/01.hyp.0000015612.73413.91. [DOI] [PubMed] [Google Scholar]
- Treiber F, Harshfield G, Davis H, Kapuku G, Moore D. Stress responsivity and body fatness: links between socioeconomic status and cardiovascular risk factors in youth. Ann N Y Acad Sci. 1999;896:435–438. doi: 10.1111/j.1749-6632.1999.tb08163.x. [DOI] [PubMed] [Google Scholar]
- Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation. 2004;110:3488–3492. doi: 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Kimball TR, Morrison JA, Khoury P, Witt S, Meyer RA. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation. 1995;92:3249–3254. doi: 10.1161/01.cir.92.11.3249. [DOI] [PubMed] [Google Scholar]
- Kupper N, Ge D, Treiber FA, Snieder H. Emergence of novel genetic effects on blood pressure and hemodynamics in adolescence: the Georgia Cardiovascular Twin Study. Hypertension. 2006;47:948–954. doi: 10.1161/01.HYP.0000217521.79447.9a. [DOI] [PubMed] [Google Scholar]
- Snieder H, Dong Y, Barbeau P, Harshfield GA, Dalageogou C, Zhu H, Carter ND, Treiber FA. Beta2-adrenergic receptor gene and resting hemodynamics in European and African American youth. Am J Hypertens. 2002;15:973–979. doi: 10.1016/s0895-7061(02)02991-6. [DOI] [PubMed] [Google Scholar]
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantification of the left ventricle by two- dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. et a. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- de Simone G, Daniels SR, Devereux RB, Meyar RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults:Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- Cook BB, Treiber FA, Mensah G, Jindal M, Davis HC, Kapuku GK. Family history of hypertension and left ventricular mass in youth: possible mediating parameters. American Journal of Hypertension. 2001;14:351–356. doi: 10.1016/s0895-7061(00)01275-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96:1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic; Dordrecht, Netherlands: 1992. [Google Scholar]
- Kendler KS, Heath AS, Neale MC, Kessler RC, Eaves LJ. A population based twin study of alcoholism in women. Journal of the American Medical Association. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Tregouet DA, Ducimetiere P, Tiret L. Testing association between candidate-gene markers and phenotype in related individuals, by use of estimating equations. Is J Hum Genet. 1997;61:189–199. doi: 10.1086/513895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–2406. doi: 10.1161/01.cir.91.9.2400. [DOI] [PubMed] [Google Scholar]
- Janz KF, Dawson JD, Mahoney LT. Predicting heart growth during puberty: The Muscatine Study. Pediatrics. 2000;105:E63. doi: 10.1542/peds.105.5.e63. [DOI] [PubMed] [Google Scholar]
- Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- Harshfield GA, Grim CE, Hwang C, Savage DD, Anderson SJ. Genetic and environmental influences on echocardiographically determined left ventricular mass in black twins. J Human Hypertens. 1990;3:538–543. doi: 10.1093/ajh/3.7.538. [DOI] [PubMed] [Google Scholar]
- Schieken RM, Schwartz PF, Goble M. Tracking of Left Ventricular Mass in Children: Race and Sex Comparisons: The MCVTwin Study. Circulation. 1998;97:1901–1906. doi: 10.1161/01.cir.97.19.1901. [DOI] [PubMed] [Google Scholar]
- Malcolm DD, Burns TL, Mahoney LT, Lauer RM. Factors affecting left ventricular mass in childhood: The Muscatine Study. Pediatrics. 1993;92:703–709. [PubMed] [Google Scholar]
- Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-american youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- Mahoney LT, Schieken RM, Clarke WR, Lauer RM. Left ventricular mass and exercise responses predict future blood pressure: The Muscatine study. Hypertension. 1988;12:206–213. doi: 10.1161/01.hyp.12.2.206. [DOI] [PubMed] [Google Scholar]
- Innes BA, McLaughlin MG, Kapuscinski MK, Jacob HJ, Harrap SB. Independent genetic susceptibility to cardiac hypertrophy in inherited hypertension. Hypertension. 1998;31:741–746. doi: 10.1161/01.hyp.31.3.741. [DOI] [PubMed] [Google Scholar]
- Baessler A, Kwitek AE, Fischer M, Koehler M, Reinhard W, Erdmann J, Riegger G, Doering A, Schunkert H, Hengstenberg C. Association of the Ghrelin receptor gene region with left ventricular hypertrophy in the general population: results of the MONICA/KORA Augsburg Echocardiographic Substudy. Hypertension. 2006;47:920–927. doi: 10.1161/01.HYP.0000215180.32274.c8. [DOI] [PubMed] [Google Scholar]
- Poch E, Gonzalez D, Gomez-Angelats E, Enjuto M, Pare JC, Rivera F, de La Sierra A. G-Protein beta(3) subunit gene variant and left ventricular hypertrophy in essential hypertension. Hypertension. 2000;35:214–218. doi: 10.1161/01.hyp.35.1.214. [DOI] [PubMed] [Google Scholar]
- Kupari M, Hautanen A, Lankinen L, Koskinen P, Virolainen J, Nikkila H, White PC. Associations between human aldosterone synthase (CYP11B2) gene polymorphisms and left ventricular size, mass, and function. Circulation. 1998;97:569–575. doi: 10.1161/01.cir.97.6.569. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Hense HW, Holmer SR, Stender M, Perz S, Keil U, Lorell BH, Gunter GAJ. Association between a deletion polymorphism of the angiotensin-converting-enzyme and left ventricular hypertrophy. N Engl Med. 1994;330:1634–1638. doi: 10.1056/NEJM199406093302302. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int J Androl. 2006;29:241–246. doi: 10.1111/j.1365-2605.2005.00575.x. discussion 286-290. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Obarzanek E, Barton BA, Kimm SY, Similo SL, Morrison JA. Sexual maturation and racial differences in blood pressure in girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 1996;129:208–213. doi: 10.1016/s0022-3476(96)70244-5. [DOI] [PubMed] [Google Scholar]
- Ge D, Dong Y, Wang X, Treiber FA, Snieder H. The Georgia Cardiovascular Twin Study: influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Twin Res Hum Genet. 2006;9:965–970. doi: 10.1375/183242706779462877. [DOI] [PubMed] [Google Scholar]