Abstract
5-HT1B receptors are densely expressed on terminals of medium spiny neurons projecting from the nucleus accumbens shell (NAccSh) to the ventral tegmental area, where 5-HT1B receptors modulate GABA release directly, and firing of dopaminergic neurons indirectly. While interactions between NAccSh 5-HT1B receptors and stress have been reported in early stages of psychostimulant-induced neuroadaptations, specifically psychomotor sensitization, the effect of this interaction on later stages of drug seeking is currently unknown. Here, we examined the effect of herpes simplex virus (HSV)-mediated overexpression of NAccSh 5-HT1B receptors on reinstatement of cocaine seeking induced by exposure to stress or a cocaine prime. Rats were trained to self-administer cocaine (0.75 mg/kg/infusion) and the operant response was extinguished. Rats were then injected with viral vector expressing 5-HT1B and green fluorescent protein (GFP) or GFP alone into the NAccSh. The effect of 5-HT1B receptor overexpression was assessed on reinstatement induced by intermittent footshock (0.5 mA for 15 minutes) or a cocaine prime (10 mg/kg, ip). Results indicate that NAccSh 5-HT1B receptor overexpression had no effect on footshock reinstatement while significantly decreasing cocaine priming-induced reinstatement. We also found that NAccSh overexpression of 5-HT1B receptors had no effect on saccharin intake following social defeat stress. These results suggest that the efficacy of pharmacological agents targeting 5-HT1B receptors for the treatment of cocaine relapse will depend largely on the nature of the reinstating stimulus. Taken together with previous results it appears that NAccSh 5-HT1B receptors influence stress responses in early, but not in the later stages of psychostimulant-induced neuroadaptations.
Keywords: Cocaine, Herpes simplex virus-mediated gene transfer, Nucleus accumbens shell, Reinstatement, Serotonin, Stress
1. Introduction
Addiction to psychostimulant drugs such as cocaine has become a worldwide epidemic with major social and economic burdens on society. An important problem in the treatment of addiction to cocaine and other drugs of abuse is the vulnerability of individuals to relapse to drugs months or even years after cessation of drug use (Dackis and O’Brien, 2001; Gossop et al., 1989). Indeed, up to 90% of previously addicted individuals relapse to drug use within twelve months of abstinence (DeJong, 1994). Relapse in addicts can be induced by exposure to the drug itself, drug-associated cues or by exposure to stressful stimuli (Childress et al., 1993; Childress et al., 1988; Sinha et al., 2000; Sinha and Li, 2007).
The serotonergic neuronal system plays an important role in relapse to cocaine seeking (Filip et al., 2010; Filip et al., 2005). Interestingly, both enhancing (Burmeister et al., 2003) and decreasing (Tran-Nguyen et al., 2001) serotonin (5-HT) levels attenuates reinstatement of cocaine seeking induced by exposure to cues previously associated with cocaine self-administration, and has variable effects on priming-induced reinstatement of cocaine seeking. These inconsistencies in delineating the effects of serotonergic neuronal modulation on relapse to cocaine seeking are most likely due to the complexity of 5-HT receptor sub-types (Filip et al., 2010).
5-HT1B receptors are known to regulate the behavioral effects of cocaine (Miszkiel et al., 2011). These receptors are Gi/o-coupled receptors that negatively couple with adenylate cyclase, resulting in an inhibitory effect on neuronal activity (Morikawa et al., 2000). In situ hybridization histochemistry studies indicate that 5-HT1B receptors are expressed on medium spiny neurons throughout the striatum (Bruinvels et al., 1994); these GABAergic neurons project to several brain regions including the ventral tegmental area (VTA), ventral pallidum, and globus pallidus externa (Groenewegen et al., 1999). Physiological studies indicate that 5-HT1B receptors on these neurons are translocated to axon terminals where they negatively regulate GABA release (O’Dell and Parsons, 2004). The level of expression for these receptors is quite dynamic and is differentially regulated by stress, novelty, and both cocaine exposure and withdrawal (Furay et al., 2011; Hoplight et al., 2007; Neumaier et al., 2002a; Neumaier et al., 2009). Stimulation of VTA 5-HT1B receptors potentiates the effects of cocaine, likely via disinhibition of GABA release (O’Dell and Parsons, 2004; Yan et al., 2004). Previously we demonstrated in a conditioned place preference assay, that increased expression of 5-HT1B receptors in nucleus accumbens shell (NAccSh) neuronal terminals in the VTA shifts the cocaine dose-response curve to the left (Neumaier et al., 2002b). However, we later observed that these receptors can enhance or inhibit cocaine preference depending on whether the procedure was optimized to detect preference or aversion, respectively (Barot et al., 2007), suggesting a more complicated role of these receptors.
While many investigators have examined the effect of 5-HT1B receptor modulation on reinstatement of cocaine seeking induced by a cocaine prime or conditioned cues (Acosta et al., 2005; Pentkowski et al., 2009; Przegalinski et al. 2007; Przegalinski et al. 2008), the effect of 5-HT1B receptor modulation on stress-induced reinstatement is currently unknown. We have previously observed an interaction between coincident exposure to mild stress and increased 5-HT1B receptor expression in NAccSh projection neurons that facilitates the behavioral effects of exposure to drugs of abuse (Ferguson et al., 2009), suggesting an interplay between the stress response and 5-HT1B receptor overexpression. These interactions between 5-HT1B receptors and stress have been observed in the early stages of psychostimulant-related behavioral plasticity (Neumaier et al., 2002b; Ferguson et al., 2009; Neumaier et al., 2009; Hoplight et al., 2007; Furay et al., 2011) and we are now examining the effect of 5-HT1B receptors in stress-induced reinstatement of cocaine seeking.
In the present study, we examined the effect of transiently increasing 5-HT1B receptor expression in the NAccSh using herpes simplex virus-mediated gene transfer on footshock stress-induced cocaine reinstatement. The optimal way to ascertain the behavioral specificity of an experimental manipulation is to determine its effect on more than one reinstating stimulus (Nair et al., 2009). Hence, we also examined the effect of NAccSh 5-HT1B receptor expression on cocaine-priming induced reinstatement of cocaine seeking. To further study the role of NAccSh 5-HT1B receptors in stress responses, we examined the effect of 5-HT1B overexpression on saccharin preference following exposure of rats to social defeat stress.
2. Materials and Methods
2.1 Subjects
For cocaine reinstatement experiments, male Long-Evans rats (Charles River, Raleigh, NC), weighing 350-425 g were used. These rats were initially double-housed and allowed to acclimate for at least one week to the vivarium prior to the experiment. The temperature- and humidity-controlled vivarium was under a 12-h light-dark cycle (lights on at 6 a.m.). Following the acclimation period, rats were handled every other day for at least 6 days prior to surgery. After surgery, the rats were housed individually in the vivarium. For the social defeat experiment, rats were procured from Harlan Laboratories (Indianapolis, IN). Resident pairs consisted of one male (375-425g) and one female Long-Evans rat (150-200g). No dependent measures were collected from these rats. Saccharin preference was performed on male, Sprague-Dawley rats (225-250g), which were individually housed throughout the experiment. Food and water were available ad libitum for all rats, with the exception of the 3h training, extinction and reinstatement test duration/day for cocaine reinstatement experiments when rats had access to water ad libitum, but not food. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee and were conducted in accordance to the guidelines of the “Principles of Laboratory Animal Care” (NIH publication no. 86-23, 1996). A total of 72 rats were used for these experiments, of which 22 were excluded due to failure to learn to self-administer cocaine (n=5), virus-induced gene expression outside the target brain region (n=12), active lever responding that was more than 3 standard deviations away from the mean (n=1) or failure to meet an extinction criterion of less than an average of 20 active lever presses per 3 h over 3 consecutive days (n=4).
2.2 Intravenous surgery
Intravenous catheters were made in the laboratory using silastic tubing (Dow Corning, Midland, MI) with a silicon ‘bead’ at the proximal end of the catheter to aid in anchoring to the jugular vein. Rats were anesthetized with isoflurane (3% for induction and 1-3% for maintenance of anesthesia). The neck and intrascapular regions were shaved and prepared with 10% povidone iodine and 70% ethanol. An incision (~10 mm) was made in the skin and fascia overlying the right jugular vein. The fascia and tissue surrounding the vein was cleared using two pairs of straight forceps, following which the vein was lifted using a pair of curved forceps. An incision (~0.5 mm) was made in the jugular approximately 5mm proximal to the point where the vein enters the pectoralis major muscle, the catheter was fed into the vein under strict aseptic conditions and held in place with 2-3 surgical knots using 4-0 silk. Optimal positioning of the catheter was verified by drawing blood into it with negative pressure. A second incision (~25 mm) was then made in the intrascapular region. A pair of straight hemostats was used to form a subcutaneous path from this incision ventrally around the right forelimb to the neck incision. The intravenous catheter was fed through this subcutaneous path to the intrascapular region where it was connected to a vascular access harness (Instech Laboratories Inc., Plymouth Meeting, PA). Both the incisions were closed and treated with 10% povidone iodine topically. Rats were closely monitored following surgery and kept warm until ambulatory. Buprenorphine (0.1 mg/kg, s.c.) (Exp.1) or meloxicam (0.2 mg/kg, s.c.) (Exp. 2) was administered for analgesia and rats were allowed to recover for 7-10 days before cocaine self-administration training. During the recovery and training phases, catheters were flushed every 24-48 h with sterile gentamicin (0.08 mg/ml). To verify catheter patency, rats received intravenous injections of methohexital sodium (10 mg/ml; injection volume: 0.12 ml), a short-acting barbiturate that induces a rapid loss of muscle tone when administered intravenously. We performed the catheter patency test in 3 rats when their lever responding during self-administration training became erratic. These rats did not demonstrate momentary loss of muscle tone and were excluded from the study.
2.3 Intracranial surgery and virus-mediated gene transfer
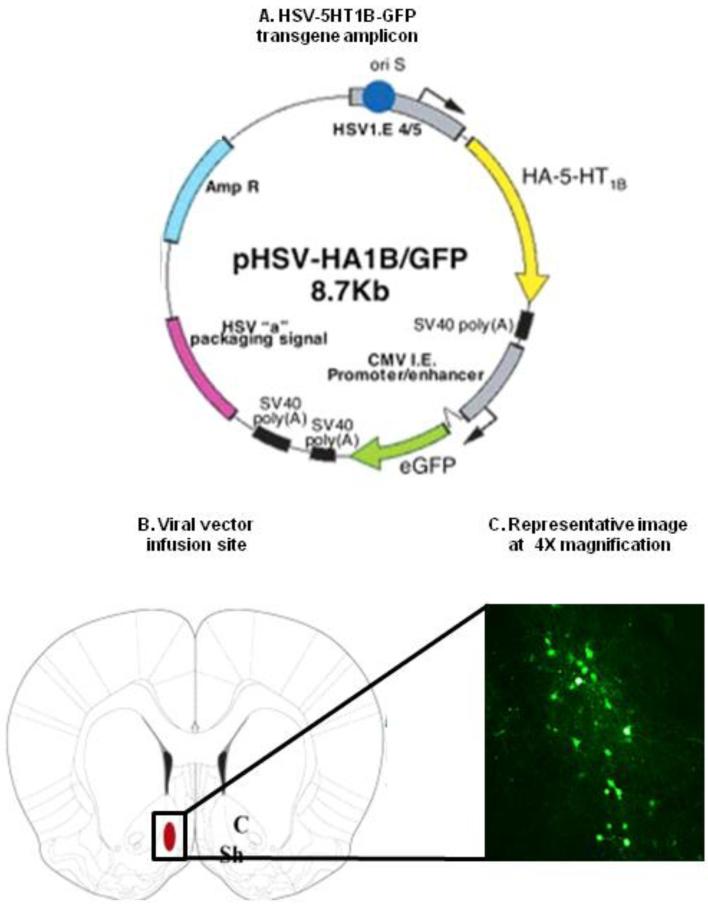
We used replication-deficient herpes-simplex viral vectors to modulate 5-HT1B receptor expression. The experimental viral vector contains two cassettes: one that expresses fully functional hemagglutinin-tagged 5-HT1B and one that expresses green fluorescent protein (5HT1B/GFP); a virus expressing GFP alone was used as the control vector (Clark et al., 2002). We have previously confirmed that this viral vector system produces hemagglutinin-tagged 5-HT1B receptors in NAccSh neurons and not glia, is translocated to axon terminals in the VTA, and shows peak receptor expression in the terminals at around four days after infection (Barot et al., 2007). Viral vectors were injected using surgical procedures previously described (Barot et al., 2007; Ferguson et al., 2009; Neumaier et al., 2002b). Briefly, each rat was placed in a Stoelting stereotaxic apparatus, the scalp incised, skull landmarks visualized by scraping the periosteum and burr holes were drilled at the injection sites. A 27 gauge needle was directed to the NAccSh stereotaxically (AP: + 1.8 mm, ML: ± 0.8 mm with injector bevel pointing inwards, DV: − 6.8 mm) (Paxinos and Watson, 2005). 5-HT1B/GFP or GFP viral vector (2 μl containing ~108 infective units) was injected over 10 minutes, after which the needle was left in place for 5 minutes and then slowly withdrawn. This volume of viral vector was chosen based on previous studies in our laboratory to induce discrete infection in the target region (Mitchell et al., 2007; Neumaier et al., 2002a) producing infection rates of approximately 20% (Clark et al., 2002). The skin incision was closed with 3-0 monofilament nylon sutures in Exp. 1 and in Exps. 2 and 3 skin closure was augmented with sterile n-butyl cyanoacrylate glue. Buprenorphine (0.1 mg/kg, s.c.) (Exp. 1) or meloxicam (0.2 mg/kg, s.c.) (Exp. 2 and 3) was administered for post-surgical analgesia. To confirm the injection site, rats were perfused as previously described (Eskenazi and Neumaier, 2011), brains were dissected and post-fixed in 4% paraformaldehyde for 4 hr, after which they were placed in phosphate-buffered saline. Coronal sections (40 μm) were made on a Leica VT 1000S microtome, mounted and coverslipped with Vectashield mounting medium (VectorLabs, Burlingame, CA). The sections were subsequently examined for GFP immunofluorescence on a Nikon Eclipse E600 microscope. A representative image is depicted in Figure 1. We examined NAccSh sections for GFP immunofluorescence instead of measuring the number of 5-HT1B receptors since these receptors are translocated away from the site of injection and hence cannot be reliably quantified.
Figure 1. Virus-mediated gene transfer.
(A) Illustration of the herpes simplex virus 5-HT1B green fluorescent protein transgene amplicon (B) Illustration of rat brain coordinates (Paxinos plate 11, +1.7 mm) used for viral vector infusion. The red oval depicts the target zone for the viral vector unilaterally. C: Nucleus accumbens core, Sh: Nucleus accumbens shell (C) Representative histological plate of GFP expression from a coronal section of the NAccSh four days after viral vector infusion.
2.4 Behavioral testing
2.4.1 Apparatus
For experiments 1 and 2, rats were trained and tested in standard Med Associates operant chambers (Med Associates, Georgia, VT). Each chamber was equipped with two levers located 9 cm above the grid floor. Lever-presses on the active lever activated the infusion pump whereas lever-presses on the inactive lever had no programmed consequences. The grid floors of the operant chambers were connected to electrical shock generators (Med Associates, Georgia, VT). All chambers were kept in sound-attenuating boxes equipped with fans for temperature regulation and to provide white noise. All chambers were connected to a Med Associates interface, and experimental data were collected using Med-PC software.
2.4.2 Procedures
For experiments 1 and 2, the procedure consisted of 3 phases: self-administration training (10-12 d), extinction training (12-17 d), and tests for footshock stress or cocaine-priming-induced reinstatement of cocaine-seeking (2 d).
Cocaine self-administration training and extinction of cocaine reinforced operant responding
Rats were trained to self-administer cocaine for 3-hr/day (5 min interval after every hr) for 10-12 days. Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile 0.9% saline and infused in a volume of 0.1ml at a dose of 0.75 mg/kg/infusion. Each session started with the turning on of a white house-light and introduction of levers into the self-administration chamber. During training, cocaine infusions were earned under a fixed-ratio-1 (FR1), 20 sec timeout reinforcement schedule and were accompanied by a compound tone-light cue for 5 sec. During the 20 sec timeout period, lever presses were recorded but did not result in cocaine delivery. A maximum of 20 cocaine infusions/hour was set to prevent cocaine overdose. At the end of each session, the house-light was turned off and the levers retracted. During the extinction phase, the procedures were identical to those of self-administration training, with the exception that the drug syringes were removed. After rats met the extinction criterion described above, they were injected with GFP or 5-HT1B/GFP into the NAccSh. Rats were given regular extinction sessions before reinstatement tests on days 3 and 4 following intracranial injection. The different groups of rats that were injected intracranially with GFP or 5-HT1B/GFP were matched for their cocaine intake and number of active lever-presses during training and for the number of active lever-presses during the extinction phase.
Tests for reinstatement of cocaine seeking
Exp. 1: Effect of viral overexpression of 5-HT1B receptors in the NAccSh on footshock-stress induced reinstatement of cocaine seeking
Two groups of rats were used. Rats were trained to self-administer cocaine and the operant response was extinguished as described above. GFP (n= 8) or 5-HT1B/GFP (n= 9) vectors were injected into the NAccSh. Each rat was exposed to either intermittent footshock that was delivered at random intervals with a mean interval of 40 seconds (ranging from 10-70 seconds) for 15 minutes prior to the start of the test session (0.5 mA for 0.5 seconds each) (Shaham and Stewart, 1995, Erb et al., 1996) or no footshock. The ‘No Footshock’ and ‘Footshock’ tests were conducted on days 3 and 4, respectively following NAccSh injections of the viral vector. We used a mixed experimental design with a between-subjects factor of Viral Vector (5-HT1B/GFP or GFP) and within-subjects factor of Stressor (Footshock or No footshock) and Session Hour. The order of the footshock and no footshock conditions were not counterbalanced because the viral expression of this transgene peaks at approximately 4 days post-injection (Barot et al., 2007).
Exp. 2: Effect of viral overexpression of 5-HT1B receptors in the NAccSh on cocaine-priming induced reinstatement of cocaine seeking
Two groups of rats were used. Rats were trained to self-administer cocaine and the operant response was extinguished as described above. On the last three extinction days rats were given sham vehicle injections to habituate them to the injection procedure. GFP (n= 7) or 5-HT1B/GFP (n=10) vectors were injected into the NAccSh. Each rat was injected with either vehicle (sterile saline) or cocaine (10 mg/kg, i.p.) on days 3 and 4 respectively, following intracranial injections of the viral vector. We used a mixed experimental design with between-subjects factor of Viral Vector (5-HT1B/GFP or GFP) and within-subjects factor of Cocaine Priming (Vehicle or Cocaine) and Session Hour. The order of vehicle and cocaine injections was not counterbalanced (see Experiment 1 for details). Cocaine (10 mg/kg, ip; injection volume: 1 mg/ml) or vehicle was injected immediately prior to the test session.
Exp. 3: Effect of viral overexpression of 5-HT1B receptors in the NAccSh saccharin preference following social defeat stress
Here, the procedures were performed in the following order: 1) two saccharin training sessions (one week apart); 2) injection with either GFP (n= 8) or 5-HT1B/GFP (n= 8) viral vectors into the NAccSh; 3) four consecutive social defeat sessions (over four days); 4) final saccharin preference test. The procedures for social defeat and saccharin preference are described below.
Social defeat
Resident pairs of Long-Evans rats (one male and one female) were housed for at least four breeding cycles (Females were tubally ligated to prevent pregnancy while maintaining hormonal tone and sexual receptivity). Males were then screened for aggression. Only males that consistently displayed aggressive behavior toward intruder stimulus animals (which were not used in the following experiments) were included in the study.
Social defeat consisted of procedures that were identical to a previous study (Furay et al., 2011) based on the method described by Covington and Miczek (Covington and Miczek, 2005) and took place between 07:30 and 11:00 h. Briefly, females were removed from residents’ cages; an intruder (Sprague Dawley) was placed in the resident’s cage under a mesh protective enclosure for a 10 min instigation period, to elicit aggressive behavior from the resident. The mesh enclosure was then removed, and the animals were allowed to interact for five minutes or until the resident defeated the intruder. Defeat occurred when the resident pinned the intruder in a supine position for at least five consecutive seconds or the resident bit the intruder five times in a row. Time to defeat ranged between 10 and 60 s. After defeat, the intruder was placed under the mesh enclosure and remained in the resident’s cage for an additional 30 min. Intruders were then returned to their home cages; females were returned to the respective resident’s cage. All animals were monitored for health during the experiment, including assessment of coat condition, body weight, and visual inspection for wounds. None of the intruders sustained wounds that required stitches and none of the intruders were excluded from the experiment for health reasons. Rats were injected with either GFP (n= 8) or 5-HT1B/GFP (n= 8) viral vectors into the NAccSh.
Saccharin preference testing
Under our experimental conditions, social defeat stress reliably decreases saccharin preference (Furay et al., 2011). The saccharin preference testing procedure consisted of two - one hour training sessions prior to social defeats and one – one hour challenge session after the final defeat. All saccharin testing started at 16:30 h (1.5 h before lights-off). Training sessions were one week apart with one week of recovery before defeats began. The final saccharin challenge took place on the last day of defeat (defeat on this day occurred at 07:30 h). For all saccharin procedures, two sippers, one containing tap water and the other containing 0.1% saccharin in tap water were placed in rats’ home cages at 16:30 h and removed from cages one hour later. Bottles were weighed before and after the test.
2.5 Statistical analyses
Data were analyzed with the statistical program SPSS (GLM procedure). Data from the cocaine reinstatement experiments (Exp. 1 & 2) were analyzed for total (non-reinforced) lever presses on the previously active lever. The experimental manipulations had minimal effects on inactive lever presses, a potential measure of non-directed activity and/or response generalization (Shalev et al., 2002). Thus the data for inactive lever presses are not reported in the results section. Data from the social defeat experiment (Exp. 3) were analyzed for saccharin preference.
3. Results
3.1 Cocaine reinstatement experiments (Exp 1 and Exp 2)
Training and extinction
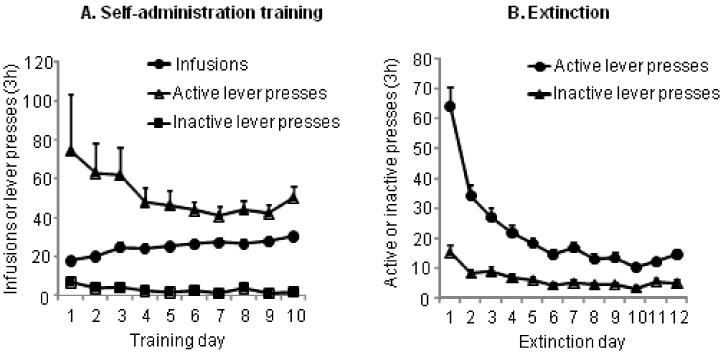
Fig. 2A depicts the mean±SEM number of cocaine infusions and presses on the active and inactive levers for all rats that were subsequently tested in Exp. 1 & 2. The rats demonstrated reliable cocaine self-administration. Fig. 2B depicts the mean±SEM number of lever presses on the previously active and inactive levers during the first 12 extinction sessions for these rats. As anticipated, during the extinction phase, response rates decreased over time.
Figure 2. Cocaine self-administration training and extinction of cocaine-reinforced responding.
(A) Self-administration training: Mean ± SEM number of infusions and active and inactive lever responses during the first 10 days of self-administration training (B) Extinction: Mean ± SEM number of presses on the previously active lever and on the inactive lever during the first 12 days of extinction, conducted in the absence of cocaine and the presence of tone and light cues previously associated with operant cocaine self-administration. Data are from all rats in Exp. 1 and 2.
Tests for reinstatement: Exp 1
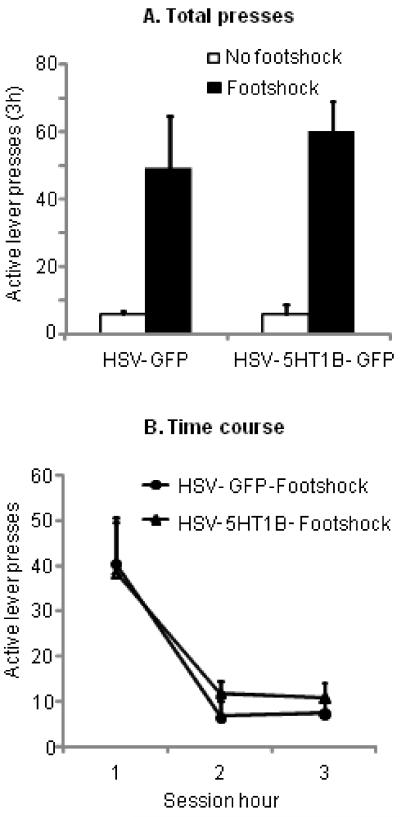
Exposure to footshock stress significantly reinstated lever responding. Viral overexpression of 5-HT1B receptors in the NAccSh had no effect on footshock stress-induced reinstatement of cocaine seeking (Fig. 3). The statistical analysis included the between-subjects factor of Viral Vector (5-HT1B/GFP or GFP) and within-subjects factor of Stress (Footshock or No footshock) and Session Hour. The ANOVA revealed a significant effect of Stress on cocaine reinstatement (F1,15=48.8, p<0.001). There was no interaction of Viral Vector × Stress or Viral Vector × Stress × Session Hour (p>0.05).
Figure 3. Viral infusions into the NAccSh have no influence on footshock-stress induced reinstatement of cocaine seeking.
(A) Total responses: Mean ± SEM number of presses on the active lever in rats that received NAccSh infusions of HSV-GFP or HSV-5-HT1B/GFP three to four days prior to exposure to no footshock or intermittent footshock (0.5 mA for 15 minutes prior to the start of the test session) respectively (n= 8 for HSV-GFP, and n=9 for HSV-5-HT1B/GFP) (B) Time-course: Time-course of data depicted in (A) over hours 1, 2, 3 of the test session. Different from no footshock group, p>0.05
Exp 2
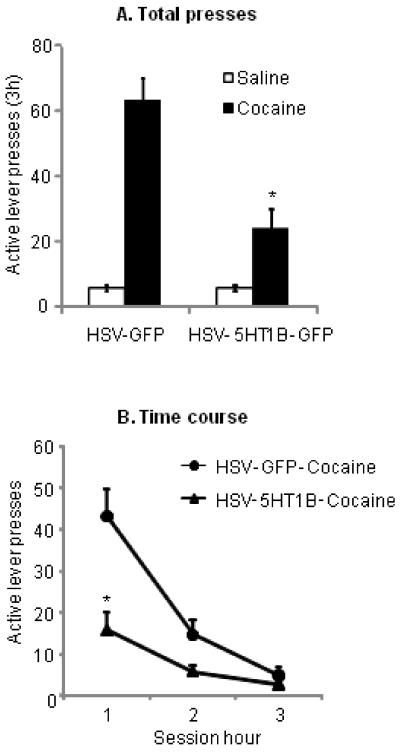
A single injection of cocaine (10 mg/kg i.p.) significantly reinstated operant responding on the active lever. Viral overexpression of 5-HT1B receptors in the NAccSh attenuated cocaine-priming induced reinstatement of cocaine seeking (Fig. 4). The statistical analysis included the between-subjects factor of Viral Vector (5-HT1B/GFP or GFP) and the within-subjects factors of Treatment (Vehicle or Cocaine) and Session Hour. The ANOVA revealed a significant effect of Cocaine Priming (F1,15=89.7, p<0.001) and a significant Viral Vector × Cocaine Priming × Session Hour interaction (F2,30= 17.9, p<0.05). In contrast, in rats with missed NAccSh injections of 5-HT1B-GFP (n=3, 2 in the nucleus accumbens core, 1 ventral to the NAccSh) behavioral responses were similar to controls (Mean ± SEM of active lever presses: HSV-GFP: 63 ± 8, HSV-5HT1B: 72 ± 11; p > 0.05).
Figure 4. Viral infusions into the NAccSh decrease cocaine-priming induced reinstatement of cocaine seeking.
(A) Total responses: Mean ± SEM number of presses on the active lever in rats that received NAccSh infusions of HSV-GFP or HSV-5-HT1B/GFP three to four days prior to systemic injections of vehicle or cocaine (10 mg/kg, ip), respectively (n= 7 for HSV-GFP, and n=10 for HSV-5-HT1B/GFP). Different from HSV-GFP group, p<0.05 (B) Time-course: Time-course of data depicted in (A) over hours 1, 2, 3 of the test session. Different from HSV-GFP group, p<0.05
Social defeat (Exp 3)
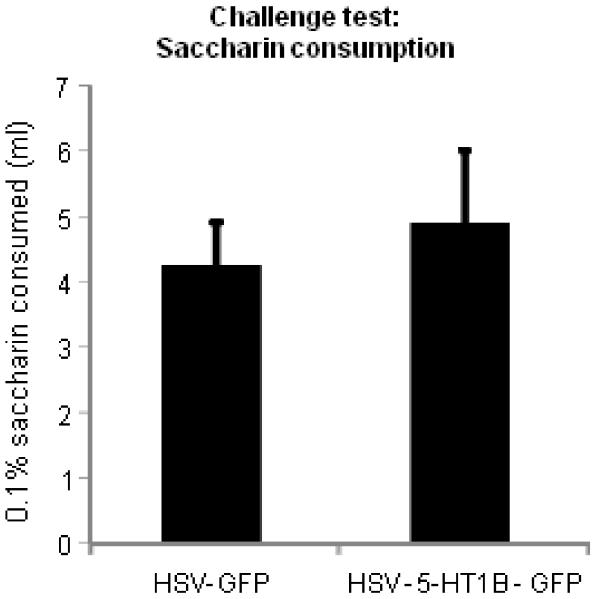
Viral overexpression of 5-HT1B receptors in the NAccSh had no effect on water (data not shown) or saccharin preference following social defeat stress (Fig. 5). The statistical analysis included the between-subjects factor of Viral Vector (5-HT1B/GFP or GFP). The ANOVA revealed no interaction of Viral Vector × Saccharin preference (p>0.05).
Figure 5. Viral infusions into the NAccSh have no influence on saccharin preference following social defeat stress.
Mean ± SEM of saccharin preference in rats that received NAccSh infusions of HSV-GFP (n= 8) or HSV-5-HT1B/GFP (n= 8) prior to test session.
4. Discussion
In the present study, we examined the effect of herpes simplex virus-mediated overexpression of 5-HT1B receptors in the NAccSh on reinstatement of cocaine seeking in a rat operant reinstatement model. We found that NAccSh 5-HT1B receptor overexpression had no influence on reinstatement of cocaine seeking induced by exposure to intermittent footshock. 5-HT1B receptor overexpression also had no effect on saccharin preference following social defeat stress. In contrast, enhanced expression of 5-HT1B receptors in the NAccSh significantly decreased reinstatement of cocaine seeking induced by a non-contingent priming injection of cocaine. These data suggest that serotonergic signaling via 5-HT1B receptors in NAccSh projection neurons is not a critical component of the neuronal circuitry of stress-induced reinstatement of cocaine seeking.
4.1 Role of 5-HT1B receptors in stress-induced reinstatement of cocaine seeking
Our data clearly indicate that 5-HT1B receptor overexpression in the NAccSh had no effect on the reinstatement of cocaine seeking induced by exposure to intermittent footshock. For further confidence in our results, we assessed the effect of enhanced NAccSh 5-HT1B receptor expression on saccharin intake following a distinct stressor-social defeat stress and found that NAccSh 5-HT1B receptor expression had no effect on saccharin intake following social defeat. A methodological issue that should be taken into consideration in the interpretation of these data is the lack of non-defeated control groups. We interpret our results as suggesting that 5-HT1B receptors in NAccSh neurons do not modulate motivation for natural rewards and do not influence the hedonic effects of social defeat stress. Interestingly, it has also been previously demonstrated that 5-HT1B receptor overexpression in the NAccSh fails to alter anxiety-like behaviors in the elevated plus maze (Pentkowski et al., 2012). These results are somewhat surprising since 5-HT1B receptors have been previously demonstrated to play a critical role in the interaction between stress and drugs. Specifically, herpes simplex virus-mediated 5-HT1B receptor overexpression in the NAccSh paired with a mild stressor enhances the psychomotor activating effects of amphetamine, as well as amphetamine sensitization (Ferguson et al., 2009), indicating an interaction between 5-HT1B receptor overexpression in the NaccSh and stress. These data, taken together with our current findings suggest that the 5-HT1B receptors in NAccSh neurons interact with stress to alter the early stages of stimulant-induced plasticity (i.e. sensitization) but not later events after abstinence (i.e. reinstatement).
4.2 Role of 5-HT1B receptors in cocaine priming-induced reinstatement of cocaine seekin
Our finding on the effect of viral overexpression of 5-HT1B receptors on cocaine-priming induced reinstatement of cocaine seeking is consistent with results from a recent report by Pentkowski and colleagues demonstrating that enhancing 5-HT1B receptor-mediated signaling using virus-mediated gene transfer in the NAccSh decreases reinstatement of cocaine seeking induced by a priming injection of cocaine (Pentkowski et al., 2012). It is very unlikely that the decrease in responding is due to non-specific behavioral disruption since NAccSh 5-HT1B receptor overexpression had no effect on footshock stress-induced reinstatement.
Results from some studies where investigators have administered 5-HT1B receptor ligands to examine their effect on cocaine priming-induced reinstatement are consistent with our result. For instance, systemic injection of the 5-HT1B receptor agonists RU 24969 (Acosta et al., 2005) and CP 94253 (Pentkowski et al., 2009) attenuates cocaine priming-induced reinstatement of cocaine seeking. Further, pretreatment with the 5-HT1B receptor antagonist GR 127935 reverses the inhibitory effect of RU 24969 (Acosta et al., 2005). However, results from other studies that have focused on 5-HT1B receptors and cocaine-priming induced reinstatement are inconsistent. Whereas Acosta et al. (Acosta et al., 2005) demonstrated that GR 127935 had no effect on cocaine priming-induced reinstatement, Przegalinski et. al. (Przegalinski et al., 2008; Przegalinski et al., 2007) found that GR 127935 or SB 216641 alone, completely blocked this reinstatement. In addition, while pretreatment with a 5-HT1B receptor antagonist GR 127935 reversed the inhibitory effect of RU 24969 (Acosta et al., 2005), pretreatment with the 5-HT1B receptor antagonist SB 216641 had no influence on CP 94253-induced attenuation of cocaine reinstatement (Przegalinski et al., 2008). A possible explanation for these discrepancies is the different doses of cocaine (2.5-10 mg/kg) used to induce reinstatement, as well as differing selectivities of the ligands used for the 5-HT1B receptor. In addition, complex distribution of 5-HT1B receptors on multiple, different neurons with potentially opposing effects on cocaine seeking behavior can also account for these discrepancies. In contrast to the pharmacological studies, results from the present study taken together with the findings of Pentkowki and colleagues (Pentkowski et al., 2012) indicate that viral overexpression of NAccSh 5-HT1B receptors reliably decreases cocaine-priming induced reinstatement.
4.3 Concluding remarks
Our findings clearly demonstrate that overexpression of 5-HT1B receptors in the NAccSh has no influence on cocaine reinstatement induced by exposure to stressful stimuli, while selectively decreasing the reinstatement of cocaine seeking induced by a priming injection of cocaine. It has been previously demonstrated that 5-HT1B receptor mediated signaling in the NAccSh enhances the rewarding properties of cocaine during active drug taking, but reduces the reinforcing and reinstating properties after forced abstinence or extinction (Pentkowski et al., 2009). Taken together, these results indicate a double dissociation in the effects of NAccSh 5-HT1B receptor-mediated signaling on (i) stress-induced versus cocaine-priming reinstatement of cocaine seeking and (ii) operant cocaine self-administration versus reinstatement of cocaine seeking. These neurochemical double dissociations potentially suggest that (i) that different neuronal mechanisms underlie relapse to cocaine seeking induced by stress versus acute re-exposure to the self-administered drug (ii) distinct mechanisms underlie operant cocaine self-administration and relapse to cocaine seeking. These conclusions are broadly in agreement with previous findings from pharmacological studies in which drug self-administration and reinstatement models were used to access mechanisms underlying drug reinforcement and relapse (De Vries and Shippenberg, 2002; Kalivas and Volkow, 2005; Shalev et al., 2002) and demonstrate that serotonergic interventions may have opposite impacts during active drug use vs. during prolonged abstinence.
Highlights.
Nucleus accumbens shell viral overexpression of 5-HT1B receptors attenuates cocaine priming-induced reinstatement of cocaine seeking
Nucleus accumbens shell viral overexpression of 5-HT1B receptors has no influence on footshock stress-induced reinstatement of cocaine seeking
Nucleus accumbens shell viral overexpression of 5-HT1B receptors has no influence saccharin preference following social defeat stress
Acknowledgments
This work was supported by the National Institutes of Health DA106432 grant to J.F. N. The authors thank Dr. Yavin Shaham for advice on cocaine reinstatement procedures and Dr. Scott Ng-Evans for invaluable technical assistance. The authors also thank Denis Smirnov for proof-reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Boynton FA, Kirschner KF, Neisewander JL. Stimulation of 5-HT1B receptors decreases cocaine- and sucrose-seeking behavior. Pharmacol. Biochem. Behav. 2005;80:297–307. doi: 10.1016/j.pbb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur. J. Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA research monograph. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA research monograph. 1988;84:25–43. [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J. Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J. Subst. Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J. Neurosci. 2002;22:3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong W. Relapse prevention: an emerging technology for promoting long-term drug abstinence. Int. J. Addict. 1994;29:681–705. doi: 10.3109/10826089409047904. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Eskenazi D, Neumaier JF. Increased expression of 5-HT receptors in dorsolateral striatum decreases habitual lever pressing, but does not affect learning acquisition of simple operant tasks in rats. Eur. J. Neurosci. 2011;34:343–351. doi: 10.1111/j.1460-9568.2011.07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Sandygren NA, Neumaier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptibility to amphetamine. Eur. J. Neurosci. 2009;30:1576–1584. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict. Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57:685–700. [PubMed] [Google Scholar]
- Furay AR, McDevitt RA, Miczek KA, Neumaier JF. 5-HT1B mRNA expression after chronic social stress. Behav. Brain Res. 2011;224:350–357. doi: 10.1016/j.bbr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br. J. Psychiatry. 1989;154:348–353. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann. N. Y. Acad. Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52:444–449. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Filip M, Przegalinski E. Role of serotonin (5-HT)1B receptors in psychostimulant addiction. Pharmacol. Rep. 2011;63:1310–1315. doi: 10.1016/s1734-1140(11)70695-8. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–1530. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol. Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog. Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mRNA regulation in two animal models of altered stress reactivity. Biological psychiatry. 2002a;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, McDevitt RA, Polis IY, Parsons LH. Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. Journal of neurochemistry. 2009;111:217–227. doi: 10.1111/j.1471-4159.2009.06313.x. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J. Neurosci. 2002b;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J. Pharmacol. Exp. Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic co-ordinates. 5th edition Elesevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict. Biol. 2009;14:419–430. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL. Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biological psychiatry. 2012;72:396–404. doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Filip M. Effects of serotonin (5-HT)(1B) receptor ligands on cocaine-seeking behavior in rats. Pharmacol. Rep. 2008;60:798–810. [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur. J. Pharmacol. 2007;559:165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological reviews. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and alcohol review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res. 2004;1021:82–91. doi: 10.1016/j.brainres.2004.06.053. [DOI] [PubMed] [Google Scholar]