Abstract
Objective
To determine whether HLA-B27 expression alters the response of bone marrow monocytes (BMMo) from HLA-B27/human β2-microglobulin transgenic (B27-Tg) rats to tumor necrosis factor-α (TNFα), and whether this affects cells involved in bone homeostasis.
Methods
BMMo were treated with receptor activator of NF-κB ligand or TNFα to promote osteoclast formation. Osteoclasts were quantified by counting. Gene expression was measured using quantitative polymerase chain reaction, and protein was detected by enzyme-linked immunosorbent assay, immunoblotting, or immunofluorescence. Effects of endogenously produced cytokines on osteoclast formation were determined with neutralizing antibodies.
Results
TNFα enhanced osteoclast formation 2.5-fold in HLA-B27-expressing cells compared to either wild type or HLA-B7/human β2-microglobulin expressing monocytes. TNFα induced approximately 4-fold upregulation of HLA-B27, which was associated with accumulation of misfolded heavy chains, binding of the ER chaperone BiP, and activation of an ER stress response, which was not seen with HLA-B7. No differences were seen with RANKL-induced osteoclastogenesis. Enhanced interleukin-1α (IL-1α) production from ER stressed B27-Tg BMMo was found to be necessary and sufficient for enhanced osteoclast formation. However, B27-Tg BMMo also produced more interferon-β (IFNβ), which attenuated the effect of IL-1α on osteoclast formation.
Conclusions
HLA-B27-induced ER stress alters the response of BMMo from B27-Tg rats to TNFα, which is associated with enhanced production of IL-1α and IFNβ, cytokines that exhibit opposing effects on osteoclast formation. The altered response of cells expressing HLA-B27 to pro-inflammatory cytokines suggests that this MHC class I allele may contribute to the pathogenesis of spondyloarthritis and its unique phenotype through downstream effects involving alterations in bone homeostasis.
Keywords: Rodent, MHC, inflammation
INTRODUCTION
The role of HLA-B27 in the pathogenesis of spondyloarthritis remains poorly understood (1). Most hypotheses focus on HLA-B27 as an upstream factor that is envisioned to trigger an aberrant immune response – either adaptive or innate - resulting in chronic inflammation. For example, peptide-loaded HLA-B27-β2-microglobulin (β2m) complexes could be targeted by autoreactive CD8+ T cells (2), or β2m-free heavy chain homodimers might trigger the survival and activation of CD4+ Th17 T cells (3). HLA-B27 and human β2m expression in transgenic (B27-Tg) rats has been linked to dendritic cell (DC) dysfunction (4) and increased apoptosis (5, 6), that could contribute to enhanced DC-mediated activation of CD4+ Th17 T cells (7) and/or failure to induce tolerance. In addition, the propensity of HLA-B27 heavy chains to misfold and generate endoplasmic reticulum (ER) stress (8, 9), initiates intracellular signals through the unfolded protein response (UPR) that converge with Toll-like receptor (TLR) signaling pathways to promote cytokine production, including interleukin (IL)-23 (IL-23) (10–12). Systemic IL-23 expression has recently been shown to activate an unusual population of enthesis-resident T cells in mice that produce pro-inflammatory cytokines such as IL-17 and IL-22, providing an explanation for some of the unique anatomical features of the spondyloarthritis phenotype (13). The relative contribution of these mechanisms to human disease remains an area of intense investigation.
Despite evidence implicating HLA-B27 as an upstream factor in spondyloarthritis, its tendency to misfold and generate ER stress, particularly when upregulated (8, 9, 14), raises the possibility that HLA-B27 may have a downstream impact on disease pathogenesis by altering the response of cells to pro-inflammatory cytokines or other factors.
One aspect of spondyloarthritis that is poorly understood is the excessive bone loss prominent in vertebral bodies, juxtaposed with pathologic bone formation that occurs along the spine of many patients with ankylosing spondylitis (AS) (15–17). One of the several features of spondyloarthritis reproduced in B27-Tg rats is trabecular bone loss in vertebral bodies and long bones (18). Since bone integrity is regulated by osteoclasts and osteoblasts we asked whether HLA-B27 expression could influence osteoclast formation in response to known stimulators of osteoclastogenesis, receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-alpha (TNFα).
MATERIALS and METHODS
Animals
Rats were housed in AAALAC-approved facilities on the Bethesda NIH campus, and all animal experiments were pre-approved by the NIAMS Animal Care and Use Committee. Lewis HLA-B*27:05 and human β2m transgenic rats (B27-Tg) were generated by backcrossing the 33-3 transgene locus from Dark Agouti (DA) B27-Tg, onto the Lewis background for over 10 generations. The 33-3 transgene locus contains 55 copies of HLA-B27 and 66 copies of human β2m (19). Lewis HLA-B*07:02 and human β2m transgenic (B7-Tg) rat breeders carrying the 120-4 transgene locus were obtained from Joel Taurog (Dallas, TX). The 120-4 locus contains 26 copies of HLA-B7 and 5 copies of human β2m (20). Hemizygous B27-Tg rats were used in this study, while homozygous B7-Tg rats were used (i.e. 52 copies of HLA-B7 and 10 copies of human β2m). Transgene negative littermates or Lewis rats purchased from Taconic (Rockville, MD) were used as wild type (WT).
Bone marrow-derived monocytes and osteoclast development
Bone marrow was obtained from tibias and femurs of euthanized 6–9 week old rats. Mononuclear cells were isolated using Lympholyte-Rat (Cedarlane Laboratories Inc., Burlington, NC), washed twice, and re-suspended at 5×106 cells/ml in Gibco DMEM high glucose medium (Invitrogen, Grand Island, NY) containing 10% FCS (Invitrogen), 10−8 M 1α,25-dihydroxyvitamin D3 (Enzo Life Sciences, Inc., Farmingdale, NY), and 20 ng/ml rat macrophage colony stimulating factor (M-CSF) (Peprotech Inc., Rocky Hill, NJ), and cultured for two days at 37°C in a 5% CO2 humidified atmosphere. To evaluate effects of cytokines on osteoclast formation, non-adherent bone marrow monocytes (BMMo) were collected, adjusted to 106 cells/ml in media, and seeded at 105 cells per well in 96 well plates with M-CSF (20 ng/ml final concentration) to examine the effects of RANKL (receptor activator of NF-κB ligand) and TNFα (tumor necrosis factor-alpha) on osteoclast differentiation. Cytokines were obtained from Peprotech Inc. (Rocky Hill, NY), and used at final concentrations indicated in the figures. BMMo were treated with RANKL for 3 days, or with TNFα for 5 days, which were established in preliminary experiments as optimal conditions for osteoclast formation. Mature osteoclasts were visualized by tartrate resistant acid phosphatase (TRAP) staining (Kamiya Biomedical Company, Seattle, WA), photographed, and quantitated by counting as TRAP-positive cells with ≥ 3 nuclei normalized to area.
Reagents
Neutralizing antibodies were goat anti-rat IL-1α (AF500, R&D Systems, Minneapolis, MN), hamster anti-rat IL-1β (4-7012, eBioscience, San Diego, CA), and rabbit anti-rat IFNβ (CL 9143AP, Cedarlane Laboratories Inc., Burlington, NC). All antibodies were used at a final concentration of 1 µg/ml during the entire 5 days of TNFα induced osteoclast development. Thapsigargin (Sigma-Aldrich, St. Louis, MO) was used at a final concentration of 300 nM and tunicamycin (Sigma-Aldrich) at 10 µg/ml.
RNA isolation and quantitation
For evaluation of gene expression, 106 cells per sample were collected by centrifugation (400 × g for 5 min), lysed in Trizol (Life Technologies, Grand Island, NJ) and stored at −80°C. RNA was isolated according to the manufacturers protocol. Complementary DNA (cDNA) was generated using iScript (Biorad, Hercules, CA), and quantitative polymerase chain reaction (qPCR) measurements of specific genes were carried out using a MyiQ cycler with the SsoFast EvaGreen Supermix (Bio-Rad Laboratories, Hercules, CA) with primers (500 nM) and cDNA corresponding to 20–50 ng of starting RNA. Three house keeping genes were used for normalization of target gene expression levels: peptidylprolyl isomerase (Ppia), hypoxanthine phosphoribosyl transferase (Hprt1), and TATA binding protein (Tbp). Fold changes were calculated by comparing normalized CT values of treated to normalized untreated samples using the qBasePlus software (Biogazelle, Ghent, Belgium). For determining Xbp1 mRNA splicing, reverse transcriptase PCR products were separated on 3% agarose gels and visualized with ethidium bromide (Bio-Rad) under ultraviolet light. Primer sequences are listed below.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| Hprt1 | GAC TTT GCT TTC CTT GGT CA | AGT CAA GGG CAT ATC CAA CA |
| Ppia | CTG GTG GCA AGT CCA TCT AC | CCC GCA AGT CAA AGA AAT TA |
| Tbp | CGA TAA CCC AGA AAG TCG AA | AGA TGG GAA TTC CAG GAG TC |
| HLA-B | GTC CAC CGT CCC CAT CG | ACG CAG CCT GAG AGT AGC |
| Xbp1 | ACA CGC TTG GGG ATG AAT GC | CCA TGG GAA GAT GTT CTG GG |
| Hspa5 (Bip) | TCA GCC CAC CGT AAC AAT CAA G | TGT CTT CCT CAG CAA ACT TCT CG |
| Il1a | ACA GTT CTG CCA TTG ACC AT | TGT TTG ATG TTG CTG ACA CC |
| Ifnb1 | TGA ATG GAA GGC TCA ACC TCA G | CGT GGA TGT CAC CCA AGT CAA TC |
Immunoblotting and immunoprecipitation
BMMo (2.5 × 107 cells) were harvested, re-suspended in 1 ml ice cold PBS with 10 mM methyl methanethiosulfonate (MMTS) (Thermo Scientific, Rockford, IL) on ice for 20 min to prevent spontaneous sulfhydryl bond formation and reduction. Cells were then spun down and lysed (500 µl per 107 cells) for 30 min on ice in 20 mM Tris, pH 7.8, 100 mM NaCl, 10 mM EDTA, 1% Triton and the Halt Protease Inhibitor Cocktail (Thermo Scientific, Rockford, IL). Nuclei were removed by centrifugation at 13,000 × g for 5 min, and supernatants were collected and used for immunoprecipitation or SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. For SDS-PAGE, lysates were diluted 1:1 with Tris-Glycine SDS sample buffer (Novex, Invitrogen, Carlsbad, CA) with or without NuPage Sample Reducing Agent (Invitrogen), and subjected to electrophoresis on 4–20% Tris-glycine gels (Novex) followed by immunoblotting. The following primary antibodies were used: HC10 to detect HLA class I heavy chains, rabbit anti-BiP (ab21685, Abcam, Cambridge, MA), and mouse anti-GAPDH (sc-32233, Santa Cruz). Appropriate species-specific secondary antibodies were alkaline phosphatase-conjugated (Southern Biotech, Birmingham, AL), and the 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Thermo Scientific, Rockford, IL) was used to visualize proteins. HLA-B27 immunoprecipitation from 500 µl of cell lysate was performed as described previously (21). Immunoprecipitates were subjected to SDS-PAGE and immunoblotting as described above, except that HLA-class I heavy chains were detected using 3B10.7 (22).
Immunofluorescence
Immunofluorescence staining was used to identify BiP and HLA-B27-expressing cells during osteoclast development. Four days after the addition of RANKL or TNFα to BMMo, cells were fixed with 4% formaldehyde, washed twice with BD Perm/Wash buffer (BD Biosciences, San Jose, CA) and then incubated in the same buffer for 15 min at 4°C to permeabilize the cells. Primary antibodies (anti-BiP, ab21685 [Abcam, Cambridge, MA] or HC10 (23)) were added at 1 µg/ml and cells were incubated for an additional hour at 4°C. Cells were then washed 3 times with Perm/Wash buffer and incubated with secondary antibodies (Alexa Fluor 594-labeled chicken anti-rabbit; Invitrogen, Grand Island, NY, or FITC-labeled chicken anti-mouse IgG; LifeSpan Biosciences Inc., Seattle, WA) diluted 1:100 in Perm/Wash buffer for 30 min at 4°C in the dark. Cells were washed again and visualized with an Axiovert Zeiss 40 CFL fluorescence microscope at 20× magnification.
Statistical analysis
Values are expressed as means with standard deviation (SD) depicted by error bars, unless otherwise noted. Statistical analysis was performed using Student’s t test. P values less than 0.05 were considered significant. For ratios, the mean and 95% confidence intervals are shown.
RESULTS
HLA-B27 expression promotes osteoclast formation
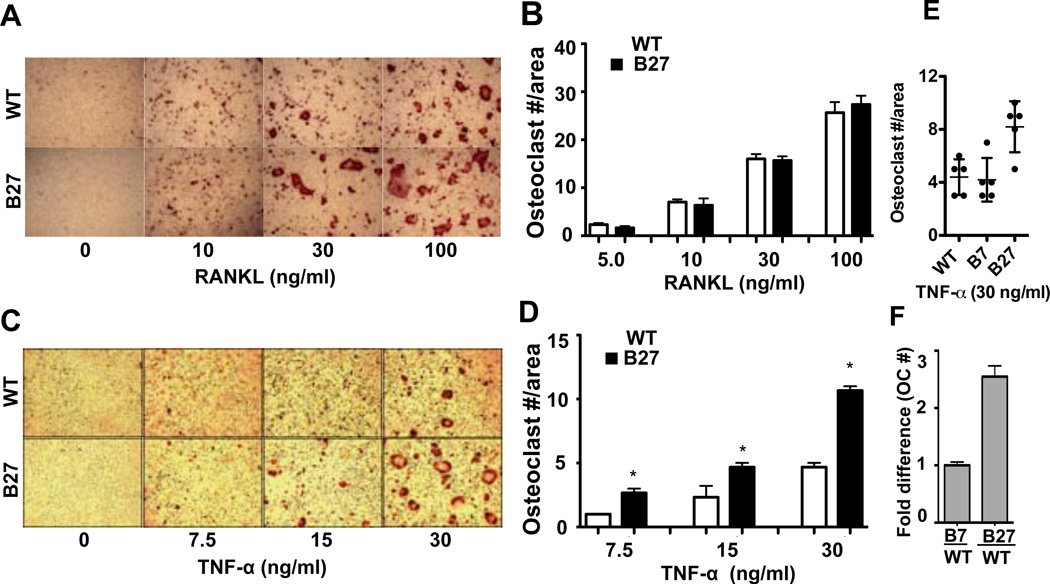
To determine whether HLA-B27 has an influence on osteoclast development, BMMo from WT and B27-Tg rats were differentiated with RANKL or TNFα. There was no difference in the number of osteoclasts formed in the presence of RANKL (Figure 1A, B), although we observed a trend toward larger osteoclasts with more nuclei in the presence of HLA-B27 and HLA-B7 (Figure 1A and unpublished observations). In contrast, TNFα treatment consistently resulted in greater osteoclast formation in BMMo derived from B27-Tg animals (Figure 1C–F). Representative experiments are shown in Figure 1C–E, with the average fold change from several experiments shown in Figure 1F. The average increase in osteoclastogenesis in HLA-B27 expressing cells treated with TNFα was 2.5 fold (Figure 1F). Experiments with BMMo derived from B7-Tg rats used as a control for HLA class I overexpression revealed no difference in osteoclast formation compared with WT rats (Figure 1E, F), indicating the effect is specific for HLA-B27. Osteoclasts formed from WT and B27-Tg BMMo were active and exhibited similar resorption patterns on calcium phosphate-coated slides, indicating they were functional (unpublished observations).
Figure 1.
Augmentation of TNFα-induced osteoclast formation by HLA-B27. BMMo from WT and B27-Tg (B27) rats were treated with M-CSF and (A, B) increasing concentrations of RANKL for three days, or (C, D) TNFα for five days. A, C, TRAP staining from a representative experiment (original magnification 5×). B, D, Number of osteoclasts, defined as TRAP positive cells with ≥ 3 nuclei per unit area. E, Representative results from an experiment comparing TNFα-induced osteoclast formation with BMMo derived from WT, B7-Tg (B7) and B27-Tg (B27) animals. P < 0.05 for B27 vs. B7 or WT. F, Fold increase (compared to WT) in number of osteoclasts induced by TNFα (30 ng/ml) using BMMo derived from B7-Tg (B7) and B27-Tg (B27) animals. Values in B and D represent means with SD indicated by error bars of triplicate wells from a representative experiment. Results in F represent averages, with 95% confidence intervals indicated by error bars, from 3 (B7) and 6 (B27) independent experiments. * = P < 0.05.
The roles of IL-1α and IFNβ in HLA-B27-induced osteoclast formation
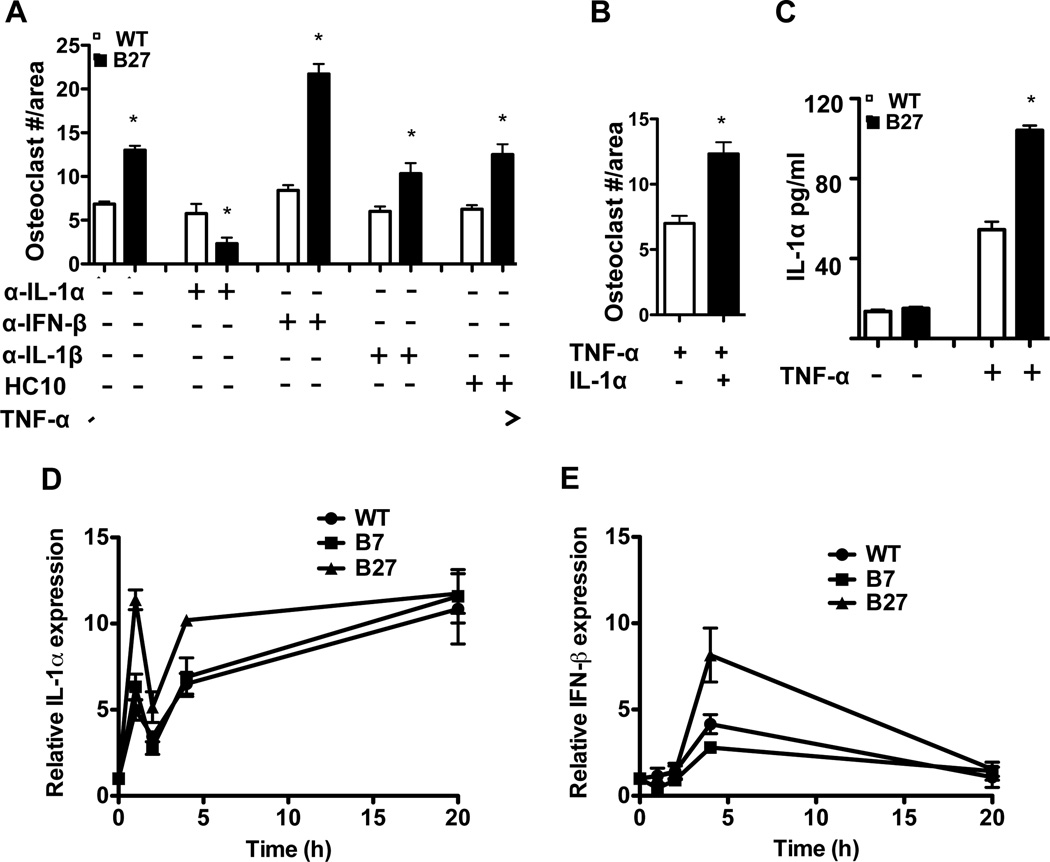
IL-1α and IFNβ have been shown to regulate osteoclast formation. IFNβ is a potent inhibitor (24), while IL-1α promotes TNFα induced osteoclastogenesis (25). To determine whether these cytokines were involved in mediating the effect of HLA-B27 expression on TNFα-induced osteoclastogenesis, each cytokine was blocked with a neutralizing antibody. Blocking IL-1α completely inhibited the effect of HLA-B27 on TNFα-induced osteoclastogenesis, whereas an IL-1β neutralizing antibody had no effect (Figure 2A). The addition of HC10, which recognizes HLA-B27 homodimers as well as free heavy chains, also had no effect on osteoclast formation (Figure 2A). To test whether additional IL-1α was sufficient to promote osteoclastogenesis, IL-1α was added to TNFα-treated WT BMMo, where it enhanced osteoclast formation approximately 2-fold (Figure 2B). HLA-B27-expressing cells produced more immunoreactive IL-1α protein (Figure 2C) and exhibited greater induction of Il1a mRNA in response to TNFα (Figure 2D), consistent with this cytokine being responsible for the effect of HLA-B27 on osteoclastogenesis.
Figure 2.
Enhanced TNFα-induced osteoclast formation in HLA-B27-expressing cells is IL-1α dependent and inhibited by IFNβ. A, Neutralizing antibodies (1 µg/ml) against IL-1α (α-IL-1α), IFNβ (α-IFNβ), IL-1β (α-IL-1β), or HLA-B class I heavy chains (HC10) were added to WT and B27-Tg BMMo during TNFα (30 ng/ml) induced osteoclastogenesis. Osteoclast formation was quantitated as described for Figure 1. B, The effect of IL-1α (0.1 ng/ml) on TNFα (30 ng/ml) induced osteoclast formation in WT BMMo. C, IL-1α production in culture supernatants from TNFα treated (30 ng/ml for 3 days) WT and B27 BMMo as measured by ELISA. D, E, WT and B27-Tg BMMo were treated with TNFα (30 ng/ml) for varying lengths of time and (D) Il1a and (E) Ifnb1 mRNA expression was measured. Data are representative of a minimum of three independent experiments with each performed in triplicate. Data points show the mean ±SD. * = P < 0.05.
In contrast to the results with IL-1α, neutralizing IFNβ further enhanced osteoclastogenesis in cultures of HLA-B27 expressing cells (Figure 2A). This effect was associated with a several-fold induction of Ifnb1 mRNA in HLA-B27-expressing cells following exposure to TNFα, whereas WT and HLA-B7-expressing BMMo exhibited only a small increase (Figure 2E). We have not found a reliable ELISA for rat IFNβ, and thus are unable to quantitate protein production in BMMo cultures. Taken together with the results for IL-1α, these data suggest that HLA-B27-expressing cells produce more IL-1α and IFNβ with opposing effects on osteoclastogenesis, although under these experimental conditions the net result is greater osteoclast formation.
TNFα induces ER stress in HLA-B27-expressing BMMo
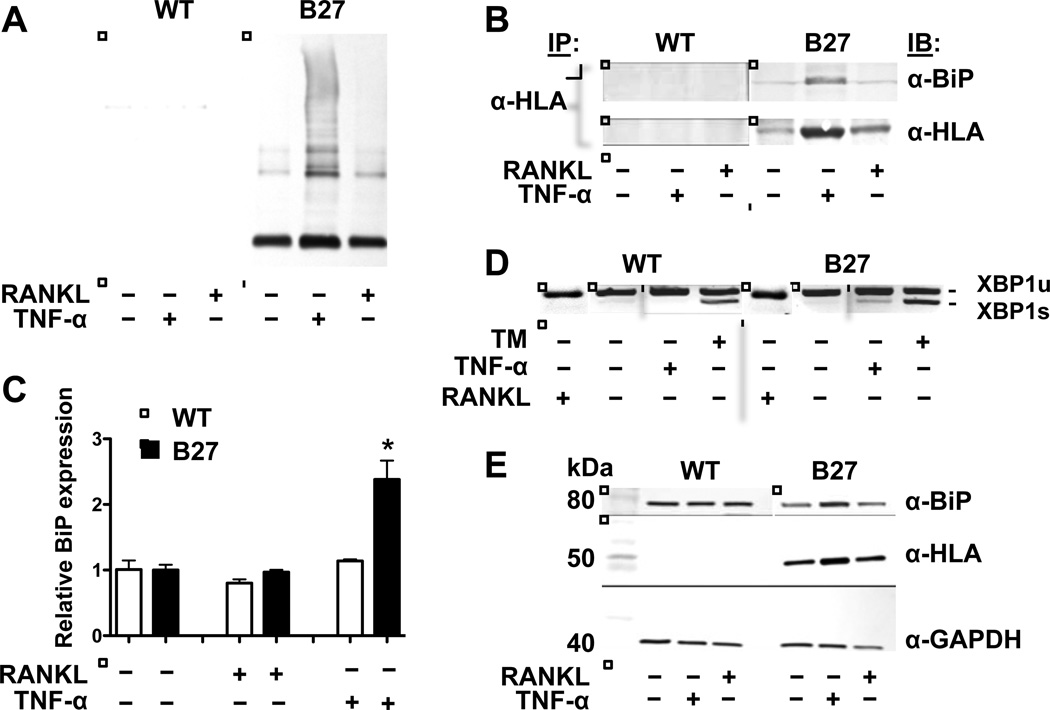
To explore differences between B27-Tg and WT BMMo that might contribute to greater TNFα induced osteoclastogenesis, we compared effects of TNFα and RANKL on HLA-B27 expression and misfolding. TNFα treatment resulted in 4-fold upregulation of HLA-B27 mRNA, with a comparable increase in heavy chain expression, while RANKL had no effect (Figure 3A, B, E and data not shown). TNFα-induced HLA-B27 heavy chains accumulated as disulfide-linked dimers and multimers, as well as conventional monomers (Figure 3A). There was also an increase in co-immunoprecipitation of BiP with HLA-B27 heavy chains (Figure 3B).
Figure 3.
HLA-B27 expression, misfolding, and activation of the unfolded protein response in BMMo cultures. WT and B27-expressing BMMo were treated with M-CSF (20 ng/ml), and then without or with RANKL (100 ng/ml) or TNFα (30 ng/ml) for 3 days (A, B, E) or 20 hours (C, D), followed by harvest. A, Cell lysates were subjected to non-reducing SDS-PAGE and HLA-B27 heavy chains visualized by immunoblotting with the antibody HC10. The lowest band represents monomers, while the higher molecular weight material constitutes dimers and multimers. B, HLA-B27 heavy chains were immunoprecipitated using HC10 and subjected to SDS-PAGE under reducing conditions, then visualized by immunoblotting with 3B10.7 (α-HLA). Co-preciptating BiP was visualized by immunoblotting (α-BiP). C, BiP mRNA expression is shown relative to untreated cells. Bars show the mean ±SD of three experiments. D, Xbp1 mRNA splicing in untreated and RANKL or TNFα treated cells. Tunicamycin (TM) treated cells serve as a positive control. The spliced form of Xbp1 mRNA (XBP1s) runs slightly faster than the unspliced form (XBP1u) due to the removal of 26 nucleotides by activated inositol-requiring 1 alpha (IRE1α). E, Immunoblots of cell lysates subjected to SDS-PAGE under reducing conditions, visualizing BiP (α-BiP), HLA-B27 (α-HLA; HC10), and GAPDH (α-GAPDH) as a loading control.
The accumulation of disulfide-linked HC10-reactive forms of HLA-B27 with enhanced BiP binding in response to TNFα is indicative of heavy chain misfolding, and reminiscent of previous results demonstrating that IFNγ upregulation of HLA-B27 exacerbates misfolding in macrophages (9). This prompted us to look for evidence of ER stress. HLA-B27-expressing BMMo exhibited increased Bip mRNA and protein, and Xbp1 mRNA splicing (Figure 3C–E) when treated with TNFα, but not RANKL. These data demonstrate that HLA-B27-expressing BMMo exhibit ER stress and activate the UPR when treated with TNFα, but not RANKL.
ER stress upregulates IL-1α and IFNβ
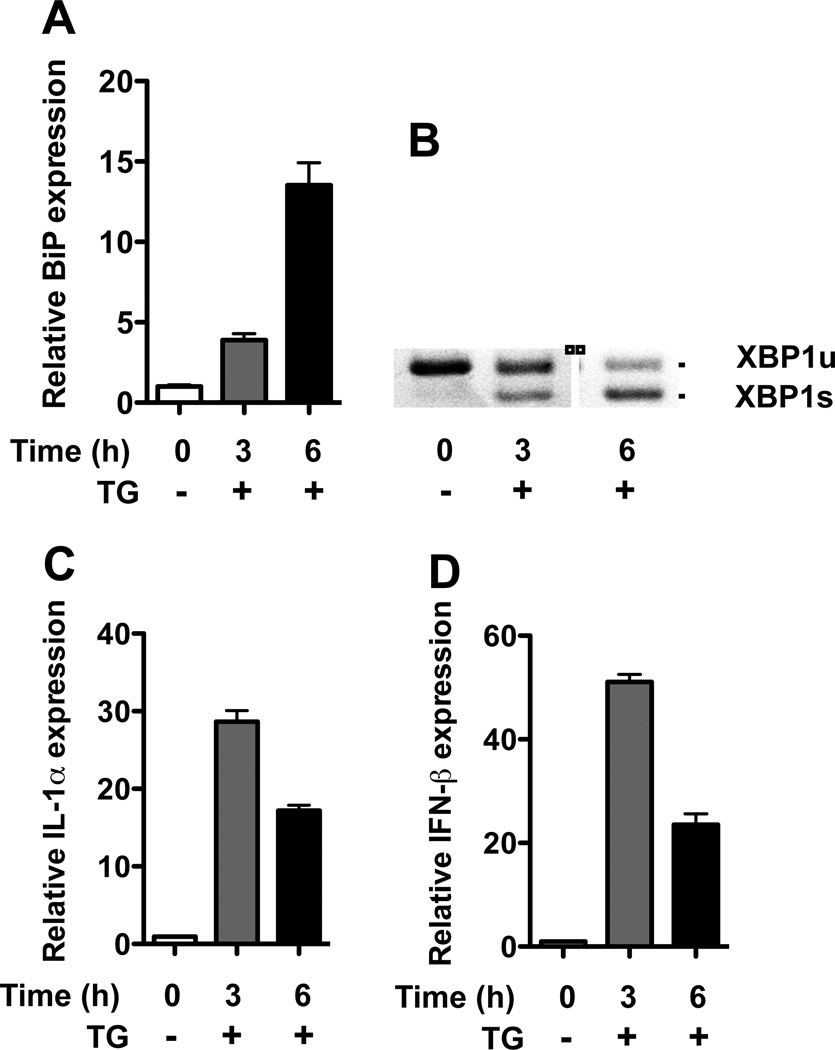
To determine whether ER stress alone is sufficient to induce IL-1α and IFNβ expression, BMMo from WT rats were treated with thapsigargin, an inhibitor of the sarco/endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA) that prevents autophagosome-lysosome fusion and induces ER stress (26). UPR activation resulting in Bip mRNA upregulation and Xbp1 splicing was readily apparent within 3 hours of treatment of cells (Figure 4 A, B), and was accompanied by strong upregulation of Il1a and Ifnb1 mRNAs (Figure 4 C, D).
Figure 4.
ER stress induces Il1a and Ifnb1 mRNA in BMMo. BMMo derived from WT rats were treated with thapsigargin (TG) (300 ng/ml) for 3 and 6 hours. A, Expression of BiP mRNA relative to untreated cells. B, Xbp1 mRNA splicing. C, D, Expression of Il1a (IL-1α) (C) and Ifnb1 (IFNβ) (D) mRNA relative to untreated cells.
Cellular localization of TNFα-induced HLA-B27 and BiP
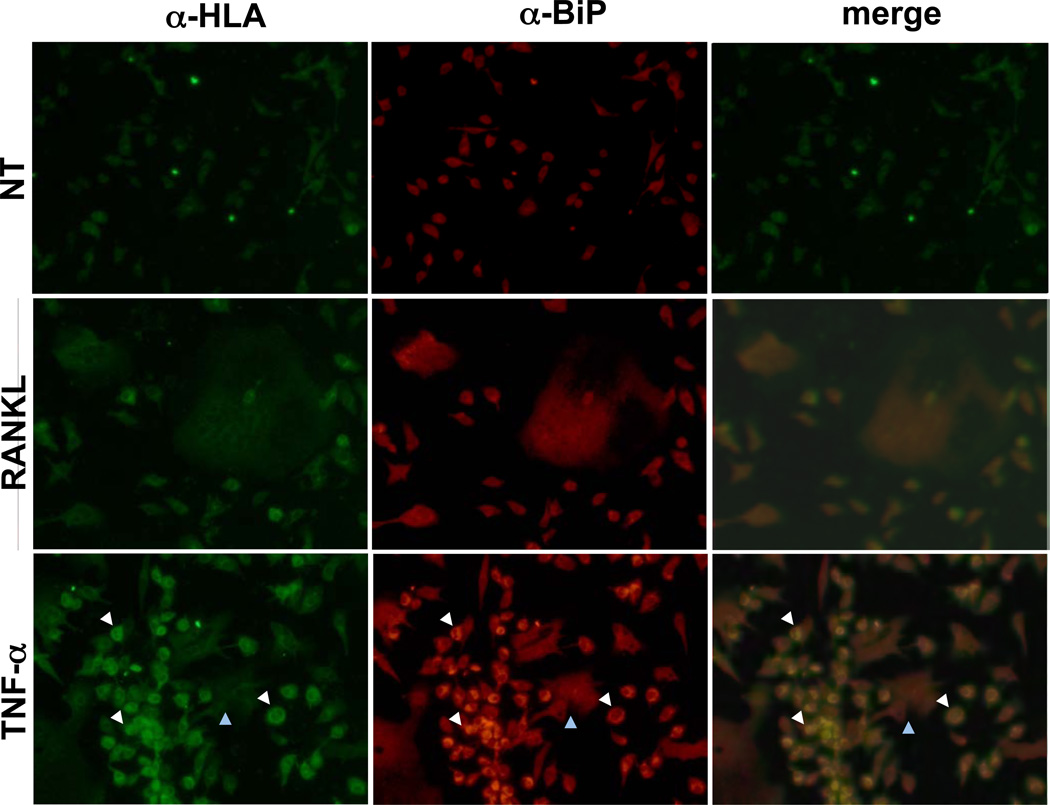
To determine whether BMMo (osteoclast precursors) or mature osteoclasts were exhibiting effects of TNFα on HLA-B27 expression and UPR activation, BMMo treated with RANKL or TNFα were assessed by immunofluorescence microscopy for BiP and HLA class I heavy chain expression. Increased BiP and HLA class I heavy chain staining was noted only with TNFα treatment and not RANKL, and was most prominent in small round/oval cells with single nuclei (BMMo; white arrows), rather than large osteoclasts with multiple nuclei (blue arrows) (Figure 5). In contrast, RANKL had no effect on HLA-B27 or BiP staining in B27-Tg cells, and neither TNFα nor RANKL had any effect on BiP staining in WT BMMo (Figure 5). These data are consistent with the immunoblot analyses shown previously (Figure 3), and further indicate the predominant effect of TNFα on HLA-B27-induced ER stress is in osteoclast precursors.
Figure 5.
Immunofluorescence visualization of HLA-B27 and BiP-expressing cells. BMMo were treated with M-CSF (20 ng/ml) alone (NT), or M-CSF plus RANKL (100 ng/ml) or TNFα (30 ng/ml) for 3 days. Cells were fixed with formaldehyde and permeabilized, then stained with antibodies against BiP (α-BiP) or HC10 (α-HLA). Texas Red or FITC-labeled secondary antibodies were used to detect α-BiP and HC10, respectively. White arrows identify monocytes showing increased HC10 and BiP staining with TNFα treatment, and blue arrows identify an osteoclast. Original magnification 20×.
DISCUSSION
The propensity of HLA-B27 to misfold, generate ER stress, and activate the UPR has the potential to exert biological effects in many different cell types expressing MHC class I molecules. We previously documented effects of HLA-B27 misfolding in rat macrophages where UPR activation after upregulation of HLA-B27 with IFNγ promotes increased expression of IL-23 and IFNβ in response to TLR agonists (1, 8, 10). In earlier work we found no biochemical evidence, such as heavy chain oligomerization or prolonged BiP binding, to suggest that HLA-B7 misfolds (1). Furthermore, in these studies upregulation of HLA-B7 did not activate the UPR, even with heavy chain expression comparable to that of HLA-B27 (8, 10). Since these earlier comparisons were made with IFNγ-treated macrophages, we also examined HLA-B7-expressing monocytes treated for 20 hours with TNFα (e.g. Fig. 2D, E), and found no UPR activation (unpublished observations). Thus, comparable overexpression in rat cells of another HLA-B allele that is closely related to HLA-B27 but does not misfold, does not appear to cause ER stress, at least under the conditions examined to date.
Here, we show that HLA-B27-expressing rat monocytes cultured with M-CSF and stimulated with TNFα alone, also exhibit ER stress and UPR activation linked to HLA-B27 misfolding, with enhanced expression of IL-1α and IFNβ, while these effects are not seen with HLA-B7. More importantly, the autocrine/paracrine effects of IL-1α result in enhanced differentiation of monocytes into osteoclasts. Interestingly, the effect of TNFα is almost doubled when IFNβ is neutralized (Figure 2A). Taken together, these results indicate that HLA-B27-expressing rat monocytes produce more pro- and anti-osteoclastogenic cytokines when stimulated with TNFα, with IL-1α promoting the effects of TNFα, with IFNβ attenuating the stimulatory effect of IL-1α. The counter-regulatory effect of IFNβ is insufficient to prevent the pro-osteoclastogenic effect of IL-1α, at least under current experimental conditions.
ER stress-induction of IFNβ has been shown to occur via the UPR transcription factor XBP1 (10). The active transcription factor produced from the spliced Xbp1 mRNA (XBP1s) binds to an enhancer to promote transcription of the Ifnb gene (27). The greatest effect of ER stress on Ifnb mRNA induction is seen when macrophages are exposed to TLR4/3 agonists that already induce IFNβ, such as lipopolysaccharide (LPS) and double stranded RNA (10, 27). However, even in the absence of TLR agonists, HLA-B27-expressing rat macrophages exhibit low-level (~3-fold) Ifnb mRNA upregulation when stimulated with IFNγ (1). Our current results extend these previous findings in an important way by showing TNFα can act as an inducer of low-level IFNβ expression in monocytes experiencing ER stress due to HLA-B27 expression. This response is fundamentally different from what is seen in WT or HLA-B7-expressing cells, where Ifnb mRNA is increased only 2–4-fold) in response to TNFα (Figure 2E). Moreover, our results establish that even a low-level of IFNβ produced from HLA-B27-expressing cells can be biologically significant as an inhibitor of osteoclastogenesis.
Effects of ER stress on IL-1α production have not, to our knowledge, been reported previously. We had observed Il1a mRNA induction in a pilot experiment using tunicamycin (unpublished observations), which activates the UPR by inhibiting protein glycosylation. We show here that thapsigargin has a similar effect on Il1a mRNA, indicating that Il1a induction is a consequence of ER stress, and unlikely due to some other effect of HLA-B27 expression. We have not further investigated the mechanism of Il1a upregulation, although enhanced ER stress-induced NF-κB and/or AP-1 activation could contribute. Interestingly, ER stress has been shown to activate the NLRP3 inflammasome and increase IL-1β production by a mechanism independent of classical UPR activation (28). However, in contrast to IL-1α, we do not see increased IL-1β production by HLA-B27-expressing rat monocytes (unpublished observations), so inflammasome activation seems unlikely to be playing a role.
Opposite effects of IL-1α and IFNβ on osteoclast development have been documented previously (24, 25). Kobayashi et al. demonstrated IL-1α was required for the formation of fully functional osteoclasts induced by TNFα in a RANKL/RANK-independent mechanism (25). The inhibitory effect of IFNβ on osteoclast formation was demonstrated in the context of RANKL-induced osteoclast formation, where small amounts of IFNβ induced by RANKL and signaling through an autocrine/paracrine loop, inhibit c-Fos expression which is essential for osteoclast formation (24). We have not further investigated the mechanism by which IFNβ inhibits TNFα-induced osteoclastogenesis in HLA-B27-expressing rat BMMo, but since TNFα also induces c-Fos, it is plausible to expect that the mechanism would be similar. It is important to note that in our system, TNFα-induced IFNβ does not appear to be limiting osteoclast formation in WT BMMo, since there is little difference with and without α-IFNβ (Figure 2A) and little IFNβ mRNA induction in WT or HLA-B7-expressing cells (Figure 2E).
Trabecular bone loss resulting in osteopenia and osteoporosis is a significant problem in SpA and particularly in AS (17, 29–31). Bone loss often begins early in disease, prior to physical immobilization (15). Osteoclastic bone resorption occurs in the sacroiliac and zygapophyseal joints of patients with AS (32, 33), and is even present in long-standing disease where osteoclasts can be found adjacent to fibrous tissue (34). It is most severe in the spine where it increases the risk of vertebral fractures, and in many individuals is anatomically juxtaposed with areas of abnormal bone formation such as syndesmophytes. TNFα inhibition significantly increases bone mineral density in SpA (35) and AS (36), implicating this cytokine in bone loss, while effects of TNF inhibitors on syndesmophyte growth remain unclear. Indeed, whether or not trabecular bone loss is coupled with bone formation is the subject of much debate (37).
B27-Tg rats carrying the 33-3 transgene locus serving as a model for SpA-like disease also exhibit substantial loss of trabecular bone in vertebral bodies, large bones of the leg, and reduced bone strength (18). Bone loss in this model occurs primarily as a consequence of increased bone resorption rather than reduced formation (38), and is associated with increased RANKL expression relative to OPG (39). TNFα also contributes to the inflammatory disease phenotype in B27-Tg rats, although a role in bone loss has not been established. Our results raise the possibility that HLA-B27 expression contributes to TNFα-induced bone loss in this model and in human SpA including AS, by promoting local production of IL-1α. Since these transgenic rats have multiple copies of the HLA-B27 transgene, they overexpress HLA-B27 heavy chain relative to human cells. Although results with HLA-B7 indicate that heavy chain misfolding is required to generate ER stress in this model, it may still occur more readily than in humans due to greater expression. It is noteworthy that Feng et al. have recently shown that peripheral blood mononuclear cells (PBMC) from HLA-B27 positive AS patients, but not healthy controls, generate sufficient ER stress to activate the UPR when treated with IFNγ (14). In previous studies, HLA-B27-expressing PBMC-derived macrophages from AS patients did not exhibit UPR activation when treated with IFNγ (40, 41). This may have been due to a lack of (40) or insufficient (41) upregulation of HLA-B in macrophages derived ex vivo. It is also possible that monocytes and macrophages respond differently to cytokine treatments. Indeed, monocyte to macrophage differentiation is itself associated with UPR activation, which in turn protects cells against further ER stress (42). It will be important to better characterize the response of PBMCs and isolated monocytes from HLA-B27 positive individuals to cytokines that upregulate MHC class I, including TNFα.
In conclusion, we demonstrate that HLA-B27 alters the response of rat bone marrow-derived monocytes to TNFα, and promotes osteoclastogenesis by causing ER stress and enhancing IL-1α production. This also increases production of the anti-osteoclastogenic cytokine, IFNβ. While the net effect under the conditions studied here is to promote osteoclastogenesis, this balance could be altered in vivo by other mediators of inflammation. These findings suggest that this unusual MHC class I molecule may exert downstream effects in the pathogenesis of spondyloarthritis with implications for bone remodeling and the unique spondyloarthritis phenotype.
ACKNOWLEDGEMENT
We thank Kristina Zaal and Evelyn Ralston, NIAMS, for their help with immunofluorescence microscopy
This work was supported by NIH grants R01 AR046177, AR048372, and the NIAMS Intramural Research Program, Z01 AR041184.
Non-standard abbreviations used in this paper
- β2m
β2-microglobulin
- BMMo
bone marrow monocyte
- ER
endoplasmic reticulum
- hβ2m
human β2-microglobulin
- UPR
unfolded protein response
- TG
thapsigargin
- XBP1
X box binding protein-1
REFERENCES
- 1.Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233:181–202. doi: 10.1111/j.0105-2896.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin R, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 3.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacquard-Bouder C, Chimenti MS, Giquel B, Donnadieu E, Fert I, Schmitt A, et al. Alteration of antigen-independent immunologic synapse formation between dendritic cells from HLA-B27-transgenic rats and CD4+ T cells: selective impairment of costimulatory molecule engagement by mature HLA-B27. Arthritis Rheum. 2007;56:1478–1489. doi: 10.1002/art.22572. [DOI] [PubMed] [Google Scholar]
- 5.Dhaenens M, Fert I, Glatigny S, Haerinck S, Poulain C, Donnadieu E, et al. Dendritic cells from spondylarthritis-prone HLA-B27-transgenic rats display altered cytoskeletal dynamics, class II major histocompatibility complex expression, and viability. Arthritis Rheum. 2009;60:2622–2632. doi: 10.1002/art.24780. [DOI] [PubMed] [Google Scholar]
- 6.Utriainen L, Firmin D, Wright P, Cerovic V, Breban M, McInnes I, et al. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondylarthritis. Arthritis Rheum. 2012;64:3199–3209. doi: 10.1002/art.34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatigny S, Fert I, Blaton MA, Lories RJ, Araujo LM, Chiocchia G, et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 2012;64:110–120. doi: 10.1002/art.33321. [DOI] [PubMed] [Google Scholar]
- 8.Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–2448. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 9.Turner MJ, Delay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: Implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 2007;56:215–223. doi: 10.1002/art.22295. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol. 2008;38:1194–1203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci USA. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Ding J, Fan CM, Zhu P. Interferon-gamma contributes to HLA-B27-associated unfolded protein response in spondyloarthropathies. J Rheumatol. 2012;39:574–582. doi: 10.3899/jrheum.101257. [DOI] [PubMed] [Google Scholar]
- 15.Will R, Palmer R, Bhalla AK, Ring F, Calin A. Osteoporosis in early ankylosing spondylitis: a primary pathological event? Lancet. 1989;2:1483–1485. doi: 10.1016/s0140-6736(89)92932-2. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly S, Doyle DV, Denton A, Rolfe I, McCloskey EV, Spector TD. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann Rheum Dis. 1994;53:117–121. doi: 10.1136/ard.53.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingberg E, Lorentzon M, Mellstrom D, Geijer M, Gothlin J, Hilme E, et al. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. doi: 10.1186/ar3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhter MP, Jung LK. Decreased bone strength in HLA-B27 transgenic rat model of spondyloarthropathy. Rheumatol. 2007;46:1258–1262. doi: 10.1093/rheumatology/kem104. [DOI] [PubMed] [Google Scholar]
- 19.Hammer RE, Maika SD, Richardson JA, Tang J-P, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2-m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 20.Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 21.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 22.Lutz PM, Cresswell P. An epitope common to HLA class I and class II antigens, Ig light chains, and β2-microglobulin. Immunogen. 1987;25:228–233. doi: 10.1007/BF00404692. [DOI] [PubMed] [Google Scholar]
- 23.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus H-chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 24.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42:731–743. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death & Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Maghraoui A. Osteoporosis and ankylosing spondylitis. Joint, bone, spine: revue du rhumatisme. 2004;71:291–295. doi: 10.1016/j.jbspin.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L, et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44:772–776. doi: 10.1016/j.bone.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Magrey M, Khan MA. Osteoporosis in ankylosing spondylitis. Curr Rheum Rep. 2010;12:332–336. doi: 10.1007/s11926-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 32.Francois RJ, Neure L, Sieper J, Braun J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor {alpha} in two patients with early disease and transforming growth factor {beta} in three more advanced cases. Ann Rheum Dis. 2006;65:713–720. doi: 10.1136/ard.2005.037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel H, Kuhne M, Spiekermann S, Ebhardt H, Grozdanovic Z, Kohler D, et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum. 2006;54:2845–2851. doi: 10.1002/art.22060. [DOI] [PubMed] [Google Scholar]
- 34.Neidhart M, Baraliakos X, Seemayer C, Zelder C, Gay RE, Michel BA, et al. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Ann Rheum Dis. 2009;68:1334–1339. doi: 10.1136/ard.2008.092494. [DOI] [PubMed] [Google Scholar]
- 35.Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008;35:855–861. [PubMed] [Google Scholar]
- 36.Visvanathan S, van der Heijde D, Deodhar A, Wagner C, Baker DG, Han J, et al. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:175–182. doi: 10.1136/ard.2007.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maksymowych WP, Elewaut D, Schett G. Motion for debate: the development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum. 2012;64:1713–1719. doi: 10.1002/art.34442. [DOI] [PubMed] [Google Scholar]
- 38.Papet I, El Yousfi M, Godin JP, Mermoud AF, Davicco MJ, Coxam V, et al. HLA-B27 rats develop osteopaenia through increased bone resorption without any change in bone formation. J Musculoskel Neur Int. 2008;8:251–256. [PubMed] [Google Scholar]
- 39.Rauner M, Stupphann D, Haas M, Fert I, Glatigny S, Sipos W, et al. The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANKL to osteoprotegerin mRNA ratio. J Rheumatol. 2009;36:120–126. doi: 10.3899/jrheum.080475. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Barnes MD, Hong D, DeLay ML, Inman RD, Colbert RA. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008;58:1640–1649. doi: 10.1002/art.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng L, Lindstrom MJ, Smith JA. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 2011;63:3807–3817. doi: 10.1002/art.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickhout JG, Lhotak S, Hilditch BA, Basseri S, Colgan SM, Lynn EG, et al. Induction of the unfolded protein response after monocyte to macrophage differentiation augments cell survival in early atherosclerotic lesions. FASEB J. 2011;25:576–589. doi: 10.1096/fj.10-159319. [DOI] [PubMed] [Google Scholar]