Abstract
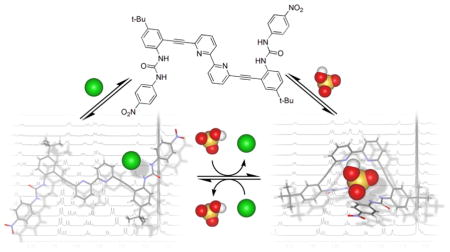
An anionic three-way switch
A bipyridyl bisurea-based anion receptor that is highly selective for dihydrogen phosphate demonstrates spectroscopically distinct anion bound conformations toward halides and select oxoanions. 1H NMR studies show the differing anion induced conformations are reversible allowing this system to function as a three-way molecular switch.
Keywords: supramolecular chemistry, molecular recognition, receptors, host-guest systems, anions
Molecular switches have received considerable attention due to interest in studying the miniaturization of macroscopic machines and the desire to emulate biological systems.[1] A firm understanding of cationic molecular interactions has resulted in numerous examples of supramolecular switches where cations serve as the major stimulus.[2] As the subtle interactions of anions in supramolecular systems have become better understood, anion-stimulated conformational changes and supramolecular switches have also gained notoriety.[3]
Several supramolecular systems have utilized conformational changes to target anions,[4] inspired by the concept of the induced-fit model proposed for enzymatic selectivity.[5] This has been implemented by engineering molecules with controlled rotational freedom in conjuction with strategic placement of specific anion-coordinating functional groups.
The phosphate anion is known to significantly affect biological and ecological systems, and a great deal of interest focuses on further understanding the impact. Phosphorylated molecules are key components in biological processes such as energy storage and signaling pathways. Phosphate has significant environmental impact with its role in the eutrophication process.[6] As a result, phosphate-binding receptors have become a highly desirable target in supramolecular chemistry, with several recent reports of systems designed to selectively coordinate phosphate.[7]
Herein we report a bisurea-based anion receptor bearing a bipyridine core unit capable of selective dihydrogen phosphate coordination. This receptor features an order of magnitude increase in association constant for dihydrogen phosphate over halides and a series of other competing oxoanions. The presence of two different induced-fit binding conformations is in part responsible for this selectivity. Receptor 1 was designed to coordinate anions by exploiting the potential of the bisureas to converge multiple binding units on a single guest. Replacement of the pyridine core in our previously reported class of arylethynyl anion receptors[8] with a bipyridal unit is expected to provide a flexible structural unit to aid in forming a discrete binding pocket, offering two hydrogen bond acceptors to specifically target protic oxoanions. This design results in a receptor with divergent and guest-specific anion binding conformations, allowing this receptor to function as a three-way molecular switch influenced strictly by anions with an “off”, or unbound state, and two spectroscopically distinct “on” states. Additionally, we show that it is possible to reversibly switch between the bound conformational states by altering the relative equivalents of chloride and hydrogen sulfate anions.
Receptor 1 was prepared according to Scheme 1. The known compounds 6,6′-dibromo-2,2′-bipyridine[9] and 2-alkynyl-4-tert-butyl aniline[10] were reacted in a double Sonogashira cross-coupling reaction yielding 2. Direct coupling of two equivalents of 4-nitrophenylisocyanate to diamine 2 produced bisurea product 1 as a light yellow powder. Repeated precipitation of 1 from hot THF gave analytically pure product in up to 83% yield. The final product could be further purified via flash column chromatography using CH2Cl2 containing 3% v/v of a 9:1 v/v MeOH:NH4OH mixture.
Scheme 1.
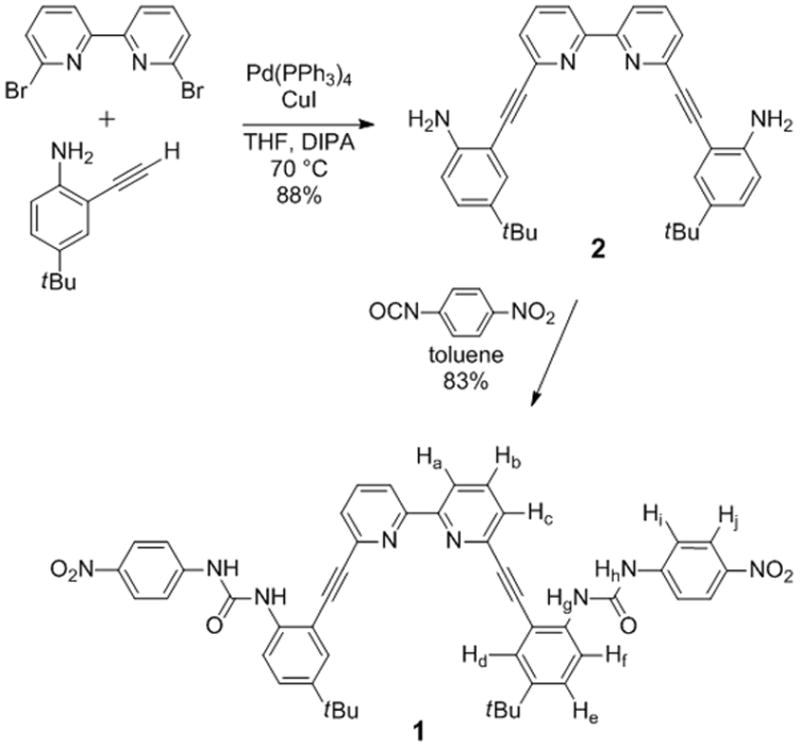
Synthesis of receptor 1. Assignments determined via 1H-1H COSY, 1H-13C HSQC and 1H-13C HMBC NMR spectroscopy.
The ability of receptor 1 to bind anions in solution was initially probed via 1H NMR titrations in 10% [D6]DMSO/CDCl3 or by UV/Vis titrations in 10% DMSO/CHCl3 (Table 1). In all cases association constants (Ka) were determined from 1H NMR titrations by simultaneous fitting of the downfield shifting of the urea protons, and in the case of titrations with Cl− and Br−, the Hc aryl resonances were included in the fit. UV/Vis binding studies were carried out by measuring the bathochromic shifting of the host absorbance. Binding curves were fit using the Hyperquad 2006 suite of nonlinear curve fitting software.[11] All titrations of the bipyridine-based receptor were fit to a 1:1 host:guest model, which was confirmed by Job’s plot analysis.
Table 1.
Association constants (Ka) obtained by UV/Vis or 1H NMR titrations of 1 with various anions added as the tetrabutylammonium salts in 10% DMSO/CHCl3 or the perdeutero-equivalent at 298 K and data fit to an apparent 1:1 binding model using HypNMR 2006 or Hyperquad 2006.[a] Association constants represent an average of at least three titrations.
| Halides (M−1) | Oxoanions (M−1) | ||
|---|---|---|---|
|
| |||
| 1 | 1 | ||
| Cl− | 140±10 | H2PO4− | 78000±2000[b] |
| Br− | 60±5 | HSO4− | 170±20 |
| I− | 40±15 | OAc− | 3200±500 |
| — | — | NO3− | 60±5 |
Data were fit to a 1:1 binding model based on goodness of fit, results of Job plots and extensive non-linear least squares modelling (see ESI). Based on increased goodness of fit it is clear that 1:2 association does occur for HSO4− and the halides, specifically Cl−, at least to some extent during titrations (see Figures S27, S28 for 1:2 fits). We report only 1:1 apparent Ka’s since the 1:2 fits are less reliable due to the need to fit too many unknown variables. Nevertheless, association constant fits (β11, β12) are provided and discussed for 1:2 association in the ESI for the interested reader.
Association constant obtained from UV/Vis titrations.
The halides in general exhibited minimal affinity toward 1 trending Cl− > Br− > I−. Acetate (OAc−) anion gave an association constant of 3200 M−1, whereas HSO4− and NO3− had binding constants of 170 and 60 M−1, respectively. Initial attempts to determine association constants from 1H NMR titrations of 1 with H2PO4− resulted in broadening of the urea resonances to approx. 1 equiv. of guest, and an eventual sharpening of the urea peaks beyond 1 equiv. was observed (Figure S8). The broadening of the urea resonances is likely a result of either exchange on the NMR time scale or a coordination-induced proton exchange event, similarly observed by Gale et al.[7a] As a result, reliable binding constants of 1 and H2PO4− (Ka = 78,000 M−1) were ultimately obtained via UV-Vis titrations (Figure S21). Taken as a whole, the observed anion binding trend is: H2PO4− > OAc− > HSO4− ≈ Cl− > Br− ≈ NO3− > I−. The most noteworthy results are the high affinity of receptor 1 for dihydrogen phosphate: H2PO4− is bound over an order of magnitude more stongly than the more basic OAc−, and over two orders of magnitude greater than similarly shaped HSO4−. This selectivity may result from a short-strong hydrogen bond between guest and host, or perhaps even a proton transfer to the host and concomitant hydrogen bonding between the resulting protonated bipyridine and HPO42−.
Further analysis of the binding data revealed distinctly different proton shifting patterns of 1 upon addition of various anionic guests. The addition of halides as the tetrabutylammonium salts expectedly caused steady downfield shifting of the urea resonances Hg and Hh, but surprisingly the Hc aryl resonance residing on the bipyridine unit also exhibited a drastic shift downfield by 0.64 ppm. The response of Hc to increasing halide concentration suggests the involvement of Hc in hydrogen bonding toward halide guests (Figure 2a). Though unexpected, the importance of aryl C–H hydrogen bonding by neutral receptors to halides in solution has been thoroughly demonstrated by Flood, et al.[12] and a similar interaction was observed by Jeong, et al.[7f] This phenomenon was most prominent for Cl− followed by Br−. The binding of I− was too weak to determine if shifting trends definitively followed that of the other halides. The synchronous downfield movement of the urea and aryl protons upon addition of the spherical halides are consistent with a binding conformation similar to Z (Figure 2c), where a cleft formed by the urea unit rotates ~180° away from the bipyridine nitrogen atoms about the alkyne bond. This binding conformation would be expected to offer two equivalent binding sites. A 1:2 host:guest complex is suggested from representative fits and speciation diagrams of Cl− at high equivalents of guest (Figure S27).
Figure 2.
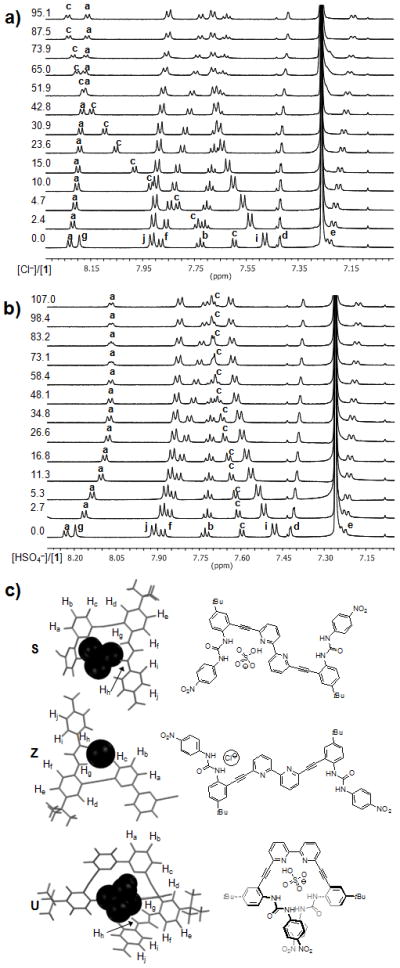
Partial 1H NMR spectra of receptor 1 upon titration with (a) TBACl and (b) TBAHSO4. Numbers next to NMR spectra represent [anion]/[1] (c) Chemdraw representations and calculated (ωB97X-D/6-31G(d,p))[13] wireframe representations of possible anion binding in the putative S, Z, (truncated for clarity) and U conformations.
1H NMR studies of 1 with oxoanions (H2PO4−, HSO4−, OAc−, and NO3−) produced distinctly different shifting patterns from those with the halides (Figure 2b). The urea resonances of 1 experienced the most intense shifts when treated with oxoanions, as was seen with the halides. The most significant differences in the shifting pattern of halides and oxoanions pertained to the Ha and Hc protons. In the case of the oxoanions, the Hc resonance does not exhibit the same magnitude of downfield shift that was observed with the halides suggesting that the aryl proton is no longer significantly involved in the complexation of guests. The Ha resonance of 1 also shifts much farther upfield with H2PO4−, HSO4−, and OAc− versus the halides. The binding of NO3− is too weak to induce a significant perturbation of the Ha resonance. The lack of Hc shifting is consistent with binding conformations similar to either S or U (Figure 2c). The upfield shifting of the 2,2′-bipyridine cleft resonance has been shown to be indicative of rotation about the bipyridine bridging bond during the transition of an “anti” to a “syn” conformation with respect to the bipyridine nitrogens, which is more suggestive of a U-like conformation.[14] Additionally, DFT calculations[13] indicate that the most stabilized anion complex is in a U-like conformation (see the Supporting Information). An S-like conformation is expected in formation of 1:2 complexes in large excesses of guest though reliable evidence for this currently exist only with the HSO4− system. Unfortunately, NOESY and ROESY NMR experiments did not yield any additional information on the nature of the anion-bound conformation.[15] The differences in the trends of the NMR data between halides and oxoanions are definitive evidence for the emergence of a binding conformation for oxoanions that is distinct spectroscopically from that of the halides. This yields a unique system with the possiblity of acting as a three-way molecular switch influenced solely by anionic stimuli with an “off” or unbound state and two different “on” or bound states that are identifiable as either halide- or oxoanion-bound.
Single crystals suitable for X-ray analysis were obtained from slow evaporation of solutions of 1 containing excess tetrabutylammonium bromide in 10% [D6]DMSO/CDCl3 (Figure 3).[16] The solid state studies revealed that the receptor adopts the anticipated “anti” conformation with respect to the bipyridine unit. The receptor is able to coordinate bromide in the solid state forming a 1·2 Br− host-guest complex in the expected Z conformation (Figure 2c). Receptor 1 forms two weak hydrogen bonds via the urea moieties, and another weak C–H hydrogen bond with the bipyridine unit to each bromide with atom distances Ng–Br 3.432(1) Å, Nh–Br 3.232(1) Å, and Cc–Br, 3.85(1) Å and bond angles Ng–Hg•••Br 156.8(4)°, Nh–Hh•••Br 167.1(7)°, and Cc–Hc•••Br 141.8(4)°. Though it is often dangerous to correlate solid state and solution phase data, the correlation of the X-ray hydrogen bonding network and the previously described solution studies are in excellent agreement. Both sources of data provide a convincing picture for halide binding in this receptor.
Figure 3.
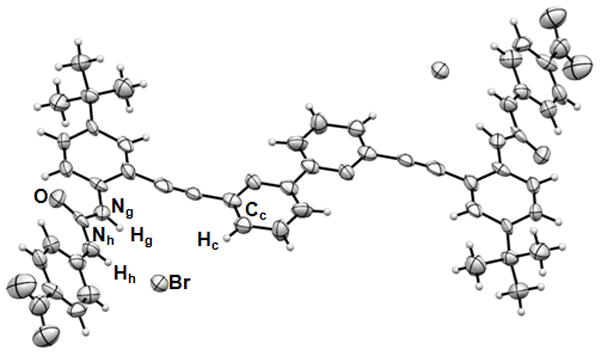
ORTEP representations of X-ray crystal structure of 1·2 Br− hydrogen bonded complex. TBA+ counter cations have been omitted for clarity.
We were interested in the ability of this system to act reversibly with regard to the two “on” states. As a result, the interconversion of the binding conformations was determined by a series of competition experiments. Chloride and hydrogen sulfate were chosen as the stimuli since they yielded differing conformations by 1H NMR spectroscopy and produced similar association constants. A sample of free receptor was incrementally saturated with Cl−. As mentioned previously, this caused significant downfield shifting of the urea resonances (Hg and Hh) and the bipyridine aryl proton (Hc). The chloride saturated system was then treated with up to ~3 equiv. of HSO4− relative to Cl−, which caused a retreating of urea and bipyridine Hc resonances back upfield as well as continued upfield shifting of the Ha resonance suggesting complexation of HSO4− over Cl− (Figure 4). Figure 4b shows the speciation diagram of free host 1, 1•Cl− and 1•HSO4− during the course of this competition experiment. The reverse experiment was also conducted where the free receptor was initially treated with HSO4− to saturation of the receptor with the oxoanion causing significant upfield shifting of the Ha resonance and moderate downfield shifting of the urea protons (Figure S38). This system was then slowly treated with an up to ~3 fold excess of Cl− to HSO4− which resulted in increased downfield shifting of the urea and Hc resonances as well as an overall downfield shift of the Ha resonance. These results show the proton resonances diagnostic of the varying conformations can be influenced reversibly by Cl− and HSO4−. Furthermore, this system is able to revert back to the “off” state from the chloride complex by the addition of 1 equivalent of NaBPh4 (Figure S31), suggesting these conformational states can indeed be reversibly achieved.
Figure 4.
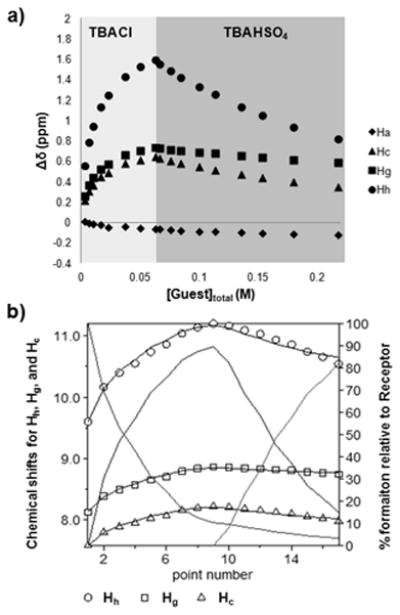
(a) Plot of the chemical shift changes of select protons on the 1·anion complexes as first Cl−, and then a second anion (HSO4−), are titrated in as the TBA salt during competition experiments. For the reverse competition (HSO4− then Cl−) see Figures S35–S38. Anion concentration range spans from 0 to over 200 equivalents of total anion (see Figures S29, S30 for raw data). (b) Titration curves (solid black lines) from the competition experiments were fit using apparent Ka’s from Table 1 to both 1:1 (shown) and 1:2 (see Figures S34, S37) host:guest models. Grayscale background traces show the speciation diagram of free host 1, 1•Cl− and 1•HSO4− throughout the competition experiment based on the 1:1 fit. The x-axis corresponds to the data “point number” in the competition titration shown in 4a.
In conclusion we have demonstrated through binding studies a supramolecular receptor that preferentially coordinates the biologically and environmentally relevant dihydrogen phosphate anion an order of magnitude greater than the more basic acetate, and several orders of magnitude greater than other anions tested. Binding studies also revealed the emergence of spectroscopically different halide and oxoanion dependent binding conformations. The halide conformation in solution is further confirmed by solid state data. The distinctly different conformations demonstrate the ability of this receptor to function as a strictly anion-influenced supramolecular three-way switch. Furthermore, it has been shown that the different bound conformations can be reversibly modulated by changing the identity of the anionic stimuli between chloride and hydrogen sulfate. The various conformational outcomes exhibited by this receptor influenced only by the subtle differences in anionic guests represents a potentially interesting system for use in supramolecular machinery, and shows that it is possible to allow conformationally flexible hosts to “select” their preferred anionic guests.
Supplementary Material
Footnotes
This work was supported by NIH grant R01-GM087398, which also funded early stage intellectual property that was licensed by SupraSensor Technologies, a company co-founded by the PIs. The authors appreciate extremely helpful conversations with Prof. Pablo Ballester at ICIQ, Spain in revising some of the complex solution speciation presented herein. University of Oregon NMR facilities are supported by a grant from the National Science Foundation (CHE-0923589).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.20130xxxx.
Contributor Information
Jesse V. Gavette, Department of Chemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Prof. Dr. Nancy S. Mills, Department of Chemistry, Trinity University, San Antonio, TX 78212 (USA)
Dr. Lev N. Zakharov, CAMCOR – Center for Advanced Materials, Characterization in Oregon, University of Oregon, Eugene, OR 97403-1443 (USA)
Dr. Charles A. Johnson, II, Department of Chemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA).
Prof. Dr. Darren W. Johnson, Email: dwj@uoregon.edu, Department of Chemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Prof. Dr. Michael M. Haley, Email: haley@uoregon.edu, Department of Chemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
References
- 1.a) Zhang M, Zhu K, Huang F. Supramolecular Chemistry From Molecules to Nanomaterials. John Wiley And Sons; West Sussex, United Kingdom: 2012. pp. 2425–2496. [Google Scholar]; b) Balzani V, Credi A, Raymo FM, Stoddart JF. Angew Chem. 2000;112:3484–3530. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2000;39:3348–3391. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; c) Kay ER, Leigh DA, Zerbetto F. Angew Chem. 2007;119:72–196. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2007;46:72–191. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]
- 2.Leung KCF, Chak CP, Lo CM, Wong WY, Xuan S, Cheng CHK. Chem Asian J. 2009;4:364–381. doi: 10.1002/asia.200800320. [DOI] [PubMed] [Google Scholar]
- 3.a) Jones IM, Hamilton AD. Angew Chem. 2011;123:4693–4696. [Google Scholar]; Angew Chem, Int Ed. 2011;50:4597–4600. doi: 10.1002/anie.201100144. [DOI] [PubMed] [Google Scholar]; b) Sato K, Nishina Y, Shiga K. J Biochem. 1992;111:359–365. doi: 10.1093/oxfordjournals.jbchem.a123762. [DOI] [PubMed] [Google Scholar]; c) Guo L, Wang QL, Jiang QQ, Jiang QJ, Jiang YB. J Org Chem. 2007;72:9947–9953. doi: 10.1021/jo701823d. [DOI] [PubMed] [Google Scholar]; d) Huang YL, Hung WC, Lai CC, Liu YH, Peng SM, Chiu SH. Angew Chem. 2007;119:6749–6753. doi: 10.1002/anie.200702197. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2007;46:6629–6633. doi: 10.1002/anie.200702197. [DOI] [PubMed] [Google Scholar]; e) Lin CF, Lai CC, Liu YH, Peng SM, Chiu SH. Chem Eur J. 2007;13:4350–4355. doi: 10.1002/chem.200601432. [DOI] [PubMed] [Google Scholar]; f) Chmielewski MJ, Jurczak J. Chem Eur J. 2006;12:7652–7667. doi: 10.1002/chem.200501471. [DOI] [PubMed] [Google Scholar]; g) Serpell CJ, Chall R, Thompson AL, Beer PD. Dalton Trans. 2011;40:12052–12055. doi: 10.1039/c1dt10186b. [DOI] [PubMed] [Google Scholar]; h) Lin TC, Lai CC, Chiu SH. Org Lett. 2008;11:613–616. doi: 10.1021/ol802638k. [DOI] [PubMed] [Google Scholar]; i) Makuc D, Hiscock JR, Light ME, Gale PA, Plavec J. Beilstein J Org Chem. 2011;7:1205–1214. doi: 10.3762/bjoc.7.140. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Suk J, Naidu VR, Liu X, Lah MS, Jeong KS. J Am Chem Soc. 2011;133:13938–13941. doi: 10.1021/ja206546b. [DOI] [PubMed] [Google Scholar]; k) Jeong KS, Chae MK, Suk J. Pure Appl Chem. 2012;84:953–964. [Google Scholar]
- 4.a) Swinburne AN, Paterson MJ, Beeby A, Steed JW. Chem Eur J. 2010;16:2714–2718. doi: 10.1002/chem.200903293. [DOI] [PubMed] [Google Scholar]; b) Kim MJ, Lee HW, Moon D, Jeong KS. Org Lett. 2012;14:5018–5021. doi: 10.1021/ol3022148. [DOI] [PubMed] [Google Scholar]; c) Filby MH, Dickson SJ, Zaccheroni N, Prodi L, Bonacchi S, Montalti M, Paterson MJ, Humphries TD, Chiorboli C, Steed JW. J Am Chem Soc. 2008;130:4105–4113. doi: 10.1021/ja711012d. [DOI] [PubMed] [Google Scholar]; d) Pierro T, Gaeta C, Troisi F, Neri P. Tetrahedron Lett. 2009;50:350–353. [Google Scholar]; e) Wallace KJ, Belcher WJ, Turner DR, Syed KF, Steed JW. J Am Chem Soc. 2003;125:9699–9715. doi: 10.1021/ja034921w. [DOI] [PubMed] [Google Scholar]; f) Moerkerke S, Ménand M, Jabin I. Chem Eur J. 2010;16:11712–11719. doi: 10.1002/chem.201001193. [DOI] [PubMed] [Google Scholar]
- 5.Koshland DE. Angew Chem. 1994;106:2468–2472. [Google Scholar]; Angew Chem, Int Ed. 1994;33:2375–2378. [Google Scholar]
- 6.For a recent review on the importance and examples of coordination of phosphate and phosphorylated guest by supramolecular receptors see the following: Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Chem Rev. 2011;111:6603–6782. doi: 10.1021/cr100242s.
- 7.a) Gale PA, Hiscock JR, Moore SJ, Caltagirone C, Hursthouse MB, Light ME. Chem Asian J. 2010;5:555–561. doi: 10.1002/asia.200900230. [DOI] [PubMed] [Google Scholar]; b) Gale PA, Hiscock JR, Lalaoui N, Light ME, Wells NJ, Wenzel M. Org Biomol Chem. 2012;10:5909–5915. doi: 10.1039/c1ob06800h. [DOI] [PubMed] [Google Scholar]; c) Kondo S, Hiraoka Y, Kurumatani N, Yano Y. Chem Commun. 2005:1720–1722. doi: 10.1039/b417304j. [DOI] [PubMed] [Google Scholar]; d) Saeed MA, Powell DR, Hossain MA. Tetrahedron Lett. 2010;51:4904–4907. doi: 10.1016/j.tetlet.2010.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Gong W, Bao S, Wang F, Ye J, Ning G, Hiratani K. Tetrahedron Lett. 2011;52:630–634. [Google Scholar]; f) Kwon TH, Jeong KS. Tetrahedron Lett. 2006;47:8539–8541. [Google Scholar]; g) Kondo S, Takai R. Org Lett. 2013;15:538–541. doi: 10.1021/ol3033626. [DOI] [PubMed] [Google Scholar]
- 8.a) Berryman OB, Johnson CA, Zakharov LN, Haley MM, Johnson DW. Angew Chem. 2008;120:123–126. doi: 10.1002/anie.200703971. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2008;47:117–120. [Google Scholar]; b) Johnson CA, Berryman OB, Sather AC, Zakharov LN, Haley MM, Johnson DW. Cryst Growth Des. 2009;9:4247–4249. [Google Scholar]; c) Carroll CN, Berryman OB, Johnson CA, Zakharov LN, Haley MM, Johnson DW. Chem Commun. 2009:2520. doi: 10.1039/b901643k. [DOI] [PubMed] [Google Scholar]; d) Carroll CN, Coombs BA, McClintock SP, Johnson CA, II, Berryman OB, Johnson DW, Haley MM. Chem Commun. 2011;47:5539. doi: 10.1039/c1cc10947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Weber E, Josel HP, Puff H, Franken S. J Org Chem. 1985;50:3125–3132. [Google Scholar]; b) Amb CM, Rasmussen SC. J Org Chem. 2006;71:4696–4699. doi: 10.1021/jo060557i. [DOI] [PubMed] [Google Scholar]
- 10.Wan WB, Haley MM. J Org Chem. 2001;66:3893–3901. doi: 10.1021/jo010183n. [DOI] [PubMed] [Google Scholar]
- 11.a) Gans P, Sabatini A, Vacca A. Talanta. 1996;43:1739–1753. doi: 10.1016/0039-9140(96)01958-3. [DOI] [PubMed] [Google Scholar]; b) Frassineti C, Ghelli S, Gans P, Sabatini A, Moruzzi MS, Vacca A. Anal Biochem. 1995;231:374–382. doi: 10.1006/abio.1995.9984. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Flood AH. Top Heterocycl Chem. 2012;28:85–108. [Google Scholar]
- 13.a) Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al. Gaussian 09, Revision C01. Gausian, Inc; Wallingford CT: 2010. [Google Scholar]; b) Chai JD, Head-Gordon M. Phys Chem Chem Phys. 2008;10:6615–6615. doi: 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]; c) Swart M, Sola M, Bickelhaupt FM. J Chem Phys. 2009;131:094103-1–094103-9. doi: 10.1063/1.3213193. [DOI] [PubMed] [Google Scholar]
- 14.a) Calder IC, Spotswood TM, Tanzer CI. Aust J Chem. 1967;20:1195–1212. [Google Scholar]; b) Spotswood TM, Tanzer CI. Aust J Chem. 1967;20:1213–1225. [Google Scholar]; c) Spotswood TM, Tanzer CI. Aust J Chem. 1967;20:1227–1242. [Google Scholar]
- 15.A combination of the high symmetry of the receptor, borderline molecular weight for NOESY and ROESY experiments, significant overlap of aryl peaks of the strongest complex (1·H2PO4−), and weak binding in all of the other systems prevented any conclusive conformational assignment of anion complexes in solution by these methods.
- 16.X-ray data for 1·(TBABr)2: C80H114Br2N10O6, Mr = 1471.63, crystal size 0.34 x 0.10 x 0.01 mm3, Triclinic, space group P-1, a = 8.496(8), b = 12.527(12), c = 19.598(18) Å, α = 100.299(16)°, β = 93.450(15)°, γ = 92.059(14)°, V = 2046(3) Å3, Z = 1, Z′ = 0.5, ρcalc = 1.194 g cm−3, μ = 1.045 mm−1, F(000) = 782, MoKα radiation, λ = 0.71073 Å, T = 173(2) K, 2Θmax = 45.00°, 11442 reflections measured [Rint=0.1774], 5324 reflections observed, 443 refined parameters, R1 = 0.0926, wR2 = 0.1626, and GOF = 1.003 for reflections with I > 2σ(I), R1 = 0.2375, wR2 = 0.2146, and GOF = 1.003 for all data, max/min residual electron density +0.490/−0.714 e Å−3. CCDC 930545 contains the supplementary crystallographic data for the structure. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


