Abstract
Papers in this volume investigate when and how putative risk factors influence development of first-onset, painful temporomandibular disorders (TMD). The results represent first findings from the OPPERA prospective cohort study which monitored 2,737 men and women aged 18–44 years recruited at four U.S. study sites. During a median 2.8 year follow-up period, 260 participants developed TMD. The average incidence rate of 4% per annum was influenced by a broad range of phenotypic risk factors including sociodemographic characteristics, health status, clinical orofacial factors, psychological functioning, pain sensitivity and cardiac autonomic responses. A novel method of multivariable analysis used random forest models to simultaneously evaluate contributions of all 202 phenotypic variables. Variables from the health status domain made the greatest contribution to TMD incidence, followed closely by psychological and clinical orofacial domains. However, only a few measures of pain sensitivity and autonomic function contributed to TMD incidence, and their effects were modest. Meanwhile, age and study site were independent predictors of TMD incidence, even after controlling for other phenotypes. Separate analysis of 358 genes that regulate pain found several novel genetic associations with intermediate phenotypes which, themselves, are risk factors for TMD, suggesting new avenues to investigate biological pathways contributing to TMD.
Keywords: Temporomandibular Disorders, Cohort Studies, Epidemiology, Psychological Factors, Clinical Pain, Comordid Conditions, Pain Sensitivity, Genetics
Introduction
This volume presents first findings from the OPPERA prospective cohort study of first-onset, painful temporomandibular disorders (TMD). The investigation extends OPPERA baseline case-control findings 14 reporting which phenotypic and genetic characteristics occurred more frequently in people with chronic TMD compared to TMD-free people. Here we address causality in asking when and how those characteristics influence development of first-onset TMD.
By measuring risk factors at enrollment in a cohort with no history of TMD and monitoring the cohort prospectively, we establish a temporal sequence between putative cause and development of first-onset TMD. Thus the papers in this volume fulfill a prominent criterion for causal inference.11 This is an important advancement over case-control findings that cannot distinguish bi-directional or reverse relationships between TMD and putative risk factors and thereby can spuriously inflate estimates of association. We noted this limitation in the OPPERA baseline case-control study,16 citing the example of parafunctional behaviors and speculating that the observed, strong association with odds of chronic TMD might be due, at least in part, to parafunctional behaviors caused by pain. As summarized below, several papers in this volume address this conundrum using the data from the OPPERA prospective cohort study.
In determining how a putative risk factor exerts an effect, it is essential to isolate its effect from those of other risk factors. Implicit in determining “independent” influences of individual risk factors is the notion of a “web of causation”.13 Like the biopsychosocial model of disease, the web of causation concept rejects the view of one necessary and sufficient cause of a complex disease or disorder such as TMD. We noted previously8 that multivariable statistical models provide a method to dissect such independent influences. However, these models were not used when analyzing the OPPERA baseline case-control study because TMD had already developed, clouding interpretations of the models. In this volume, putative risk factors within each phenotypic domain were evaluated using two multivariable statistical methods. Another paper examines the combined influences of risk factors across multiple domains on first-onset TMD.
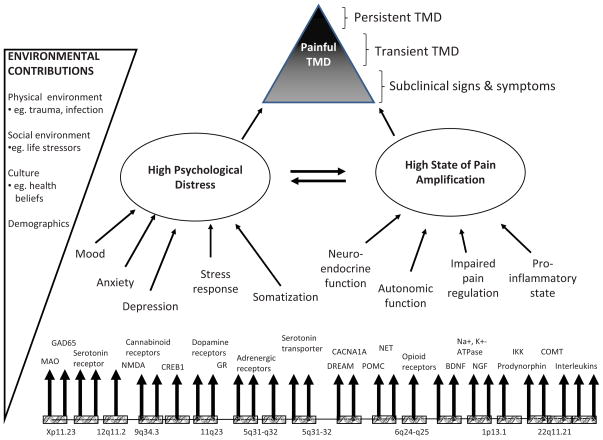
The search for intermediate effects of putative risk factors addresses the question of how TMD develops. For example, the OPPERA heuristic model (Figure 1) proposes that the role of genetic factors in TMD development is mediated by phenotypes representing intermediate processes of pain amplification and psychological distress. In this volume, both intermediate phenotypes and TMD onset are evaluated for their associations with a panel of 358 genes encoding proteins known to contribute to nociceptive pathways, inflammation, and affective distress.
Figure 1.
This model displays two principal intermediate phenotypes (psychological distress and pain amplification) that contribute to onset and persistence of TMD. Each intermediate phenotype represents a constellation of more specific risk factors, all of which are subject to genetic regulation. Interactions between intermediate phenotypes take place in the presence of environmental contributions that further contribute to onset and persistence of painful TMD. Time is not shown in the model, because its effects occur implicitly on a third dimension that is not readily shown in the diagram. Reproduced with permission from Maixner W, et al, 2011.14
This paper summarizes and integrates the major findings within this volume, focusing on evidence as to when and how putative risk factors influence development of painful TMD in a cohort of adults who had no significant history of TMD when enrolled.
Bair and coauthors: Study design, methods and loss-to-follow-up
In describing the study methods, Bair and coauthors2 emphasize the importance of frequent monitoring of symptoms throughout follow-up to fully enumerate TMD incidence. They concede that loss-to-follow-up is almost inevitable in population-based studies and they direct attention to potential biases created by such loss. The target population for the OPPERA prospective cohort study was adults aged 18–44 years with no significant history of TMD. Between May 2006 and November 2008, 3,263 community-based volunteers were recruited at four study U.S. sites and examined using OPPERA’s adaptation of the Research Diagnostic Criteria for TMD (RDC/TMD) to exclude people who had ever received a TMD diagnosis.5 Furthermore, enrollees reported no orofacial pain in the month before enrollment and, prior to that period, no more than four days of orofacial pain in any month. Once enrolled, participants completed questionnaires and physical assessments that measured putative risk factors, and examiners confirmed absence of TMD. During a median 2.8 year follow-up, study participants completed quarterly questionnaires that screened for TMD pain symptoms experienced during the preceding three months, thus permitting continuous surveillance. A total of 2,737 participants completed one or more questionnaires. Those reporting TMD pain symptoms were invited to a follow-up clinical visit where they were examined using the RDC/TMD-based examination.5 Specifically, the 260 incident cases satisfied two criteria for first-onset TMD: 1) symptoms of orofacial pain reported for ≥5 days during the preceding month; and 2) examiner findings of TMD myalgia, arthralgia, or both.
Based on several measures of data quality, follow-up methods performed well: the quarterly screening questionnaires had excellent test-retest reliability and validity as a screening measure. In annual evaluations, examiners had excellent inter-examiner reliability compared to the study’s reference examiner. Yet, 16% of participants completed no follow-up assessments, some did not complete all quarterly questionnaires, and methods used to enumerate cases of first-onset TMD were imperfect. Conventional methods of complete case-analysis, which use only observed data, ignore potential biases created by missing data. To quantify the degree of bias, the authors used hot-deck multiple imputation to account for loss-to-follow-up and they developed a two-stage, multiple imputation procedure to address problems arising from non-examination - or from dubious examination - of some symptomatic episodes. The results revealed a small, although not ignorable, degree of bias when data were analyzed using complete-case analysis. Based on these findings, the two-stage multiple imputation procedure were adopted in all subsequent papers that evaluated risk factors for first-onset TMD.
Slade and coauthors: Signs and symptoms of first-onset TMD and sociodemographic predictors
Levels of pain and related symptoms reported by the 260 participants who developed first-onset TMD were similar to values reported in other studies of acute TMD although, as expected, they were lower than levels reported in studies of chronic TMD.19 For example, one quarter of OPPERA’s incident cases recorded levels of II or more using the Graded Chronic Pain Scale,22 compared to 50% of chronic TMD cases in the OPPERA baseline case-control. Two thirds described having experienced “recurrent bouts” of facial pain symptoms, and most of the incident cases reported two or more months of symptoms prior to clinical classification of TMD. A noteworthy finding was that nearly one quarter of people with first-onset TMD described their onset-pain as headache, not jaw pain. The authors speculated that inclusion of headache among the screening symptoms in quarterly follow-up questionnaire might be one of the reasons that the overall incidence rate of first-onset TMD in OPPERA (3.9% per annum) was nearly twice the rate reported in two previous prospective cohort studies. Other proposed explanations were OPPERA’s greater frequency of symptom-monitoring and underlying differences in the populations studied.
Among these 18–44 year olds, greater age and lifetime U.S. residence were the two demographic characteristics associated most strongly with increased incidence of TMD. Relative to Whites, African-Americans had somewhat greater incidence while Asians had markedly lower incidence of TMD. In contrast to earlier case-control findings, females had only slightly greater incidence than males. Measures of socioeconomic status were not associated with TMD incidence, although incidence was greater in people who reported dissatisfaction with material standard of life – a measure of subjective socio-economic status. In multivariate analysis, an interaction emerged between age and race/ethnicity, such that older age was more strongly associated with TMD incidence in racial minorities than in Whites. The authors noted that social and economic disadvantage is thought to accelerate the development of some other age-related diseases in minorities, and recommended that this process of “weathering” be investigated in studies of TMD.
The paper further investigated multivariable predictors of TMD incidence using random forest models. This novel method of data mining, which was also used in subsequent papers, had two aims: a) to identify the most important risk factors for first-onset TMD; and b) to generate plots depicting adjusted association between each variable and TMD incidence, with adjustment for the effects of other variables and including considerations of non-linear associations. Using the demographic variables, the random-forest model confirmed that age was the most important demographic predictor of TMD incidence, and it showed a threshold effect in the inverse relationship between material satisfaction and TMD incidence. As summarized in the following sections, separate random forest models were developed for other sets of phenotypic predictors and for multivariable analysis of all 202 phenotypic predictors.3
Several of these demographic associations were in stark contrast to findings from the OPPERA case-control study of chronic TMD. Specifically, Whites had markedly greater odds of chronic TMD than African-Americans, whereas they had lower incidence of first-onset TMD. Females had markedly greater odds of chronic TMD than males, although their incidence of first-onset TMD was only marginally greater. Finally, lifetime U.S. residence was not significantly associated with chronic TMD, but was strongly associated with greater incidence of TMD. The one consistent demographic association was that greater age was associated with both greater odds of chronic TMD and greater incidence of first-onset TMD. However, the predominant impression was that demographic characteristics contribute differentially to first-onset TMD versus chronic TMD.
The authors caution that these findings should not be extrapolated beyond the study population or the condition studied: this was a generally healthy cohort of 18–44 year-olds and most participants who developed first-onset TMD had experienced symptoms for only one or two months. Nonetheless, the results challenge a widely-held view that TMD is predominantly a condition of females in early adulthood, and they suggest that, even in early adulthood, ill-health or other experiences related to aging could be important etiological influences on risk of developing TMD.
Ohrbach and coauthors: Clinical orofacial predictors
Findings from clinical assessment of masticatory tissues are intuitively appealing as prognostic indicators of TMD. Ohrbach and coauthors15 tested whether clinical orofacial characteristics measured at enrollment contribute to development of first-onset TMD. Their investigation is a sequel to the OPPERA baseline case-control study which found striking differences between chronic TMD cases and controls in both self-reported symptoms and clinical measures of jaw function, muscle and joint tenderness, and oral parafunctions. 16 However they cautioned that any causal inferences should await the findings now reported from the prospective cohort study.
In univariate analysis, TMD incidence was associated with symptoms of oral parafunctions, non-specific orofacial symptoms, reported TMJ noises and jaw locking and prior facial pain related life-impacts. Clinically-assessed measures that predicted TMD incidence were pain on jaw opening and pain from palpation of masticatory, neck, and body muscles. Meanwhile, self-reported history of external trauma and examiner assessments of TMJ noise and tooth wear facets did not predict incidence. Using factor analysis, a majority of the clinical domain variables were reduced to three latent variables underlying bodily palpation pain, pain with jaw mobility, and self-reported TMJ problems. In multivariate Cox regression models utilizing the 3 factors as well as other primary variables, the strongest predictors of first-onset TMD were examiner-elicited pain from jaw opening, non-specific orofacial symptoms, and oral parafunctions. Multivariable analysis with random forest models confirmed predominant effects on TMD incidence of oral parafunctions, reported inability to open the jaw wide, and non-specific orofacial symptoms. There was a threshold effect of oral parafunctions, such that TMD incidence was elevated only in people who reported either multiple- or frequent-parafunctional behaviors.
The authors speculated that this density of parafunctions probably signified underlying central dysregulation in the form of overactive motor activation, underactive motor inhibition, loss of normal proprioception, and/or persistent psychophysiologic reactivity. The hypothesized central dysregulation may be specific to the masticatory system or it may include more general motor activation. In either case, mediation via CNS dysregulation is an obvious further research area. Conversely, the authors were surprised that TMD onset was influenced by only a few (e.g., palpation pain, pain from range of jaw motion) of the numerous examination-assessments specific to the masticatory tissues. They concluded that the most pronounced influences on TMD incidence appeared to reflect alterations to systems beyond the masticatory tissues.
Sanders and coauthors: General health status predictors
In the OPPERA case-control study, aspects of general health status were reported in the same paper that described symptoms and signs of orofacial pain. Based on the observed strength of association and central importance of comorbidity in TMD, general health status is evaluated in this volume as a separate domain of putative risk factors for first-onset TMD.17 As discussed at the conclusion to this paper, the subdivision also aided a conceptual distinction when distinguishing effects of comorbid characteristics and pre-clinical findings from underlying etiological influences on pain.
As hypothesized, TMD incidence was increased in the presence of regional pain conditions reported at baseline: headache, irritable bowel syndrome, low back, and genital pain symptoms were all significant predictors of first-onset TMD in univariate analysis. Univariate associations were also observed with specific health conditions that are not primarily painful in nature, including respiratory conditions and neurosensory conditions. Likewise, a global self-rating of overall health was a strong predictor, as was a 20-item checklist of non-specific comorbid conditions. The latter included some pain conditions (e.g., irritable bowel syndrome and fibromyalgia) as well as many non-painful conditions (e.g., tendency to faint, insomnia, depression, ringing in the ears). Similarly, physical and mental health scales from the Short-Form Health Survey (SF-12) were strong predictors.
Other noteworthy predictors of TMD incidence were cigarette smoking, sleep quality and digit length ratio, a marker of intra-uterine exposure to sex hormones. Furthermore, these factors persisted as independent predictors when evaluated in a multivariable Cox regression model that controlled for specific regional pain conditions and specific health conditions. Likewise, in the random forest model that adjusted for all health status variables, including non-specific and global measures, current or former cigarette smokers had significantly greater incidence of TMD than people who had never smoked. The authors concluded that sleep disturbance, smoking and other aspects of general health could be targeted in future therapeutic approaches to prevent TMD.
Greenspan and coauthors: Pain sensitivity and cardiac autonomic function
Quantitative sensory testing at enrollment measured pressure pain thresholds, cutaneous mechanical pain sensitivity, and heat pain sensitivity. In addition, cardiac autonomic function was assessed by measuring blood pressure, heart rate, and heart rate variability, both at rest and in response to orthostatic and psychological challenge. Fourteen of the 39 quantitative sensory testing variables were significant univariate predictors of TMD incidence, most notably pressure pain thresholds measured at cranial sites. However, none of the individual measures exhibited strong associations with TMD incidence, and when all quantitative sensory testing variables were reduced to five principal components, none of the five derived components were significant univariate predictors of TMD incidence. There was, however, some evidence of an interaction between two principal components of heat pain responses: greater temporal summation of heat pain was associated with increased incidence of TMD among people with relatively low ratings of single-thermal stimuli, but not for people who had moderate- or high-ratings of single-thermal stimuli. When the individual measures were considered collectively in random forest models, the results confirmed generally weak associations with TMD incidence. However, the relationships often were non-linear, such that the gradient between pain sensitivity and TMD incidence was apparent only at the upper or lower quintiles of the distribution of pain sensitivity. Meanwhile, only three of the 59 measures of cardiac autonomic function were nominally associated with TMD incidence, and none of the five latent constructs of autonomic function derived using principal components analysis predicted TMD incidence.
Overall, the findings suggest that heightened sensitivity to pressure pain at cranial sites is a modest predictor of TMD incidence. Other aspects of pain sensitivity have more subtle influences, including non-linear effects and interactions between components of pain sensitivity. This was in marked contrast to the OPPERA baseline case-control study, where 29 of the 36 measures of pain sensitivity and several domains of autonomic function were associated with chronic TMD. In noting that pain sensitivity and autonomic function appear to be more important for chronic TMD than for first-onset TMD, the authors speculated that such measures might play an important role in the transition from acute TMD to chronic TMD.
Fillingim and coauthors: Psychological characteristics
Many of the 26 psychological characteristics measured at enrollment were significant predictors of TMD incidence when evaluated in the univariate analysis.6 The strongest effects were seen for measures of somatic symptoms, general psychological symptoms, negative mood, and measures of stress, including symptoms of post-traumatic stress and perceived stress. In many respects, these univariate findings mirrored associations with chronic TMD observed in the OPPERA baseline case-control study. Exceptions were measures of catastrophizing and active pain coping - well-established constructs associated with chronic pain - that were not significant predictors of first-onset TMD.
Variable reduction with principal component analysis identified four latent constructs: stress and negative affectivity; global psychological and somatic symptoms; passive pain coping; and active pain coping. Using multivariable Cox regression models, global psychological and somatic symptoms emerged as one significant predictor that was independently associated with TMD incidence, although when interactions were considered, there was some evidence that TMD incidence was additionally influenced by stress and negative affectivity among people with relatively low levels of global psychological and somatic symptoms. Associations between each latent construct and TMD incidence generally were consistent among demographic subgroups.
When all 26 measures were evaluated using random forest models, the strongest predictor of TMD incidence was a measure of somatic awareness, the Pennebaker Inventory of Limbic Languidness (PILL). However, the relationship was strikingly non-linear: greater incidence was associated with higher PILL scores only in the upper tercile of its distribution. After accounting for somatic awareness, some other variables in the random forest model had a modest influence on TMD incidence, including: Perceived Stress Scale; State Anxiety; anxiety, somatization, and obsessive-compulsive subscales of the Symptom Checklist (SLR-90); and the Ignoring Pain Sensations Scale of the Coping Strategies Questionnaire. This latter scale did not emerge as a predictor in the univariate model, suggesting that this coping strategy may provide some predictive value after adjusting for the effects of other psychological variables. As observed for the PILL, several of these associations appeared to be non-linear.
It was somewhat surprising that many of the psychological characteristics associated with chronic TMD observed in the OPPERA case-control study were likewise predictive of TMD incidence. Previously,7 the authors had speculated that psychological characteristics such as anxiety and perceived stress might occur as much as a consequence of TMD as a cause of it. They therefore expected psychological characteristics to exhibit fewer associations with TMD incidence than with chronic TMD. These findings refute that expectation. Indeed, given that first-onset TMD developed some years after enrollment for many of these incident cases, the findings suggest that influence of these psychological characteristics extends over a long time period.
Smith and coauthors: Genetic predictors of first-onset TMD and its intermediate phenotypes
Unlike Mendelian genetic disorders, complex conditions such as TMD appear to be influenced by multiple genetic variants of relatively high minor allele frequency. Generally, the genetic variants display stronger associations with phenotypes that are intermediaries in the causal pathway than with the complex clinical condition itself. This has prompted the search for associations between genes and intermediate phenotypes, sometimes referred to as endophenotypes, and defined as “measurable components unseen by the unaided eye along the pathway between disease and distal genotype”.9 As proposed in the OPPERA heuristic model, intermediate phenotypes relevant to TMD include neural processes subserving pain transmission, inflammation, and psychological distress.14
In the OPPERA prospective cohort study, DNA obtained from blood samples collected at baseline was used to measure variation in 3,295 single nucleotide polymorphisms (SNPs) representing 358 genes known to be involved in systems relevant to pain perception. The included genes putatively contributing to TMD and chronic pain states such as nociceptive transmission, inflammation, and mood and affect. The SNPs selected for the panel prioritize functional variants such as those that code for non-synonymous changes or result in differences in expression or alternative splicing, while others were selected as representative markers of regions with high linkage disequilibrium, containing many correlated SNPs that are inherited in blocks, in order to “tag” untyped variation. In this volume,20 Smith and coauthors evaluate associations between these genetic variants and two sets of outcomes: clinically-classified first-onset TMD; and eight intermediate phenotypes measured at enrollment and then found to be predictors of first-onset TMD. The intermediate phenotypes were: 1) the number of comorbid health conditions17 2) the number of non-specific orofacial symptoms 15; 3) the number of masticatory muscle groups painful during examination procedures,15 4) a global score of sleep quality, 17 5) global psychological and somatic symptoms 6; 6) stress and negative affectivity 6; and 7) heat pain temporal summation,10 and 8) pressure pain threshold.10 The latter four measures were latent constructs obtained from principal component analysis, as reported in the respective papers.
In the association analysis with clinically-classified TMD, no single SNP was significantly associated with risk of TMD onset. After correction for multiple testing, however, several SNPs exceeded false discovery rate thresholds for association with intermediate phenotypes. A SNP within a gene encoding a sodium channel protein (SCN1A, rs6432860) was significantly associated with non-specific orofacial symptoms, as was a SNP within a gene encoding an angiotensin enzyme (ACE2, rs1514280). Another SNP within a gene encoding enzymes that catalyze the conversion of arachidonate to prostaglandin (PTGS1 also labelled COX-1, rs3842803) was significantly associated with global psychological and somatic symptoms, while a SNP within a gene encoding an amyloid precursor protein (APP, rs466448) was associated with the stress and negative affectivity factor. Finally, temporal summation of heat pain with significantly associated with a SNP within a gene encoding multiple PDZ domain protein (MPDZ, rs10809907).
The authors commented on the value of analyzing intermediate phenotypes, noting that the observed genetic associations with intermediate phenotypes were stronger than for clinically-classified TMD. Furthermore, many intermediate phenotypes are measured as continuous variables, potentially increasing statistical power to detect associations. They emphasized that the observed associations with intermediate phenotypes should be examined in other cohorts. They also suggested that the five implicated genes should become high priorities for investigation with acute and chronic TMD in large, case-control studies. Notwithstanding this need for additional studies, the authors noted the value of dissecting the genetic architecture underlying the physiological and behavioral domains of pain susceptibility, in that novel etiological pathways and therapeutic approaches may be revealed.
Bair and coauthors: Multivariable analysis of phenotypic predictors
The final paper3 extended the investigation of “how” risk factors contribute to development of TMD by evaluating the combined effects of all phenotypic measures from the six domains described above: sociodemographics, clinical orofacial characteristics, general health status, pain sensitivity, cardiac autonomic function and psychological characteristics. Genetic variables were not used. In addition to the random forest models described above, a multivariable Lasso model used penalized Cox regression to evaluate the full set of 202 phenotypic predictor variables. Both methods identified the most important variables and quantified their contribution to first-onset TMD relative to the other predictor variables. The random forest models also evaluated non-linear effects and interactions, producing partial dependence plots depicting the relationship between each variable and TMD incidence after adjusting for all other observed variables.
Findings from the two modeling approaches were consistent in revealing that variables from the health status domain made the greatest contribution to predicting TMD incidence, followed closely by the psychological and clinical orofacial domains. Socio-demographic, pain sensitivity and cardiac autonomic function domains made smaller contributions. Among the individual variables, four stood out as the most important predictors: a greater number of comorbid conditions; a greater number of non-specific orofacial symptoms; indicator variables for study site; and higher scores on the bodily pain scale from the SF-12. The first two measures are notable because they are simple checklists of commonly-occurring experiences, rather than precise, psychometrically-validated measures of an identified construct. Specifically, the checklist of 20 comorbid conditions was created for the OPPERA study and includes both painful conditions (e.g., irritable bowel syndrome) and other conditions that are not primarily painful (e.g. depression). The list of six nonspecific orofacial symptoms includes aversive sensations of the face and jaw that were not primarily painful (e.g., fatigue and stiffness) or that reflect sub-pain symptoms (e.g., soreness or tenderness). These measures emerged as the most important predictors despite the random forest model’s inclusion of more specific and better validated measures, such as the ROME-II questions about irritable bowel syndrome, the SCL-90R depression subscale, and the Jaw Functional Limitation Scale. Likewise, TMD incidence was strongly associated with the SF-12 bodily pain scale, a single question about the degree to which overall pain interferes with work, but not with more comprehensive measures of orofacial pain (e.g., clinical measures of palpation tenderness).
Other important predictors of TMD incidence were oral parafunctions, symptoms of limited jaw movement, clinical findings of masticatory muscle tenderness, and the PILL score of somatic awareness. Partial dependence plots confirmed several of the non-linear associations seen in the within-domain analyses described above. Meanwhile, the sociodemographic characteristics of Asian race and lifetime US residence were less pronounced predictors, implying that their associations with TMD incidence observed in univariate analysis were confounded by variables from other domains. Yet, age and study site differences in TMD incidence were observed even after adjusting for other OPPERA variables, suggesting that as yet unidentified environmental/cultural factors also contribute to the development of TMD. Conversely, some measures that were not associated with TMD incidence in the univariate analyses emerged as modestly important predictors in the random forest model. They included marital status and some measures of cardiac autonomic function. In general, standardized hazard ratios associated with the variables in the lasso model were noticeably attenuated compared to the corresponding univariate HR’s. This suggests that the etiology of first-onset TMD is strongly multifactorial and that any single factor is likely confounded to some degree (although not completely) by other factors.
Random forests proved to be a valuable method for evaluating cross-cutting phenotypic domains, each comprising multiple variables, many of which were strongly correlated. The method identified the most important predictors of TMD incidence in this cohort, with partial dependence plots often revealing non-linear relationships. Because random forest models are complex and may be unfamiliar, the introduction by Bair et al3 discusses underlying concepts and the benefits of the method as a data mining tool for managing large numbers of predictor variables. However, the authors note that the models are impractical for the purpose of predicting individuals who are most likely to develop TMD, since the method requires data from all 202 variables. Instead, future research is needed to create a clinically-useful prediction model. While random forest models provided valuable insights into the most important predictors of first-onset TMD, the authors also caution that other variables should not be discounted. Such variables may have been excluded simply because they were strongly correlated with another variable.
Study limitations
While previous analysis of baseline data18 found that characteristics of OPPERA participants were consistent with population benchmarks, the volunteer-participants were not selected at random from the population. Hence, TMD incidence rates and associations reported in these papers may not be generalizable to the U.S. adult population. Nonetheless, the sample comprised major U.S. demographic groups, suggesting broad applicability of these OPPERA findings to generally healthy U.S. adults aged 18–44 years who have no history of TMD. The restricted age range is an important caveat, especially in view of cross-sectional data from the U.S. population showing that prevalence of TMD in women peaks around the fifth decade18. Inevitably during follow-up, there were problems of loss-to-follow-up and, as reported by Bair et al, 2 problems of incomplete or dubious data created some bias that had to be addressed using multiple imputation, adding to the complexity of data analysis. The 260 incident cases limited power to detect associations, a problem that was most notable when evaluating genetic associations using over 3,000 genetic markers. Finally, even after accounting for the extensive array of predictor variables measured at baseline, there were large differences in TMD incidence between the four study sites, suggesting that there are other important cultural or environmental variables not measured in OPPERA that contribute to first-onset TMD.
Implications and future directions
By showing that incidence of first-onset TMD is influenced by a multitude of risk factors, the papers in this volume confirm that TMD is a complex condition best viewed within a biopsychosocial model of illness. Indeed, the biopsychosocial model was implicit in the design of the OPPERA project which used a heuristic model4 integrating evidence from previous biological and clinical studies of pain. Findings from the baseline case-control study led to refinement of the model14 in which genes are depicted as an upstream biological determinant of several intermediate phenotypes. Together with environmental influences, they produce downstream phenotypic changes that increase individuals’ risk of developing TMD. Much of the evidence used to formulate and refine the heuristic model derived predominantly from cross-sectional studies of chronic pain in humans, clinical- and animal-experimental studies of experimental pain, and in-vitro studies of nociception.
With their focus on incidence of first-onset TMD, these papers begin to fill a gap in knowledge about factors that initiate the condition. The results confirm the salience of upstream genetic influences and intermediate phenotypes as determinants of first-onset TMD. The findings also clarify contributions of additional intermediate phenotypes that previously might have been overlooked either as pre-clinical or comorbid conditions of little prognostic significance. In particular, development of TMD was strongly influenced by global constellations of orofacial symptoms and non-specific measures of general health status. Meanwhile, other results suggest that measures of pain amplification are probably more critical determinants of the transition to chronic TMD than initial onset of the condition. Taken together, these discoveries contribute to current concepts of TMD etiology and have implications for clinical management of chronic pain. There are additional implications for future research, including a need for new studies that focus on cumulative effects of biological parameters and intermediate phenotypes over time.
The effects of time were not depicted explicitly in the OPPERA heuristic model.14 There was implicit recognition of a temporal continuum from pre-clinical signs and symptoms to first-onset TMD and then to persistent TMD. In this implicit temporal model, pre-clinical symptoms such as jaw stiffness or fatigue represent early indicators of clinical TMD that are intermediate variables in the etiologic pathway connecting underlying causes of TMD to clinically-classified TMD. In other words, the pre-clinical symptoms are proximal consequences of the underlying causes, not independent causes of TMD. Three aspects of the current findings call for a reassessment of that type of causal thinking. Many people without pre-clinical symptoms developed TMD, implying that other factors were also capable of causing painful TMD without first causing the non-specific symptoms. Second, most episodes of first-onset TMD occurred some years after enrollment, implying a lengthy period of dormancy if the nonspecific symptoms were merely pre-clinical. Third, the association between symptoms and TMD incidence persisted in multivariable models that adjusted for the intermediate phenotypes that are depicted as primary causes of TMD. If the non-specific symptoms were solely intermediaries between causal effects and TMD onset, adjustment for the intermediate phenotypes should nullify the association between the symptoms and clinical TMD.
Instead, the finding of a strong, independent contribution of nonspecific orofacial symptoms to first-onset TMD suggests that they might be a marker, rather than consequence, of dysregulation in another causal domain. Somatic awareness was speculated as one such type of dysregulation, but that seems unlikely given that the non-specific orofacial symptoms had independent effects in the multivariable models that simultaneously adjusted for measures such as the PILL.3 Alternatively, an intriguing possibility comes from the analysis of genes associated with intermediate phenotypes20 with the results showing that the non-specific orofacial symptoms were associated with variants in genes encoding a sodium channel protein and an angiotensin enzyme. If so, those biological processes and the symptoms they elicit might represent a new domain of intermediate phenotypes in an expanded OPPERA heuristic model.
Comorbid conditions are another group of characteristics that need to be understood within the context of time. Co-occurrence of multiple conditions is common in patients with a given chronic illness1. It requires an arbitrary judgment to label one of the conditions as an “index” condition, and others as “comorbid” conditions, although the labels do not clarify the causal relationship (if any) between the conditions.21 Importantly, if comorbid conditions are regarded as “collateral damage” caused by the same underlying risk factors that cause the index condition, then it follows that adjustment for the underlying factors will nullify the association between comorbid and index conditions. This was not the case in the multivariable analysis that found a disparate set of health conditions to be the strongest predictors of TMD incidence, even after adjustment for all putative risk factors. An important caveat is that there likely were important, unmeasured risk factors for TMD, as evidenced by the finding that there were substantial, residual differences in TMD rates between study sites in the multivariate random forest model. 3 Potentially, those risk factors might explain the associations of TMD incidence and comorbid conditions observed here. They might also reveal a distinct domain of intermediate phenotypes with associated genetic influences that collectively could be included in an expanded heuristic model of TMD etiology.
One strategy to investigate these temporal effects is to measure risk factors and protective factors repeatedly over appropriate time intervals, evaluating their cumulative effects. Studies could also select participants who have reached one phase, and follow them prospectively to evaluate persistence of the condition, transition to another phase, or remission. In addition to addressing questions about preclinical and comorbid conditions, such studies might shed light on the cumulative effects on chronic pain of vulnerability and resilience factors that interact over time. New research undoubtedly will produce evidence to expand the OPPERA heuristic model, providing additional insights as to the etiology of TMD and prognosis for patients who develop it.
We have made some steps in that direction by extending the follow-up of this cohort. OPPERA’s ongoing investigations will include more detailed assessment of injury and life stressors than the baseline measures reported here, thereby enabling us to detect a greater contribution of environmental influences on first-onset TMD. We also intend to extend investigation of the biological domain through genome-wide association studies, evaluation of some rarely-occurring genetic variants, and assessment of circulating inflammatory mediators.
For now, the results have important clinical implications for our understanding of pain “as a condition in its own right”.12 The findings to date from OPPERA’s studies of first-onset TMD and chronic TMD show unequivocally that TMD is a complex disorder that must be envisaged within a biopsychosocial model of illness. It is a misnomer and no longer appropriate to regard TMD solely as a localized orofacial pain condition. Likewise, it is pointless to envisage a single cause, nor even to expect that any one cause might be necessary or sufficient. For the majority of people with chronic TMD, the condition is a multisystem disorder with overlapping co-morbidity. One of the clinical challenges is to distinguish incidental findings from those that have prognostic or etiological significance. These papers suggest that a diverse set of symptoms and health-related characteristics are strong predictors of first-onset TMD, and not merely consequences of TMD or “collateral damage” from its underlying causes.
Perspective.
Collectively, the papers in this volume demonstrate that TMD is a complex disorder with multiple causes consistent with a biopsychosocial model of illness. It is a misnomer and no longer appropriate to regard TMD solely as a localized orofacial pain condition.
Acknowledgments
The authors would like to thank the OPPERA research staff for their invaluable contributions to this work. In addition, we express our gratitude to the participants who have devoted time and effort in support of this research.
Footnotes
This work was done at: University of North Carolina at Chapel Hill, NC; University at Buffalo, NY; University of Maryland-Baltimore, MD; University of Florida, FL; and Battelle Memorial Institute, NC.
Disclosures
This work was supported by NIH grant U01DE017018 and P01NS045685. The OPPERA program also acknowledges resources specifically provided for this project by the respective host universities: University at Buffalo, University of Florida, University of Maryland-Baltimore, and University of North Carolina-Chapel Hill. Gary Slade and Roger Fillingim are consultants and equity stock holders, and William Maixner and Luda Diatchenko are cofounders and equity stock holders in Algynomics, Inc., a company providing research services in personalized pain medication and diagnostics. Other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med. 2001;134:868–81. doi: 10.7326/0003-4819-134-9_part_2-200105011-00011. [DOI] [PubMed] [Google Scholar]
- 2.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon S, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study design, methods, sample characteristics and loss-to-follow-up: the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.06.006. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, Helgeson E, Knott C, Maixner DW, Slade GD. Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.09.003. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin S, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 6.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological Factors Associated with Development of TMD: the OPPERA Prospective Cohort Study. J Pain. doi: 10.1016/j.jpain.2013.06.009. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T46–60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, Ohrbach R, Maixner W. Summary of findings from the OPPERA baseline case-control study: implications and future directions. J Pain. 2011;12:T102–7. doi: 10.1016/j.jpain.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W. Pain sensitivity and autonomic risk factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.06.007. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 13.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Social science & medicine. 1994;39:887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 14.Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. Orofacial pain prospective evaluation and risk assessment study--the OPPERA study. J Pain. 2011;12:T4–11. e1–2. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohrbach R, Bair E, Fillingim RB, Gonzalez Y, Gordon S, Lim PF, Ribeiro-Dasilva M, Diatchenko L, Dubner R, Greenspan JD, Knott C, Maixner DW, Smith SB, Slade GD. Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.07.018. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.06.001. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12:T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and socio-demographic predictors of its development: the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.07.014. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Weir B, Maixner W, Diatchenko L. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. J Pain. doi: 10.1016/j.jpain.2013.09.004. in press (in this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7:357–63. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]