Abstract
CCN1, also known as CYR61, is a survival and pro-angiogenic factor overexpressed in about 30% of invasive breast carcinomas, and particularly in triple negative breast carcinomas (TNBC). CCN1 expression in breast cancer promotes tumorigenicity, metastasis, antihormone and chemo-resistance. TNBCs often develop bone metastasis, thus the vast majority of patients receive bisphosphonate treatment as a companion to chemotherapy. Zoledronic acid (ZOL), a bisphosphonate currently in use, inhibits bone resorption, prevents development of new osteolytic lesions induced by tumor metastasis, and has a direct anti-tumor activity in breast cancer cells and tumors. We have shown that ZOL inhibits anchorage independent growth as well as branching and morphogenesis in CCN1 overexpressing cells. However, the mechanism is not yet well understood. In this study, we investigate the effect of ZOL in breast cancer cells with high and with undetectable CCN1 expression levels. We demonstrate that CCN1 expressing cells are more sensitive to ZOL, that ZOL induces downregulation of the CCN1 promoter activity and CCN1 protein expression in a dose dependent manner, and that ZOL is associated with a decrease in phosphorylated Akt and translocation of FOXO3a, a negative regulator of CCN1 expression, to the nucleus. Deletion of the FOXO3a binding site in the CCN1 promoter prevents ZOL inhibition of the CCN1 promoter activity demonstrating that FOXO3a transcriptional activation is necessary for ZOL to induce CCN1 inhibition. This study provides evidence that ZOL targets the pro-angiogenic factor (CCN1) through FOXO3a, and reveals a new mechanism of ZOL action in breast cancer cells.
Keywords: CYR61/CCN1, bisphosphonates, Zoledronic acid, growth, breast cancer
INTRODUCTION
Human breast cancers are a heterogeneous group of tumors displaying significant diversity in clinical behavior, outcome, and response to therapy (1, 2). The prognosis and management of breast cancer are influenced by the status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) within the tumor. The mortality rate in breast carcinomas is decreasing due to recent developments in diagnostic techniques and therapies; however, the mortality in triple-negative breast carcinomas (TNBC) that are ER negative, PR negative and HER2 negative remains high (3,4). In addition to being a clinically heterogeneous disease, breast cancer is a molecularly heterogeneous disease making it even more difficult to find a single treatment. CCN1 is an extracellular matrix-associated protein of the cysteine rich 61/connective tissue growth factor/nephroblastoma overexpressed (CCN) family which includes also CCN2 (CTFG), CCN3 (NOV), CCN4 (WISP-1), CCN5 (WISP-2), and CCN6 (WISP-3). All CCN proteins, including CCN1, mediate functions as diverse as cell proliferation, migration, adhesion, cell survival, differentiation, and extracellular matrix formation. CCN1 is unique among the CCN proteins in its ability to induce angiogenesis and tumorigenesis (5–7). CCN1 is a survival and pro-angiogenic factor overexpressed in about 30% of triple negative breast carcinomas (6, 8). Previously, we demonstrated that CCN1 is highly expressed in metastatic cell lines and that its expression is inversely correlated with ER expression, response to Estrogen (E2), and sensitivity to antiestrogens (9). The expression of CCN1strongly correlates with vimentin expression, a known marker for invasiveness (10), and is associated with the ability of breast cancer cells to invade in vitro and metastasize in vivo. Sampath et al. (11) demonstrated that CCN1 is overexpressed in 70% of breast cancer patients with infiltrating ductal carcinomas of the breast and that CCN1 was localized exclusively in hyperplastic ductal epithelial cells. These studies have shown a significant correlation between elevated levels of CCN1and an advanced stage of the primary tumor and lymph node involvement at the time of removal of the primary tumor. Patients with advanced breast cancer often develop bone metastases that can cause pathological fractures. Most patients receive bisphosphonate treatment as a complementary treatment to the chemotherapy. ZOL, a third generation bisphosphonate, is a bisphosphonic acid, a heterocyclic nitrogen-containing bisphosphonate that has an imidazole-ring side chain. The imidazole ring contains two critically positioned nitrogen atoms (Fig. 1). ZOL inhibits osteoclast activity and interrupts the cycle of osteoclast-tumor cell interactions (12). ZOL also appears to directly and indirectly inhibit tumor growth (13–15) alone or in combination with chemotherapeutic drugs, molecular targeted agents and other biological agents (16–18). In vitro ZOL inhibits the growth of breast cancer cells via apoptosis and cytostasis, exhibits synergistic cytostatic effects with other anticancer drugs, displays anti-angiogenesis effects, stimulates T-lymphocyte activity against tumor cells, and modulates tumor cell adhesion and invasion(17,19–24).
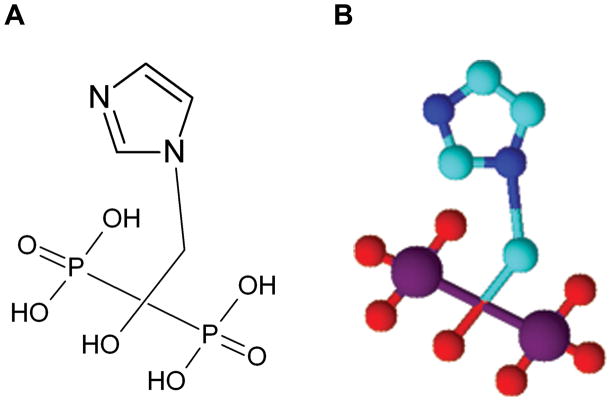
Figure 1.
Chemical structure of zoledronic acid, a new-generation heterocyclic aminobisphos-phonate compound: 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid. A) Skeletal structure of ZOL. It shows the imidazole ring containing the two nitrogen atoms, and B) 3-dimensional structure of ZOL created using ACD/ChemSketch, Version 12.01.
We recently demonstrated that ZOL decreases the transcriptional activity of the CCN1 promoter in androgen-independent prostate cancer cells (25). As a result, we anticipate that CCN1 could be a suitable target to enhance the anti-tumor effect of ZOL in a subpopulation of breast cancer tumors. CCN1 is overexpressed in aggressive and metastatic breast cancer cells, including MDA-MB-231, HS578T and BT549 cells, all of which are derived from TNBC (26). Coincidentally, ZOL treatment of MDA-MB-231 cells results in anti-proliferative effects in vitro and anti-osteolytic effects in vivo (27).
In this study we used cells that express high levels of CCN1 to analyze the inhibitory effect that ZOL had on growth, branching and morphogenesis, CCN1 expression, and its possible mechanism of action. Cells overexpressing CCN1 were shown to be specifically inhibited by ZOL, and that CCN1 was down-regulated at the protein and transcriptional level in our experimental models. We also assessed the mechanisms by which ZOL downregulates CCN1 thus promoting tumor growth inhibition. Our data suggest a relationship between the anti-tumor activity of ZOL and CCN1 expression. This association can be explored as a therapeutic initiative for treating the sub-population of breast cancer patients with high levels of CCN1 expressing tumors.
MATERIAL AND METHODS
1. Cell culture
Human derived breast cancer cell lines MDA-MB-231 and MCF-7, were obtained from American Type Culture Collection on 1987and maintained in monolayer in IMEM medium supplemented with 5% FBS (Fetal Bovine Serum) at 37°C in a humidified 5% CO2 atmosphere. Cells were last time genotyped on August 13th, 2010 to ensure their identity using the short tandem repeat profiling method provided by the Genotyping Shared Resource, Mayo Clinic Rochester, MN.
2. Generation of MCF-7 cells expressing CCN1 full-length cDNA
MCF-7/CCN1 breast cancer cell line was developed by transducing MCF-7 cells with retroviruses containing CCN1 full-length cDNA. A cell line containing the retroviral vector alone was generated as control (MCF-7/pBabe). Both cell lines were established by their resistance to puromycin and by having high level of CCN1 expression (MCF-7/CCN1) or low or undetectable expression levels of CCN1 (MCF-7/pBabe).
3. Zoledronic acid
Pure crystalline ZOL, in the form of Zometa® was obtained from the Rochester Mayo Clinic Pharmacy. Zometa® is produced by Novartis Pharma AG (Stein, Switzerland).
4. Cell viability
Drug sensitivity of breast cancer cells growing in monolayer was determined using a standard colorimetric MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] reduction assay (Promega, Madison, WI). Cells were plated at a density of 3,000 cells/100μl/well in 96-well microtiterplates and allowed 24 hours to attach. The medium was then removed and fresh medium containing increasing concentrations of ZOL was added. Control cells were cultured using the same conditions but in the absence of ZOL. When the untreated controls reached confluence the medium was carefully aspirated, MTT dye solution was added and incubated for 4h at 37°C. MTT-formazan crystals were dissolved in dimethyl sulphoxide (100μl/well) and the absorbance was measured at 570nm in a multi-well plate reader Wallac Victor™ III (PerkinElmer, Waltham, MS). Cell viability effects from exposure to ZOL were analyzed plotting concentration-effect curves of the fraction of unaffected (surviving) cells versus drug concentration. For each treatment, cell viability was calculated as a percentage using the following equation: A570 of treated sample/A570 of untreated sample × 100 (A570-Absorbance at 570nm). Drug sensitivity was expressed in terms of the concentration of drug required for 50% (IC50) decrease in cell viability. Since the percentage of control absorbance was considered to be the surviving fraction of cells, the IC50 values were defined as the concentration of drug that produced 50% reduction in control absorbance (by interpolation).
5. Soft-agar colony formation assays
The efficiency of colony formation in liquid culture was determined by monitoring anchorage-independent cell growth in soft-agar in the presence of increasing concentrations of ZOL. A bottom layer of 1.5ml (2X) IMEM containing 1.2% agar and 10% FBS was prepared in 6 well-plates. After the bottom layer solidified, cells (10,000/well) were added in a 1ml top layer of 0.7% agar containing increasing concentrations of ZOL (10, 15, and 25 μM). All samples were prepared in triplicate. Plates were incubated in a humidified 5% CO2 incubator at 37°C. At day eight colonies measuring ≥50μm were counted using a cell colony counter (Optronix GelCount™; Oxford Optronic, Milton Park, Oxford, OX14 4SA, United Kingdom). With this assay we determined that ZOL inhibits the anchorage-independent growth typical for the metastatic MDA-MB-231 cells.
6. Annexin V-FITC/PI assay of apoptotic cells
Annexin V-FITC kit (Promega, Madison WI) was used to assess apoptotic cell death. The assay was performed as directed by the manufacturer. Briefly, cells were harvested and resuspended in Annexin V binding buffer. Then 10μl of Propidium Iodine (PI) (50μg/ml) and 5μl of Annexin V-FITC were added per reaction. Samples were incubated 15 min in the dark and then analyzed by flow cytometry. The samples were analyzed in a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cell Quest software (Becton Dickinson, Franklin Lakes, NJ) was used for data acquisition and analysis.
7. Three-dimensional culture on Matrigel
The 3D cell culture assays were performed as previously described (28). Briefly, single-cell suspensions of cells were prepared using trypsin. In an eight-well chamber slide, on top of a polymerized layer of 100% Matrigel, 2×103 cells/well were plated in 0.4mL of 2% Matrigel in IMEM medium containing increasing concentrations of ZOL (5, 10 and 25μM). Cultures were kept for 5 days. Phase-contrast images were obtained under ×100 magnification.
8. CCN1-transcription assay
Cells were seeded (2×104) in 24well-plates in IMEM medium containing 10% FBS. After 24 hours cells were washed and then transiently transfected with luciferase reporter constructs using FuGene6 reagent (Roche Biochemicals, Indianapolis, IN) according to the manufacturer’s instructions. Briefly, cells were transiently transfected with: a) an internal control plasmid pRL-CMV (50ng/well); b) empty vector (pGL3) (500ng/well); CCN1-promoter (pGL3-CCN1-Luc) (500ng/well); c) CCN1 promoter containing a FOXO3a deletion (pGL3-ΔFOXO3a-Luc) (500ng/well); d) CCN1 promoter containing a deletion in the −176 region (pGL3-Δ176-Luc) (500ng/well) and e) CCN1 promoter containing a AP-1 deletion (pGL3-ΔAP-1-Luc) (500ng/well). The internal control plasmid was used to correct for transfection efficiency. After 24h of the transfection cells were washed with fresh media, treated and incubated in fresh medium containing 10% FBS supplemented with increasing concentrations of ZOL 10, 15 and 25μM in IMEM containing 10%FBS. Twenty four hours after ZOL treatments the cell extracts were prepared and luciferase activity was measured using a Dual Luciferase Assay kit (Promega, Madison, WI) according to the manufacturer protocol using a luciferase multi-well plate reader Wallac Victor™ III (PerkinElmer). Quadruplicate luciferase activities were normalized to Renilla luciferase (pRL-CMV)and the ratios between Luciferase/renilla activities were determined.
9. Immunoblotting analysis
Cells were seeded in 6-well plates and allowed an overnight period for attachment. The medium was then removed and fresh medium, along with various concentrations of ZOL, was added. Control cells without the agent were cultured using the same conditions with comparable medium changes. Cells were treated for 24h. The next day, cells were harvested, washed twice with PBS and then lysed with 1XCell lysis buffer (Cell signaling, Danvers, MA) for 30 minutes on ice with vortexing every 5 minutes. The lysates were then cleared by centrifugation (10 minutes at 14,000 rpm - 4°C). Protein concentration was determined using the Pierce® BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein (30μg) were resuspended in 5x Lane marker reducing sample buffer (Thermo Scientific, Rockford, IL) and denaturated for 5 minutes at 99°C. Then the proteins were resolved by electrophoresis on 10% polyacrylamide gel (Criterion XT Bis-Tris precast Gel; Bio-Rad; Hercules, California), and transferred onto PVDF membrane (Amershand Biosciences; Buckinghamshire, HP7 9NA UK). The membranes were blocked with TBS-T (TBS and 0.5%Tween 20) containing 5% (w/v) non-fat dry milk for 1h and incubated with a rabbit anti-CCN1 (H78) polyclonal antibody (1:2,000) (Santa Cruz Biotechnology, Santa Cruz, CA) for 1h at room temperature. Different membranes were blocked with 5% BSA for 1h and incubated with the polyclonal anti-MAPK, anti-phospho-MAPK, anti-Akt, anti-phospho Akt antibodies (1:2,000) (Cell signaling, Danvers, MA) overnight at 4°C. The membrane to detect FOXO3a was blocked with 5% milk and incubated overnight with a polyclonal anti-FOXO3a antibody (1:1,000) (Upstate, Billerica, MA). After three washings with TBS-T, blots were incubated with a 1:2,000 dilution of horseradish peroxidase-linked donkey anti-rabbit IgG secondary antibody. Proteins were detected by the enhanced chemoluminescence reaction using Hyperfilm (Amershand-Pharmacia, Piscataway, NJ).
10. Statistical Analysis
Data are expressed as mean ± SEM. ANOVA and Tukey-Kramer Multiple Comparisons were used to determine statistical significance using GraphPad Prism Version 4.0 statistical package (San Diego, CA, USA). A P-value of less than 0.05 was considered significant.
RESULTS
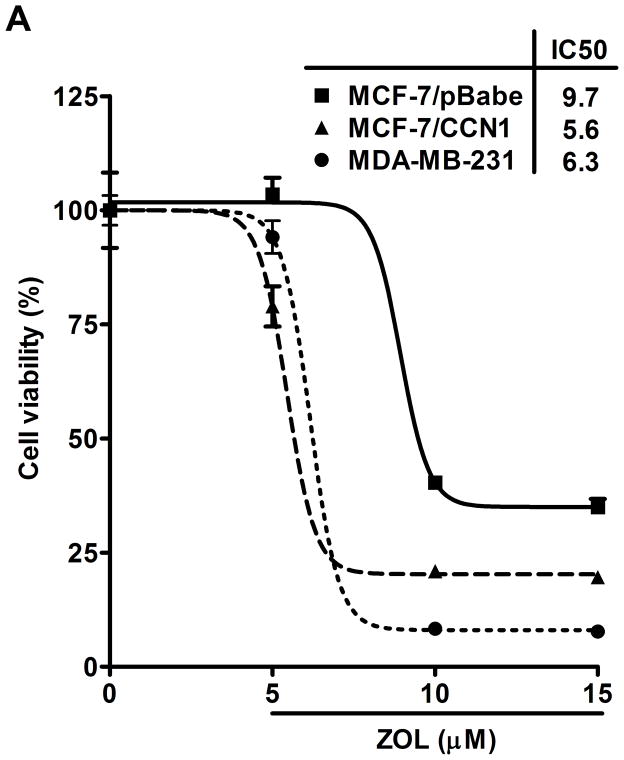
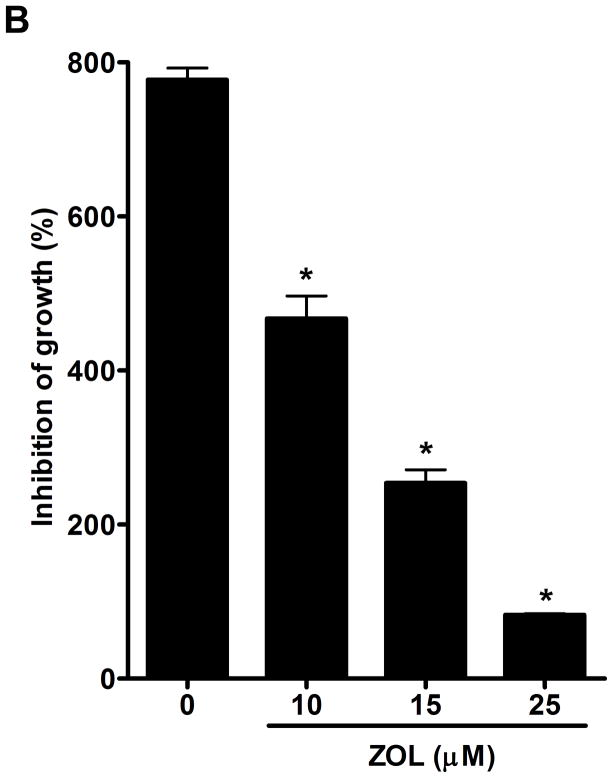
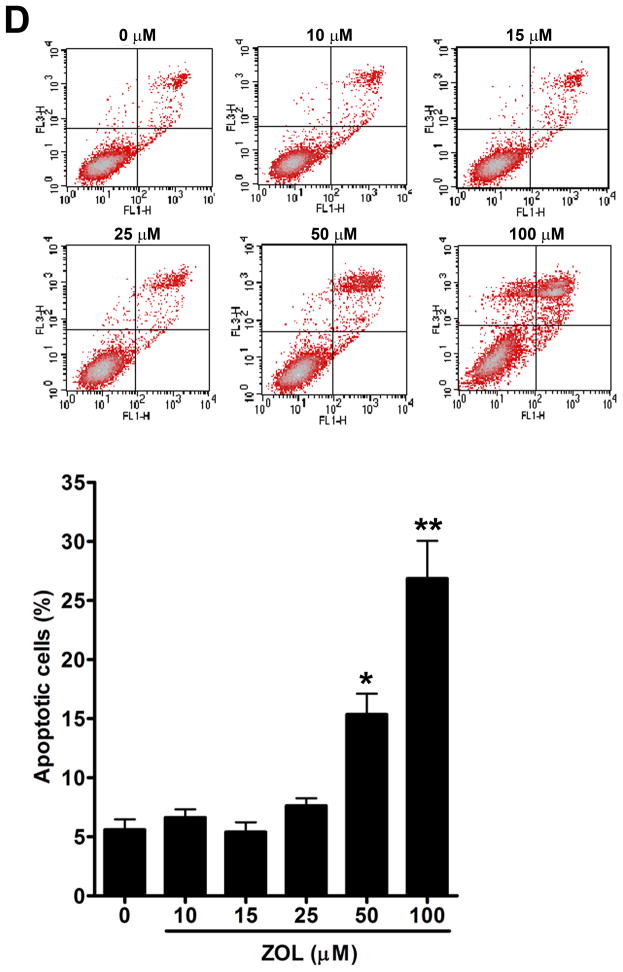
ZOL inhibits growth and induces apoptosis of CCN1-overexpressing breast cancer cells: The viability of MDA-MB-231 cells, which express high levels of endogenous CCN1 (7), was considerably reduced after the treatment of ZOL in a dose dependent manner (Fig. 2A). Additionally, we tested the effect of ZOL on a cell line engineered to overexpress CCN1 (MCF-7/CCN1) and their vector control cell line (MCF-7/pBabe). Strikingly, we observed that MCF-7/CCN1 cells were as sensitive as the MDA-MB-231 cells to ZOL.(Fig. 2A) (the IC50 for MDA-MB-231 was 6.3μM and the IC50 for the MCF-7/CCN1 was 5.6μM). Remarkably, ZOL inhibited anchorage-dependent and anchorage–independent growth of MDA-MB-231 cells by about 90% (p<0.05) (Fig. 2B for anchorage-independent growth). ZOL had no effect on the growth of MCF-7/pBabe cells (data not shown). Cell culture in 2D suggests that ZOL treatment affected the cell growth of MDA-MB-231 and MCF-7/CCN1 cells in a dose dependent manner (Fig.2C). To assure that the effect of ZOL was not due to toxicity, we determined whether ZOL induced apoptotic cell death in MDA-MB-231 cells. Apoptosis was determined by Annexin V-FITC staining and PI labeling. Early apoptotic cells were positive for Annexin V and negative for PI and then analyzed by flow cytometry. ZOL treatment of MDA-MB-231 cells resulted in increased staining for Annexin V in a dose-dependent manner (Fig. 2D). The number of apoptotic cells (7% – 35%) augmented with increased doses of ZOL (0–100μM) (p<0.05). Necrosis induced by ZOL was observed only at 50 and 100μM of ZOL (Fig. 2D). Overall our results suggest that breast cancer cells expressing CCN1 are sensitive to ZOL treatment inducing cell death by apoptosis.
Figure 2. ZOL inhibits the growth and induces apoptosis in cells overexpressing CCN1.
A). In order to evaluate how ZOL affects metastatic breast cancer cell viability MTT-based metabolic assays (as described in Material and Methods) in cells overexpressing endogenous CCN1(MDA-MB-231) and CCN1 overexpressing cells (MCF-7/CCN1) were cultured in the absence or presence of equimolar concentrations of ZOL for 3 days. B). ZOL inhibits the anchorage-independent growth of MDA-MB-231 cells. The ability of MDA-MB-231 cells to growth in soft agar in the presence/absence of ZOL was assessed using anchorage-independent colony-formation assay. Subconfluent breast cancer cells were plated (10,000 cells/well) in triplicate in 6 well plates for anchorage-independent soft agar assay as previously described (49). Cells were cultured in the absence or in the presence of different concentrations of ZOL (5, 10, 15 and 25 μM) for around 8 days at 37°C and 5%CO2. Colonies were counted using the colony counter machine. Data are representative from three independent experiments. C). ZOL induces cells death in cells overexpressing CCN1. The effect of different concentrations of ZOL on MDA-MB-231 and MCF-7/CCN1 compared with MCF-7/pBabe (empty vector) cells were observed in 2D culture and photographed in an inverted microscope. D). ZOL induces apoptosis in MDA-MB-231 cells in a dose dependent manner. The cell death induced by ZOL in MDA-MB-231 cells was assessed by Annexin V. Briefly, MDA-MB-231 cells were treated with different concentrations of ZOL (10, 15, 25, 50 and 100μM) for 48h. Then cells were harvested and stained for Annexin V following the instructions of the kit. After staining, cells were analyzed by FACScalibur flow cytometry. Cell Quest software was run for data acquisition and Modfit software for analysis. Data are shown as mean ± SEM of 3 independent experiments. *P< 0.05 from control cells. **P <0.01 from control cells.
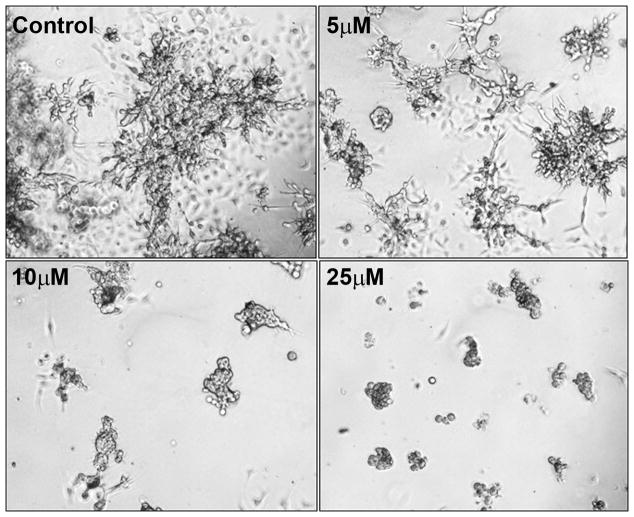
ZOL inhibits branching and morphogenesis of CCN1 expressing cells: To explore whether ZOL affects the growth of MDA-MB-231 cells in 3D cell culture, we cultured these cells on Matrigel in the presence of increasing concentrations of ZOL (5, 10 and 25μM) for 5 days. We found that ZOL inhibited branching and morphogenesis of MDA-MB-231 cells in a dose-dependent manner reaching maximum effect at 10μM (Fig. 3). Our results show that ZOL perturbs morphogenesis and further suggest the likelihood that ZOL could also inhibit tumor invasion and metastasis. Similar results were obtained with other CCN1 expressing cells including HS578T and BT549 (data not shown).
Figure 3. ZOL inhibits MDA-MB-231 growth in three-dimensional Matrigel culture.
Cells (1×103) in 0.4mL of 2% Matrigel in 1X IMEM medium were plated per well of an eight-well chamber slide on top of a polymerized layer of 100% Matrigel, as described (50). Cells were treated with different concentrations of ZOL (5, 10, 25μM) and the cultures were fed every 3 days. Controls cells were kept in medium containing 5% FBS. Phase-contrast images were obtained under x100 magnification.
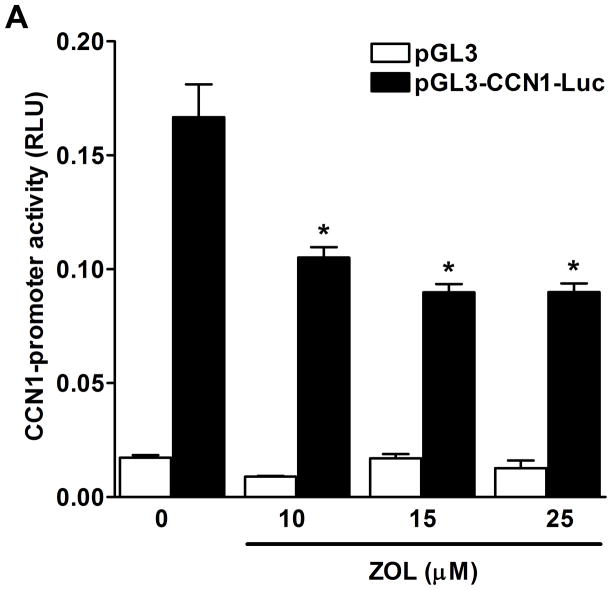
ZOL inhibits CCN1-promoter activity and CCN1-protein expression: To determine whether ZOL regulates CCN1-promoter activity MDA-MB-231 cells were transfected with a Luciferase-CCN1 reporter containing 884bp [from −1093 to −209 upstream of translation start site] and assessed the CCN1 transcriptional activity in response to increasing concentrations of ZOL for 24h (Fig. 4A). Parallel studies were performed to assess the regulation of CCN1 at the protein level. Cells plated in a 6 well/plate were treated with increasing concentrations of ZOL. Cells lysates were prepared and blotted using an anti-CCN1 antibody (Fig. 4B). Results demonstrate that ZOL inhibits CCN1 promoter activity in a dose dependent manner and that it inhibits CCN1 expression at the protein level.
Figure 4. ZOL inhibits CCN1 expression in MDA-MB-231 cells.
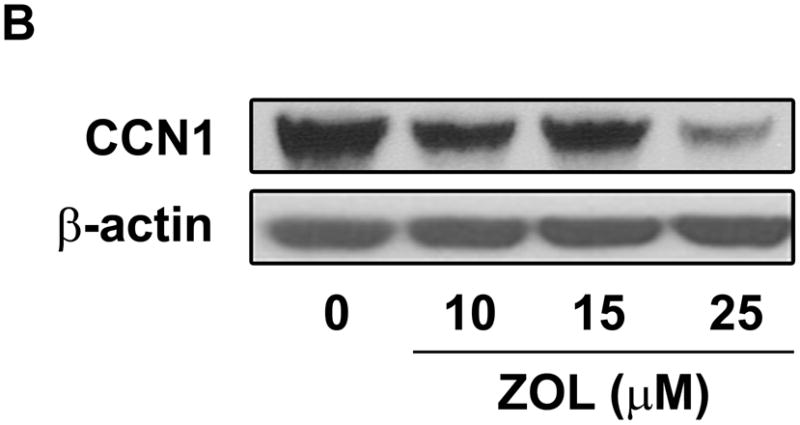
A). MDA-MB-231 cells were transfected with the wild type CCN1-promoter (pGL3-CCN1-Luc) and after 24h cells were treated with increased concentrations of ZOL (10, 15, 25μM) for 24 hours and CCN1-promoter activity was measured. Luciferase activity was normalized by pRL-CMV control plasmid, which was co-transfected to normalize the plasmid transfection efficiency. The data represent means ± SE for 3 independent assays. *P< 0.05 from control cells. B). Subconfluent cells were treated with different concentrations of ZOL (10, 15 and 25μM) for 24h. Cells were lysed and total protein (30μg) was resolved by 10% polyacrylamide gel (Criterion XT Bis-Tris precast Gel) and analyzed by Western blotting with a rabbit polyclonal anti-CCN1antibody. Blots were reprobed with an antibody for β-actin as a control for protein loading.
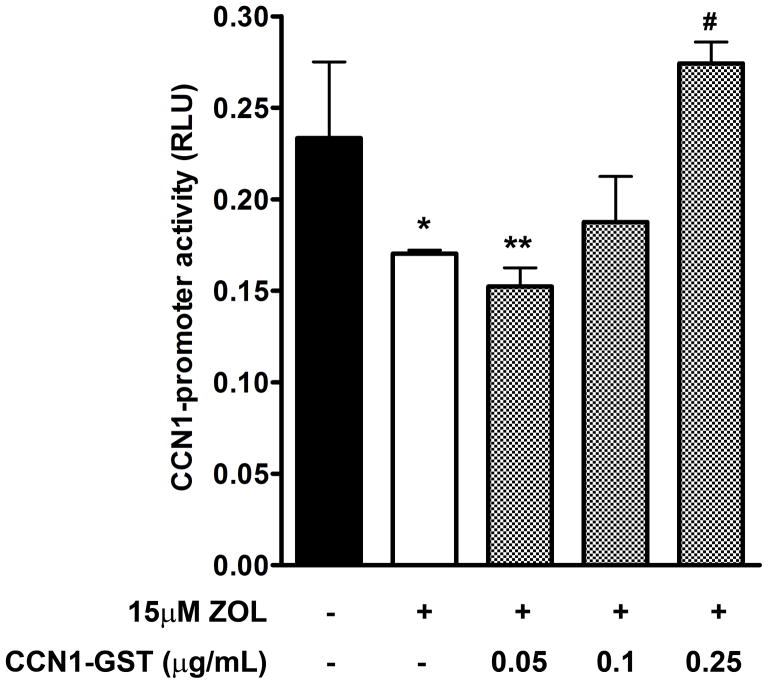
Exogenous CCN1 impair the ZOL effect on CCN1-promoter activity: In additional experiments, we determined whether exogenous CCN1 can modulate the ZOL effect on CCN1-promoter. MDA-MB-231 cells were transfected with pGL3-CCN1-Luc promoter and the activity was measured in the presence or absence of increasing concentrations of CCN1 recombinant protein and the optimal dose of ZOL (15μM). Our results demonstrate that exogenous CCN1 protein reverts ZOL inhibition of CCN1 transcription (Fig. 5), therefore suggesting that ZOL has a specific effect on CCN1–promoter.
Figure 5. Exogenous CCN1 impair the ZOL effect on CCN1-promoter activity in MDA-MB-231 cells.
MDA-MB-231 cells were transfected with the wild type CCN1-promoter (pGL3-CCN1-Luc) and after 24h cells were treated with 15μM ZOL or in the presence or absence of increasing concentrations of CCN1 recombinant protein (CCN1-GST). *P<0.05 versus control cells (untreated); **P< 0.01 versus control cells; #P<0.05 versus cells treated with 15μM ZOL.
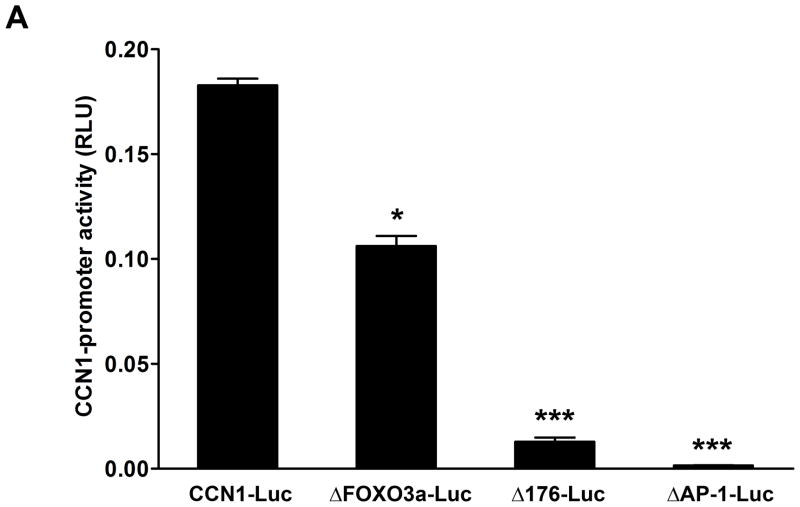
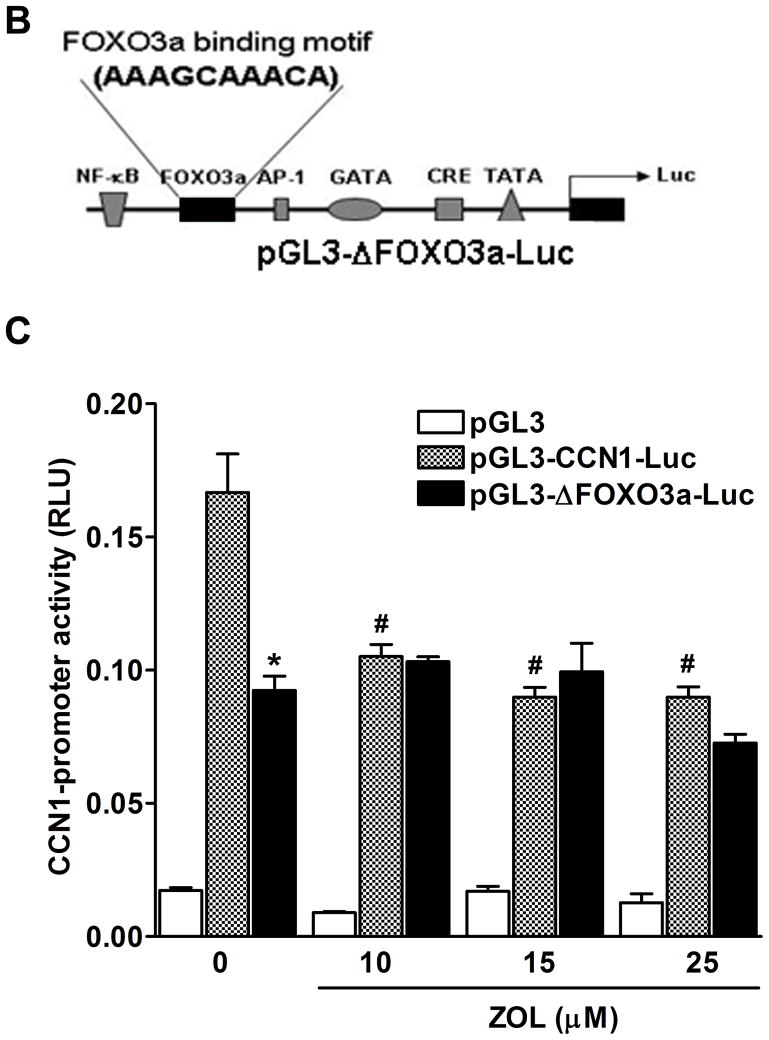
Transcriptional regulation of CCN1 gene by ZOL is dependent on FOXO3a: CCN1 contains different binding sites for transcription factors that regulate its expression (29).Transcriptional regulation of CCN1 promoter wild type and CCN1-promoter containing different deletions was assessed using Luciferase-reporter assay. The constructs used were: 1) −565 (pGL3-ΔFOXO3a-Luc), 2) −176 (pGL3-Δp-176-Luc), and 3) deletions to positions −936 (pGL3-ΔAP1-Luc). Results showed that deletions at the positions −176 (pGL3-Δp-176-Luc) and −936 (pGL3-ΔAP1-Luc) resulted in a strong inhibitory effect in the CCN1 promoter activity even in the absence of ZOL treatment (Fig. 6A). Previous studies showed that the promoter of the human CCN1 gene contains a forkhead 3a binding motif (FOXO3a) (30). To study the role of FOXO3a in the transcriptional regulation of CCN1, a deletion mutant of the CCN1 promoter was generated (from −565 to −209 upstream of ATG; pGL3-ΔFOXO3a-Luc) (Fig.6B). Then the transcriptional activity of CCN1-promoter-Luc reporter constructs pGL3, pGL3-CCN1-Luc, and pGL3-ΔFOXO3a-Luc were analyzed after treatment with increasing concentrations of ZOL (Fig. 6C). As we expected increasing concentrations of ZOL reduced the transcriptional activity of the wild type CCN1-promoter.
Figure 6. Inhibition of CCN1-promoter activity by ZOL is dependent on FOXO3a binding site.
A). Transcriptional regulation of CCN1 promoter wild type and CCN1-promoter containing different deletions was assessed, using Luciferase-reporter assay. The constructs used were: 1) −565 (pGL3-ΔFOXO3a-Luc), 2) −176 (pGL3-Δp-176-Luc) and 3) −936 (pGL3-ΔAP1-Luc). *P<0.05 versus control cells (transfected with CCN1 full length) and ***P<0.001 versus control cells. B). Diagrammatic representation of the promoter of CCN1 with various selected transcription-binding sites. The FOXO3a binding motif (AAAGCAAACA) is showed in the schema. The plasmid vector containing luciferase reporter gene was used in all the reporter assays. pGL3-CCN1-Luc is the plasmid with full sequence of CCN1 promoter. C). The inhibitory effect of ZOL on CCN1-promoter is mediated by FOXO3a binding site. MDA-MB-231 cells were transfected with pGL3-CCN1-Luc promoter or pGL3-ΔFOXO3a-Luc containing the FOXO3a deletion and then treated with increases concentrations of ZOL for 24h. *P<0.05 versus control cells (untreated) transfected with pGL3-CCN1-Luc; #P<0.05 in cells transfected with pGL3-CCN1-Luc and treated with different concentrations of ZOL relative to control cells (untreated) transfected with the pGL3-CCN1-Luc.
ZOL inhibition of CCN1 results in a significant decrease in Akt signaling: MDA-MB-231 cells were treated with increasing concentrations of ZOL for 24h. The cells were then lysed and the proteins were resolved using a 10% acrylamide gel and visualized by western blotting analysis using specific antibodies against Akt, pAkt, MAPK, pMAPK and FOXO3a. Our results demonstrate that ZOL treatment blocks activation of Akt (Fig. 7A) and induces FOXO3a expression in the nuclear fraction (Fig. 7B), thus suggesting a molecular mechanism for the cellular and molecular effects mediated by ZOL on CCN1-overexpressing breast cancer cells.
Figure 7. Zoledronic acid decreases the activation status of Akt and increase the nuclear fraction of FOXO3a in MDA-MB-231 cells.
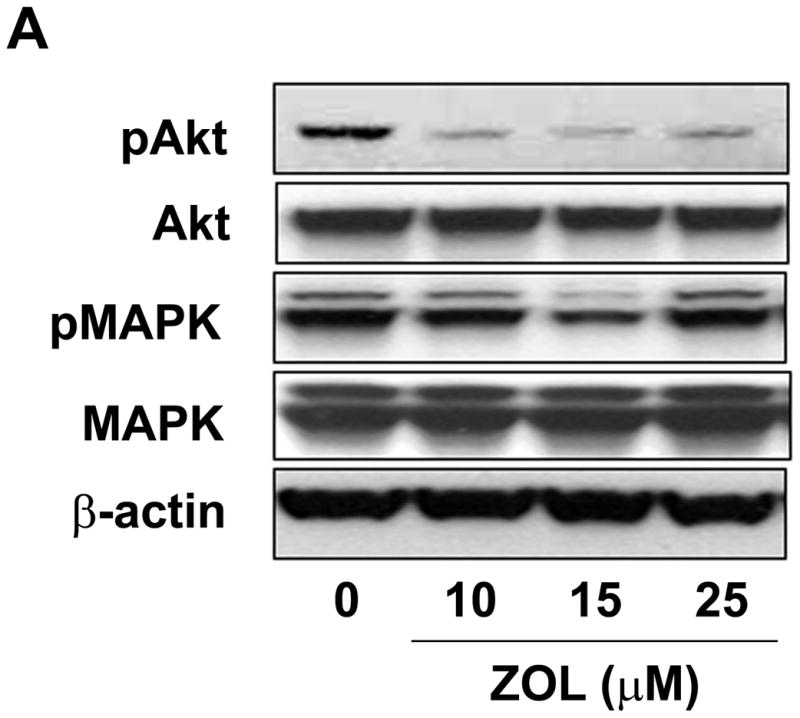
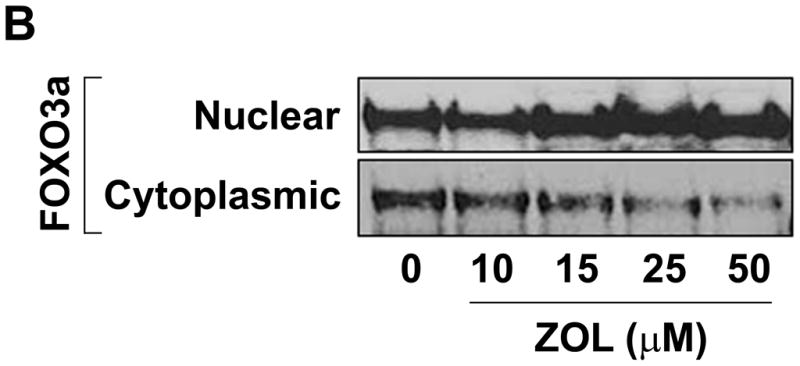
A) Cells were treated for 24h with micromolar concentrations of ZOL (10, 15, 25 and 50μM). Then cell lysates were loaded in a 10% polyacrylamide gel (Criterion XT Bis-Tris precast Gel; Bio-Rad) and analyzed by immunoblot for MAPK, phospho-MAPK, Akt and phospho-Akt. Blots were reprobed with an antibody for β-actin as a control for protein loading and transfer. B) ZOL increases the localization of FOXO3a in the nuclear fraction of MDA-MB-231 cells. Cells were treated for 24h with increased concentrations of ZOL (10, 15, 25 and 50μM) and the nuclear and cytosolic extracts were prepared using the Nuclear Extract kit from Active Motif. Then total proteins (30μg) from both cell fractions were resolved by 10% acrylamide gel and analyzed by immunoblot for FOXO3a.
DISCUSSION
Our early studies using MDA-MB-231, HS578T, and BT549 breast cancer cells demonstrated that CCN1, an angiogenic factor overexpressed in these cells, is actively involved in the acquisition of an estrogen-independent breast cancer phenotype (8,31). CCN1 was found to be overexpressed in a subgroup of breast cancer cell lines derived from triple negative breast carcinomas, which have a unique molecular profile, aggressive behavior, distinct patterns of metastasis, and lack of targeted therapies (8,32). Moreover, forced expression of CCN1 in MCF-7 cells induced their progression to become hormone-independent, similar to triple negative breast cancer cells (7).
Metastatic bone disease is the main cause of morbidity in breast cancer patients (33). Bisphosphonates are potent antiresorptive drugs that inhibit the action of osteoclasts. Interestingly, bisphosphonates seem to have a direct anti-tumor effect in addition to their indirect effect through the inhibition of osteoclasts decreasing the concentration of tumor growth promoting molecules. Emerging results indicate significant clinical benefit especially when combined with other drugs e.g. chemotherapeutic agents (34). ZOL belongs to the latest generation of bisphosphonates and is considered to be the most potent one clinically available. Therefore, it is suitable to use ZOL as a reference substance when evaluating new bisphosphonates derivatives to estimate their relative efficacy. The recognized mechanism of action for ZOL is known to be mediated through the inhibition of farnesyl pyrophosphate synthase (35) and protein geranylgeranylation (36). We recently demonstrated that CCN1 enhances the antitumor effects of ZOL in prostate carcinomas (25). These findings motivated us to investigate whether the effect of ZOL observed in other carcinomas is also found in breast cancer cells overexpressing CCN1.
In this study we demonstrated that treatment with ZOL specifically decreased viability of cells overexpressing CCN1, including MDA-MB-231 and MCF-7/CCN1 cells, as compared to the control MCF-7 cells which do not express CCN1 (Fig. 2A). We also demonstrated that ZOL inhibits anchorage-independent growth in a dose dependent manner (Fig. 2B) exclusively on CCN1 overexpressing breast cancer cells. Our results are consistent with other studies demonstrating that ZOL inhibits MDA-MB-231 cell viability by affecting changes in cell growth and apoptosis (37). More importantly, studies in human myeloma cells demonstrate that nitrogen-containing bisphosphonates, including ZOL, inhibit cell proliferation (38). The apoptotic effect of bisphosphonates such as pamidronate, incadronate, and ZOL on cancer cells has been reported in several systems including macrophages, osteoclasts and myeloma cells (38–40). In addition, we demonstrated that ZOL inhibits branching and morphogenesis of triple negative breast cancer cells in a 3D Matrigel cell culture assay (Fig. 3) and that ZOL induces apoptotic cell dead at higher concentrations (50–100μM) (Fig. 2D). Our results are analogous to previous reports showing that higher doses of ZOL are required to induce apoptosis of endothelial progenitor cells [>25μM in the present study, 100μM in the study using mature endothelial cells (41) and in breast cancer cells (37)] which implies that perhaps ZOL affects both angiogenesis and vasculogenesis.
Previously, we demonstrated that CCN1 induces metastasis and drug resistance in breast cancer cells (31). Following our reported findings we then studied the mechanism by which ZOL induces growth inhibition and apoptosis of CCN1 overexpressing cells. Initially we demonstrated that ZOL regulated the expression of CCN1 at the protein level (Fig. 4B). These data is consistent with our previous reported results which demonstrate that ZOL downregulates CCN1 in prostate cancer (25). We then demonstrated that increasing concentrations of ZOL inhibited the transcriptional activation of CCN1 in triple negative-derived breast cancer cells. To ascertain that the inhibitory effect of ZOL was directed against the CCN1-promoter activity and the translation of CCN1, we performed a competitive experiment between ZOL and CCN1. MDA-MB-231 cells were incubated with 15μM ZOL and with increased concentrations of CCN1-GST protein for 24h. Results showed that CCN1 reverts the inhibitory effect of ZOL (Fig. 5). Our findings reveal that ZOL specifically inhibits CCN1 expression which results in inhibition of cell proliferation and induction of apoptotic cell death in cells overexpressing CCN1.
With this study we demonstrate that cell proliferation of CCN1-overexpressing breast cancer cells that spread and create osteolytic metastasis are inhibited by ZOL. A concentration of 10μM is required to induce significant inhibition of anchorage independent growth concomitantly with downregulation of CCN1. Concentration greater than 50μM of ZOL were necessary to induce apoptosis which is consistent with another in vitro study (37) showing that in the local bone microenvironment higher doses than in the serum may be needed. Previous studies show that ZOL alone does not inhibit tumor growth of MDA-MB-231 xenografts. Combination treatment of ZOL and doxorubicin (42) or Doxycycline (43) or specific antibodies (27) inhibits the growth of breast tumors
CCN1 contains the forkhead factor binding motif AAAGCAAACA in its promoter region. It has been shown that FOXO3a is a negative regulator of CCN1 in the muscle (30). Since ZOL inhibited CCN1 expression at transcriptional level affecting the CCN1-promoter activity, we speculated that a specific binding site at the CCN1 promoter is the molecular mechanisms by which ZOL induces inhibition of breast cancer growth and induces apoptosis. Our studies revealed that all the transcription binding sites mutated at the CCN1 promoter (Fig. 6A) were critical for the CCN1 promoter activity. Nevertheless, the deletion of the FOXO3a binding site was the only deleted site on CCN1 promoter that showed a partial activity and that completely abolished ZOL inhibition of CCN1 transcriptional activity (Fig. 6B and 6C). This was remarkable, because forkhead transcription factors are key players, under regulation of Akt, including a series of biological properties such as cellular proliferation, morphogenesis, and apoptosis (44). In view of the previous results we next determined that ZOL induced a significant decrease in Akt activation and concomitantly induced the accumulation of FOXO3a in the nucleus (Fig. 7A–B, respectively). No changes in MAPK activation were observed (Fig. 7A), thus demonstrating that blockage of CCN1 by ZOL is specifically driven through the FOXO3a/Akt survival pathway.
Although ZOL has clear antiproliferative and apoptotic effects in vitro and in vivo, a critical issue to its potential clinical use is the transient maximum concentration in circulation. ZOL reaches 2μM in the blood in a very short time (45). We showed that ZOL at 10μM induces growth inhibition. We anticipate that 95μg/kg ZOL will be the appropriate dose for in vivo (athymic nude mice) treatments which closely corresponds to 3.8mg/m2 ZOL, the dose currently used for patients treatment. In humans, following intravenous infusion, ZOL concentrations decline in a triphasic fashion: the early distribution half-life is 0.23 hours; the elimination half-life is 1.75 hours and the terminal elimination half-life is 167 hours. Here we show that ZOL targets CCN1, CCN1 is expressed in the tumor site, thus, it is a strong probability that 2μM ZOL would fully reach the tumor site.
When ZOL is used as a single agent for cancer treatment its high bone mineral affinity decreases significantly its bioavailability weakening its in vivo antitumor potential. Several pre-clinical studies showing that ZOL alone does not inhibit the growth of MDA-MB-231 xenografts have been published (27, 42, 43). One can envision treatment strategies where zoledronic acid is combined with synergistic chemotherapies or with agents able to decrease the bone mineral affinity of bisphosphonates. The rapid post infusion decline of zoledronic acid to 1% of peak plasma concentrations by 24 hours post dose, followed by prolonged, very low drug plasma concentrations, is typical of all bisphosphonates allowing for synergistic chemotherapy combination when given simultaneously. In addition, because zoledronic acid and other bisphosphonates are very slowly released from a deep storage compartment (presumably bone) over an extended period of time, terminal half-lives of zoledronic acid have never been accurately determined (46–48). This fact allows for a potential use of bisphosphonates, such as zoledronic acid, in scenarios of minimal residual disease such as in the adjuvant setting.
Overall our results suggest that CCN1 may be a specific target for ZOL in triple negative breast carcinomas. ZOL is already a drug relative well tolerated with a low incidence of adverse effects, and already used clinically. Our data provides the rationale for the use of ZOL as an adjuvant in the treatment of cancer, especially for tumors rich in angiogenesis and vasculogenesis.
Acknowledgments
The authors thank Drs. Hyo-Soo Kim and Brahim Chaqour for kindly providing the constructs for the CCN1 promoter. In addition, we would like to acknowledge Dr. Michele Caraglia and Julian Molina for scientific discussion, and Dr. Christian R. Gomez for critical review of the manuscript. Dr. Cheol H. Park and Mr. Alejandro Lupu are acknowledged for editing the manuscript.
Financial Support: NIH grant R01 CA118975 (R. Lupu). During this study all the authors were supported by the NIH grant RO1 CA118975.
Abbreviations List
- CYR61
cysteine-rich 61
- ZOL
Zoledronic Acid
- ER
Estrogen Receptor
- PR
progesterone Receptor
- E2
17-β-Estradiol
- PVDF
Polyvinylidene Diflouride
- TBS
Tris Buffered Saline
- RT
Room temperature
- BSA
Bovine Serum Albumin
- AP-1
activator protein-1
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest are disclosed.
References
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Mattie MD, Benz CC, Bowers J, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 5.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–66. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie D, Miller CW, O’Kelly J, et al. Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. JB Chem. 2001;276:14187–94. doi: 10.1074/jbc.M009755200. [DOI] [PubMed] [Google Scholar]
- 7.Tsai MS, Bogart DF, Li P, Mehmi I, Lupu R. Expression and regulation of Cyr61 in human breast cancer cell lines. Oncogene. 2002;21:964–73. doi: 10.1038/sj.onc.1205131. [DOI] [PubMed] [Google Scholar]
- 8.Tsai MS, Hornby AE, Lakins J, Lupu R. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer Res. 2000;60:5603–7. [PubMed] [Google Scholar]
- 9.Hijazi MM, Thompson EW, Tang C, et al. Heregulin regulates the actin cytoskeleton and promotes invasive properties in breast cancer cell lines. Int J Oncol. 2000;17:629–41. doi: 10.3892/ijo.17.4.629. [DOI] [PubMed] [Google Scholar]
- 10.Thompson EW, Paik S, Brunner N, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 11.Sampath D, Zhu Y, Winneker RC, Zhang Z. Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17 beta-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J Clin Endocrinol Metab. 2001;86:1707–15. doi: 10.1210/jcem.86.4.7423. [DOI] [PubMed] [Google Scholar]
- 12.Rachner TD, Singh SK, Schoppet M, Benad P, Bornha€user M, Ellen-rieder V, et al. Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Lett. 2009;287:109–16. doi: 10.1016/j.canlet.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Guan H, Duan X, Kleinerman ES. Zoledronic acid inhibits primary bone tumor growth in Ewing sarcoma. Cancer. 2005;104:1713–20. doi: 10.1002/cncr.21383. [DOI] [PubMed] [Google Scholar]
- 14.Daubine F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99:322–30. doi: 10.1093/jnci/djk054. [DOI] [PubMed] [Google Scholar]
- 15.Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. MolCancer Ther. 2007;6:3263–70. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- 16.Caraglia M, D’Alessandro AM, Marra M, et al. The farnesyl transferase inhibitor R115777 (Zarnestra) synergistically enhances growth inhibition and apoptosis induced on epidermoid cancer cells by Zoledronic acid (Zometa) and Pamidronate. Oncogene. 2004;23:6900–13. doi: 10.1038/sj.onc.1207814. [DOI] [PubMed] [Google Scholar]
- 17.Ullen A, Lennartsson L, Harmenberg U, et al. Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol (Stockholm, Sweden) 2005;44:644–50. doi: 10.1080/02841860510029617. [DOI] [PubMed] [Google Scholar]
- 18.Vordos D, Paule B, Vacherot F, et al. Docetaxel and zoledronic acid in patients with metastatic hormone-refractory prostate cancer. BJU Int. 2004;94:524–7. doi: 10.1111/j.1464-4096.2004.04919.x. [DOI] [PubMed] [Google Scholar]
- 19.Budman DR, Calabro A. Zoledronic acid (Zometa) enhances the cytotoxic effect of gemcitabine and fluvastatin: in vitro isobologram studies with conventional and nonconventional cytotoxic agents. Oncology. 2006;70:147–53. doi: 10.1159/000093006. [DOI] [PubMed] [Google Scholar]
- 20.Bellahcene A, Chaplet M, Bonjean K, Castronovo V. Zoledronate inhibits alphavbeta3 and alphavbeta5 integrin cell surface expression in endothelial cells. Endothelium. 2007;14:123–30. doi: 10.1080/10623320701347187. [DOI] [PubMed] [Google Scholar]
- 21.Soltau J, Zirrgiebel U, Esser N, et al. Antitumoral and antiangiogenic efficacy of bisphosphonates in vitro and in a murine RENCA model. Anticancer Res. 2008;28:933–41. [PubMed] [Google Scholar]
- 22.Nagamine I, Yamaguchi Y, Ohara M, Ikeda T, Okada M. Induction of gamma delta T cells using zoledronate plus interleukin-2 in patients with metastatic cancer. Hiroshima J Med Sci. 2009;58:37–44. [PubMed] [Google Scholar]
- 23.Coxon JP, Oades GM, Kirby RS, Colston KW. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int. 2004;94:164–70. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- 24.Hasmim M, Bieler G, Ruegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost. 2007;5:166–73. doi: 10.1111/j.1538-7836.2006.02259.x. [DOI] [PubMed] [Google Scholar]
- 25.Marra M, Santini D, Meo G, et al. Cyr61 downmodulation potentiates the anticancer effects of zoledronic acid in androgen-independent prostate cancer cells. Int J Cancer. 2009;125:2004–13. doi: 10.1002/ijc.24648. [DOI] [PubMed] [Google Scholar]
- 26.Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24:761–79. doi: 10.1038/sj.onc.1208238. [DOI] [PubMed] [Google Scholar]
- 27.Peterschmitt J, Bauerle T, Berger MR. Effect of zoledronic acid and an antibody against bone sialoprotein II on MDA-MB-231(GFP) breast cancer cells in vitro and on osteolytic lesions induced in vivo by this cell line in nude rats. Clin Exp Metastasis. 2007;24:449–59. doi: 10.1007/s10585-007-9082-x. [DOI] [PubMed] [Google Scholar]
- 28.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods (San Diego, Calif) 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Ren J, Ooi LL, Chong SS, Lee CG. Dinucleotide repeats negatively modulate the promoter activity of Cyr61 and is unstable in hepatocellular carcinoma patients. Oncogene. 2005;24:3999–4008. doi: 10.1038/sj.onc.1208550. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Chung JW, Youn SW, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. CircRes. 2007;100:372–80. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 31.Tsai MS, Bogart DF, Castaneda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:8178–85. doi: 10.1038/sj.onc.1205682. [DOI] [PubMed] [Google Scholar]
- 32.Sampath D, Winneker RC, Zhang Z. Cyr61, a member of the CCN family, is required for MCF-7 cell proliferation: regulation by 17beta-estradiol and overexpression in human breast cancer. Endocrinology. 2001;142:2540–8. doi: 10.1210/endo.142.6.8186. [DOI] [PubMed] [Google Scholar]
- 33.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane database of systematic reviews (Online) 2005:CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Clyburn RD, Reid P, Evans CA, Lefley DV, Holen I. Increased anti-tumour effects of doxorubicin and zoledronic acid in prostate cancer cells in vitro: supporting the benefits of combination therapy. Cancer Chemother Pharmacol. 2010;65:969–78. doi: 10.1007/s00280-009-1106-6. [DOI] [PubMed] [Google Scholar]
- 35.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–42. [PubMed] [Google Scholar]
- 36.Goffinet M, Thoulouzan M, Pradines A, et al. Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer. 2006;6:60. doi: 10.1186/1471-2407-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 38.Shipman CM, Croucher PI, Russell RG, Helfrich MH, Rogers MJ. The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res. 1998;58:5294–7. [PubMed] [Google Scholar]
- 39.Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–87. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 40.Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–8. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–61. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 42.Ottewell PD, Deux B, Monkkonen H, et al. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clin Cancer Res. 2008;14:4658–66. doi: 10.1158/1078-0432.CCR-07-1545. [DOI] [PubMed] [Google Scholar]
- 43.Duivenvoorden WC, Vukmirovic-Popovic S, Kalina M, Seidlitz E, Singh G. Effect of zoledronic acid on the doxycycline-induced decrease in tumour burden in a bone metastasis model of human breast cancer. Br J Cancer. 2007;96:1526–31. doi: 10.1038/sj.bjc.6603740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–36. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 46.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745–51. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 47.Green JR. Chemical and biological prerequisites for novel bisphosphonate molecules: results of comparative preclinical studies. SemOncol. 2001;28:4–10. doi: 10.1016/s0093-7754(01)90259-3. [DOI] [PubMed] [Google Scholar]
- 48.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 49.Guerra-Vladusic FK, Scott G, Weaver V, et al. Constitutive expression of Heregulin induces apoptosis in an erbB-2 overexpressing breast cancer cell line SKBr-3. Int J Oncol. 1999;15:883–92. doi: 10.3892/ijo.15.5.883. [DOI] [PubMed] [Google Scholar]
- 50.Soule HD, Maloney TM, Wolman SR, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]