Abstract
Purpose
We identified masseter muscle fiber type property differences in subjects with dentofacial deformities.
Patients and Methods
Samples of masseter muscle were collected from 139 young adults during mandibular osteotomy procedures to assess mean fiber areas and percent tissue occupancies for the 4 fiber types that comprise the muscle. Subjects were classified into 1 of 6 malocclusion groups based on the presence of a skeletal Class II or III sagittal dimension malocclusion and either a skeletal open, deep, or normal bite vertical dimension malocclusion. In a subpopulation, relative quantities of the muscle growth factors IGF-I and GDF-8 gene expression were quantified by real-time polymerase chain reaction.
Results
Fiber properties were not different in the sagittal malocclusion groups, but were very different in the vertical malocclusion groups (P ≤ .0004). There were significant mean fiber area differences for type II (P ≤ .0004) and type neonatal—atrial (P = .001) fiber types and for fiber percent occupancy differences for both type I–II hybrid fibers and type II fibers (P ≤ .0004). Growth factor expression differed by gender for IGF-I (P = .02) and GDF-8 (P < .01). The ratio of IGF-I:GDF-8 expression associates with type I and II mean fiber areas.
Conclusion
Fiber type properties are very closely associated with variations in vertical growth of the face, with statistical significance for overall comparisons at P ≤ .0004. An increase in masseter muscle type II fiber mean fiber areas and percent tissue occupancies is inversely related to increases in vertical facial dimension.
Malocclusion is a relatively recent human trait that appeared after transition to a modern diet of processed food, which reduced masticatory functional requirements. The trait may appear rapidly within 1 human generation when there is a transition from primitive rural diets to more industrialized diets.1 This may happen with family immigration to industrialized nations,2 in different locations of a geographic area for populations with a similar genetic makeup,3 or within a population that changes diet to align with modern customs.4 This trend was first identified by the dentist, Westin Price, who compared the craniofacial morphology and dental malocclusion in residents of Australia, New Zealand, and South America with their prehistoric ancestors.5 He associated the modern increased variability in occlusal relationships and jaw shape to the decreased functional demand for chewing.
Experimentally introduced soft diets from animal experiments, which result in changes of craniofacial morphology,6 altered masticatory muscle fiber type composition, and decreased muscle fiber size, all affirm these associations.7 In rodents, soft diets result in a smaller layer of mandibular condylar cartilage, decreased condylar length, and mandibular height, while hard diets result in increased mandibular skeleton weight, volume, and thickness.8–10 Nonhuman primates with experimentally induced soft diet develop crowded teeth, and a narrow and longer maxilla with increased overjet between maxillary and mandibular anterior teeth, which replicates some aspects of human malocclusion.11 Because these changes can occur within 1 generation, and in animal experiments over short periods, they are most likely the result of changes in gene expression by bone, cartilage, and muscle rather than from genetic variation in offspring. Where the outcome is severe malocclusion, orthodontic treatment alone is usually unable to correct the skeletal deviations, and orthognathic surgery is also needed. It is estimated that approximately 1.5 million Americans have dentofacial deformities requiring complex diagnosis and treatment management by orthodontists and oral surgeons.12 In the English National Health Service, where dental provision is closely monitored, there is a 1.7:1 ratio of women:men undergoing these corrective surgical procedures.13 At the University of Lille clinic there was a 1.5:1 ratio for women to men as surgical candidates. This gender predominance, however, may represent a greater preference for surgical and orthodontic treatment by women, rather than real differences in the population at large.
Currently, the mechanisms by which nonsyndromic deviations in craniofacial morphology develop are not fully understood, but recent work has shown that altered masticatory functioning has significant effects on the face and neurocranium.14 For example, mice with a hypermuscular phenotype due to loss of myostatin, a growth factor which negatively regulates muscle fiber size, have more brachycephalic and smaller cranial vaults, decreased maxillary length, and a distinctive mandibular shape, which replicates the “rocker mandible” described previously in some humans.15–18 The rocker morphology appears during the adolescent growth period when increasing muscle forces influence mandibular shape,17 and a similar morphology seen in Inuit mandibles is attributed to the vigorous chewing characteristic of Eskimo populations.18 Masticatory muscle function is a key environmental influence on these bony craniofacial areas through force application during growth.6
Human craniofacial skeletal dimensions correlate with masticatory muscle volume because this is directly related to the loads generated during normal functioning.19 The intensity of masticatory activity in young adults in modern human populations (eating a highly processed diet) is extremely limited, with teeth estimated to be in occlusion for only about 6 to 7 minutes in a 24-hour cycle.20 Therefore masticatory activity per se contributes relatively little to normal function of masticatory muscles in the population. By contrast, muscle tone for postural purposes appears to be more important as, even though the force is small and difficult to quantitate,21 it undoubtedly maintains the freeway space between the teeth and helps to protect the airway—activities that have a much higher duty cycle. Perhaps the best demonstration of how variation in muscle activity affects craniofacial growth is the example of children with Duchenne muscular dystrophy who develop severe open bite malocclusions due to decreasing muscle forces transmitted to the face as the condition progresses.22
An aspect of relevance to orthognathic surgical treatment of malocclusions is whether individual variations in muscle tone could also influence the long-term stability of the surgery. Given the large number of surgical options and the necessity to determine the long-term effects of skeletal muscle on treatment outcome, retrospective clinical studies have sought to characterize the relative stability of intervention. We have a general understanding of the relative stability of the various surgical procedures, either alone or in combination.23 For the most part, these procedures are relatively stable, with only modest relapse of some anatomic areas (less than 1 to 4 mm change) in approximately 80% to 85% of the subjects.24,25 In the remaining 15% to 20%, however, relapse may be greater than 4 mm, which is often noticeable to the patient and clinically significant. These findings are similar to earlier conclusions from bite force studies, which showed enhancement of function in most cases, but an unexpected force decrease in some subjects.26 Even though our experience allows us to limit changes after orthognathic surgery, we still cannot predict which subjects will have clinically significant relapse.
In human limb muscle, fiber type proportions (type II = fast-twitch and type I = slow-twitch) and mean fiber areas have been shown to be significantly correlated with muscular strength and power.27–29 Other contractile properties, such as speed of shortening and tension cost (energy consumed per unit force developed), also vary with fiber “type” at single fiber level.30 Variations in masticatory muscle fiber types have been associated with differences in craniofacial morphology in previous studies,31,32 but have yet to be assessed in a large number of young subjects over the full range of sagittal and vertical skeletal malocclusion relationships that characterize human malocclusion. This report aims to show how masticatory muscle fiber types (the main functional phenotypic attribute), and gene expression for 2 growth factors, which regulate muscle fiber size (insulin-like growth factor-I [IGF-I] and myostatin [GDF-8]), relate to craniofacial skeletal morphology in a large subject population with malocclusion severe enough to require orthognathic surgery.
Patients and Methods
Masseter muscle samples were obtained from 139 subjects undergoing orthognathic surgery for treatment of malocclusion at the University Hospital of Lille, France. One surgeon (J.F.) excised the tissue, and a second (G.R.) collected information regarding clinical diagnosis and surgical procedures. The age range of the population was 18 to 40 years old (mean = 20), and subjects over the age of 40 were excluded, because this is the customary surgical age range for the study population. A second consideration for excluding subjects over 40 is the inherent tendency for changes in lean muscle mass, influenced by variations in metabolism, disability, and morbidity. The population was almost equally divided for sagittal skeletal malocclusion, with 56% having a skeletal Class II malocclusion and 44% having a skeletal Class III malocclusion. For the vertical malocclusion groups 42% had normal anterior vertical facial dimension, 36% had a skeletal open bite malocclusion, and 20% had a skeletal deep bite malocclusion. All subjects required both orthodontics and orthognathic surgery to correct growth dentofacial deformations. Consent for subject participation was obtained according to research ethics committee regulations at the University of Lille and the Institutional Review Board of Temple University. Subjects with systemic conditions, facial trauma, tumor, condylar hypertrophy, rheumatoid or osteoarthritis, congenital craniofacial syndromes, or developmental conditions that might influence craniofacial growth were excluded. Masseter samples were collected approximately 1.5 cm from the lowest point of the mandible’s angle and processed for histologic analysis. The size of the sample was approximately 0.5 cm3. Tissue was snap-frozen and cryosectioned serially at 10 μm, and sections were mounted on glass microscope slides for immunostaining (indirect immunoperoxidase method) with antibodies specific for myosin heavy chain isoforms. These antibodies were specific for myosin heavy chain isoforms type I (BA-F8), all type II (MY-32), type IIA only (SC-71), neonatal (a polyclonal antibody prepared by A.R.), and α-cardiac (MAS 366) (also termed atrial myosin) and have been described previously.33 Malocclusion classification was based on the sagittal and vertical jaw repositioning required to execute the surgical treatment plan. The surgeon who performed the procedure also summarized the presurgical treatment planning malocclusion diagnosis, which was based on the amount of required jaw repositioning necessary to achieve the treatment plan. Subjects were classified into 1 of 6 craniofacial morphologic groups that included 1 variation of sagittal skeletal jaw malocclusion, either Class II or III, and 1 variation of vertical skeletal jaw malocclusion, open, deep, or normal bite relationship.
HISTOLOGICAL ANALYSIS OF MUSCLE
We classified masseter fibers into 4 fiber type groups as described previously.33 Type I fibers contained only type I myosin; type II fibers contained only type IIA and/or IIX myosin; type I–II hybrid fibers contained both type I and II isoforms in varying amounts; and type neonatal--atrial fibers that contained the neonatal and/or α-cardiac myosin in combination with other type I and II myosin isoforms. For fiber type classification, only tissue section series with consistent antibody reactions from all stains and acceptable morphology of muscle fibers were used. A specific area was identified on each of the serially immunostained sections that was clearly in transverse section and had both adequate morphology and staining to type the fibers. This area was photographed in all relevant stained sections, so that individual fibers could be identified on serial images. All fibers within the selected areas were type-classified by 1 operator, and this classification was checked by a second operator before their cross-sectional areas were measured with image-analysis software. Tests for measurement error included intrarater reliability in determination of fiber area (by repeating morphometric tracing of all fiber areas in 1 biopsy by 1 examiner), which resulted in an R2 value of 0.9452, and interrater reliability in determination of all fiber areas error in 1 biopsy by a second examiner, which resulted in an R2 value of 0.9752.
QUANTIFICATION OF GROWTH FACTOR mRNA
RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) from the tissue that remained after morphometric analysis of masseter muscles and quantified by absorbance at A260 as described previously.34 Muscle from 18 female and 15 male subjects yielded sufficient amounts of RNA for quantification of IGF-I and GDF-8 in triplicate. The average age, as well as both the distribution and the mean fiber areas for all vertical dimension classes in these 33 subjects, approximated those of the overall population. Quantification was performed by TaqMan (Applied Bio-systems, Foster City, CA) real-time polymerase chain reaction. Reactions contained RNA from masseter muscle or an adult skeletal muscle reference standard (Ambion, Austin, TX), TaqMan RNA-to-CT 1-step reagent, and an Applied Biosystems expression assay set for either GDF-8 (Hs00976237_m1), IGF-I (Hs01547656_m1), or internal control 18S rRNA (Hs03003631_g1) for normalization. RNA was expressed as relative quantities determined by the comparative CT (ΔΔCT) method that measures fold difference between normalized quantities of target in the sample and in the reference standard. Relative quantities of IGF-I and GDF-8 RNA were compared with mean fiber areas in the same tissue from which mRNA was extracted.
STATISTICAL ANALYSIS
To determine interactions among gender, sagittal dimension malocclusion groups, vertical dimension malocclusion groups, and fiber type properties, a multivariate analysis of variance MANOVA for a 2 × 2 × 3 × 4 mixed between-within design with 2 dependent variables was used to compare differences in muscle phenotype with the 6 malocclusion groups. Significant interactions were then tested using the univariate ANOVA (analysis of covariance) to determine significant interactions between mean fiber type area and the muscle fiber type percent occupancy (the percent tissue area composition) values. Muscle fiber type percent occupancy is derived from the product of the average fiber number multiplied by average fiber diameter for each fiber type. The total fiber area for all the fiber types is summed, and each fiber type is calculated to be a specific fraction of the total sum, which determines the overall percent tissue composition. Post-hoc tests for simple main effects were then used to determine significance levels for individual group comparisons. Significant interactions for simple main effects were subsequently tested for simple--simple main effects using Bonferroni multiple comparisons.
For gene expression studies, a MANOVA was used to compare differences between muscle fiber properties, gender, age, and malocclusion groups with the quantities of IGF-I and GDF-8 mRNA for between subject effects. Age initially was considered as a possible covariate in the analysis, but it did not correlate significantly with the dependent variables and was excluded from the design.
Results
GENERAL COMPARISONS
Muscle fiber properties were significantly different when compared for differences between each other (P ≤ .0004) and within a fiber type group when compared between the 3 vertical malocclusion groups (P ≤ .0004). There was also a significant difference between gender for fiber type properties (P = .032). There were, however, no significant differences between the Class II and III sagittal skeletal malocclusion groups (P = .281), or age, for muscle properties.
DIFFERENCES FOR GENDER
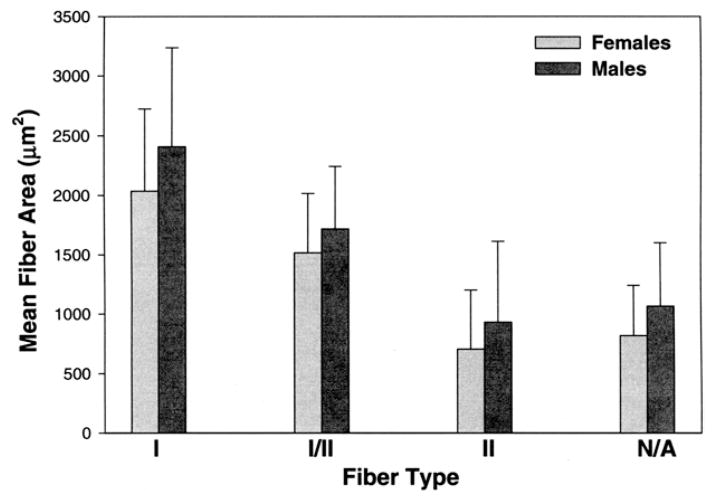
There were very large size differences in fiber types, with type I fibers having by far the largest average fiber diameter at 2,159 μm2. This was followed by the type I–II hybrid fibers at 1,583 μm2. The type II and neonatal--atrial fibers had much smaller sizes of 781 μm2 and 900 μm2, respectively. Males consistently had larger diameter fibers than females (Fig 1). Mean percent tissue occupancy for fiber types was not significantly different for gender.
FIGURE 1.
Gender differences for mean fiber area compared by fiber type. There were overall large differences in mean fiber areas for the different fiber types, with types I and I/II hybrid being the largest and type II fibers being the smallest. In all comparisons male fiber types were significantly larger than female fiber types (P = .032).
Sciote et al. Human Masseter Muscle Fiber Type Properties. J Oral Maxillofac Surg 2012.
GENERAL COMPARISONS FOR MEAN FIBER AREA
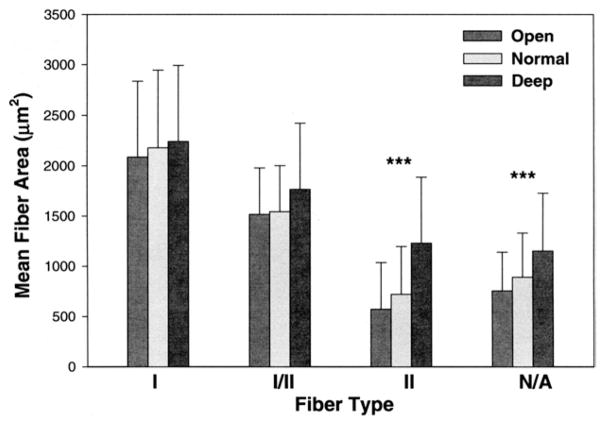
After the MANOVA, a univariate ANOVA was used to test for differences between the mean fiber area measurement in comparison with vertical malocclusion groups, gender, and fiber type. The test identified significant differences for mean fiber area between gender (P = .014) and between vertical malocclusion groups (P = .008) and fiber types (P ≤ .0004). Post-hoc tests for simple main effects for differences in fiber type and mean fiber area, compared between normal, open, and deep bite malocclusion groups, found significant differences for type II (P ≤ .0004) and type neonatal--atrial (P = .001) fiber types. The differences for type I fibers and type I–II hybrid fibers were not significant at P = .648 and P = .083, respectively (Fig 2).
FIGURE 2.
Comparison of mean fiber areas of masseter muscle fiber types in male and female subjects by vertical skeletal malocclusion groups. Data for mean fiber area values in μm2 for skeletal open bite (14 males and 36 females), normal vertical dimension (16 males and 42 females), and deep-bite malocclusion categories (16 males and 15 females). Bars represent 1 standard deviation of the mean. Significant differences in mean fiber type area compared between normal, open, and deep bite malocclusion groups were found for type II (P ≤ .0004) and type neonatal—atrial (P = .001) fiber types.
Sciote et al. Human Masseter Muscle Fiber Type Properties. J Oral Maxillofac Surg 2012.
GENERAL COMPARISONS FOR FIBER TYPE PERCENT TISSUE OCCUPANCY
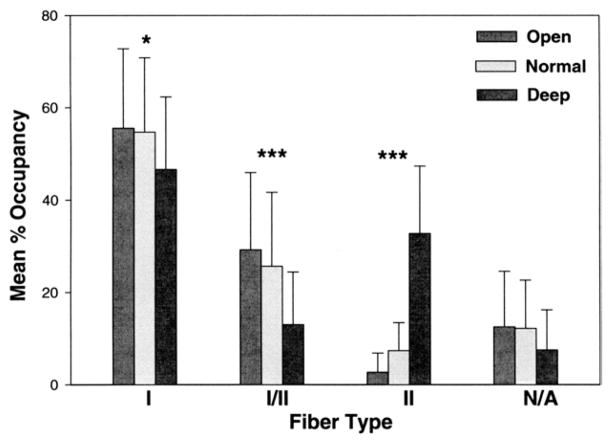
An ANOVA was also used in a similar comparison for muscle fiber type percent tissue occupancy. Here there were significant differences in fiber type percent tissue occupancy for vertical malocclusion groups (P ≤ .0004) and fiber types (P ≤ .0004), but not for gender. The simple main effects test for differences in fiber type percent tissue occupancy, compared between normal, open, and deep bite malocclusion groups, found a significant difference for type I percent occupancy (P = .043), and for type I–II hybrid fiber (P ≤ .0004) and type II (P ≤ .0004) percent occupancy (Fig 3).
FIGURE 3.
Comparison of mean percent occupancies of fiber types in male and female subjects by vertical skeletal malocclusion group. Data for overall muscle tissue percent composition of each fiber type for skeletal open bite (14 males and 36 females), normal vertical dimension (16 males and 42 females), and deep-bite mal-occlusion categories (16 males and 15 females). Bars represent 1 standard deviation of the mean. Significant differences were found for type I percent occupancy (*P = .043), and very significant differences for type I/II hybrid and type II fiber percent occupancy (***P ≤ .0004).
Sciote et al. Human Masseter Muscle Fiber Type Properties. J Oral Maxillofac Surg 2012.
MULTIPLE COMPARISONS FOR MEAN FIBER AREAS
Tests for simple-simple main effects were subsequently conducted for mean fiber area measurements, which were found to be significantly different in the first set of post-hoc tests. Type II and type neonatal--atrial mean fiber areas had significant multiple comparisons. Type II mean fiber areas were significantly different between normal bite and deep bite malocclusion groups (P ≤ .0004), open bite, and deep bite malocclusion groups (P ≤ .0004), but not between the normal bite malocclusion group compared with open bite malocclusion. For neonatal--atrial mean fiber areas there were significant differences between the normal bite malocclusion and deep bite malocclusion groups (P = .034) and between the deep bite and open bite malocclusion groups (P = .001). As with the type II fibers, the neonatal—atrial mean fiber areas were not significantly different between the normal bite malocclusion group compared with the open bite malocclusion group.
MULTIPLE COMPARISONS FOR FIBER TYPE PERCENT OCCUPANCY
There were no significant multiple comparisons for type I percent tissue occupancy, but type I–II hybrid and type II fibers had significant multiple comparisons. Type I–II hybrid fiber percent tissue occupancy was significantly different between the normal bite and deep bite malocclusion groups (P = .001) and between the open bite and deep bite malocclusion groups (P ≤ .0004), but not between the normal bite malocclusion group compared to the open bite malocclusion group. Type II percent tissue occupancy was very significantly different between the normal bite and deep bite malocclusion groups (P ≤ .0004), the open bite and deep bite malocclusion groups (P ≤ .0004), but not between the normal bite malocclusion group compared with the open bite malocclusion group.
Overall, these multiple comparisons for fiber type differences between skeletal open bite, deep bite, and normal bite malocclusion groups show that the greatest differences are found in people with skeletal deep bite malocclusions. Differences are much smaller between people with skeletal open bite or normal bite malocclusions. Type II fibers were significantly different between the vertical malocclusion groups for both mean fiber area and percent tissue occupancy (Fig 4). The mean fiber area for the neonatal—atrial fiber types and the percentage tissue occupancy for I–II hybrid fiber types were also different between the vertical malocclusion groups.
FIGURE 4.
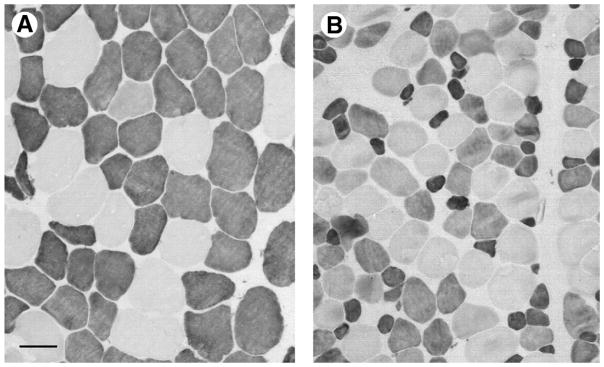
Mean fiber area differences compared between a deep bite and normal bite malocclusion subject. Sections of masseter muscle immunostained with antibody to fast (type II) myosin (Sigma clone my32), photographed at the same magnification (scale bar = 50 μm). A, Deep bite patient, B, normal bite patient. Strongly stained fibers are type II; pale stained are type I. Note the difference in type II fiber areas in A compared with B, and the large population of intermediate stained fibers in B, many of which were type I/II hybrid fibers.
Sciote et al. Human Masseter Muscle Fiber Type Properties. J Oral Maxillofac Surg 2012.
MUSCLE GROWTH FACTOR GENE EXPRESSION
Relative quantities of the muscle growth factors IGF-I and GDF-8 mRNA gene expression were obtained from 33 of the subject tissue samples using a real-time polymerase chain reaction method on extracted RNA. The 33 samples were representative of the genders, with the mean fiber type area gender differences (females:males in μm2) of 1,949.5:2,366 for type I fibers and 500.2:1,097 for type II fibers. Percent tissue occupancy measurements were not significantly different for the whole population when compared for gender. Males had higher growth factor gene expression values (IGF-I = 7.26; GDF-8 = 9.24) than females (IGF-I = 5.69; GDF-8 = 6.16). MANOVA analysis confirmed significant differences between growth factor RNA amounts for gender (Wilks’ lambda P = .003), but not for age or sagittal and vertical malocclusion groups. Tests of between-subjects effects for gender indicated significant differences for IGF-I expression (P = .02) and for GDF-8 expression (P < .01).
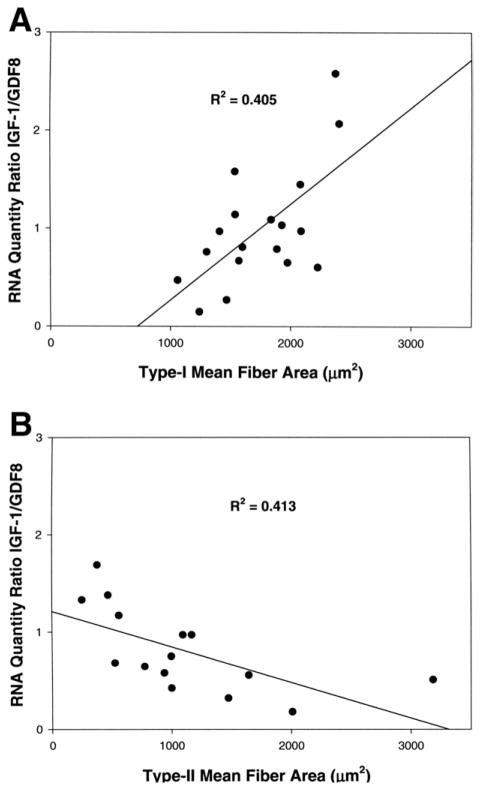
To test if this pilot data on growth factor gene expression might relate to fiber type morphology from histological analysis, we plotted individual regression lines for IGF-I and GDF-8 gene expression levels and the mean fiber areas of either type I or type II fibers for all 33 subjects. R2 values of these regression lines were in the order of 0.005. Plotting the ratio of relative quantities of IGF-I to GDF-8 against the mean fiber areas for either type I or type II fibers separated by gender improved the association and showed a potential biologically relevant trend (Fig 5). In females, type I mean fiber areas increased as the ratio of IGF-I to GDF-8 increased (R2 = .405) and in males type II mean fiber areas decreased as the ratio of IGF-I to GDF-8 increased (R2 = .413). These plots suggest that the size of fiber types are differentially affected between gender by proportionate quantities of the growth factors, but larger subject numbers will be needed to explore these relationships, especially as the trend in males (fiber size decreased as the ratio of IGF-I:GDF-8 increased) was counter to expectation.
FIGURE 5.
Linear regression analyses of relative quantities of IGF-1 and GDF-8 RNA with type I and type II mean fiber areas. A, Plot of the ratio of relative quantities of RNA for IGF-I to GDF-8 growth factor ratios and type I mean fiber area values for 18 female masseter muscles. B, Plot of the ratio of relative quantities of RNA for IGF-I to GDF-growth factor ratios and type II mean fiber area values from 15 male masseter muscles. R2 values represent the correlation coefficient of each plot.
Sciote et al. Human Masseter Muscle Fiber Type Properties. J Oral Maxillofac Surg 2012.
Discussion
The results found highly significant differences for muscle fiber type properties when compared for differences between open bite, deep bite, and normal bite malocclusion groups (P ≤ .0004), but no differences for either Class II or III sagittal malocclusion groups. There was also a significant gender difference for fiber type properties and for expression of IGF-I and GDF-8 growth factor gene message. Therefore, a very close association exists between muscle fiber type size and tissue percent occupancy and vertical development of the face.
MASSETER FIBER TYPES IN RELATION TO MALOCCLUSION TYPES
From our population data there is no evidence that variations in masticatory muscle fiber type size or proportions influence condylar growth or mandibular length (which are reflected in the sagittal malocclusion). Rather, both fiber type properties are very closely associated with variations in vertical growth of the face. An increase in type II mean fiber areas and percent tissue occupancies is inversely related to increases in vertical facial dimension (Figs 1, 2). The fiber type differences in the range we observed between the different vertical malocclusion groups are known to produce significant differences in muscle power and force–velocity characteristics when tested in limb muscles.35 Both the large number of subjects examined and the strength of statistical differences between the vertical dimension groups from our study indicate that masticatory muscle function can influence growth of midfacial sutures, alveolar bone, and dental eruption. Therefore, when growth is completed, total facial height is produced by quite strong interactions between muscle function and vertical growth of bone.
The present findings give us some clues as to which influences are most important in the interaction between muscle and bone during craniofacial growth. Variations in type I muscle fiber size do not influence differences in vertical facial dimension, and type I fiber proportions in masseter muscle have only a modest influence. The size of type neonatal—atrial fibers and the overall percent tissue occupancy of type I–II hybrid fibers appear to modulate growth of vertical facial dimension. Although the physiologic properties of neonatal—atrial skeletal muscle fibers are not yet fully described, the presence of the α-cardiac (or atrial) myosin in masseter muscle fibers when tested in rabbit muscle produce a contraction speed that is intermediate between type I and type II fibers.36 The presence of the hybrid fibers expressing more than 1 myosin isoform is also a normal feature of masseter.37–39 The most important interaction, however, results from variation in both the size and the percent tissue occupancy of type II fibers (Figs 2 and 3). For fiber size and percent tissue occupancy, the deep bite group averages were always significantly different from both the open bite and the normal bite group averages, and no significant differences were found between the open bite and normal bite groups.
MUSCLE GROWTH REGULATORS
In addition to being the target of systemically acting growth promoters such as growth hormone, muscle also produces its own growth factors, which act in a paracrine fashion to regulate fiber hypertrophy and muscle volume. GDF-8 is a negative regulator of muscle growth, and disruption of its gene produces increased muscle fiber size in mice and the “double-muscling” phenotype.40 However, variations in its gene expression have not been studied in human masticatory muscles. Muscle also expresses different forms of the positive growth regulator IGF-1, one very similar to that produced in the liver, IGF-I, and an alternatively spliced gene product called mechano-growth factor.41 In laboratory animals, there is a correlation between the expression of these growth regulators and fiber type composition of muscle.41 It has been shown that GDF-8 knockout mice have significantly different craniofacial morphology in the neurocranium, midface, and mandible compared with wild-type controls,15 and differences in masseter muscle size16 and proportion of type I fibers relative to type II fibers throughout the growth period.42
In this study we have begun to test how variations in IGF-I and GDF-8 growth factors may influence masseter muscle fiber types. Our findings suggest that there are gender differences between fiber type size and growth factor gene expression levels (Fig 5). The positive correlation seen between the IGF-I:GDF-8 ratio and type I fiber area in females is consistent with the known role of IGF-I as a growth promoter. The males also had a trend toward positive correlation for type I fiber areas, but the R2 values were much lower at about 0.2. The negative correlation of this ratio with type II fibers in males was unexpected and so far unexplained. For females, by contrast, the IGF-I:GDF-8 ratio to type II fiber area correlation was positive, but with a low R2 value of 0.1. Therefore, larger sample sizes will be necessary to fully describe these differences.
One possible reason for the surprising differences between males and females of type II fiber size and growth factor expression levels may be the differential effect of GDF-8 for gender. Some GDF-8 gene mutations in mice only affect males, and the reason for gender specificity is as yet unknown.43 Overall we could identify significant differences for masseter muscle fiber type size for gender, but not for the percent tissue occupancy. It is known that age, when subjects in the 40- to 80-year range are included, and gender influence maximal bite force and masticatory muscle thickness.44 In our study males consistently had larger mean fiber type area than females, but very large differences in fiber type muscle percent occupancy between the vertical dimension malocclusion classes most likely obscured gender differences.
Orthognathic surgery results in changes in tensile strength or compression in masticatory muscles, which may contribute to stability, or instability and relapse over the long term.45 In a study of 10 subjects of mixed gender, equally distributed for Classes II and III malocclusion with normal vertical facial dimensions, Gedrange et al identified changes of myosin expression in masseter muscle from the time of surgical correction to a 6-month surgical follow-up.46 In both genders there were significantly decreased levels of both type I and II myosin expression, which may have resulted from decreased muscle activity. In a follow-up study in a similar population of 30 subjects, type I myosin mRNA decreased at 6 months, while type II myosin increased.47 These changes occurred as the number of occlusal tooth contacts increased. This association indicates that the establishment of normal masticatory function coincides with changes in myosin gene expression. Most recently in these series of investigations, Maricic et al found a similar transition in types I and II myosin in 29 subjects, but here they also compared expression levels for mechanogrowth factor--IGF and GDF-8.48 Mechanogrowth factor levels were significantly increased 6 months after surgery. GDF-8 levels were unchanged for males, but significantly decreased for females. These findings, together with the differences we found for differential growth factor association to fiber type area measurements by gender, need to be investigated further with regard to surgical relapse. Presurgical masticatory fiber type distributions and postsurgical transitions in both growth factor gene expression and fiber types most likely affect long-term prognosis for stability and these differences may be gender specific.
References
- 1.Corruccini RS. An epidemiologic transition in dental occlusion in world populations. Am J Orthod. 1984;86:419. doi: 10.1016/s0002-9416(84)90035-6. [DOI] [PubMed] [Google Scholar]
- 2.Goose DH. Maxillary dental arch width in Chinese living in Liverpool. Arch Oral Biol. 1972;17:231. doi: 10.1016/0003-9969(72)90153-7. [DOI] [PubMed] [Google Scholar]
- 3.Corruccini RS, Kaul SS, Chopra SR, et al. Epidemiological survey of occlusion in North India. Br J Orthod. 1983;10:44. doi: 10.1179/bjo.10.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Corruccini RS, Whitley LD. Occlusal variation in a rural Kentucky community. Am J Orthod. 1981;79:250. doi: 10.1016/0002-9416(81)90073-7. [DOI] [PubMed] [Google Scholar]
- 5.Price WA. A Comparison of Primitive and Modern Diets and Their Effects. Paul B. Hoeber, Inc; New York: Medical Book Department of Harper and Brothers; 1939. Nutrition and Physical Degeneration. [Google Scholar]
- 6.Abed GS, Buschang PH, Taylor R, et al. Maturational and functional related differences in rat craniofacial growth. Arch Oral Biol. 2007;52:1018. doi: 10.1016/j.archoralbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Kiliaridis S, Engström C, Thilander B. Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Arch Oral Biol. 1988;33:187. doi: 10.1016/0003-9969(88)90044-1. [DOI] [PubMed] [Google Scholar]
- 8.Watt DG, Williams CH. The effects of the physical consistency of food on the growth and development of the mandible and the maxilla of the rat. Am J Orthod. 1951;37:895. doi: 10.1016/0002-9416(51)90101-7. [DOI] [PubMed] [Google Scholar]
- 9.Bouvier M, Zimny ML. Effects of mechanical loads on surface morphology of the condylar cartilage of the mandible in rats. Acta Anat (Basel) 1987;129:293. doi: 10.1159/000146418. [DOI] [PubMed] [Google Scholar]
- 10.McFadden LR, McFadden KD, Precious DS. Effect of controlled dietary consistency and cage environment on the rat mandibular growth. Anat Rec. 1986;215:390. doi: 10.1002/ar.1092150409. [DOI] [PubMed] [Google Scholar]
- 11.Corruccini RS, Beecher RM. Occlusal variation related to soft diet in a nonhuman primate. Science. 1982;218:74. doi: 10.1126/science.7123221. [DOI] [PubMed] [Google Scholar]
- 12.Bailey LJ, Proffit WR, White R., Jr Assessment of patients for orthognathic surgery. Semin Orthod. 1999;5:209. doi: 10.1016/s1073-8746(99)80015-2. [DOI] [PubMed] [Google Scholar]
- 13.Moles DR, Cunningham SJ. A national review of mandibular orthognathic surgery activity in the National Health Service in England over a nine year period: Part 2—patient factors. Br J Oral Maxillofac Surg. 2009;47:274. doi: 10.1016/j.bjoms.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat Rec. 2006;288A:1225. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- 15.Vecchione L, Miller J, Byron C, et al. Age-related changes in craniofacial morphology in GDF-8 (Myostatin)-deficient mice. Anat Rec. 2010;293:32. doi: 10.1002/ar.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vecchione L, Byron C, Cooper GM, et al. Craniofacial morphology in myostatindeficient mice. J Dent Res. 2007;86:1068. doi: 10.1177/154405910708601109. [DOI] [PubMed] [Google Scholar]
- 17.Houghton P. Rocker jaws. Am J Phys Anthropol. 1977;47:365. doi: 10.1002/ajpa.1330470303. [DOI] [PubMed] [Google Scholar]
- 18.Hylander WL. In: The adaptive significance of Eskimo craniofacial morphology. Dahlberg AA, Graber TM, editors. Orofacial Growth and Development; Chicago, Mouton: 1977. p. 129. [Google Scholar]
- 19.Boom HP, van Spronsen PH, van Ginkel FC, et al. A comparison of human jaw muscle cross-sectional area and volume in long-and short-face subjects, using MRI. Arch Oral Biol. 2008;53:273. doi: 10.1016/j.archoralbio.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Ishizuka Y, Tanne K. Changes in masseter muscle activity during orthodontic treatment evaluated by a 24-hour EMG system. Angle Orthod. 1996;66:223. doi: 10.1043/0003-3219(1996)066<0223:CIMMAD>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Mew JRC. The postural basis of malocclusion: A philosophical overview. Am J Orthod Dentofac Orthop. 2004;126:729. doi: 10.1016/j.ajodo.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Eckardt L, Harzer W. Facial structure and functional findings in patients with progressive muscular dystrophy (Duchenne) Am J Orthod Dentofac Orthop. 1996;110:185. doi: 10.1016/s0889-5406(96)70107-5. [DOI] [PubMed] [Google Scholar]
- 23.Proffit WR, Turvey TA, Fields HW, et al. Orthodontic surgery: A hierarchy of stability. Int J Adult Orthodon Orthognath Surg. 1996;11:191. [PubMed] [Google Scholar]
- 24.Proffit WR, Bailey LJ, Phillips C, et al. Long-term stability of surgical open-bite correction by le Fort I osteotomy. Angle Orthod. 2000;70:112. doi: 10.1043/0003-3219(2000)070<0112:LTSOSO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Busby BR, Bailey LT, Proffit WR, et al. Long-term stability of surgical class III treatment: A study of 5-year postsurgical results. Int J Adult Orthod. 2002;17:159. [PubMed] [Google Scholar]
- 26.Proffit WR, Turvey TA, Fields HW, et al. The effect of orthognathic surgery on occlusal force. J Oral Maxillofac Surg. 1989;47:457. doi: 10.1016/0278-2391(89)90277-2. [DOI] [PubMed] [Google Scholar]
- 27.Froese EA, Houston ME. Torque-velocity characteristics and muscle fiber type in human vastus lateralis. J Appl Physiol. 1985;59:309. doi: 10.1152/jappl.1985.59.2.309. [DOI] [PubMed] [Google Scholar]
- 28.Gerdle B, Wretling ML, Henriksson-Larsén K. Do the fibre-type proportion and the angular velocity influence the mean power frequency of the electromyogram? Acta Physiol Scand. 1988;134:341. doi: 10.1111/j.1748-1716.1988.tb08501.x. [DOI] [PubMed] [Google Scholar]
- 29.Suter E, Herzog W, Sokolosky J, et al. Muscle fiber type distribution as estimated by Cybex testing and by muscle biopsy. Med Sci Sports Exer. 1993;25:363. [PubMed] [Google Scholar]
- 30.Reggiani C, Bottinelli R, Stienen GJ. Sarcomeric myosin iso-forms: Fine tuning of a molecular motor. News Physiol Sci. 2000;15:26. doi: 10.1152/physiologyonline.2000.15.1.26. [DOI] [PubMed] [Google Scholar]
- 31.Tuxen A, Bakke M, Pinholt EM. Comparative data from young men and women on masseter muscle fibres, function and facial morphology. Arch Oral Biol. 1999;44:509. doi: 10.1016/s0003-9969(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 32.Rowlerson A, Raoul G, Daniel Y, et al. Fiber type differences in masseter muscle associated with different facial morphologies. Am J Orthod Dentofac Orthop. 2005;127:37. doi: 10.1016/j.ajodo.2004.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciote JJ, Rowlerson AM, Hopper C, Hunt NP. Fibre type classification and myosin isoforms in the human masseter muscle. J Neurol Sci. 1994;126:15. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton MJ, Rosen C, Close JM, Sciote JJ. Quantification of myosin heavy chain RNA in human laryngeal muscles: Differential expression in the vertical and horizontal posterior cricoarytenoid and thyroarytenoid. Laryngoscope. 2008;118:472. doi: 10.1097/MLG.0b013e31815c1a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trappe S, Costill D, Gallagher P, et al. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol. 2009;106:1159. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 36.Sciote JJ, Kentish JC. Unloaded shortening velocities of rabbit masseter muscle fibres expressing skeletal or alpha-cardiac myosin heavy chains. J Physiol (Lond) 1996;492(Pt 3):659. doi: 10.1113/jphysiol.1996.sp021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringqvist M. Fibre sizes of human masseter muscle in relation to bite force. J Neurol Sci. 1973;19:297. doi: 10.1016/0022-510x(73)90093-2. [DOI] [PubMed] [Google Scholar]
- 38.Ringqvist M. Fiber types in human masticatory muscles. Relation to function. Scand J Dent Res. 1974;82:333. doi: 10.1111/j.1600-0722.1974.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 39.Yu F, Stal P, Thornell L-E, et al. Human single masseter muscle fibers contain unique combinations of myosin and myosin binding protein C isoforms. J Musc Res Cell Motil. 2002;23:317. doi: 10.1023/a:1022061706126. [DOI] [PubMed] [Google Scholar]
- 40.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Alnaqeeb M, Simpson H, et al. Cloning and characterisation of an IGF-I isoform expressed in skeletal muscle subjected to stretch. J Musc Res Cell Motil. 1996;17:487. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- 42.Salerno MS, Thomas M, Forbes D, et al. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am J Physiol Cell Physiol. 2004;287:C1031. doi: 10.1152/ajpcell.00492.2003. [DOI] [PubMed] [Google Scholar]
- 43.Reisz-Porszasz S, Bhasin S, Artaza JN, et al. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 44.Palinkas M, Salles M, Nassar P, et al. Age and gender influence maximal bite force and masticatory muscle thickness. Arch Oral Biol. 2010;55:797. doi: 10.1016/j.archoralbio.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Jäger A, Kubein-Meesenburg D, Luhr HG. Longitudinal study of combined orthodontic and surgical treatment of class II malocclusion with deep overbite. Int J Adult Orthodon Orthognath Surg. 1991;6:29. [PubMed] [Google Scholar]
- 46.Gedrange T, Büttner C, Schneider M, et al. Change of mRNA amount of myosin heavy chain in masseter muscle after orthognathic surgery of patients with malocclusion. J Cranio-Maxillo-fac Surg. 2006;34(suppl):110. doi: 10.1016/S1010-5182(06)60023-1. [DOI] [PubMed] [Google Scholar]
- 47.Harzer W, Worm M, Gedrange T, et al. Myosin heavy chain mRNA isoforms in masseter muscle before and after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:486. doi: 10.1016/j.tripleo.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Maricic N, Stieler E, Gedrange T, et al. MGF- and myostatin-mRNA regulation in masseter muscle after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:487. doi: 10.1016/j.tripleo.2008.01.039. [DOI] [PubMed] [Google Scholar]