Abstract
The discovery of corticotropin-releasing factor (CRF) or CRH defining the upper regulatory arm of the hypothalamic-pituitary-adrenal (HPA) axis, along with the identification of the corresponding receptors (CRFRs 1 and 2), represents a milestone in our understanding of central mechanisms regulating body and local homeostasis. We focused on the CRF-led signaling systems in the skin and offer a model for regulation of peripheral homeostasis based on the interaction of CRF and the structurally related urocortins with corresponding receptors and the resulting direct or indirect phenotypic effects that include regulation of epidermal barrier function, skin immune, pigmentary, adnexal, and dermal functions necessary to maintain local and systemic homeostasis. The regulatory modes of action include the classical CRF-led cutaneous equivalent of the central HPA axis, the expression and function of CRF and related peptides, and the stimulation of pro-opiomelanocortin peptides or cytokines. The key regulatory role is assigned to the CRFR-1α receptor, with other isoforms having modulatory effects. CRF can be released from sensory nerves and immune cells in response to emotional and environmental stressors. The expression sequence of peptides includes urocortin/CRF→pro-opiomelanocortin→ACTH, MSH, and β-endorphin. Expression of these peptides and of CRFR-1α is environmentally regulated, and their dysfunction can lead to skin and systemic diseases. Environmentally stressed skin can activate both the central and local HPA axis through either sensory nerves or humoral factors to turn on homeostatic responses counteracting cutaneous and systemic environmental damage. CRF and CRFR-1 may constitute novel targets through the use of specific agonists or antagonists, especially for therapy of skin diseases that worsen with stress, such as atopic dermatitis and psoriasis.
-
Introduction
In memory of Dr Wylie Vale: life journey from CRF to the CRF receptors
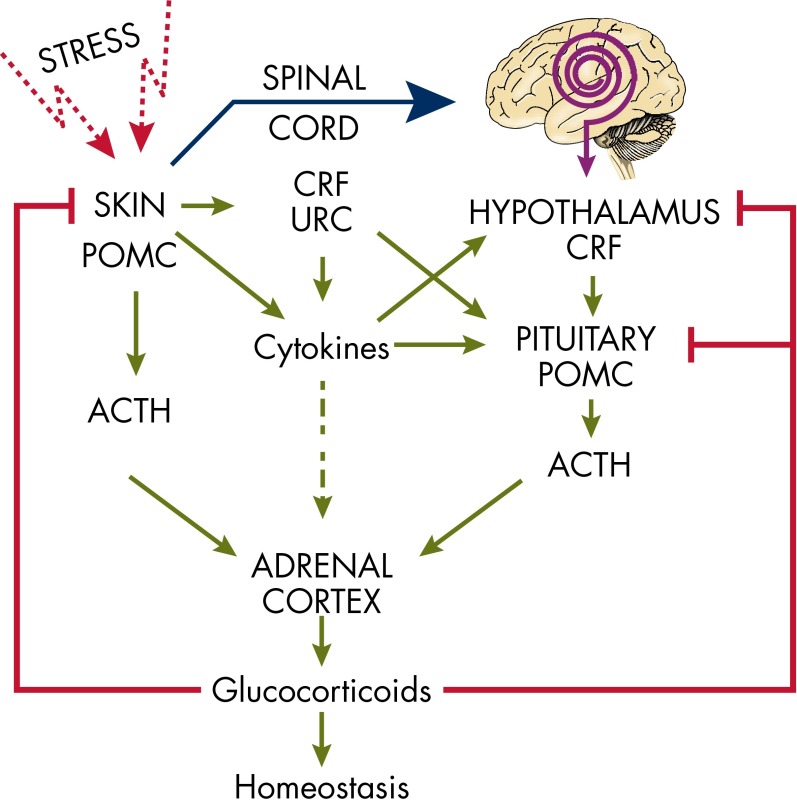
Organization of the central response to stress “in a nutshell”
-
Central Role of CRF in the Systemic Response to Stress: An Overview
Corticotropin-releasing factor (CRF) and urocortins 1–3 (Urc 1–3)
CRF receptors: CRFR-1 and CRFR-2
Hypothalamic-pituitary-adrenal axis
-
Structural Organization and Biological Role of the Skin “in a Nutshell”
Structure and function
Cutaneous systems and their role in homeostasis
Skin neuroendocrine system as a coordinator and integrator of peripheral responses to stress
-
CRF Signaling in the Skin: Its Expression and Organization
CRF and Urc 1–3 expression
CRF receptors (CRFR-1 and CRFR-2) in the skin
Cell type-dependent coupling to signal transduction systems
Alternative splicing of CRFR-1 and CRFR-2 and its physiological relevance
Neuropeptide and cytokine regulation of CRFR expression
Environmental regulation of the cutaneous CRF signaling with a focus on UV radiation and skin bacteria
Comparison with other peripheral organs
-
Skin Equivalent of the HPA Axis
Skin pro-opiomelanocortin (POMC) signaling system
Skin corticosteroidogenic system
Structural and spatiotemporal organization of skin HPA axis
Pathophysiological relevance of skin HPA axis and its departure from the central algorithm
Implications for other peripheral organs
Modes of communication between skin and the central HPA axis
-
CRF and Urc Function as Pleiotropic Cytokines
Regulation of skin barrier function
Regulation of skin pigmentary system
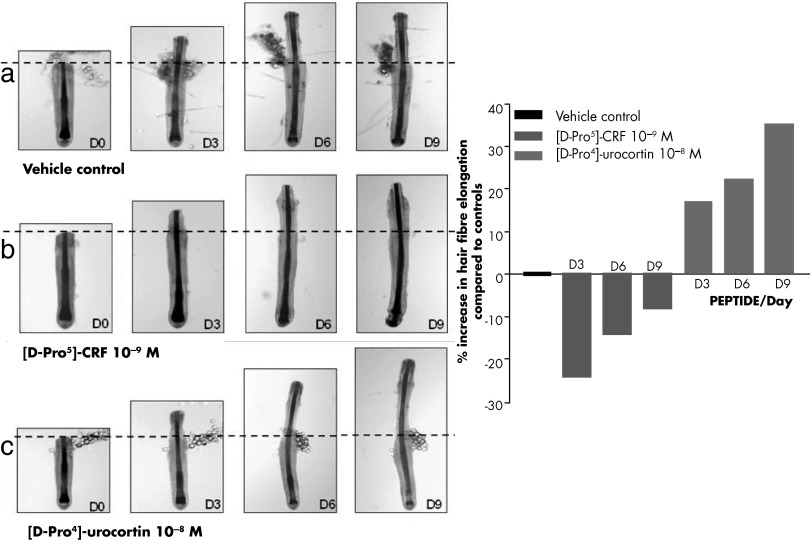
Regulation of adnexal structures with focus on the hair follicle
Regulation of the dermal compartment
Regulation of the skin immune system
Systemic implications
-
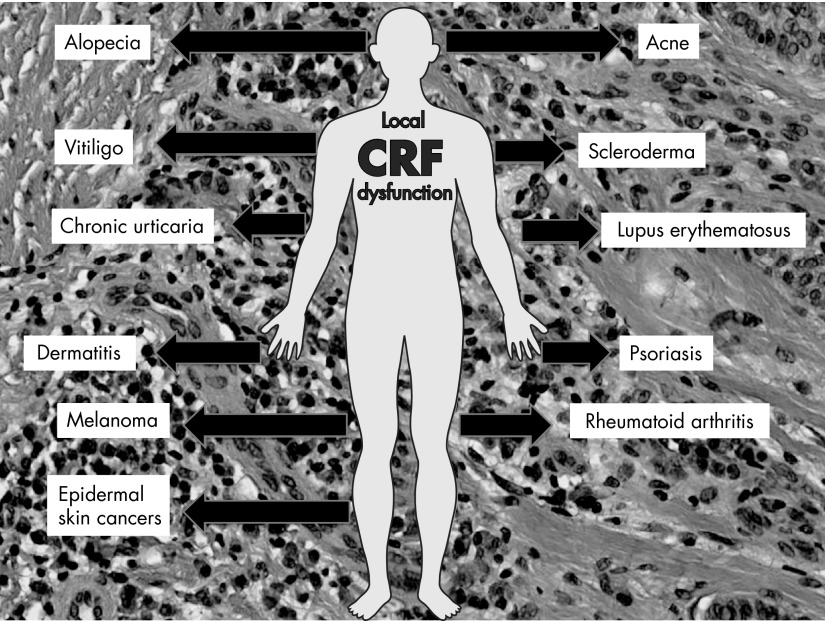
Skin Pathology Associated With Dysregulation of the Cutaneous CRF Signaling System
Proliferative disorders: psoriatic arthritis and psoriasis
Pigmentary disorders with emphasis on vitiligo
Disorders of adnexal structures including alopecia
Skin cancers including melanoma
Proposed unified mechanism of skin pathology secondary to dysregulation of local CRF signaling
Quest for Novel Treatments of Cutaneous Disorders Based on Interventions Into Local CRF Signaling System
-
Theory on the Origin of CRF-Led Stress Response System
Differences and similarities between the central and cutaneous HPA axis organization
Hypothesis on the integumental origin of CRF-led HPA-like organization
Integration of local and central CRF signaling systems in regulation of global homeostasis
Final Comments and Future Directions
I. Introduction
The work of Hans Selye has been fundamental in defining the hypothalamic-pituitary-adrenal (HPA) axis as the one of the body's main coordinators of responses to stress (1, 2). The functional structure of the HPA axis was finally defined after identification and sequencing of the hypothalamic neuropeptide corticotropin-releasing factor (CRF) and by defining its role in the pituitary production of adrenocorticotropic hormone (ACTH; corticotropin) and β-endorphin (3, 4).
A. In memory of Dr Wylie Vale: life journey from CRF to the CRF receptors
1. CRF peptide family
As a member of the hypothalamic factor family, CRF was the first factor whose existence was validated experimentally but the penultimate one to be chemically characterized. In 1948, Harris (5) proposed that the neuroregulation of ACTH might be mediated by a substance originating in the hypothalamus that reaches the adenohypophysis by the hypothalamic-hypophyseal portal system. Early experimental observations by Guillemin and Rosenberg (6) and Saffran and Schally (7) using in vitro and organ culture systems supported the presence of such a factor in the hypothalamus that would increase the rate of ACTH secretion by the pituitary gland (8).
This takes us back to 1955, when there was a great hypothesis but limited supporting data. Over the subsequent 14 years, Roger Guillemin at Baylor and Andrew Schally at Tulane University invested in collecting hundreds of thousands of sheep and porcine hypothalami, respectively. Processing these tissues took years, and additional activities in the extracts were identified with the availability of quantitative and selective in vivo and in vitro assays developed by Wylie Vale, a graduate student and then postdoctoral fellow in Guillemin's laboratories. In succession, the tripeptide amide TRH was isolated, characterized, and synthesized independently by Guillemin's group (9) and Schally's group (10). Definitive luteinizing releasing factor/LHRH/GnRH was fully characterized within a couple of years by Schally and colleagues (11), and the discovery of the tetradecapeptide somatostatin was not far behind (12).
The search for CRF expression started in earnest with the availability of an ACTH antibody provided by Drs Felber and Aubert in Lausanne, Switzerland, and the development of an RIA by C. Rivier, who had joined Guillemin's team in Houston in 1969 as a graduate student supervised by W. Vale. By then, CRF was referred to as “elusive” and was partially purified using classic chromatographic methods including ion exchange, gel permeation, and partition. By 1976, the power of reverse phase HPLC was identified (13), and by 1978, the use of triethylammonium phosphate (14) and trifluoroacetic acid-containing buffers (15), and derivatized (C4, CN, C18) large pore (300 Å) silicas led to the isolation of ovine CRF (4), and later of rat CRF (16), that were sequenced and synthesized by 1980 and 1983, respectively. Subsequently, the amino acid sequence of the protein precursor of human CRF was deduced from the cDNA (17), and the sequence of the mature peptide was found to be identical to that of rat CRF. Other members of the CRF family include frog sauvagine (18), the fish urotensins (19), and the urocortins (Urc-1, -2, and -3) (20, 21).
It is interesting to note that a straight line is obtained when plotting the time of discovery vs molecular weights of the hypothalamic factors including the CRF. In other words, the greater the difficulties associated with isolation—HPLC, sequencing (microsequencing), and solid-phase peptide synthesis of the hypothalamic hormones—the longer it took to duplicate their structures synthetically. This emphasizes the importance of technological development for the advancement of scientific discoveries.
Historically, the elusive CRF activity was referred to as resulting from the hypothalamic secretion of a CRF acting specifically and exclusively on the anterior pituitary. The word factor was used to describe a molecule that did not circulate as a result of its instability toward blood enzymes. On the other hand, stability to blood enzymes is a condition sine qua non to justify the hormone qualifier. In this review, we continue using the term CRF instead of CRH because of its wide expression in different tissues where it acts locally as a cytokine, growth factor, or immunomodulator.
2. CRF receptors
A milestone advancement in understanding CRF actions was the cloning and characterization of two CRF/Urc receptors, encoded by different genes and existing in multiple forms: CRFR-1 (22–24) and CRFR-2 (25–29). These have encouraged the development of synthetic CRFs, CRF/CRFR antibodies, and competitive antagonists (peptides and nonpeptides) that allow the study of various aspects of CRFs, their distribution, and the multiple activities of CRFs. These include, but are not limited to, the study of mood/anxiety/depression and stress, mania or obsessive-compulsive behaviors, substance abuse, food intake and satiety, feeding disorders, Alzheimer's disease, reproduction/parturition, immune function, cardiovascular function, somatic disorders, inflammation, rheumatoid arthritis (RA), gastrointestinal (GI) motor function, irritable bowel syndrome (IBS), sleep, analgesia, migraine, skin physiology and pathology, and cancer, among others.
In a few words, Dr Wylie Vale (Figure 1) initiated and was further involved in a series of discoveries that made the CRF/Urc signaling system one of the central elements of each important regulatory axis in the body. One of them is represented in this review.
Figure 1.
Dr Wylie Vale. The photograph was taken by Ms Kristen Peelle on September 4, 2010, at the wedding of Dr Vale's daughter at The Bishop's School Chapel.
B. Organization of the central response to stress “in a nutshell”
As proposed by Cannon, external psychological or physical danger (stress) stimulates the animal (human) to respond both consciously and unconsciously (30). Multiple levels of systems are present that differentiate noxious from benign stimuli. The most widely recognized system is an autonomic system composed of the sympathetic and parasympathetic components (31). The sympathetic system participates in the so-called “fight and flight response” (32). The inputs to this system come from the senses, sensory nerves, and other organs and tissues (31). The immune system is responsible for recognition of self from nonself. The master regulator/switchboard transcription factor that integrates and regulates inflammatory inputs is nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (33). Τhe “innate” immune system is generally the first to be encountered by foreign antigens (34, 35). The adaptive immune system recognizes antigens and mounts a specific, targeted response (34). The actions of the immune and autonomic systems are coordinated by the HPA axis (36, 37). Many of those systems act in concert and balance the actions of other systems to preserve homeostasis. The HPA system is evolutionarily conserved and has additional functions such as the regulation of pigmentation, which itself is a protective mechanism, as well as skin barrier functions (37–42). Inflammation can develop as a consequence of CRF and immune system activation, with cytokines such as IL-1 and IL-6 producing positive (feedback) CRF stimulation along with CRF secreted outside the central nervous system (CNS) (43–45).
Last but not least, the concept of stress was recently extended by Sterling and Eyer (46) and was further extended by McEwen (47, 48). According to these authors, allostasis (“achieving stability through change”) is a sum of actions that the body undertakes to cope with daily stressors to preserve internal milieu. Allostatic overload refers to the situation when those actions exceed what is essential for the organism and instead begins to be harmful and unneeded. Interaction between inflammation, sympathetic and parasympathetic systems, and the HPA axis forms a framework for the concept of allostasis or a system of “checks and balances” that may also be applied to the skin (49).
II. Central Role of CRF in the Systemic Response to Stress: An Overview
A. Corticotropin-releasing factor (CRF) and urocortins 1–3 (Urc 1–3)
CRF is coded by a gene located on the long arm of chromosome 8 (8q13) that has 2349 bases (50). Its promoter has binding sites among others for activating transcription factor (ATF) 2, adaptor protein 1 (AP-1), neuron-restrictive silencer factor, NF-κB, cAMP responsive element (CRE) binding protein (CREB), and v-jun avian sarcoma virus 17 oncogene homolog (51). Several molecules affect expression of CRF including IL-1, IL-6, TNF-α, serotonin, acetylcholine, histamine, norepinephrine, epinephrine, arginine vasopressin, angiotensin II, neuropeptide Y, cholecystokinin, activin, encephalin, estrogens, γ-amino butyric acid, dynorphin, substance P (SP), somatostatin, galanin, and last but not least, glucocorticoids (52). The CRF propeptide is composed of 196 amino acids (molecular mass, 21.4 kDa) that are processed by proconvertases (PCs) and by post-translational modifications in the endoplasmic reticulum and Golgi apparatus to yield a final product that is composed of 41 amino acids (4.7 kDa) (50). The homology of nucleotide sequences between human and chimpanzee is 99.66%; mouse, 83.60%; rat, 83.42%; and zebrafish, 76.98% (53). The highest levels of CRF outside the HPA axis are found in the heart and placenta (25, 54). It is also expressed in the uterus, GI system, immune system, adrenal gland, and skin (55–57). Abnormalities in CRF levels are linked to a wide range of conditions including adenoma, prolonged pregnancy, chronic fatigue syndrome, anorexia nervosa, asthma, IBS, obesity, migraines, amenorrhea, depression, fibromyalgia, and interstitial cystitis/bladder-pain syndrome (58–62).
Urc-1, also known as stresscopin-related protein, is coded by a gene located on the short arm of chromosome 2 (2p23) that has 1049 bases (50). Its promoter binds E2 transcription factors (E2Fs), specificity protein 1 (SP1), neuron restrictive silencing factor (NRSF), and signal transducer and activator of transcription 3 (STAT3) (51). The propeptide is composed of 124 amino acids that result in a molecular mass of 13.45 kDa. It is processed to the final 40 amino acid peptide. The homology of the nucleotide sequences between the human and mouse gene is 84.31% (53). Urc-1 is expressed in numerous tissues, but the highest levels occur in the brain, pancreas, bone marrow, heart, skeletal muscle, kidney, and lung (52). Abnormality in Urc-1 levels is linked to preterm delivery, pre-eclampsia, endometriosis, heart failure, and pheochromocytoma (58).
Urc-2, known as Urc-related peptide, is coded by a gene located on the short arm of chromosome 3 (3p21) that has 2056 bases (20, 50). Its promoter binds acute myeloid leukemia 1 protein. The peptide is composed of 112 amino acids (12.15 kDa). It is expressed in the brain, kidney, and skin. Abnormalities in Urc-2 levels are linked to obesity, anxiety, depression, ulcerative colitis, and dermatomyositis.
Urc-3, also known as stresscopin, is coded by a gene located on the short arm of chromosome 10 (10p15) that has 9198 bases. Its promoter has binding sites among others for the nuclear factor of activated T-cells, homeobox protein NK-3 homolog A, forkhead-related transcription factor 2, cell division cycle 5, POU class 3 homeobox 2, and ecotropic viral integration site 1 (51). The peptide is composed of 161 amino acids (17.96 kDa). The homology of nucleotide sequences between the human and mouse genes is 75.42%; chicken, 69.09%; and zebrafish, 59.34% (53). It is expressed in the pancreas, urinary bladder, and skin (52). It is also expressed in mouse skin (20). Abnormalities in Urc-3 levels are linked to obesity, heart failure, pheochromocytoma, and IBS (58).
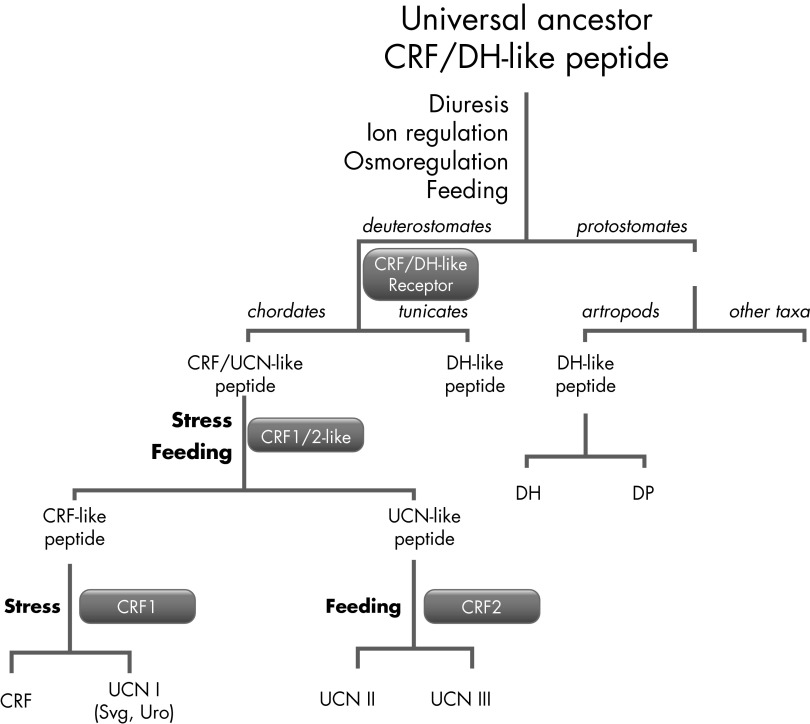
A schematic of the evolution of CRF, related peptides, and corresponding receptors is presented in Figure 2 (see Refs. 63–65).
Figure 2.
Schematic evolution of CRF and related peptides and receptors. The progenitor peptide for CRF and antidiuretic hormone had evolved before separation of chordates because CRF analogs were not found in tunicates or protostomates. The novel function CRF/DH (diuretic hormone)-like peptides in multicellular organisms required development of specific receptors, and those receptors once appeared evolved with peptides and were subjected to genome duplications (64). In the case of CRF precursor, the duplication occurred twice, whereas for CRFR it occurred only once. UCN, urocortin.
B. CRF receptors: CRFR-1 and CRFR-2
CRFR-1 and -2 belong to class B (Secretin and Adhesion receptors) of the G protein-coupled receptor (GPCR) family (66, 67). The other members of class B of GPCRs include receptors for secretin, calcitonin, glucagon, glucagon-like hormone, GHRH, PTH, and pituitary adenylate-cyclase-activating peptide (66, 68). These receptors bind to short peptides and interact with multiple G proteins (67). The main structural elements for this class are the substrate binding domain with three highly conserved disulfide bridges (69, 70) and 7-transmembrane domains (7-TMs) including three internal coils (1–3) and three external coils (1–3) followed by the C terminus. Interestingly, internal coil 3, which is responsible for interaction with multiple G proteins, was shown to be identical for all CRFRs. The 7-TM domain is highly conserved among GPCRs, with homology of at least 80%. On the other hand, the N-terminus extracellular domain (ECD) is the most variable region of the CRFRs with only 40% of homology, most probably reflecting different substrate preferences (71, 72). The CRFR-1 has a high affinity to CRF and Urc-1 and does not bind Urc-2 or Urc-3. The CRFR-2 preferentially binds Urc-2 and Urc-3, but it can also be activated by CRF, although with weaker binding compared to CRFR-1. Binding of the ligand to the CRFRs results in signal transduction and activation of adenylate cyclase, phospholipase C, and/or calcium channels followed by phenotypic changes (49, 73).
High levels of CRFR-1 mRNA can be detected in the pituitary; several areas of the cortex, amygdala, cerebellum, and hypothalamus; parts of the hippocampus; and the olfactory bulbs of the human, mouse, rat, and other mammalian species (69, 70, 74, 75). Recent analysis of CRFR-1 expression, using fusion constructs with green fluorescent protein (GFP) (75) or β-galactosidase (76), confirmed previous in situ hybridization studies (76, 77). These findings also explained why CRFR-1−/− mice exhibited elevated auditory thresholds and impaired hearing (78). CRFR-1 is also expressed in peripheral organs and tissues such as the testis, ovary, uterus, placenta, GI system, endocrine organs, immune systems, skeleton-muscular system, and vascular system (70, 79), as well as in the skin (54, 55, 62, 80–86).
In contrast to CRFR-1, CRFR-2 is present to only a limited extent in the brain structures, while being widespread in the periphery (87). Interestingly, CRFR-2 isoforms CRFR-2α and CRH2β are expressed in different regions of the rat brain and are usually not coexpressed in peripheral organs. The presence of CRFR-2α mRNA was detected in the hypothalamus, lateral septum, and hippocampus, whereas CRH2β expression was restricted to non-neuronal structures such as the choroid plexus and arterioles (88). In the periphery, CRFR-2β is abundant in the mucosa of the GI tract and in the heart, lung, and skeletal muscle, whereas CRFR-2α is low or undetectable in many peripheral tissues (49, 89) but is strongly expressed by mast cells (90). CRFR-2 was found to be expressed in the myocardial cells, and this expression correlates with high expression of Urc-1 and Urc-3 (91).
Taken together, the expression and activity of CRFRs play an essential role in regulation of the brain and peripheral organ functions, and the pattern of their expression reflects such functional diversity.
1. Gene and protein structure and alternative splicing
The ancestral forerunner of the CRFR must have evolved from the first GPCRs, which occurred around 1.2 billion years ago, just before animals, plants, and fungi diverged into separate kingdoms (92). Interestingly, CRFRs are found only in chordates; thus, they appeared relatively late in evolutionary time. Arthropods possess a receptor for the diuretic hormone that shares some homology with the CRFR, but it seems that arthropods and other invertebrates do not have an HPA axis analog (93, 94). Still, there is circumstantial evidence that a common ancestor for CRF and diuretic hormone did exist and was involved in osmoregulation, but its receptor appeared much later in evolution (64, 92–94). The CRF/Urc precursor underwent two rounds of duplication resulting in four genes coding CRF and Urc-1–3. The stepwise evolution might be reflected by the single CRF-like receptor that was found in the sea squirt, Ciona (95), and two receptors (CRFR-1 and CRFR-2) in higher vertebrates (Figure 2).
2. CRFR-1
The human CRFR-1 coded by the CRFR-1 gene is located on chromosome 17 (17q12-q22) (96). It consists of 14 exons and 13 introns spanning about 20 kb and possesses 1 additional exon when compared with the rat or mouse CRFR-1 homolog (23, 97–99). The sequence of the main isoform, CRFR-1α, is 415 amino acids in length with the first 23 amino acids forming a signal peptide, which is subsequently cleaved (100).
The structure of the first ECD of CRFR-1 (101, 102) resembles the ECD of CRFR-2β (103). It consists of two antiparallel β-sheets (α-β-αβ fold) stabilized by three disulfide bonds between Cys30–Cys54, Cys44–Cys87, and Cys68–Cys102 forming a characteristic Sushi domain (101). Also at least 6/5 N-glycosylation, but not of O-glycosylation sites were detected in the mammalian CRFR-1 ECD (104, 105). This was confirmed experimentally, and it was proposed that glycosylation of CRFR-1 is responsible for proper intracellular trafficking and functions of the receptor (106–109). Interestingly, recent structural studies revealed that a single amino acid, Glu-104 of CRFR-1, is responsible for a selective interaction with Arg35 of CRF/Urc-1, but not with Ala in Urc-2 and Urc-3; this fully explains the selective affinity of CRFR-1 toward CRF and Urc-1 (110).
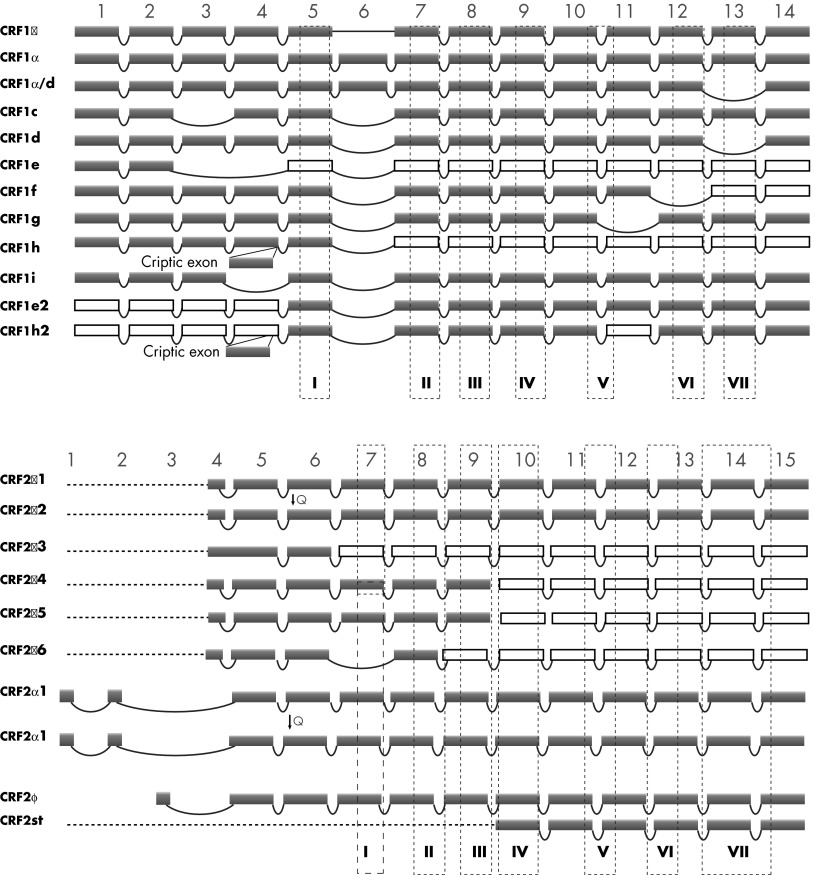
Alternative splicing of CRFRs is the obvious consequence of a multiexonal structure of the genes, and this feature is characteristic of a majority of human genes. The expression of multiple alternatively spliced CRFR-1 mRNAs was found in several organisms, including human, green monkey, rat, mouse, and hamster (67, 70, 80, 81, 111–113). Moreover, the pattern of alternative splicing is not only preserved in CRFR-1 homologs from different organisms, but is also found in other receptors belonging to class B1 of GPCRs, such as the characteristic deletion of exon 13 in CRFR-1 isoform d and calcitonin receptor (109, 114–116). In humans, at least 10 variants (α, β, β/d, c, d, e, f, g, h, and i) of CRFR-1 transcripts were found (70, 81, 111, 117–119) (Figure 3, upper panel). We believe that alternative splicing of CRFR-1 mRNA is conserved in evolution because a similar pattern of exonal excision was found in human, mouse, and hamster (81). For instance, in addition to the main CRFR-1 isoform (CRFR-1α), homologs of isoform c, e, and f where found in human and mice (111). Although the hamster has three unique isoforms with deletions within the C terminus, the homologs of human isoforms d, e, f, and h were also detected (112). Interestingly, new data received from high-throughput sequencing and transcriptome studies revealed multiple CRFR-1 isoforms in human's close relatives, including great apes (NCBI DNA database), which is consistent with our findings in green monkey (113). It has to be noted that in rodents, the CRFR-1 gene lacks a homolog of human exon 6 (49, 111, 112).
Figure 3.
Alternative splicing of CRFR-1 and CRFR-2 pre-mRNA leads to production of several isoforms of the receptors. A, Human CRFR-1 protein sequence is coded by 14 exons that could be subjected to alternative splicing. Expression of at least 12 isoforms was detected so far in humans and rodents (49, 80, 111). Protein coding exons are shown in violet. Alternative splicing results occasionally in the introduction of termination codon, and some exons no longer code protein (white squares). B, Human CRFR-2 gene contains 15 exons with 3 alternative transcription start codons located in different exons. Three main isoforms were described (CRFR-1α, β, and γ), and at least 7 others were detected, including “headless” isoform. Interestingly, some isoforms (CRFR-2α3, CRFR-2α4, CRFR-2α5) show the retention of intronic segment in the coding sequence. Isoforms CRFR-2α2 and CRFR-2β2 have deletion of 3 nucleotides coding glutamine: CRFR-2β (desQ126) and CRFR-2β-2 (desQ126) (227). Positions of exon 1–14 for CRFR-1 and 1–15 for CRFR-2 are marked on the top of each panel. 7-TM domains are shown as dashed-line squares numbered I–VII (49, 80, 81).
3. CRFR-2
The human CRFR-2 receptor is coded by the CRFR2 gene with 15 exons and 14 introns and was mapped to chromosome 7 (7p14.3) (55, 70). Given the similar structure of the CRFR-2 and CRFR-1 genes and their high degree of homology, CRFR-2 should also have the capacity for generating similar splicing variants. However, only CRFR-2α, -β, -γ and soluble sCRFR-2 isoforms of CRFR-2 have been characterized so far (71, 120) (Figure 3, lower panel). Moreover, alternative splicing of CRFR-2 mRNA is connected with at least three alternative transcription starting codons, which appears to be a unique feature of CRFR-2. The CRFR-2 gene also possesses alternative exons located on the 5′-end of the gene, whereas a soluble isoform of CRFR-2 (sCRFR-2a) was detected in mouse brain (121), and the so-called “headless” isoform of CRFR-2 was found in the stomach (GenBank accession no. E12750; patent no. JP199707289-A). The soluble sCRFR-2 (121) and “headless” isoform is similar to CRFR-1e2 and CRFR-1h2 isoforms and contains only the 7-TM region of the protein (49, 111).
C. Hypothalamic-pituitary-adrenal axis
The hypothalamus is stimulated by various stressors, including inflammatory cytokines, either directly (in situations where the blood-brain barrier is weakened) (122) or indirectly (eg, through locally produced histamine or prostaglandins) (32, 37, 43). Various medications, including psychotropic drugs, also affect the activation of the hypothalamus (123). Neurons of the medial dorsal parvocellular region of paraventricular nucleus of the hypothalamus produce CRF and arginine-vasopressin, which are transported by local vascular network to the anterior pituitary. CRF stimulates corticotrophs through CRFR-1, and arginine-vasopressin potentiates this effect by V1b receptors. The pituitary in turn produces ACTH, which stimulates the function of the zona fasciculata of the adrenal glands, which produce cortisol (humans) or corticosterone (rodents) as the main end effectors of the HPA axis. Those glucocorticosteroids bind to the glucocorticoid receptors, which after binding ligand translocate through nuclear pores to reach their respective binding elements in the regulatory regions of DNA (123, 124).
The HPA axis is regulated by a circadian clock at the central (hypothalamic) and peripheral levels (125, 126). This circadian clock is based on the action of Clock-BmaI1 heterodimers that in turn interact with proteins of Period, Cryptochrome, retinoic acid receptor-related orphan receptor α, and V-erbA-related protein 1-related families (126, 127). At the periphery, Clock-BmaI1 decreases glucocorticoid receptor activity via acetylation (128). Similarly to the presence of central, “master” (located at the suprachiasmatic nucleus), and peripheral (“slave”) circadian clocks, the HPA axis functions at these two levels, too. Apart from recognized anatomic locations of both circadian and HPA systems, their mediators function in the tissues or in individual cells. In our work, we focus on the HPA axis in the skin (129, 130), but other peripheral locations such as the cochlea (78), eye (131), and perhaps the GI tract (85, 132) may also operate based on HPA effectors.
III. Structural Organization and Biological Role of the Skin “in a Nutshell”
A. Structure and function
Skin is the largest body organ, representing 15% of body weight with an average surface of about 2 m2 (133), and serves as the homeostatic barrier with the external environment (49, 134). It is composed of three distinct compartments: epidermis, dermis with adnexal structures, and sc fat. In humans, skin is formed during the first 1–2 weeks of gestational age, and the epidermis derives from the ectoderm, as does the brain. The progenies of both structures are separated during neurogenesis, although the epidermis and hair follicles are later populated with neural crest-derived melanocytes and Merkel cells.
The epidermis is predominantly composed of keratinocytes, which either self-replicate/regenerate (basal layer) or differentiate (suprabasal layers) in an organized fashion toward the skin surface, forming on their way the spinous, granular, and a solid lipid-rich cornified layers (135–137). Other cellular populations include melanocytes, which reside in the basal layer and produce the protective melanin pigment that is transferred to neighboring keratinocytes (38). The main mesenchymal components of the epidermis, Langerhans cells, derive from the bone marrow. They present foreign antigens through their major histocompatibility complex system to T lymphocytes initiating adaptive immune responses.
Adnexal structures are of epidermal origin, the most prominent of which are the hair follicles with hairs covering most of the body surface (138). Hair follicles undergo cyclic changes of growth (anagen), involution (catagen), and resting (telogen), with significant differences between humans and other mammals including rodents. In the former, different hair follicles are in different (sub) phases of the growth cycle at any one time, whereas in rodents their hair cycling is synchronized. The associated sebaceous glands release a protective substance into the follicular infundibulum through a holocrine mechanism, whereas eccrine glands release sweat to the epidermal surface (139). In humans, sweat glands play important thermoregulatory functions, whereas apocrine sweat glands (present in axillae, genitals, ears, and eyelids) have predominantly vestigial functions.
The dermis is predominantly composed of connective tissue, of which the extracellular components collagen and elastic fibers and proteoglycans are produced by fibrocytes/fibroblasts. They give the skin its strength and elasticity and cushion the body from impact stress. The dermis is separated from the epidermis by a basement membrane, which controls molecule and cell trafficking between both compartments. Immune cells (lymphocytes, macrophages, mast cells, and dendrocytes) predominantly reside in the dermis, the number and activity of which change depending on environmental or internal homeostasis. The dermal vasculature is crucial for the viability/survival of structural elements of the skin, as well as for efficient communication between the external and internal environment using structural elements of the skin and a variety of mediators (129). It forms superficial and deep dermal plexuses connected by collaterals with glomus bodies involved in local thermoregulation. The sc adipose tissue is an important element of the skin and is composed of fat lobules separated by fibrous septae rich in vasculature. Its structural role lies in isolation, cushioning, and energy storage.
All skin structures are innervated by an extensive neural network of somatosensory and autonomic nerve fibers (49). Cutaneous afferent nerve terminals transduce sensory stimuli, generated in response to temperature, pH, pressure, physical, chemical, and biological insults or inflammation, with projections to the specific areas of the brain. In addition, orthodromic and antidromic conductions lead to simultaneous signal transduction and release of neurotransmitters at the local level. The major ascending routes for sensory cutaneous inputs transmit to the thalamus, which sends neural signals to the somatosensory cortex, midbrain, and hypothalamus. The cutaneous stimuli from the face are transmitted via the trigeminal nerve (140). There are also intraepidermal and intra- adnexal nerve fiber termini in skin (reviewed in Refs. 49, 129, 133, 141, and 142). Dermal and epidermal neural density varies with age and in different pathologies or after exposure to UV radiation. Autonomic nerve fibers are distributed almost exclusively in the dermis and subcutis, where they supply arterioles, glomus bodies, hair follicles, pilosebaceous units, hair erector muscles, and apocrine and eccrine glands. They derive from sympathetic (cholinergic, catecholaminergic, nonadrenergic/noncholinergic) and parasympathetic (cholinergic) neurons.
B. Cutaneous systems and their role in homeostasis
The skin's location at the interface between external and internal environments determines its structural and functional organization, which plays a fundamental role in the regulation of local and global homeostasis. The latter is amplified by the size of this organ (reviewed in Refs. 49 and 129). In physical terms, its main function is to preserve enthalpy and to reduce the entropy of the organism in the environment. For this function, the skin forms the protective barrier against water and nutrient loss and against harmful actions of numerous external chemical, physical, and biological factors. Additional functions include thermo- and electrolyte regulation, insulation, absorption of chemicals, and production of vitamin D, folates, lipids with various biological functions, sterols/oxysterols, steroid production/activation/inactivation, xenobiotic metabolism, hormones, cytokines, growth factors, as well as neurotransmitter production, and metabolism including biogenic amines (reviewed in Refs. 40, 49, 136, 137, and 143–152). The role of skin, including its adnexal structures in social and behavioral communication, also cannot be underestimated (42, 49).
The above skin functions are regulated and maintained mainly by autonomous innervation, however, closely interacting with each other epidermal (153, 154), adnexal (138, 155, 156), pigmentary (38, 157), local skin immune (158, 159), fibroblastic, vascular, adipose, and neural systems (49, 141, 160). Their activities (either stochastic or organized) are determined by the type of the cells, their level of differentiation, receptors expressed and metabolic and secretory activities, spatial location, and local direct and distant interactions. The above systems also can affect global homeostasis either through chemical messengers and immune cells entering circulation or through neural communications (reviewed in Refs. 49 and 129).
C. Skin neuroendocrine system as a coordinator and integrator of peripheral responses to stress
The diversity and complexity of the skin functions and the systems regulating them require a precise coordination and execution. These activities are mediated by a cutaneous neuroendocrine system that is integrated into central regulators of body homeostasis (49, 129, 161). Specifically, epidermal, dermal, and adnexal compartments (as well as cells within these compartments) communicate to regulate local homeostasis (49, 129). This is achieved through the production of classical stress neurotransmitters, neuropeptides, neurohormones, and hormones, with a precise response mechanism in a receptor-mediated manner (49), which can be amplified in cell type or compartment selective fashion (162, 163). The pathways and responses are modified by biological, chemical, and physical factors including UV radiation with attendant changes in physicochemical milieux. Examples of potent epidermal products include catechols and biogenic amines (catecholamines, serotonin, and N-acetyl-serotonin, histamine) (49, 144, 148, 164–166), acetylcholine (167, 168), melatonin and its metabolites (169–173), pro-opiomelanocortin (POMC)-derived ACTH, β-endorphin and MSH peptides (130, 174, 175), CRF and related Urcs (55, 59, 80, 176–178), corticosteroids and their precursor molecules (130, 179, 180), TSH, TRH, and thyroid-related hormones (49, 181–184), opioids (185, 186), cannabinoids (187), 7Δ-steroids (188, 189), and secosteroids (151, 190–194).
The production of these molecules in the skin and the following receptor-mediated responses are hierarchical and follow the algorithms of classical neuroendocrine axes such as the HPA axis (130, 181, 195), serotoninergic/melatoninergic (148, 172), catecholaminergic (165, 196), cholinergic (144, 197), and corticosteroidogenic (49, 198) systems. The etiology of many skin diseases, as well as systemic autoimmune disorders, can dysregulate these systems, with the central role played by local CRF signaling and HPA axis organization (49, 59, 80, 130, 199–201). We have proposed that these local neuroendocrine systems may restrict the effects of environmental stress to preserve the body's local and, in extreme cases, global homeostasis in order to adapt to a changing external environment (49, 129). The signals generated in the skin in response to stress activate cutaneous sensory nerve endings to alert the brain of changes in the epidermal and dermal milieu or activate other coordinating centers by spinal cord neurotransmission with or without the brain's involvement (40, 49, 129). Furthermore, the skin neuroendocrine system can imprint immune cells to serve as cellular messengers of the skin's response to the changing environment to trigger global responses (49, 129, 199).
IV. CRF Signaling in the Skin: Its Expression and Organization
A. CRF and Urc 1–3 expression
Human skin (including sc adipose tissue) expresses the genes for CRF (202–207), Urc-1 (208), and Urc-2 (209), whereas reverse phase HPLC and liquid chromatography/mass spectrometry analyses confirmed that CRF (203, 204, 210) and Urc-1 (208) peptides are indeed produced by human skin cells.
Immunocytochemistry, RIA, or ELISA has also detected these peptides in the majority of cells, if not all cells, of the epidermal, dermal, and adnexal compartments of human skin (55, 80, 117, 177, 201, 205–209, 211–216). Moreover, CRF expression is increased under pathological conditions, including autoimmune and inflammatory disorders (177, 199, 201, 205, 207, 215–217). Interestingly, serum levels of CRF were increased in patients with psoriasis and atopic dermatitis (218), skin conditions that are known to worsen with stress.
Importantly, biological insults such as lipopolysaccharide (LPS; a toll-like receptor [TLR]-4 agonist), but not TLR-2 agonists were able to stimulate CRF production, indicating that CRF mediates LPS-induced inflammation in epidermal keratinocytes (219). This has been confirmed by other studies showing stimulation of CRF production in keratinocytes by extracts from bacillus Propionibacterium acnes (220). Furthermore, UVB stimulated CRF gene expression and peptide production in epidermal melanocytes, keratinocytes (203, 214, 221) and dermal fibroblasts (DFs) (80), as did factors raising intracellular levels such as forskolin (204).
In contrast to human skin, the production of the CRF peptide in mouse skin is not accompanied by corresponding CRF gene expression (55, 203, 211, 222), suggesting an extracutaneous supply of CRF in this species, perhaps released from nerve endings (55, 211, 223). Mouse skin, however, does express the Urc-1 gene and produce the corresponding peptide (208), which can be detected in cells of the epidermis, dermis, hair follicle, and skeletal muscle (208, 209). The expression of CRF and Urc-1 in mice is modified by the phase of the hair growth cycle (208, 211, 222). Rodent (mouse and rat) skin also expresses Urc-2 (209, 224–226), as well as Urc-3 (20). Both Urc-2 and Urc-3 are detectable in keratinocytes of rat esophagus (227). CRF accumulation in mouse skin is increased after acute stress or in models of skin inflammatory diseases (201, 228–230).
Thus, mammalian skin has the capability to produce or accumulate CRF and related Urc peptides in a species-dependent fashion (human skin does express CRF gene, whereas murine skin does not), and this production can be linked to physical or biological stress, skin inflammatory disorders, and phase of the hair growth cycle (predominantly in mice).
B. CRF receptors (CRFR-1 and CRFR-2) in the skin
Human skin expresses both CRFR-1 and CRFR-2 in a compartmentalized manner (55, 80, 111, 174, 202, 204, 207, 209, 211–213, 231–233). In general, the epidermis (including epidermal keratinocytes) and melanocytes (including immortalized lines of melanocytes and melanoma cells) (55, 76, 112, 203, 209, 211, 222, 229, 234–238) express predominantly, if not exclusively, CRFR-1, with the most predominant isoform represented by CRFR-1α (80, 111, 209). However, cells of dermal, adnexal (including hair follicle and sebaceous glands), and sc compartments and mast cells expressed both CRFR-1 and CRFR-2 (178, 207, 209, 212, 213, 216, 239–241). In the case of CRFR-2, we detected preferentially CRFR-2α isoform (209). In addition, CRF binding protein (CRF-BP) expression can be seen in human DFs, sc adipose tissue, and sebaceous cells (207, 209), with in situ detection of CRF-BP in the latter (178, 207). However, we failed to detect CRF-BP in epidermal melanocytes and keratinocytes (209).
Importantly, the expression pattern of both CRFRs was affected by skin pathology including inflammatory disorders, age of the patient, and anatomical localization (55, 111, 174, 207, 209, 216, 218, 239, 242–245). Their cutaneous expression appears to be regulated also by locally produced cytokines and biological insults (80). For example, in mast cells, IL-1, IL-4, and LPS stimulated CRFR-2 but not CRFR-1 expression (239), whereas SP stimulated CRFR-1 expression while CRF inhibited it (233). More recently, it was reported that the peptide neurotensin (NT) also induced CRFR-1 expression (246). A single dose of UVB has been shown to alter the splicing pattern and induced/increased the expression of CRFR-1α in cultured skin cells, whereas continued UVB treatment resulted in a progressive increase in the number of CRFR-1 isoforms (55, 111). Also, CRFR-1 expression can be regulated by factors raising intracellular cAMP and by 12-o-tetradecanoylphorbol 13-acetate (111), whereas melanogenesis inhibited its expression in melanoma cells (204). Interestingly, UVB stimulated CRFR-2γ in human keratinocytes (247). The environmental regulation of CRFR-1 on the gene, protein, and processing levels was further confirmed using human epidermal keratinocytes, where cell density, the presence/absence of serum, or UVB showed marked regulatory effects (109). Finally, CRFRs are biologically functional and regulate a variety of skin functions (see corresponding sections of this review; also reviewed in Refs. 49, 80, 81, 178, and 201).
The expression of CRFR-1 (211, 222, 223) and CRFR-2 (209) changes during hair growth cycling. Of note is the change in the pattern of alternatively spliced isoforms for CRFR-1 during murine hair cycling with CRFR-1α absent in telogen (111, 248). At this phase of hair cycle, only CRFR-1e is expressed. In addition, in hamster melanoma cells, UVB stimulated CRFR-1α expression, and the CRFR-1 isoform pattern changed after induction of melanogenesis (112).
In conclusion, mammalian skin shows differential, spatiotemporal selectivity and species-dependent (human vs rodent) expression of CRFR-1, CRFR-2, and CRF-BP. These differences are likely to be the result of evolutionary pressure on human skin, which, because of continuous exposure to solar radiation and lack of fur, has developed the epidermal stress response system favoring CRFR-1 as a major regulator, which is also involved in the activation of a local HPA axis. Although alternatively spliced CRFR-1 soluble isoforms can negatively regulate the local availability of CRF and Urcs, the expression of CRF-BP in the dermis can also serve as an additional buffer in order to modulate CRF and Urc bioactivity and/or availability for systemic circulation. In contrast, mice are nocturnal animals (leading to very limited exposure to solar radiation) and have the skin to some degree shielded from environmental insults by fur, relieving it from evolutionary pressure to develop an epidermal CRFR-1-centered stress response system. As a result, in mice CRFR-2 distribution is equal or more prevalent to CRFR-1, and the preferred ligands for CRFR-2 (ie, Urcs) are produced locally, whereas CRF is delivered by nerves or from circulation. However, the relatively high expression of CRFR-2 in adnexal structures and dermis both in humans and mice indicates similarity in this signaling system in subepidermal compartments. For more detailed discussions on this subject, see Refs. 49, 80, and 209.
C. Cell type-dependent coupling to signal transduction systems
Most of the studies on signal transduction pathways were performed on human skin cells that solely or preferentially express CRFR-1 (80, 117, 249), and below we will focus on this receptor. CRF and Urc-1 binding to CRFR-1 increases intracellular Ca2+ concentration in epidermal HaCaT and primary keratinocytes, melanocytes, and melanoma cells via an influx of Ca2+ from the opening of voltage-activated Ca2+ ion channels (211, 250, 251). This effect can be inhibited by EGTA, d-cis-diltiazem, and verapamil, and cyclic nucleotide-gated ion channels were not involved (no effect of Mg2+) (251). Interestingly, increased Ca2+ levels were even detected at extremely low ligand concentrations (10−13 m) (250, 251). There was a selectivity for CRF in the stimulation of intracellular Ca2+ concentration in human and hamster melanoma cells, with CRF acting already at 10−12 m, whereas Urc-1 and sauvagine acted at much higher concentrations of ≥10−7 m (250). CRFR-1 activation also stimulated inositol triphosphate (IP3) production to increase intracellular Ca2+ concentrations in melanocytes, melanoma cells, keratinocytes, and fibroblasts (117, 237). Therefore, we have proposed that increases in intracellular Ca2+ concentrations are induced by stimulation of IP3, which activates release of Ca2+ from intracellular stores, and by direct coupling to voltage-activated Ca2+ ion channels leading to rapid cytosolic raises in Ca2+ (117). One remarkable finding was the differential effect of CRF vs Urc-1, with the former increasing of cytosolic Ca2+ and the latter demonstrating predominantly intranuclear and oscillatory increases of Ca2+ (251). This indicates a different mode of activation of the same receptor CRFR-1 by related, but chemically distinct, ligands. An explanation for this unexpected phenomenon could be provided by alternative splicing of CRFR-1 (80, 109, 111, 252).
Activation of CRFR-1α induces the production of cAMP in epidermal melanocytes, DFs, most melanoma cells, and immortalized HaCaT keratinocytes and squamous cell carcinoma (117, 211, 231), as well as the HSC-2 cell line (253). Interestingly, SKMEL-188 melanoma cells, which lack CRFR-1α, were not coupled to cAMP as second messengers (117). Moreover, cAMP accumulation was not detected after CRFR-1 activation in normal human primary epidermal keratinocytes (117), indicating poor coupling of CRFR-1α to Gs protein in these cells. However, CRF stimulates Gq protein in keratinocytes to activate phospholipase C, leading to IP3 accumulation and subsequent protein kinase C (PKC) activation (249). In addition to generating second messengers by cutaneous CRFR-1, the following signaling cascades lead to differential but expected activation of transcriptional regulators including AP-1, CREB, and NF-κB (117, 249, 254–257).
For example, in primary epidermal keratinocytes, CRF stimulates PKC with subsequent activation of AP-1 and then Jun D, leading to the early differentiation in these cells without involvement of protein kinase A (PKA) and MAPK pathways (249). In contrast, additional coupling to cAMP in HaCaT keratinocytes can also lead to PKA activation and CREB phosphorylation, with the subsequent binding of phosphorylated CREB to the CRE elements (80). This additional coupling could be responsible for the shapes of the CRF or Urc inhibition curves in HaCaT keratinocytes, determined by the concentration of Ca2+ in the growth medium (bell shape in medium containing high Ca2+) (80, 231, 256, 258). For example, in HaCaT keratinocytes, CRF at 10−7 m (259) or 10−8 m (260) stimulates the MAPK signaling pathway with secondary inhibition of vascular endothelial growth factor (VEGF) (259) and IL-18 production (260). Thus, the stimulation of MAPK may be responsible for the loss of the inhibitory effect of CRF on cell proliferation at concentrations of 10−7 m or higher that was observed at the range 10−11 to 10−8 m (231, 258). Another possible explanation for the abrogation of the high concentration CRF effect is an indirect stimulation of HaCaT cell proliferation by IL-6, of which production is stimulated by CRF in this cell type (199). In addition, some authors (253) claim that CRF can stimulate the proliferation of human keratinocytes. Careful analysis of these data, however, does not substantiate this hypothesis (253). First, the proliferation was tested in the immortalized HSC-2 line, but not in primary keratinocytes; second, 10−7 m of CRF had no effect on proliferation because standard errors were covering mean values of the control and experimental groups; and third, it is unclear whether increased DNA incorporation by extremely high concentrations (10−5 and 10−6 m) of peptide hormone is statistically significant because statistical analysis was not performed (253).
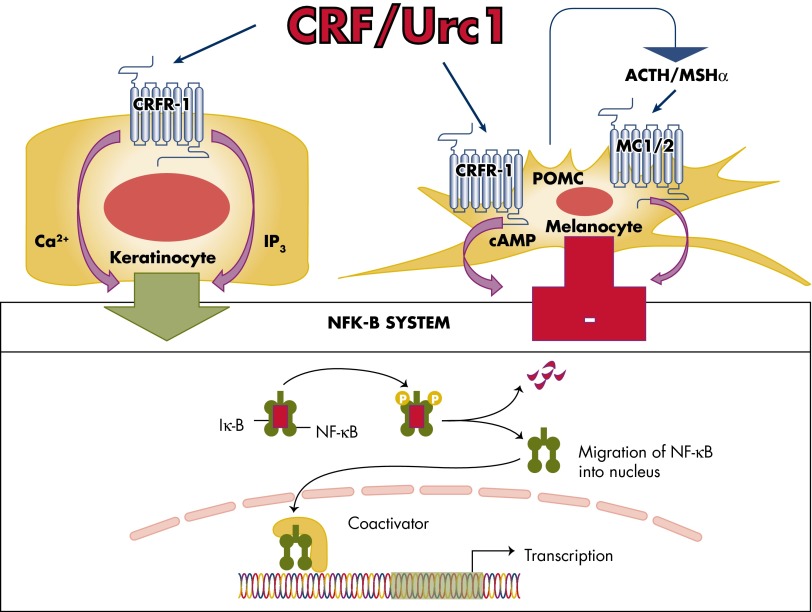
Finally, CRF both stimulates and inhibits NF-κΒ activity in HaCaT cells depending on the presence or absence of serum in the culture medium (80, 254). Thus, when cells are subjected to prolonged serum starvation (12 h), the addition of CRF stimulates NF-κΒ activity, presumably to enhance cell survival (80, 254). However, under the stress of acute serum starvation (15–30 min) that activated the NF-κΒ pathway, CRF inhibited NF-κΒ activity (80, 254). A potential mechanism for this differential effects of CRF on NF-κΒ that depends on the environmental context, with its dual coupling to pro- and anti-inflammatory responses, is discussed in detail elsewhere (80, 255). In contrast, activation of CRFR-1 in normal epidermal keratinocytes enhances NF-κΒ activity, in agreement with the observed immunostimulatory activity of CRFR-1 in keratinocytes (219, 232, 261) (Figure 4), which is consistent with CRF-induced NF-κΒ stimulation of the immune system (262). We believe that this solely stimulatory effect on NF-κΒ in normal keratinocytes is due to a lack of coupling of CRFR-1α signaling to adenylate cyclase (80, 117). In contrast, CRF inhibits in normal epidermal melanocytes the nuclear translocation of the NF-κΒ subunit p65, NF-κΒ binding to DNA, and luciferase construct activity driven by κΒ sites (257) (Figure 4). The specificity of this effect was indicated by its attenuation using the CRF-specific nonpeptide antagonist antalarmin. Further study showed that this inhibition of NF-κΒ activity reflected an indirect stimulation of downstream POMC, with POMC peptides acting directly as inhibitors of NF-κΒ activity (257).
Figure 4.
CRFR-1-coupling to the NF-κκB signaling in normal epidermal keratinocytes and melanocytes. CRF stimulates NF-κB activity in normal human keratinocytes (255) and inhibits it in epidermal melanocytes (257). Of note, in immortalized HaCaT keratinocytes, CRF both inhibits and stimulates NF-κB activity, depending on the environmental context (80, 254).
In conclusion, CRF and related peptides couple CRFR-1 to variable signal transduction pathways with a selectivity defined by skin cell type in order to regulate cell viability, proliferation, differentiation, immune functions, and secretory activity in growth condition-dependent manners. Given the diversity of skin cell types and the known pleiotropic effects of CRF and Urcs, a selectivity of signal transduction pathway systems is required, and this can be achieved by differential expression of the CRFR-1 or CRFR-2 or their isoforms. The biological context-dependent pattern or compartmentalization of this response will decide the phenotypic outcomes.
D. Alternative splicing of CRFR-1 and CRFR-2 and its physiological relevance
1. CRFR-1
In addition to the main, fully functional isoform CRFR-1α, CRFR-1 isoforms could be arranged into three groups: isoforms with modification of the N-terminal ECDs, modified 7-TM C-terminal isoforms, and soluble isoforms lacking 7-TM domains (Figure 3, upper panel). CRFR-1 isoforms with modified substrate binding domain have limited or no capacity to bind its ligands (CRF and Urc-1), and these include human and mouse isoforms including CRFR-1c (exon 3 is spliced out) and CRFR-1e (exons 3 and 4 are absent, which cause frameshift and early stop codon in exon 8), although the latter also belongs to soluble isoforms due to the absence of the entire 7-TM domain. The isoform CRFR-1c failed to bind CRF when expressed in the monkey kidney cell line COS-1 (263). Stimulation of cAMP production is observed only at high concentration of human CRF, which suggests strong inhibition of downstream signaling in cells expressing CRFR-1c in comparison to CRFR-1α (54). Moreover, recent studies using GFP-labeled CRFR-1 isoforms revealed a cell membrane localization of CRFR-1c and colocalization with CRFR-1α, but also showed strong inhibition of CRF signaling most probably through formation of nonfunctional dimers (109, 264). The isoform CRFR-1i, recently discovered in human carcinoid BON-IN, has exon 4 spliced out and possesses two alternative starting codons with or without exons 1 and 2 (CRFR-1i-a and CRFR-1i-b). Both isoforms, while expressed in HEK-293 cells, were not activated by CRF. However, CRFR-1i-b in the presence of NBI-35965 (CRFR-1 antagonist known to bind to 7-TM), was able to attenuate basal or CRF-stimulated pERK1/2 activation (119).
The second group of CRFR-1 isoforms contains isoforms with deletion of fragments of 7-TM domains that results in impaired trafficking and localization (81, 111, 161, 264). These are human CRFR-1d (exons 13 is spliced out), CRFR-1f (exon 12 is absent that results in subsequent frameshift), CRFR-1g (27 bp of exon followed by whole exon 10 exons, and 28 bp of exon 12 are absent). Additional isoforms were detected in hamsters: CRFR-1k (with deletion of exon 10), CRFR-1m (lacking exons 11 and 12), and CRFR-1n (with removed exons 10 to 12). As was predicted (80), deletion of a fragment of the 7-TM domain results in the intracellular retention of CRFR-1 isoforms with deletions in the 7-TM domain, as shown for CRFR-1d, -f, and -g using several cellular models including HaCaT keratinocytes (109), pituitary cell line AtT-20 (264), and retinal pigment epithelium line ARPE-19 (49). Moreover, the expression of CRFR-1d, CRFR-f and g with CRFR-1α resulted in the accumulation of coexpressed isoforms inside cells (264). Several functional studies showed inhibition of CRF and Urc-1 signaling in cells overexpressing CRFR-1 isoforms with impaired 7-TM domain (for review, see Ref. 81). A similar decrease was observed when CRFR-1α was coexpressed with CRFR-1d, -f, or -g, showing the dominant effect of the expression of those isoforms. The negative effects were demonstrated both by the reduced production of secondary messengers (cAMP, IP3, and Ca+2) and subsequent low or no activation of corresponding transcriptional elements (CRE, AP-1, and calcium responsive element [CARE]), followed by decreased regulatory effects on gene expression (109).
The third group of CRFR-1 isoforms are known as soluble receptors; thus, they do not possess the entire 7-TM domain. CRFR-1e has a deletion of exons 3 and 4, which results in a frameshift and premature stop codon in exon 8. The isoform CRFR-1h is unique because it contains an additional exon (cryptic exon) inserted between exons 4 and 5. The frameshift causes early termination in exon 5 of CRFR-1h mRNA, but in contrast to CRFR-1e this isoform possesses the entire first ECD, thus it may represent a soluble receptor with the capacity to bind ligands (80, 111, 252). In fact, the presence of CRFR-1h was detected in medium of cells overexpressing CRFR-1h tagged with GFP, as well as in with the endoplasmic reticulum in HaCaT keratinocytes (109). Moreover, conditioned medium from the mouse pituitary cell line AtT-20 cells, which overexpress CRFR-1h, inhibited slightly the stimulation of these cells by CRF, suggesting that soluble CRFR-1h may act as a decoy receptor (264). In contrast, CRFR-1e, in which most of the first ECD and whole 7-TM is missing due to the deletion of exons 3 and 4 and subsequent frameshift, was not detected in the culture medium, suggesting its protein and mRNA may undergo fast degradation (81, 264).
Isoform CRFR-1β, coded by all 14 exons (265), may be a by-product of CRFR-1 alternative splicing because all other CRFR-1 isoforms have exon 6 removed, and the presence of this exon was shown only in humans although it is predicted in some primates as a theoretical transcript (266). Moreover, recently this group showed the expression and function of yet another CRFR-1 variant with intact exon 6 and deletion of exon 13 (identical to CRFR-1d). It seems that the insertion of an additional loop into the first intracellular loop of the receptor alters its responsiveness to PKC-induced phosphorylation, leading to desensitization and endocytosis, and thus inhibition of downstream signaling (118).
Although there is a shortage of information on molecular factors regulating alternative splicing of CRFR-1, recent studies have shown that the small nucleolar RNA MBII-52 (SNORD 115) is involved in alternative splicing of mouse CRFR-1, and that its presence stimulates excision of substrate binding domain (267).
The DNA polymorphisms mapped to the CRFR-1 gene correlate with development or susceptibility to depression (268–272), alcohol abuse (273, 274), Parkinson's disease (275) or sensitivity to antidepressants (269, 276, 277), hypertension (278), sensitivity to corticosteroids (278, 279), or low bone densities (280) (Figure 5). More than 400 different single nucleotide polymorphisms (SNPs) have been detected within the human CRFR-1 gene so far (NCBI, dsSNP, build 130), with all but eight mapped to noncoding fragments of CRFR-1 sequence (introns). Surprisingly, all SNPs found within the CRFR-1 gene and linked to human diseases or drug sensitivity were mapped to intronic sequences, thus having no direct impact on protein product (Figure 5). Therefore, it is possible that the presence of unique SNPs might influence alternative splicing of CRFR-1 pre-mRNA, resulting in modulation of CRF-driven pathways of stress response and steroidogenesis. It is well established that both intronic and exonic SNPs can influence alternative splicing, and so shed some light on the mechanism of the observed phenomena (281–283). The position of the SNPs seems to correlate with alternative splicing sites forming “hot spots” of splicing. Ten of 14 SNPs were mapped to introns 2, 3, or 4 (Figure 5). Alternative splicing in this region may result in at least three different variants of CRFR-1 mRNA (isoforms CRFR-1, -e, or -h) (Figure 5). Two SNPs (rs1876828 and rs1876828) located in intron 13 might be involved in alternative splicing of CRFR-1 leading to isoform d. Although the SNPs are located in around 200 to 2000 base pairs from the splicing site, they might play a regulatory role in the splicing (282, 283).
Figure 5.
SNPs can affect CRFR-1 splicing. Exons are shown in green, introns are in blue, and splicing variants are in pink. The positions of selected SNPs are indicated by superscript letters that correspond to reference numbers for the following studies: a 271; b 270, 271, 291; c 275; d 268, 276–278; e,f 275; g 268, 276, 277; h 273, 274; i 278, 286, 290; j 269–271, 275, 291; k 270, 271; l 271; m 273, 274; n 268, 276–278, 280; and o 278.
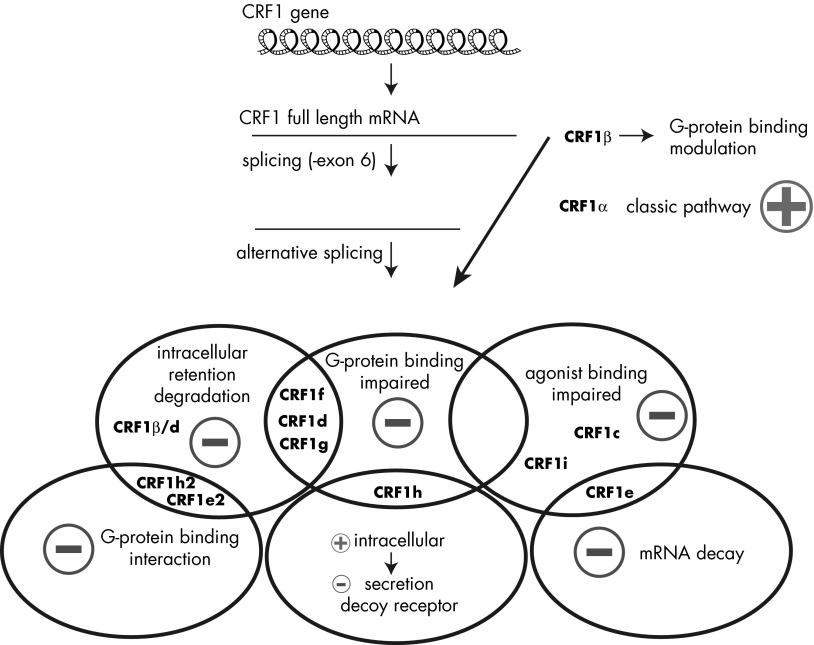
Expression of alternative splicing variants of CRFR-1 was shown to modulate CRF-driven signaling on many cellular models (109, 116, 117, 264) (Figure 6). The significance of alternative splicing of the CRFR-1 receptor may be explained by a decrease in the number of copies of mRNA coding the fully functional isoform CRFR-1α and the fast degradation of other mRNAs by a nonsense mRNA decay mechanism (284) (Figure 6). Nevertheless, there is growing evidence that the regulatory function of alternative splicing goes far beyond the nonsense mRNA decay mechanism, and several reports showed that CRF signaling is modulated by expression of multiple isoforms of the receptor (67, 80, 109, 264). In this model, the activity of the main isoform CRHR1α is regulated by coexpression of other isoforms with formation of heterodimers (80, 109, 264). Thus, alternative splicing of CRFR-1 changes not only a pool of mRNA coding CRHR1α, but also the properties of the receptors (Figure 6). Separate functions were postulated for a soluble isoform (lacking the 7-TM domain) with distinct intracellular and extracellular activity (80, 109, 264).
Figure 6.
CRF signaling is regulated by different CRFR-1 isoforms. CRFR-1 gene contains 14 exons, and only 1 isoform of the CRFR-1β receptor (also called pro-CRFR-1) is coded by all exons. Depending on external or internal factors, CRFR-1 pre-mRNA might be subjected to alternative splicing that results in the formation of at least 12 isoforms. Those proteins might regulate CRF signaling, which is mainly transduced by CRFR-1α, via modulation of its expression, localization, or activity. Minus signs indicate inhibition, and plus signs indicate stimulation of CRF signaling by expression or coexpression of multiple CRFR-1 isoforms. For details, see Ref. 81.
So far, there is a lack of definitive information on whether SNPs influence CRFR-1 expression or splicing in the skin. Recent studies indicate that CRFR-1 expression changes with age (245) or in pathological conditions such as psoriasis (218). Moreover, alternative splicing of other GPCRs was associated with the development of several human pathologies (285). The coexistence of “hot spots” of alternative splicing with SNPs suggests a potential modulatory effect of SNP on CRFR-1 pre-mRNA splicing (Figure 5). A correlation between germline variations in the CRFR-1 gene (including the rs1876828 SNP) and alternative splicing of CRFR-1 pre-mRNA, if proven, would be important in diagnostics and in the development of therapeutic strategies for diseases or abnormalities in which SNPs were detected in CRFR-1 (268–271, 273–278, 280, 286–292).
2. CRFR-2
Due to the high level of homology of CRF-2 with CRFR-1, theoretically alternative splicing of CRFR-2 mRNA could result in the formation of similar isoforms. For example, the soluble isoform (sCRHR2), similar to CRFR-1h, was detected in mouse brain (121). Interestingly, CRFR-2 pre-mRNA can be spliced into at least three unique 5′-end variants with alternative starting codons (coding isoforms CRFR-2α, -β, and -γ) (Figure 3, lower panel).
In addition, CRFR-2 splicing variants with retention of intron 3 – (CRFR-2a-3), 8 (CRFR-2a-4), or 8 and 9 (CRFR-2a-5) were reported in rat esophagus (227). Esophagus epithelium contains keratinocytes in a similar manner to skin epidermis. These insertions result in a frameshift and the introduction of a premature stop codon potentially produced truncated proteins lacking the 7-TM. Two other isoforms have unique deletion of three nucleotides coding glutamine (CRFR-2b [desQ126] and CRFR-2b-2 [desQ126]) (227). It remains to be tested whether similar CRFR-2 splicing is seen in human adnexal and dermal compartments or in rodent skin because it was suggested that CRFR-2 expression has a regulatory function in these structures, including hair cycling (209) and pigmentation (213).
E. Neuropeptide and cytokine regulation of CRFR expression
There are several well-documented effects of cytokines on CRFR expression and its isoform regulation in different organ systems. IL-1 stimulates both CRF and CRFR-1 in the paraventricular nucleus of the hypothalamus (293), whereas IL-1β increases the expression of CRFR-1, decreases expression of isoform β, and does not affect isoform d in the human myometrium (294). The effect of IL-1β on CRFR-1 is mediated by the NF-κΒ pathway. Also, overexpression of CRFR-1 is actually characterized by diminished cAMP signaling, which indicates the significance of other isoforms in this model. IL-1β and TNF-α decrease expression of CRFR-2 on mouse cardiomyocytes, mediated indirectly by the Urc-1 and possibly corticosterone (295). IL-1β decreased the expression of CRFR-1 in the pituitary (296), an effect mediated both by CRF causing desensitization (as proposed by Ref. 297) and by LPS itself, IL-1β, and also IL-6 on the receptor expression (296).
Although SP was discovered in the CNS, it has a widespread distribution and plays a role in inflammation (298), including the skin (59, 299). SP is known to stimulate mast cells to express CRFR-1, leading to the synthesis and release of IL-8, TNF-α, and VEGF (230). Neurokinin 1 receptor is engaged in this process, and the whole model is postulated to operate in psoriasis (233). In the brain, where it was hypothesized to underlie anxiety disorders, SP also engaged neurokinin 1 receptor and led to overexpression of CRFR-1 (300). A similar effect was recently reported for the brain peptide NT, which induced the expression of CRFR-1 (246). Interestingly, CRF also induced the expression of NT1 (246). Of note, mast cells respond to IL-6 with increased expression of both CRFR-1 and CRFR-2, but only of CRFR-2 to IL-4, IL-1, or LPS (301).
Taking into consideration that skin is actively producing and responding to numerous neuropeptides (including SP and NT) and cytokines (49, 59, 129, 141, 174, 186, 302, 303), these neuropeptides and cytokines will regulate CRFRs in a compartment- and context-dependent manner. In this context, the same or neighboring cells will be in bidirectional communication in an intracrine, autocrine, or paracrine manner with feedback and feed-forward instructions (49) with autoregulatory loops between CRF, neuropeptide, and cytokine signaling systems. These represent an evolutionary signature of similar interactions occurring at the central level (40, 304). This is an exciting area for exploration because CRF signaling may also be up-regulated locally by POMC-derived peptides activating self-amplifying autoregulatory loops (38, 129, 130, 174) that are beyond testing due to spatial separation of the hypothalamus and pituitary.
F. Environmental regulation of the cutaneous CRF signaling with a focus on UV radiation and skin bacteria
It has already been mentioned that LPS can affect the CRF system. LPS is an example of a biological insult that can stimulate CRF activity with subsequent activation of proinflammatory cytokines that are dependent on CRF and CRFR-1 (219). Stimulation of CRF signaling by extracts from bacillus P. acnes has been demonstrated in keratinocytes by Isard et al (220). Many skin cells, including mast cells, express TLR and respond to bacteria with release of cytokines that can go on to influence the CRF system (305, 306). A second dominant environmental stimulus of CRF signaling is UV radiation (UVR). This part of the spectrum of electromagnetic energy covering wavelengths between 100 to 400 nm includes vacuum UV (100–200 nm), UVC (200–290 nm), UVB (290–320 nm), and UVA (320–400 nm). Only UVA and UVB reach the surface of the earth and so represent a major cutaneous stressor.
It is already well documented that UVB stimulates POMC expression with the production of corresponding ACTH and α-MSH peptides by skin cells in a dose-dependent fashion (307). UVB also stimulates the expression of receptors and increases melanocyte responsiveness to MSH (307). This phenomenon was first recognized by Pawelek and colleagues (307–312), who proposed that UVB-induced melanogenesis is mediated by up-regulation of the MSH receptor system. In this manner, UVR up-regulates the expression of functional MSH receptors and amplifies the melanogenic effect of MSH, as observed in vivo and in cell culture systems (38, 307–315). Similar results have been obtained in clinical studies, which showed that topical application of a superpotent α-MSH induces skin pigmentation predominantly on the sun-exposed areas (reviewed in Ref. 38). Moreover, patients with Addison's disease (adrenal failure with increased plasma ACTH levels) exhibit hyperpigmentation, predominantly on sun-exposed body sites (reviewed in Ref. 38). In addition, it was shown that activation of MC1R by α-MSH induces additional protective mechanisms in skin melanocytes against solar radiation that are separate from the induction of melanin pigment (316–319). Thus, UVB may regulate how the skin responds against its damaging effects through a concerted activity involving the stimulation of ligand production (POMC peptides) and increased activity of the corresponding receptors (38, 129, 174).
As mentioned previously, UVB stimulates the production of CRF by cultured normal human melanocytes, keratinocytes, and fibroblasts (80, 203, 214). UVB-induced stimulation of CRF appears to be regulated by UVB-induced stimulation of PKA and phosphorylation of CREB, with subsequent binding to CRE sites in the CRF promoter (214). Because CRF can stimulate ACTH and α-MSH production in the human skin cells and hair follicle (320–323), we have investigated a potential role of CRFR-1 in this process. We found that whereas UVB activated the POMC promoter, POMC mRNA expression, and ACTH release, an antagonist of CRFR-1 abrogated this UVB-stimulated induction of POMC (214). Therefore, we proposed the hypothesis that UVB-induced CRF production activates CRFR-1 with subsequent stimulation of POMC expression (214).
Using human skin incubated ex vivo and melanocyte/keratinocyte cocultures, we have reported that CRF, POMC, MC2R, CYP11A1, and CYP11B1 gene expression can be stimulated by UVR, with wavelength- and dose-dependent effects. The greatest effects were observed for highly energetic UVC and UVB elements of the spectrum (221). However, significant production of CRF, POMC, ACTH, and CYP11A1 proteins and peptides (as measured by ELISA and Western blotting) was seen only after UVB and UVC irradiation, whereas β-endorphin expression was also stimulated by UVA. Furthermore, immunocytochemical analysis showed that UVA also increases CRF and β-endorphin levels in skin, predominantly within the epidermis with additional accumulation in the dermis (221). These led to the conclusion that highly energetic wavelengths (UVC and UVB) are predominantly responsible for activating the CRF and POMC systems. Additional corticosteroidogenic enzymes contribute as part of a local stress response, where selective activation of CRF and β-endorphin by UVA occurs as part of an alternative although potentially overlapping mechanism (221).
UVB-mediated induction of cutaneous signaling also includes regulation of CRFR-1 expression and activity. Specifically, UVB can increase CRFR-1α expression and change the pattern of CRFR-1 isoforms expressed in skin, depending on the dose and repetition of UVB exposure (109, 111, 112).
Additional environmental factors in the regulation of the cutaneous CRF signaling include cold, as demonstrated by increased cortisol production in hair follicles (324–326), chemical peeling, and 2,4 di-nitrofluorobenzene that induce POMC (234, 327).
G. Comparison with other peripheral organs
1. GI tract
There is significant interest in the exploration of peripheral equivalents of the HPA axis, not only in skin, but also the gut (328) and perhaps other adjacent organs (37), such as the bladder (60, 329, 330). In the case of the GI tract, this is driven in part by an interest to explore the role of inflammation and the gut microbiome in disorders of the gut, including IBS (331). Indeed, modulation of the gut microbiome by nutritional interventions may be able to reduce stress-induced neurogenic inflammation and peripheral tissue responses to stress. Increasingly, evidence from clinical and preclinical studies suggest a role for peripheral CRF signaling in mediating stress-induced effects on GI function (85). Modulation of the psycho-neuro-endocrine-immune systems via a so-called brain-gut axis has been proposed to play a key role in the pathogenesis of inflammatory bowel disease and IBS. This axis is thought to involve interactions between the autonomic nervous system, the CNS, the stress system (HPA axis), and the GI CRF system, as well as the intestinal barrier, microbiota, and local immune system (332). In this way, CRF-associated stress pathways can induce a change in gut motility, secretion, visceral sensitivity, intestinal permeability, and local inflammatory responses in the GI tract (333, 334). In reverse, chronic colitis appears to suppress CRF gene activation in the brain (hypothalamus) and also reduces plasma corticosterone levels (335).
Mice deficient in CRFR-2 tend to develop reduced intestinal inflammation, whereas CRF antagonists exhibit anti-inflammatory effects in murine ileitis induced by Clostridium difficile toxin A (83). These data suggest a role for CRFR-1 in this form of ileitis (336). When these antagonists are given peripherally, they prevent stress-induced GI dysfunction. Both stress and CRF can also increase permeability of rat colon (337), which can lead to an inflammatory response via enhanced bacterial antigen access to the immune system of the gut lamina propria (338). Agonists at each CRFR type apparently promote opposing effects on the gut. Whereas CRFR-1 promotes intestinal inflammation and endogenous and inflammatory angiogenesis, CRFR-2 inhibits these activities (339). By contrast, stress-induced bladder inflammation seems to be driven by CRFR-2 activation (330).
In summary, several functional studies show that the peripheral administration of CRF (or Urc) stimulates colonic transit, motility, and defecation via CRFR-1 and a decrease in ileal contractility via CRFR-2 (84, 85). The CRF system in the gut is, therefore, an interacting and balanced system where disruption to this balance favors inflammation. Thus, targeting the CRF system may be important in the management of inflammatory bowel disease and/or IBS.
2. Immune system
Much has been written about the intersection of the neuroendocrine and immune systems because both provide crucial host defenses in health and disease (340). Chronic stress generally impairs or suppresses immune function (promoting glucocorticoid release and a type-2 cytokine response), leading to increased susceptibility to cancer and infections. Stress can also worsen the outcome for some diseases, like asthma (341). In this sense, chronic stress is maladaptive in the evolutionary survival context. By contrast, acute stress (ie, short-term flight-or-flight responses) may have opposite, ie, immune-enhancing effects, and so promote survival responses in both our cardiovascular and musculoskeletal systems (342). Despite this, acute stress can worsen many inflammatory diseases, such as multiple sclerosis and psoriasis (166, 343).
CRF, as the stress-integrating peptide and stimulator of ACTH secretion, can indirectly modulate the immune system function, principally via immunosuppression. At the periphery, CRF can regulate directly immune activity via both its synthesis and the expression of CRFRs by immunocytes (344, 345).
CRF is involved in the inflamed tissues of patients with autoimmune and inflammatory diseases. For instance, both CRF and CRFRs are up-regulated in inflammatory arthritis and psoriasis. CRF can also induce the expression of the orphan nuclear receptor NURR1, a transcription factor of the steroid/thyroid superfamily, and associated ligands can be effective in psoriasis (177). Additional discussion is presented in the next paragraph.
One of the early immunoregulatory effects of CRF is its activation of mast cells (346) and in stimulating the release of VEGF and several inflammatory cytokines (122, 166, 201, 341). Stress signals from the immune system, as well as body tissues, can trigger the release of CRF from lymphoid tissue like the spleen and thymus, as well as from the inflamed tissue itself (44). In general, data from both in vivo and in vitro studies indicate that CRF in the periphery and from immune cells has proinflammatory effects. Given the widespread expression pattern of CRFRs, CRF can act in a paracrine manner by activating CRFR-1 and CRFR-2 receptors on local immune cells. Whether all of the proinflammatory activity attributed to CRF is indeed due to this peptide is not clear because increasing data suggest that CRF-like peptides and Urc itself may be involved as these are also expressed by immunocytes and can also bind CRFR-2 receptors (347). For instance, CRF (348) and Urc (349) similarly stimulate skin mast cells and lead to increased vascular renewability and inflammation (350).
3. Gestational tissues
CRF expression has been reported in a number of reproductive organs, including the ovaries, endometrial glands, decidualized endometrial stroma, placenta, decidua, and the testes (54, 70, 82, 345). Moreover, CRF is reported to regulate a number of reproductive functions including ovulation, luteolysis, decidualization, implantation, and early maternal tolerance, whereas this stress peptide also functions in pregnancy and labor onset (82). The placenta is the main source of both CRF and CRF-BP and, when in the circulation, causes physiological hypercortisolism during the second and third trimester. This is followed postpartum by transient adrenal suppression (82). The latter may participate in associated postpartum mood alterations, including depression (345).
CRF levels are elevated in women with preterm labor and other complications, such as pre-eclampsia, and has led to the suggestion that there exists a placental “clock” that determines the length of normal gestation (82, 351–354). However, spontaneous preterm delivery involves other factors, in addition to CRF level elevation, so CRF level alone, while useful, is not a robust diagnostic predictor of preterm delivery. CRF, together with progesterone, is crucial for the transition from myometrial quiescence to contractility (355). Placental CRF in particular stimulates production of cortisol (and androgens) in the adrenal glands of the developing fetus (356). Increased CRFR-1 and Urc-positive mast cells have been identified in lesions of endometriosis (357), a neuro-immuno-endocrine disease exacerbated by stress (358).
4. Cardiovascular system
The CRFR-2 receptor is highly expressed in the heart, and when administered peripherally both CRF and Urc cause significant hemodynamic effects via activation of cardiac CRFRs. These include increased arterial pressure and heart rate, via the autonomic sympathetic nervous system, and elevation of plasma catecholamine, in addition to stimulating ACTH release. ACTH increases blood pressure as a result of stimulated secretion of mineralocorticoids and glucocorticoids (359, 360). Thus, there is interest in the use of CRF and Urcs as potential therapies for heart failure (87). There is evidence that the peripheral administration of CRF and its analogs can exert effects on the heart and vasculature directly via CRFRs, even without involving the CNS and autonomic nervous system (361). For instance, direct intracoronary administration of Urc in pigs raises coronary blood flow, and this can be blocked using a nitric oxide synthase inhibitor via endothelial nitric oxide synthase (362). Furthermore, Urc can stimulate CRFR-2 receptors on aortic endothelial cells to phosphorylate ERK, protein kinase B, and p38 leading to endothelial nitric oxide synthase activation (363). Importantly, pretreatment of animals with a CRF antagonist (α-helical CRF9–41) blocks the cardiovascular and hemodynamic responses to peripherally administered CRF (364). Endogenous Urcs can suppress vascular tone in heart failure, and iv Urc increases cardiac output and reduced systemic vascular resistance (365).
CRF and its associated peptides have also been reported to exhibit direct actions on cardiac myocytes via CRFR-2 receptor activation and, importantly, can achieve these without the involvement of the nervous system (366, 367). Data from these studies support a direct Urc action via CRFR-2 in cardiac myocytes. CRF analogs, including receptor selective and nonpeptidic CRF agonists and antagonists, have significant potential as drugs that target the peripheral cardiac system given their likely inotropic, vasodilator, and diuretic actions. Acute stress can also stimulate cardiac mast cell activation, accompanied by histamine (368) and IL-6 (369) release, both of which are increased in apolipoprotein E knockout mice that are prone to atherosclerosis. Moreover, Urc can directly stimulate IL-6 release from cultured cardiomyocytes (370). Cardiac IL-6 elevation is reported in patients with acute coronary syndrome (371) and appears to derive from mast cells stimulated by stress (372).
Thus, CRF signaling systems are operating in the periphery to regulate their function in response to stressful stimuli (37, 82, 85, 122), and the organization of these responses is similar to that in the skin (49, 80).
V. Skin Equivalent of the HPA Axis
A. Skin pro-opiomelanocortin (POMC) signaling system
Since the first report on hair cycle-dependent POMC expression and β-endorphin production in the skin of the C57BL/6 mouse (373), it is now well established that skin cells produce POMC peptides and express functionally active corresponding receptors in a cell type-, species-, and context-dependent fashion (see reviews in Refs. 38, 49, 129, 139, 161, 174, 175, 317, 318, and 374). This has been a subject of extensive reviews, and we will therefore provide only a brief overview. Skin production includes ACTH, α-MSH, β-MSH, γ-MSH, β-endorphin, and β-lipotropic hormone (β-LPH). POMC processing in human skin appears to resemble that of the hypothalamus and pituitary in that ACTH, α-MSH, and β-endorphin have been found in melanocytes, keratinocytes, fibroblasts, sebocytes, and immune cells and in mammalian skin biopsies (129, 162, 174, 375–381). In addition, melanocytes and keratinocytes, as well as other skin cell types, express POMC-processing components (eg, PC1, PC2, and regulatory protein 7B2) that are dependent on the status of the skin (reviewed in Refs. 49, 130, 323, 382, and 383).
α-MSH binds to its preferred melanocortin receptor (MC1), to which ACTH can also bind in addition to its own preferred receptor, MC2, whereas β-endorphin can influence melanocytes via its preferred μ-opiate receptor. Melanin pigment produced in this way can act as a buffer molecule to antagonize the noxious effects of physical, biological, and chemical insults (38, 49). Furthermore, these peptides have immunosuppressive properties and regulate several functions in the epidermis, fibroblasts, and dermal microvasculature, as well as in hair cycling, sebaceous glands, and the secretory activities of other adnexal structures. A protective role of α-MSH signaling against oxidative and UVB-induced damage has also been emphasized recently.
Despite significant advances in this field, there are also areas that have to be clarified. Although POMC can be processed in the skin by convertases also operative in the pituitary or brain (380, 382–384), the correlation of the different signatures of POMC fragments with the kinetics of PC1 and PC2 activity in different cell types and in a compartment-restricted fashion still remains to be demonstrated. An analysis of how POMC is processed in relation to PC1, PC2, and 7B2 expression/activity should elucidate the nature of the intracellular POMC processing mechanism(s) that transform this prohormone into biologically active secreted products. Elucidation of the chemical nature of the active peptide is important because it defines the final phenotypic effects in the skin. For example, if ACTH is the predominant POMC cleavage product formed, one would expect that its actions, in addition to stimulating melanogenesis through activation of MC1, should also include stimulation of cortisol production (as occurs in the adrenal gland) with the final phenotypic effects represented by local immunosuppression. However, if the final product is instead α-MSH, the stimulation of melanin pigmentation should represent the main local (epidermis or hair follicle) phenotypic effects accompanied by immunosuppression. A similar view holds for cleavage to β-endorphin because it, too, has promelanogenic activity (385). However, the latter role may additionally include regulation of other epidermal functions including nociception; opioid receptors have been identified in epidermal and hair follicle cells, as well as in nerve endings (141, 175, 176, 385–389). Therefore, the challenge is this area is to define the context-dependent POMC processing in the skin.
B. Skin corticosteroidogenic system
Since the first demonstration that skin cells express crucial genes of the corticosteroidogenic pathway, CYP11A1, CYP17, and CYP21A, as well as the MC2 gene encoding the ACTH receptor (390), along with functional activity of these and other steroidogenic enzymes in the skin or skin cells (391–393), it has been firmly established that skin expresses corticosteroidogenic activity (reviewed in Ref. 198). Cutaneous steroidogenesis can be initiated directly from cholesterol by the action of locally expressed CYP11A1 (180). Pregnenolone is metabolized by cutaneous 3β-hydroxysteroid dehydrogenase (3β-HSD) (394–396) with subsequent steps involving the metabolism of progesterone to deoxycorticosterone and 18-hydroxy-deoxycorticosterone, with the final production of corticosterone (321, 322, 391, 392). Human hair follicles (320, 324), cultured normal epidermal melanocytes (322), and DFs (321, 397) can also produce cortisol (321, 397). The skin's capability to produce cortisol was mostly confirmed in skin cells in vitro (398–400) and by us in skin fragments incubated ex vivo (221, 401). Cutaneous corticosteroidogenesis can be regulated by CRF, ACTH, and cAMP (320–322), IL-1 and tissue injury (400), as well as by UVR (152, 221, 401). Skin cortisol can either be produced from 11-deoxycortisol by CYP11B1 or from cortisone by 11β-HSD1 (130, 398–402).
Skin also produces sex hormones from dehydroepiandrosterone (DHEA) and DHEA sulfate or androstenedione either derived from the circulation or produced locally (129, 146, 152, 394–396, 403). This involves transformation of DHEA into 4-androstenedione and 5-androstene-3β,17β-diol, and of their further transformation to T and 5α-dihydrotestosterone (139, 395, 396, 403–406). Moreover, cutaneous fibrocytes/fibroblasts and adipocytes convert T into estradiol, whereas keratinocytes can transform reversibly 17-estradiol into estrone.
In addition, cutaneous activity of CYP11A1 can generate novel 7Δ-steroids, which after exposure to UVB are transformed to corresponding secosteroids (vitamin D analogs) (151, 180, 188–190, 192, 407, 408), and can metabolize vitamin D itself to novel hydroxy-derivatives (190). Both types of molecules are biologically active (151, 188, 192, 408–418).
The nature and level of production of the final steroid products is dependent on the cell type and whether the process takes place in epidermis, dermis, adnexal structures, or adipose tissue. Locally produced glucocorticoids, androgens, and estrogens can affect functions of the epidermis and adnexal structures, as well as local immune activity and perhaps the pigmentary system. Malfunction in these steroidogenic pathways can lead to inflammatory disorders, autoimmune diseases, or defects in the functioning of adnexal structures or to local carcinogenesis (49, 150–152). The cutaneous steroidogenic system can also have systemic effects that are emphasized by significant skin contribution to circulating androgens and/or estrogens. Therefore, we conclude that mammalian skin can be defined as an independent steroidogenic organ, whose activity can affect its functions and affect also the development of local or systemic inflammatory or autoimmune diseases. Furthermore, modulation of local steroidogenic activity may serve as a new strategy for treatment of inflammatory, autoimmune, or other skin disorders (150–152).
C. Structural and spatiotemporal organization of skin HPA axis
Significant experimental evidence has accumulated in support of the original hypothesis that mammalian skin expresses a homolog of the HPA axis to regulate local response to stress (129, 130, 180, 214, 221, 223, 320–326, 398–400). The original hypothesis was based on the evidence that skin expresses POMC products (202, 223, 373, 375, 376, 419, 420), MC2 gene (223), CRF, CRFR-1 receptors (202, 203), and CYP11A1, CYP17, CYP21A2, and 3β-HSD enzymes as well as the cutaneous production of steroidal products (421). The expression of MC2 protein in skin cells was also demonstrated (422, 423). Interestingly, the same skin cells (or the cells in close contact with them) express CRF, CRFR-1, POMC, and melanocortin and opioid receptors as tested in vitro or in vivo by immunocytochemistry or in situ hybridization (38, 129, 174, 178, 206, 209, 211, 213, 215, 320–323, 380, 382, 386, 387). These findings indicated autocrine or paracrine modes of action for HPA axis components (129, 130). Furthermore, evidence was presented that mammalian skin is also an extra-adrenal site of glucocorticoid synthesis that can act in intracrine, autocrine, or paracrine fashion (40, 130, 198, 205, 424).
The expression of these HPA axis elements is not random but is organized into functional, cell type-specific, regulatory loops with a structural hierarchy similar or identical to that found at the central level (40, 130). For example, CRF-induced CRFR-1 stimulation up-regulates POMC gene transcription and production of ACTH in epidermal melanocytes and DFs, via the activation of cAMP-dependent pathway(s) (321, 322). Melanocytes also respond with a POMC-dependent enhanced production of cortisol and corticosterone (322), whereas DFs respond to CRF and ACTH with enhanced production of corticosterone only (321) while producing cortisol constitutively (397). Human hair also contains a fully functional HPA axis equivalent, including increased production of cortisol following a CRF→POMC→ACTH→cortisol order of action, with negative feedback regulation by cortisol of intraepithelial CRF and POMC expression (320). Interestingly, cold or pain stressors can trigger the rapid production of cortisol in human body hair follicles that is independent from the central HPA axis (324–326, 425). CRF also stimulates the production of POMC with further processing to α-MSH in melanocytes, which release both POMC precursor and α-MSH into the media (323). In addition, Funasaka et al (205) also observed the stimulation of POMC by CRF in melanocytes and melanoma cells. CRF induction of POMC in the skin of C57BL/6 mouse in vitro has been reported (234).
Thus, there is overwhelming evidence of a structural and functional equivalent of the HPA axis operating in the skin, and the main challenge now is to define the degree to which this cutaneous stress response system follows or departs from the central (CRF→CRFR-1→POMC→ACTH→cortisol) organization, with its direct local phenotypic consequences.
D. Pathophysiological relevance of skin HPA axis and its departure from the central algorithm
Unlike the brain and pituitary, in the skin all elements of the HPA axis are synthesized in the same organ and at close mutual proximity, including their regulatory elements such as cytokines (49). In addition, the alternative ligand for the CRFR-1, Urc-1, is also produced in the skin (208) and therefore can also act as a trigger of the CRFR-1-led stress response in this organ (40). This already represents a departure from the central algorithm and identifies CRFR-1 as the main regulatory switchboard in the skin (CRF/Urc-1/synthetic agonists→CRFR-1) and opens new possibilities for pharmacological manipulations using synthetic ligands (40, 81). Thus, a context-dependent CRFR-1 coupling to a signal transduction pathway with a possible sequence of CRFR-1→POMC synthesis→processing→final peptide products is of great clinical and biological importance.
The following scenarios can be envisioned: 1) CRF/Urc-1→CRFR-1; 2) CRF/Urc-1→CRFR-1→POMC→ACTH→cortisol/corticosterone; and 3) CRF/Urc-1→CRFR-1→POMC→ACTH+β-LPH→α-MSH+β-endorphin or departs from the CRFR-1 signaling system to a POMC→ACTH+α-MSH+β-endorphin route. The first possibility lacks POMC and glucocorticoids signals and so will lead to a strong proinflammatory response and supports the skin barrier, as discussed in the corresponding sections of this review (Figures 7 and 10) and Refs. 49, 163, and 200. The second variant of the classical HPA axis leads to immunosuppressive effects, with the additional promelanogenic activity of ACTH. The third scenario includes the stimulation of melanogenesis by α-MSH and β-endorphin with an additional direct immunosuppression and nociception effect (see corresponding section on immunology and melanin pigmentation). Finally, POMC signaling can also be activated without CRFR-1 involvement through the action of locally-produced cytokines, direct effect of UVB, or activation of cAMP signaling by membrane-bound receptors (examples of which are CRFR-2 or MC1, -2, -5). An example of this mode is the activation of POMC in the skin by chemical peeling stress without involvement of CRF (327).
Figure 7.
Stressed skin regulates the central HPA axis. Signals generated in stressed skin are delivered either by ascending nerve routes to the brain or by circulation to the hypothalamus, pituitary, or adrenal gland, which would depend on the nature and intensity of the stressor and on skin anatomy/histology. Furthermore, UVR production and secretion of final effectors of the HPA (glucocorticoids) is activated by sequential and/or alternative modes of action originating in the skin that will depend on the wavelength and dose of solar electromagnetic energy.
Figure 10.
Effects of CRF (b) and CRF-related peptides (c) on human scalp hair fiber elongation at day 0 (D0), day 3 (D3), day 6 (D6), and day 9 (D9) and compared with hair follicles grown in the vehicle control (a). Anagen or growing scalp hair follicles were microdissected from human scalp and placed in organ culture as previously described (504).
The pathophysiological implications for such a variety of signaling outcomes is discussed in depth in Sections VI and VII. Briefly, there is good evidence that dysregulation of the proposed cutaneous HPA axis or departures from it can lead to inflammatory or autoimmune disorders, eg, psoriasis, alopecia areata (AA), acne, atopic dermatitis, or RA (139, 177, 201, 215–218, 230, 244, 426–428). The effects on the pigmentary system and melanoma development are discussed in sections on pigmentation and skin pathology.
E. Implications for other peripheral organs
The immune system was one of the first extracranial sites in which the production of POMC-derived peptides was detected (reviewed in Refs. 429 and 430). Now it is well recognized that most peripheral organs—including immune, respiratory, GI, cardiovascular, and musculoskeletal systems; adipose tissue, placenta, uterus, kidney, eyes, and skin; and even endocrine organs such as pancreas, adrenals, and gonads—are producing POMC-derived ACTH, α-MSH, and β-endorphin to different degrees for local use and express corresponding receptors (35, 129, 131, 160, 174, 317, 380, 431–434). These are involved in modification of local homeostasis, fine-tuning functions of these organs; they have site-specific immunomodulatory properties (predominantly immunosuppressive) and can modulate sensory input from the peripheral organs (35, 37, 129, 131, 160, 174, 317, 380, 431–435). However, their expression under physiological conditions is relatively small. For example, in mouse skin the concentration of POMC mRNA is ≤10 000 lower than in the pituitary (223). Still, peripheral POMC signaling systems are deregulated under pathological states including cancer (reviewed in Refs. 129, 174, 317, 375, and 380), and in some cases the aberrant overexpression of POMC may have systemic effects (reviewed in Refs. 35, 375, and 380).
Although the CRF/CRF-like signaling systems are widely expressed in the same organs and tissues, there is some degree of organ and tissue selectivity for the predominant type of the ligand CRF vs Urc-1, -2, or -3 and CRFR-1 vs CRFR-2 (69, 70, 73, 82, 85, 91). Again, these ligands, by activating either CRFR-1 or CRFR-2, can have immunomodulatory effects and have regulatory functions in these organs (69, 80, 82, 85, 91, 117, 201, 436–438). These signaling systems are also deregulated in various cancers (439).
Several tissues, besides the adrenal cortex, gonads, and placenta, also express CYP11A1 and so can be considered steroidogenic (132, 198). These tissues encompass skin, GI, heart, mammary gland, prostate, and the immune system, including during cancerous conditions (132, 152, 440–450). Interestingly, the brain also expresses steroidogenic activity (132, 451, 452). The products of these local pathways most likely play autocrine or paracrine regulatory roles (132, 198); steroidogenesis in these tissues is quite modest, usually being less than 1% of that seen in adrenal and gonadal cells. The steroids produced include pregnenolone, pregnenolone sulfate, DHEA sulfate, progesterone, 3β and 5α reduced derivatives of progesterone, and corticosteroids including cortisol and corticosterone (132, 152, 445, 446, 451, 452). It is also reported that LPS can induce intestinal glucocorticoid synthesis in a TNFα-dependent manner (453), adding another level of complexity to this system.
Thus, all molecular elements of the HPA axis are expressed locally although at relatively low levels. Therefore, it is likely that CRF and POMC signaling systems, in communication with cytokines, can regulate local steroidogenic activity in a context- and organ-dependent manner following a similar mechanism based on the HPA axis.
F. Modes of communication between skin and the central HPA axis
It is unknown whether the skin can directly activate the central HPA axis. However, this possibility is likely and would depend on the intensity and nature of the stressor (40, 49, 454) (Figure 7). For example, humans and horses exposed to sunlight exhibit increased circulating levels of α-MSH and ACTH (455, 456), whereas experimental whole-body exposure to UVB increases β-LPH and β- endorphin serum levels (457, 458). It should be noted that UVB stimulates cutaneous CRF, POMC peptides (80, 221, 307, 380), and cytokine production such as IL-1, IL-6, and TNFα (380, 459, 460). The latter can enter the systemic circulation (461). Also, the systemic immunosuppressive effect of UVB is well documented (462, 463). In fact, we have noted that UVB can stimulate serum ACTH, β-endorphin, and corticosterone levels in C57BL/6 mouse exposed to radiation (C. Skobowiat and A. Slominski, manuscript in preparation).
The activation of brain and endocrine responses by skin molecules that can be induced by environmental stressors was previously proposed in this journal (129). The current hypothesis is that systemic responses to UVR originating in the skin (in addition to production of vitamin D) also involve pathways encompassing the activation of the cutaneous and systemic HPA axis (Figure 7). Depending on the wavelength and intensity of the UV irradiation, the skin will activate the systemic HPA axis via neural transmission to the brain (hypothalamus), will activate the pituitary through skin-derived factors, or will activate the adrenal cortex directly (Figure 7). In the first case, UVR regulation of systemic homeostasis via the HPA axis will start with the stimulation of CRF in the hypothalamus via neutrally transmitted signals (Figure 7). From this point the information will be transmitted through the existing HPA axis organization, with cortisol/corticosterone serving as final messengers. In the latter case, the skin can activate the HPA axis at different entry points, eg, the pituitary or adrenal gland, by skin-derived humoral messages such as CRF/Urc-1, cytokines (IL-1, IL-6, and TNF-α), or ACTH (Figure 7). The former would act on the pituitary level, whereas ACTH released after massive skin damage would act on the adrenals. The intriguing possibility that skin-derived cytokines can directly activate the adrenal cortex (Figure 7) should be considered, taking into consideration work from Bornstein's group (464–469) and others (470–475) that shows direct activation of corticosteroidogenesis by immune mediators.
There is much clinical evidence for this mechanism, ie, the well-known phenomenon of systemic immunosuppressive action of UVB (462). For example, it is known that exposure to UVR can attenuate the progression of multiple sclerosis, a phenomenon linked to increased production of vitamin D3 (476). Here, we propose an alternative/additional explanation, eg, UVR activation of the HPA axis leading to immunosuppression, because of cortisol production (Figure 7). The UVR wavelength model may provide a mechanistic explanation for the recently described phenomenon of “UVR addiction” (477, 478) because of UVR-induced cutaneous β-endorphin production (49).
VI. CRF and Urc Function as Pleiotropic Cytokines
A. Regulation of skin barrier function
As in the epidermis, CRF and functional CRFR-1 have been detected (at both the mRNA and protein levels) in cultured keratinocytes isolated from the hair follicle and sebaceous gland (55, 129, 174, 207, 209). CRFR-1α is the predominant isoform of CRFR-1 expressed in hair follicle keratinocytes. The CRFR-2α gene is also readily detectable in hair follicle keratinocytes in vitro (209) and in multiple keratinocyte lineages of the human anagen hair follicle in situ, including outer and inner root sheaths, hair bulb matrix, and the differentiating precortex. By contrast, the CRFR-2 protein is strikingly absent from epidermal keratinocytes in situ, providing further evidence that the more “stable” epidermis is differentially regulated compared with the actively cycling hair follicle. In this way, up-regulation of CRFR-2 protein expression in those components of the anagen VI hair follicle that survive the apoptosis-driven regression of the lower “transient” hair follicle during catagen, indicates that CRF/Urcs are involved in hair growth (209). Others have reported a similar distribution of CRF and CRFR-1 in human scalp hair follicles at both the gene and protein levels (212, 320). These authors further reported that CRFR-1 and CRFR-2 expression was up-regulated after CRF treatment of anagen hair follicles in ex vivo organ culture (212). This also resulted in the up-regulation of POMC mRNA gene expression and in the expression of ACTH and α-MSH peptides in the outer root sheath (212).
CRFR-1 signaling plays a role in the most important function of the skin for organismal survival: the establishment of the barrier separating the body from the external environment. This barrier is formed by epidermal keratinocytes, which after leaving the basal layer differentiate to form a corneal layer composed of cross-linked proteins (forming envelopes) and intercellular lipids (forming Odland bodies) (153, 154). The calcium gradient is recognized as the most important regulator of keratinocyte differentiation (153). Phospholipase C, PKC, transcription factors of the AP-1 family are all engaged in this process (153, 479), and CRF stimulates calcium influx in the keratinocytes through voltage-regulated calcium channels (211, 250, 251). CRF has been shown to stimulate keratinocyte differentiation both in the continuous human epidermal cell line model HaCaT and in primary adult and neonatal keratinocyte (109, 211, 249, 256, 264). Activation of CRFR-1 increases intracellular IP3 and causes increased binding of Jun D (of AP-1 family) to its respective binding regions in DNA (80, 117, 249, 256). This initiates a differentiation program involving the attenuation of cytokeratin 14 expression (characteristic of basal keratinocytes) for the enhancement of involucrin and cytokeratin 1 expression (characteristic of more differentiated keratinocytes) on both mRNA and protein levels (80, 117, 249). CRFR-1 activation causes flattening and increased granularity of the keratinocytes in vitro, as evidenced with microscopy and more quantitatively with flow cytometry by increased forward and side scatters. Differentiation of cells follows their growth arrest in the G1/0 phase of the cell cycle. This CRF-induced accumulation of keratinocytes in G1/0 (as a way to inhibit proliferation) is also characteristic for other types of cells including malignant glioma cells (480), breast carcinoma (481), endometrial adenocarcinoma (482), and melanoma (483). In keratinocytes, this growth arrest is mediated by inhibition of cyclin-dependent kinases p16 and by fewer Ki-67 positive cells (249). In the mouse model, psychological stress induces the disruption of the epidermal barrier by inhibition of keratinocyte proliferation, inhibition of differentiation, decrease of the integrity of stratum corneum, and increased transepidermal water loss (484). In this model, psychological stress inhibited keratinocyte proliferation as measured with the number of PCNA-positive cells in the basal layer. This is consistent with the findings in a human cell culture models. However, in the murine studies, psychological stress decreased involucrin expression, contradictory to the findings in the human cell culture model (40). Moreover, the effects of psychological stress in the mouse model are reversed by both RU-486 (inhibitor of glucocorticosteroids) and antalarmin (inhibitor of CRF) (484). This confirms a role for CRF in the inhibition of keratinocyte proliferation. The reported data on the effects of CRF on keratinocyte differentiation are best explained by differences of the effects of CRF locally, directly on the cells vs the effects of CRF through HPA axis (40). CRFR-1 expressed on normal human keratinocytes is not coupled to cAMP (117), and this might explain the lack of subsequent stimulation of the HPA axis effectors by those cells (321, 322). However, the situation is different in immortalized human HaCaT keratinocytes and cells of squamous cell carcinoma, where CRFR-1 is indeed coupled to cAMP (117, 231).
Local CRF can serve different roles, including by stimulating keratinocyte to better preserve epidermal homeostatic function, as well as stimulating the production of inflammatory cytokines and antimicrobial peptides, as well as via the increased expression of adhesion molecules (Figure 8). CRF can thus stimulate the production of IL-6 and IL-11 and down-regulates IL-1β, IL-2, and IL-18 by keratinocytes, but it does not affect the release of TNF-α (254, 260, 261). Although all of those cytokines are known to be overexpressed in inflammation, their precise role, including their effect on the Th1/2 balance, differs based on the cellular and microenvironmental context (eg, effects of IL-18 are summarized in Ref. 485). The precise meaning of particular CRF effects remains to be elucidated. CRF also stimulates the interferon-γ-induced expression of cell adhesion molecules and human leukocyte antigen DR in human keratinocytes (55). CRF also regulates sebaceous gland secretory activity and contributes to the transport of fat-soluble antioxidants (and various lipids and compounds) to the epidermal surface to strengthen its barrier activity (139, 207). Furthermore, the barrier can also be strengthened via CRF1 stimulation of melanin production (Figure 8) (38, 80, 117). Moreover, antimicrobial activity of the barrier is enhanced by α-MSH (it has direct antimicrobial activity [486]) secondary to stimulation of POMC by CRF (80, 117, 323).
Figure 8.
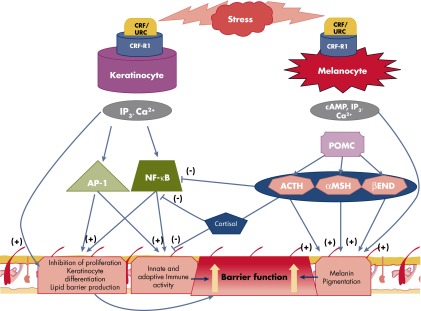
Differential phenotypic effects of CRF1 signaling in keratinocytes and melanocytes with secondary impact on skin barrier formation. In keratinocytes, CRF1 directly inhibits proliferation and stimulates differentiation plus stimulation of immune activity via stimulation of NF-κB. This enhances protective epidermal barrier function. In melanocytes, CRF1 directly and indirectly (through POMC peptides) stimulates differentiation and melanin production, and the latter enhances protective barrier function. In contrast to keratinocytes, CRF1 signaling leads indirectly (through POMC peptides) to inhibition of NF-κB with subsequent suppression of immune activity. This immunosuppressive effect can be amplified by production of cortisol by melanocytes.
In conclusion, there is ample evidence that local CRFR-1α activity plays a role in building barrier function against biological and noxious factors to protect internal homeostasis, but it may also lead to increased permeability during inflammation. On the contrary, CRF may result in barrier disruption through activation of skin mast cells. For instance, CRF can generate mast cells from hair follicle precursors (240) and CRF-induced mast cells-dependent barrier disruption (487), as well as an increased permeability of normal human colonic biopsies (488). Stress also activates brain mast cells through CRF (489) and leads to increased blood-brain barrier permeability (490) and increased brain metastases of rat mammary carcinoma cells (491).
B. Regulation of skin pigmentary system
The skin contains a local defensive melanocortin system to neutralize a wide range of external noxious stimuli (principally UVR) and consists of the pigment melanin and the crucial peptides CRF, POMC, and its associated cleaved POMC peptides. In cutaneous melanocytes, UVR stimulates both CRF and POMC formation, with resultant release of several POMC peptides via differential enzymatic cleavage of POMC by prohormone convertases. This results in the production of ACTH, α-MSH, β-MSH, γ-MSH, and other hormones that include β-endorphin and β-LPH (38). α-MSH binds to MC1, and ACTH binds MC1 or MC2, whereas β-endorphin can influence melanocytes via μ-opiate receptor. Melanin pigment produced in this way can act as a buffer molecule to antagonize the noxious effects of physical, biological, and chemical insults (38). Despite their common origin in the neural crest during embryogenesis, follicular and epidermal melanocytes diverge in many important ways both functionally and in their assignment to distinct compartments (157, 492). Briefly, an examination of the CRF/CRFR-1/2 system in the biology of the human scalp hair follicle pigmentary unit (213, 386, 493) shows that CRF, CRFR-1, and CRFR-2 were differentially expressed in cells of the human hair follicle pigmentary unit, including hair bulb melanocytes, follicular papilla fibroblasts, and hair bulb matrix keratinocytes. Moreover, there were differences in the pattern of peptide expression in melanocytes in situ compared to in vitro, suggesting the existence of important microenvironmental controls within the hair follicle resulting in delicate spatiotemporal regulation of these cells. CRF not only up-regulated follicular melanocyte cell proliferation and melanin synthesis (melanogenesis) but was also able to alter cell shape via the formation of more extensive dendrites and stimulated the expression and activity of the rate-limiting enzyme in melanogenesis, tyrosinase, tyrosinase-related protein-1 (TRP-1), and TRP-2 (dopachrome tautomerase [DCT]) (213) (Figure 9). By contrast, Urc-1 or CRFR-2 agonists down-regulated or had no effect on melanocyte phenotype. When taken together, these findings suggest that CRF can influence human scalp hair follicle melanocyte differentiation via both CRFR-1- and CRFR-2-mediated mechanisms. Whether the observed CRF effects result from the up-regulation of ACTH or MSH production in these cells or a direct effect via CRFRs and cAMP awaits further study. The involvement of ACTH and α-MSH in human skin pigmentation was first recognized by the stimulation of melanogenesis upon systemic administration of ACTH, α-MSH, and β-MSH especially in sun-exposed regions of the body (494, 495). Further evidence suggesting their involvement in cutaneous pigmentation comes from several clinical observations—for example, that elevated circulating levels of ACTH and α-MSH or prolonged therapeutic administration of ACTH induces hyperpigmentation in humans (38). α-MSH and ACTH peptides are involved in the regulation of human epidermal melanogenesis, dendricity, and proliferation via action at the melanocortin receptor (496–502).
Figure 9.
Expression of CRF, CRFR-1, and CRFR-2 in hair follicle melanocytes and the effect of CRF on follicular melanocytes. a, Human hair follicles express CRF and cognate receptors CRFR-1 (CRF1) and CRFR-2 (CRF2) (red fluorescence). These proteins were also detected in a subpopulation of melanocytes (yellow) located in the proximal/peripheral matrix region and in the outer root sheath (see arrowheads in enlargements of insets) but apparently were down-regulated in the melanogenic zone of anagen VI hair follicles. Cytoplasmic expression of CRF and its receptors was present in hair bulb keratinocytes and less so in dermal papilla cells (FP). b, CRF (10−8 m) stimulated dendricity in cultured hair follicle melanocytes. Cell dendricity was assessed by counting cells with three or more dendrites before and after stimulation of cells. c, CRF (10−7 m) stimulated melanogenesis in hair follicle melanocytes in culture. CRF modulated the expression and activity of melanogenic enzymes in hair follicle melanocytes in culture, including: d, tyrosinase protein expression and activity (dopa oxidase); e, TRP-1; and f, DCT (TRP-2). Lane 1, Molecular weight markers; lane 2, CRF 10−7 m; lane 3, unstimulated control; lane 4, negative control. [Individual panels were reproduced from S. Kauser et al: Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J. 2006;20:882–895 (213), with permission. © Federation of American Societies for Experimental Biology.]
More recently, we examined whether pigmentary effects were present in the CRF-POMC system at a POMC precleavage point (323). We found that POMC processing is incomplete in both keratinocytes and melanocytes because POMC secretion was detected in matched human epidermal keratinocytes and melanocytes in vitro. However, only keratinocytes secreted α-MSH (and then at much lower levels than for POMC), and neither cell type released/secreted ACTH. Although melanocytes and keratinocytes from human epidermis expressed POMC-processing components (eg, PC1 and PC2, and also 7B2), ACTH and small amounts of POMC and α-MSH were present only in extracts of keratinocytes. This finding suggests that the epidermal-melanin unit in epidermis relies on keratinocyte-derived peptides to stimulate MC1 on adjacent melanocytes. Remarkably, the situation in the hair follicle appears to be rather different. Here, melanocytes isolated and cultured from hair follicles secreted both POMC and α-MSH. Importantly, this secretion was enhanced in response to CRF acting primarily through the CRFR-1 (323). The modified CRF peptide d-Pro5CRF (5-fold more selective for CRFR-1 than CRFR-2) also stimulated large increases in POMC secretion. By contrast, the CRF agonist d-Pro4-r-Urc (almost wholly selective for CRFR-2) had relatively little effect on POMC release. These data suggest that CRF is acting primarily through CRFR-1 in the hair follicle melanocytes. This finding that follicular and epidermal melanocytes respond differently both in the production and secretion of α-MSH further supports the view that the epidermal-melanin unit and the follicular-melanin units are distinct, albeit linked, pigmentary systems in the skin in terms of their respective regulation (386, 492, 503, 504). Using MC1-transfected cells, we were also able to show that POMC could stimulate cAMP in these cells. Although the potency of POMC appears to be much lower than that of ACTH, α-MSH, or β-MSH, these results still show that POMC was able to increase both melanogenesis and dendricity in human pigment cells (323). Similar activity was shown previously for POMC-derived γ3-MSH in rodent melanomas (505).
Funasaka et al (205) reported the presence of CRF in melanocytic cells, with the greatest levels of expression in melanoma cells, findings that followed the first detection of CRF and CRFR-1 in normal and malignant melanocytes (202–204). Attempts were also made to assess whether CRF itself can directly influence the behavior of skin cells, including melanocytes. Support for a direct CRF effect derives from POMC knockout C57/BL6 mouse, which expresses normal hair pigmentation with eumelanin production despite a lack of ACTH, α-MSH, and β-endorphin ligands (506). Alternatively, some of the effects of CRF peptides on human melanocytes may be indirect, via activation of POMC. We have previously reported that ACTH, α-MSH, and β-endorphin are all active in stimulating proliferation of these cells in culture (38, 157, 213, 385, 493) and that in melanocytes CRF activation of CRFR-1 results in increased POMC gene expression and production of ACTH (322). Moreover, the inhibition of proliferation by CRF in some melanoma cell lines may result from uncoupling of CRFR-1 from POMC signaling (483). It could be argued that at least part of the observed CRF effect on melanocyte numbers may be due to improved melanocyte survival rather than proliferation per se, as reported previously in neural cell systems (507). In addition, a similar pro-cell survival action of CRF was observed in normal epidermal melanocytes (117). A CRF-associated up-regulation of POMC peptides may lead also to α-MSH- and ACTH-associated protection from apoptosis (316, 508).
The incubation of normal epidermal melanocytes with CRF initiates a cascade of events that is hierarchically ordered, whereby CRF activates CRFR-1, which induces cAMP accumulation and increases POMC gene expression with subsequent production of ACTH (322). Melanocytes of the hair follicle express CRF mRNA in a similar manner to melanocytes derived from the epidermis (213). Although the expression of CRF and Urc-1 was prominent in keratinocytes and fibroblasts of the hair follicle, the expression of both hormones was conspicuously down-regulated in differentiated melanotic hair bulb melanocytes (80, 117, 213) (Figure 9). Follicular melanocytes expressed low levels of CRFR-1 and CRFR-2 in situ (Figure 9), whereas their expression was up-regulated in differentiated dendritic melanocytes in vitro. CRF and its analogs [D-Pro5]-CRF, [D-Glu20]-CRF, and [D-Pro4]-r-Urc all significantly stimulated melanocyte proliferation at concentrations that varied from 10−7 to 10−10 m. Similarly, CRF (Figure 9), [D-Pro5]-CRF, [D-Glu20]-CRF, and [D-Pro4]-Urc all stimulated melanocyte dendricity with [D-Pro5]-CRF, the most active inducer of melanocyte dendricity, with a maximal dendritogenic effect observed at 10−10 m. In terms of effect on melanogenesis, CRF (Figure 9), [D-Pro5]-CRF, [D-Glu20]-CRF, and [D-Pro4]-Urc all significantly stimulated melanin production in melanocytes at 10−7 to 10−10 m (213). Furthermore, CRF (Figure 9), [D-Pro5]-CRF, and [D-Glu20]-CRF all stimulated increased expression of tyrosinase, TRP-1, and TRP-2 (DCT) proteins. However, no significant stimulation of tyrosinase, and indeed a reduction in TRP-1 and TRP-2 expression, was observed in melanocyte cultures incubated with [D-Pro4]-r-Urc (213). CRF was the most potent stimulator of tyrosinase activity, followed by [D-Pro5]-CRF and [D-Glu20]-CRF, whereas [D-Pro4]-r-Urc did not exhibit any effect on the L-3,4-dihydroxyphenylalanine-oxidase activity of tyrosinase compared to the unstimulated control (213). Interestingly, a most recent study by Watanuki et al (509) demonstrated that both CRF and Urc-1 can regulate TRP-1 gene expression via Nurr-1/Nur77 production, independent of POMC or α-MSH stimulation. This indicates a POMC-independent role of CRF signaling in regulation of melanin pigmentation.
The precise role of the CRF/CRFR-1 system in the differential regulation of cutaneous melanocytes is complicated by the significant melanocyte heterogeneity in this organ (492, 510, 511). Although melanotic melanocytes are distributed in the basal layer of the epidermis, infundibulum of the hair follicle, basal layer of the sebaceous gland, and the anagen hair bulb, other amelanotic melanocytes reside in the hair follicle outer root sheath, as well as in the most peripheral and proximal hair bulb matrix. Follicular and epidermal melanocytes share a common origin, but they diverge during hair follicle morphogenesis in many important ways (157, 492), with the most striking difference being the tight coupling of hair pigmentation to the hair growth cycle (512). By contrast, melanogenesis in the epidermis appears to be continuous (511), although this is further up-regulated by UVR. UVB radiation does not reach the melanogenic cells of the anagen hair bulb located in the hypodermis, and so UVR is unlikely to directly influence the follicular-melanin unit. Thus, this major stressor of the skin may not, at least directly, impact on the hair follicle pigmentary unit below the infundibulum.
C. Regulation of adnexal structures with focus on the hair follicle
The skin is well-equipped with both secretory and excretory functions invested in a range of different skin adnexa. These include sweat glands (merocrine and aprocrine), sebaceous glands, ceruminous glands (external ear canal), mammary glands in the breast, as well as hair follicles and nails. Moreover, skin adnexa are “hard-wired” into neuroendocrine networks (49, 175). For example, eccrine sweat gland activity is regulated via acetylcholine-associated sympathetic nerve fiber stimulation, with control residing in “sweat centers” in the hypothalamus.
1. Sweat glands
Early studies in cats revealed that preganglionic sympathetic neurons with CRF immunoreactivity were sudomotor in function. Using a retrograde tracer, Fluoro-Gold, to label postganglionic neurons projecting to the paw pads that includes cholinergic sudomotor neurons, researchers found that approximately one-third of these retrogradely labeled ganglion cells were surrounded by CRF-positive terminal baskets (513). Although the feline preganglionic sudomotor neurons contained true CRF, exogenous CRF did not have a measurable effect on postganglionic neurons or on ganglionic transmission in the cat sudomotor pathway, which appears instead to be entirely nicotinic. Thus, the function of CRF in cat sudomotor pathway remains unknown. In human skin biopsies, we have found expression of CRFR-1, CRF, and Urc-1 in sweat glands (208, 209, 231).
2. Sebaceous gland
The skin contains approximately 800 sebaceous glands per square centimeter, and this holocrine gland exhibits an endocrine function in the periphery that appears to be independent of other skin components. Sebocytes also express receptors for multiple neuropeptides, including for β-endorphin, CRF, Urc, and POMC. Indeed, a complete CRF system has been described for human sebocytes in vitro (207), and an autonomic CRF signaling system was described in sebaceous glands in vivo (139, 178, 216).
3. Hair follicle fibroblast subpopulations
Haired skin contains at least four distinct fibroblast subpopulations under normal skin resting conditions. Interfollicular DFs are the most numerous fibroblast subtype in skin and consist of distinct upper dermis “papillary” and lower dermis “reticular” fibroblasts (514). Moreover, at least two fibroblast subtypes exist in the hair follicle, namely the dermal sheath (DS) fibroblast and the dermal papilla fibroblast. The latter is the growth-inductive component of the hair follicle (515). However, there is increasing evidence to suggest a degree of polyclonality to some of these follicular mesenchymal cell populations (516).
Hair follicle and DFs are targets for α-MSH and express the relevant melanocortin receptors (517–519). We recently undertook an in vitro study to characterize the fibroblast subpopulations in hair scalp skin using fully matched DS, dermal papilla, and interfollicular DFs isolated from the same scalp tissue specimens from healthy individuals (519) (S Huq, ROS Karoo, DT Sharpe, DJ Tobin, et al, manuscript in preparation). Here we report that DFs exhibited the greatest baseline proliferation rates and DS exhibited the greatest baseline migration ability in the “scratch assay,” and that ACTH, α-MSH, and CRF (but not β-endorphin) all significantly increased both proliferation and cell migration parameters for all cutaneous fibroblast subtypes. Furthermore, under “wounded conditions,” DS secreted significantly more collagen than DF and dermal papilla, and this collagen secretion was significantly increased for DS and DF in the presence of TGF-β1. This TGF-β1-associated increase was antagonized by ACTH, α-MSH, and CRF but was unchanged by β-endorphin. Similarly, ACTH, α-MSH, and CRF (but not β-endorphin) raised cAMP levels in these fibroblast types. These data are consistent with a role for HPA axis components in activating the initial stages of wound repair, ie, cell migration and fibroplasia. The observation that hair follicle fibroblasts can contract collagen gels and that α-MSH, ACTH, and CRF potently inhibit TGF-β1-stimulated increases in collagen secretion suggests that these HPA axis peptides can regulate dermal regeneration (519) (S Huq, ROS Karoo, DT Sharpe, DJ Tobin, unpublished data). CRFR-1α and CRFR-2β are both detectable in hair follicle dermal papilla fibroblasts (undetectable in DFs) (80, 517), further supporting the view that follicular fibroblasts may be regulated differentially by local CRF-dependent activities.
4. CRF/Urc and hair follicle fiber growth
The hair follicle contains the second most rapidly dividing epithelial tissue, after gut epithelium. Specifically, the hair bulb matrix, which produces the cortical keratinocytes that make up the bulk of the hair shaft, exhibits a proliferation index of almost 100%, especially below the so-called Auber's line. Using isolated anagen VI scalp hair follicles as a model for hair growth (504), we found that CRF and [D-Glu20]-CRF that activate CRFR-1 can significantly inhibit hair fiber elongation (80, 117) (Figure 10). This CRFR-1 agonist-mediated inhibition was associated with the inhibition of keratinocyte proliferation in the anagen hair bulb and results in the premature precipitation of the anagen growing hair follicle into an apoptosis-driven catagen-like state. By contrast, treatment of human scalp hair follicles with CRFR-2 agonists does not inhibit hair fiber elongation; eg, D-Pro4]-r-Urc (selective agonist for CRFR-2) stimulates hair fiber elongation, whereas [D-Pro5]-CRF has a very minor effect or no effect, which indicates that they may even protect the hair follicle from entry in the catagen-like state (80, 117) (S Kauser, AT Slominski, ET Wei, DJ Tobin, unpublished data). Thus, CRFR-2 agonists appear to maintain keratinocytes of the hair bulb in an anagen-like state for longer, and this must be associated with both continued keratinocyte proliferation and retardation of keratinocyte differentiation in a subpopulation of undifferentiated hair bulb matrix keratinocytes. This observation suggests that, as in the epidermis, signaling via CRFR-1 leads to inhibition of keratinocyte proliferation via an induction of keratinocyte differentiation. By contrast, preferential activation with CRFR-2 maintains proliferation and/or retards the onset of keratinocyte differentiation. CRF (or ACTH) stimulation of these ex vivo hair follicle organ cultures also targets the nonhair fiber producing outer root sheath keratinocytes, resulting in the enhanced expression of cortisol in these keratinocytes and its secretion into the media (320). Conversely, hydrocortisone treatment of ex vivo scalp anagen VI growing hair follicles caused a down-regulation of CRFR-1 in the hair follicle outer root sheath, and in this may reflected its classical feedback regulatory mechanisms in the central stress axis (320).
D. Regulation of the dermal compartment
The dermis is composed primarily of collagen and other nonviable elements and is supported by rich vascular networks forming deep and superficial plexuses (133). Collagen is produced by fibroblasts through a multistep process that ends with the formation of characteristic fibrils. The activity of fibroblasts is typically stimulated by factors such as TGF-β (133). Fibroblasts also produce cytokines (such as IL-6 and IL-8), neuropeptides, and other effector molecules (129, 520). The activity of fibroblasts is of paramount importance in wound healing. Recent studies of murine wound healing performed on CRF−/− mouse, and confirmed on human DF cultures, shed light on functions of CRF in this process (237). CRF inhibited proliferation of murine DFs, stimulated production of IL-6, and inhibited migration in these cells. Cultures of human foreskin fibroblasts exposed to the CRFR-1 antagonist antalarmin recapitulated the findings in the CRF−/− cells (237). Our own studies demonstrating the presence of functionally active CRFR-1 in human DFs are consistent with the above finding, with the exception that we observed stimulation of cell cycling in growth factor-starved media and no effect when growth factors were present (117). The latter could be explained by different culture conditions and techniques used in both studies.
Human fibroblasts do not respond to CRF or ACTH with increased production of cortisol but do respond with increased production of corticosterone (321, 397). In humans, corticosterone is not considered to be the end effector of HPA axis, but rather is a precursor to aldosterone in the adrenal glands. Therefore, it is possible that CRF/ACTH affects local water/ion exchange mechanisms by inducing local mineralocorticoid activity. However, because corticosterone is a main glucocorticoid that suppresses the immune responses in many nonmammals, it may simply perform this function in human dermis reflecting its evolutionary origins. The expression of CRF, CRFR-1, ACTH, melanocortin 2 receptor (MC2R), and glucocorticoid receptor α is decreased in hypertrophic scars (243), whereas it would be expected that they would be rather overexpressed in the context of findings by Rassouli et al (237). Therefore, it is possible that effectors of the HPA axis function differently in normal vs abnormal wound healing. The direct vs indirect effects of CRF triggered secondarily by effectors may differ and simply reflect the course of the inflammatory response with its stimulation in early phases and suppression in later phases.
In conclusion, CRF signaling plays a role in the regulation of dermal functions. However, we are still in the initial stage of the understanding of CRFR-1 and CRFR-2 in these processes. However, detection of CRFR-1 in human fibroblasts and CRFR-1 and CRFR-2 in human mast cells and dermal papilla fibroblasts, and of both CRFR-1 and CRFR-2 by immunocytochemistry in smooth muscles, eccrine glands, blood vessels, hair follicles, and sebaceous glands, indicates that this line of research should be successful (139, 207, 209, 385, 521).
E. Regulation of the skin immune system
CRF is an active component and mediator of the skin immune system (59, 80, 117, 139, 176, 177, 199–201, 215–217). In brief, the skin immune system is based on both innate and adaptive responses and on the multiple types of cells that play a role in it. Cytokines mediate interactions between the cells that are traditionally accepted as part of the immune system (Langerhans cells, lymphocytes, neutrophils, etc) and resident cells in the epidermis and dermis. Epidermal keratinocytes and melanocytes actively process stimuli from the environment and then interact with cells of the skin immune system (49). CRF in this context acts as a cytokine and mediator because it is produced by both resident cells and immune cells (45, 117, 199). CRF stimulates the Th-2 arm of the immune response, ie, it up-regulates the production of IL-4 (Th-2) and down-regulates the production of IFN-γ (Th-1) and IL-10 (Treg) (428). Some of the documented inflammatory triggers for CRF release include UVB, LPS, chemical peeling (trichloroacetic acid), and P. acnes (80, 117, 214, 219, 220, 327). IL-18 acts as a proinflammatory cytokine that stimulates Th-2 responses and is considered to be at the mechanistic heart of inflammatory skin diseases including adult-onset Still's disease and atopic dermatitis (522). CRF has been shown to decrease production of IL-18 by HaCaT keratinocytes (260), although the downstream target of CRF, ACTH, stimulates production of this cytokine in these cells (423). CRF also stimulates the production of IL-1β, IL-6, and TNF-α by HaCaT keratinocytes (261) and stimulates translocation of the subunits of NF-κB to the nucleus of normal human keratinocytes (255). NF-κB is a transcription factor that binds to promoters of many proinflammatory mediators and is considered to be a crucial regulator of inflammatory responses. CRF also increases the expression of human cell adhesion molecule, intracellular adhesion molecule 1, and human leukocyte antigen DR by human keratinocytes (232). The LPS-stimulated production of IL-1β, IL-6 and TNF-α by human keratinocytes is mediated by CRF and CRFR-1 (219). Dermatofibromas, a common reactive/inflammatory lesions of the skin, exhibits increased expression of both CRF and CRFR-1 (523).
It is quite interesting that Selye published his book, The Stress of Life, in 1956 (2), and he wrote a book entitled The Mast Cells in 1965 (524), but never connected the two. Cutaneous mast cells occupy a strategic position in the brain-skin axis due to their location at the interface of the skin immune and nervous systems (59, 90, 122, 201). The activation of CRFR-1 on mast cells can lead to strong proinflammatory states and be linked with many cutaneous disorders (59, 166, 201, 233, 236, 525). Also, mast-cell dependent visceral hypersensitivity in IBS can be prevented (but not reversed) by α-helical CRF (9–41) due to the antagonist's impact on mast cell stabilization and epithelial barrier maintenance (526). Moreover, skin mast cells can be activated by acute stress or by intradermal administration of CRF, with associated increased vascular permeability and flushing that is dependent on CRF expression (348, 349, 527, 528). Thus, activity of cutaneous mast cells can be controlled via CRFR-1 antagonists to attenuate neurogenic inflammation. As far as the vascular component of the dermis is concerned, the effects of CRF are consistent with its local immediate proinflammatory function. In an elegant paper by Donelan et al (236), it was shown that CRF induced skin vessel permeability through its activation of local mast cells. The authors proposed that dorsal ganglia were the source of CRF (and also NT in their mouse model). CRF stimulates mast cells to release VEGF but inhibits the production of VEGF by HaCaT keratinocytes (90, 259). Of note, in mast cells the effect of CRF is selective because it does not stimulate release of histamine, tryptase, IL-6, IL-8, or TNF-α (90). On the other hand, CRF counteracts the effects of TNF-α on the expression of vascular endothelial adhesion molecule 1 and E-selectin (437). We also recently reported that human mast cell degranulation and TNF secretion were accompanied by mitochondrial fission and translocation to the cell surface (529) and secreted their components extracellularly, leading to augmentation of VEGF (530) and histamine (531). This predominant proinflammatory effect here is similar to other peripheral organs, including the immune system in general (44, 57, 166, 426, 438, 532, 533). However, anti-inflammatory activities of CRF and related Urcs in the periphery have also been described (436, 534), and we have already proposed an explanation reconciling these apparently conflicting results (55, 80, 117, 199). Based on recent data obtained in skin models, we propose that the proinflammatory activities of CRFR-1 agonists are secondary to the direct stimulation of NF-κB activity, whereas the anti-inflammatory effects are secondary to the inhibition of NF-κB activity indirectly by POMC peptides or glucocorticoids (secondary to activation of local HPA axis) or by the direct immunoinhibitory activity of the glucocorticoids (Figures 4 and 8). Part of this concept was discussed in depth (40, 130, 200), and it included intermediates of melanogenesis as additional immunoinhibitory factors (200) based on the ability of CRFR-1 to stimulate melanogenesis (38, 80, 117, 213, 323, 509) and on the extensive documentation for potent immunosuppressive properties of melanogenesis (49, 149, 535). Finally, it remains to be explored whether alternative splicing of CRFRs would couple CRF and Urc signals to direct immunosuppressive activity and to define the role of CRFR-2 in this process (49, 80, 81).
VII. Skin Pathology Associated With Dysregulation of the Cutaneous CRF Signaling System
The peripheral effects of CRF are a matter of ongoing debate (438). In the mouse knockout model of asthma, CRF was found to act as an anti-inflammatory agent, whereas in the similar model of colitis it was proinflammatory (57, 536). Therefore, extensive basic research is still required to find a satisfactory explanation for these apparently contradictory phenomena. Nevertheless, there is a sufficient body of information to link CRFR-1 activity with many clinical conditions, including RA and psoriasis.
A. Proliferative disorders: psoriatic arthritis and psoriasis
Psoriasis is a chronic inflammatory condition characterized by papulosquamous lesions with a symmetrical distribution on the scalp and in the intertriginous areas and extremities. The lesions typically do not resolve without treatment. Psoriatic arthritis is asymmetric and typically involves few small joints (133, 537). Regular elongation of epidermal rete ridges, dilated vessels, and neutrophilic infiltrate in the epidermis characterize the lesions by skin biopsy. The pathogenesis of psoriasis is multifactorial (537). The main role of the epidermis vs that of cellular immune response is still a matter of dispute. Histocompatability antigens (in particular HLA-Cw6) are related to a significantly increased risk of psoriasis (133, 537). Cytokines such as IL-2, IL-23, IL-17, interferon- γ, and TNF-α play a role in the development of this disease (538–540). Kono et al (206) detected CRF in psoriatic lesions. Kim et al (215) have analyzed the expression of CRF, ACTH, and α-MSH in different forms of psoriasis, including guttate, small plaque, and large plaques. Although these forms do not differ in their expression pattern of the above elements, CRF expression was increased in the upper layers of the epidermis, hair follicles, and sweat glands when compared to normal skin. Furthermore, no difference in the expression of ACTH or α-MSH was observed by these authors. In other studies, the expression of CRFR-1 was higher in psoriatic lesions as compared to normal skin (239) and correlated positively (P = .001 and r = 0.6) with the Psoriasis Area Skin Index (PASI) score (244). These receptors were found in the epidermis, the adnexal structures, and the perivascular inflammatory infiltrate. On the other hand, decreased expression of CRFR-1 was observed in psoriatic epidermis or dermis by Vasiadi et al (218), whereas decreased expression of both CRF and CRFR-1 was found by Zhou et al (217). Conflicting results on the expression of CRF and CRFR-1 in the epidermis might be explained by both different methods and different sources of antibodies used. Nevertheless, Vasiadi et al (218) found increased levels of CRF in the serum of patients with psoriasis. Also, mast cells in close proximity to psoriatic plaques express CRFR-1. Of note, the development of mast cells in the hair follicle is stimulated by CRF (240). In another study, the expression of CRFR-1 and the effects of CRF on the release of VEGF were observed on mast cells (90). It was also shown recently that serum NT levels are increased in patients with psoriasis, and NT induces VEGF release from human mast cells (541), which also have the ability to synthesize NT (542). Because mast cells release VEGF (236), which is involved in the pathogenesis of psoriatic lesions, increased serum levels of CRF might be responsible for their activation. With time, the mast cells desensitize their responsiveness to CRF by decreasing their expression of CRFR-1. Increased levels of CRF have been correlated to exposure to stress (218), providing a link between stress and HPA axis status in psoriasis. CRFR-1 antagonists are being considered as prospective therapeutic targets in this disorder (218). Although increased serum CRF was found in patients with psoriasis in this study, it is also possible that CRF might be released from local nerve endings (543). In the context of the effect of CRF on VEGF release, the inhibition of CRFR-1 in endothelial cells increases a TNF-α-induced expression of VEGF-1 (437). The somewhat contradictory effects of CRF on the release of VEGF might stem from the fact that different cells and targets were studied, ie, mast cells in Vasiadi's study (218) and endothelial cells in Inada's study (437).
There is considerable evidence of CRF involvement in RA, a symmetric arthritis typically involving the metacarpophalangeal and proximal interphalangeal joints of the hands. Levels of rheumatoid factor, anticitrullinated peptide/protein antibody, erythrocyte sedimentation rate, and C-reactive protein are elevated. Results from both laboratory and clinical studies indicate an etiological role of CRF in RA. Immunoreactivity (ie, peptide but not mRNA levels) for CRF is increased in the synovial fluid of patients with RA (426). These levels are independent of central/systemic stimulation because plasma levels of CRF in those patients are not elevated (544). The source of synovial CRF, and how it affects the synovial cells, is a matter of investigation, although neuron-derived clone 77 (NUR77)–related protein (NURR) 1 was found to mediate both inflammatory cytokine-induced expression of CRF by synoviocytes and its effect on endothelial and immune cells (545). NURR1 expression is stimulated by the cAMP/PKA/CREB/ATF-2 pathway in endothelial cells of the synovium (546). CRFR-1α, but not CRFR-1β or any CRFR-2 isoform, is engaged in RA activity (547). Effects of CRF on synovial tissue include its stimulation of prostaglandin E2, and both CRF and prostaglandin E2 act through phosphorylated CREB/ATF (548). The well-documented overproduction of CRF into the synovium of RA patients has led to an analysis of CRF promoter polymorphisms. Three variants were discovered with point mutations at position 1273 (alleles A1 and A2), 2942 (alleles B1 and B2), and 95 (alleles C1 and C2) of GenBank entry x67661 (549). The A2B1 compound allele protected against development of RA, and A1B1 was strongly associated with the development of RA (550). Please confirm or correct expansion of XXX (used once). CRF allele A2 is correlated with late-onset RA (551). The haplotype A1B1 has the biggest influence on the activity of luciferase promoter in rat pheochromocytoma cells PC12 (552). Patients with the A2B2 allele exhibited an earlier CRF response compared to A1B1-positive patients during an insulin hypoglycemia test (427). Genetic linkage analysis has revealed a significant linkage between RA and the CRF promoter region (in particular, a dinucleotide microsatellite marker, D8S1723) (553). The CRHRA1*10;CRHRA2*14 haplotype was subsequently found to be significantly associated with RA (554).
In summary, the role of CRF and CRFR-1 in the development of psoriatic lesions is emerging, and new theories on this subject are being proposed (200, 217, 218). The accumulated data are somewhat contradictory. One reason for discrepancies is the possible presence of specific isoforms of CRFR-1 or modified CRF/Urc family members that are recognized or not recognized by antibodies used in the above studies. Another reason could be differences in the sex and age of the patients and anatomical location of the lesion. Because the effects of CRF on the cell signaling mediated by different CRFR-1 isoforms may differ substantially, the development of isoform-specific antibodies and more thorough studies of psoriatic lesions represent the next logical step to investigate in this area.
B. Pigmentary disorders with emphasis on vitiligo
The release of neuropeptides (eg, CRF and associated POMC peptides) from skin cells and peripheral nerve endings is thought to synergize with cytokines to adversely affect melanocyte function and viability. Impacting on such interactions could serve as the basis for new treatments.
Vitiligo is an acquired, idiopathic, progressive (mostly), unpredictable depigmenting disorder of the skin (555, 556). Although the etiology of vitiligo remains enigmatic, several hypotheses have been proposed for the loss of functioning melanocytes in vitiligo (555). However, the close linkage between the skin, immune, and nervous systems suggests that neuroimmunological factors, which may be further influenced by psychosocial stress (525), play a role in the pathogenesis of vitiligo (49, 175, 557, 558). Nevertheless, the role of HPA axis components in vitiligo is not clear. Reduced α-MSH has been reported in vitiligo lesional skin and serum (559, 560), and this reduced α-MSH expression reflected peptide levels rather than being a function of melanocyte numbers (559). Kingo et al (561) recently assessed the expression of CRF and POMC peptides in vitiligo skin biopsies and reported that the expression of POMC, MC1, and MC4 mRNA was significantly decreased in lesional skin. Perhaps surprisingly, the levels of MC1 and MC4 were increased in uninvolved skin of vitiligo patients compared with normal healthy controls. Furthermore, no difference was detected between different subtypes of vitiligo, ie, nonsegmental vs segmental. Although the observed reduction of MC1 and MC4 expression in vitiligo lesional skin is to be expected (given the reduction in constituent melanocytes in these lesions), just why nonlesional skin in vitiligo should express higher amounts of CRF/POMC peptide receptors (but not POMC itself) than healthy control skin is less obvious, especially because the researchers controlled for relative sun exposure of the tested skin sites, as well as for skin phototype. The observation that POMC levels were similar between nonlesional vitiligo skin and healthy control skin suggests that melanocortin receptor expression may be controlled in part systemically rather than only peripherally in vitiligo skin. The preferential up-regulation of MC1 in nonlesional vitiligo skin compared with normal epidermis was mirrored by an observed statistically significant up-regulation of some melanogenesis-related enzymes including TRP-1. This change could be the result of melanocortin stimulation in nonlesional vitiligo skin, as a type of compensatory mechanism for the vitiligo insult on the epidermal melanin unit in the lesional skin. However, an activated melanocortin system in vitiligo nonlesional skin may reflect the need for greater immune modulation in this immune-mediated dermatosis and the well-known anti-inflammatory properties of melanocortins (129, 317).
Another way in which the CRF/POMC system could potentially impact on the pathomechanism of vitiligo centers on how oxidative stress in this disease can alter POMC processing to its final neuropeptides (562). Given that patients with vitiligo can accumulate hydrogen peroxide at millimolar concentrations in the epidermis (555), the impact of this level of reactive oxygen species on POMC peptide cleavage is of interest, especially because both POMC cleavage products, α-MSH and β-endorphin, lose their functionality after oxidation. Spencer et al (563) have reported reduced epidermal furin expression in the skin of patients with progressive vitiligo. However, furin levels return to normal after the lowering of epidermal hydrogen peroxide levels. Moreover, furin mRNA expression is also directly affected by hydrogen peroxide exposure. It therefore can be envisaged that a reduction or loss in the expression of POMC peptides α-MSH and β-endorphin in vitiligo (as previously reported in Ref. 564) could be due to a reactive oxygen species-associated disruption of the Ca2+-dependent proteolytic activity of this convertase, which could impact on the immune status of these lesions. In addition, the finding that CRF can inhibit starvation-induced apoptosis of melanocytes (80, 117) identifies CRFR-1 as an attractive adjuvant target for possible therapy of vitiligo. In particular, the activation of CRFR-1 can lead to the production of immunosuppressive POMC peptides and cortisol (130, 257).
It is not known whether other pigmentary disorders are impacted by the CRF-POMC system. One potential example could be melasma, with its known links with endocrine axes, with possible indirect effects through the action of MSH and ACTH peptides. However, a POMC-independent role of CRF signaling in the regulation of melanin pigmentation also represents a viable option (80, 509).
In conclusion, it is possible that dysregulation of CRF and Urc-1 signaling can play a role in pigmentary disorders including vitiligo. However, considerations for clinical implications would require further careful studies because activation of CRFR-1 in melanocytes increases their viability, and a CRFR-1-led HPA-like cascade can suppress immune attack against melanocytes.
C. Disorders of adnexal structures including alopecia
Given that skin adnexa are both the target and the source of components of the CRF-POMC axis in the periphery, researchers are interested in assessing how disruption of this axis may play a role in diseases of the adnexa.
One of the most common skin disorders affecting the pilosebaceous unit is acne vulgaris, which is characterized by sebaceous gland hyperplasia and associated increased sebum production, follicular hyperkeratinization, colonization of P. acnes, and perifollicular inflammation (565). CRF can induce lipid synthesis and steroidogenesis and also has the capacity to interact with T and GH. These observations suggest that disruption of these systems in the sebaceous gland may lead to disorders including acne, seborrhea, androgenetic alopecia, and age-associated skin xerosis (566, 567).
A recent study reported a strong up-regulation of CRF expression in sebaceous gland cells in acne-involved skin (216). CRF-BP expression was also found in the differentiating sebocytes of acne-involved glands. These glands expressed CRFR-1 and CRFR-2. By contrast, the expression of CRF was low in noninvolved and normal skin sebaceous glands. Given the up-regulation of the CRF system in acne-involved skin, it is likely that the CRF in this tissue can influence immune and inflammatory processes and that these can impact on the development of acne lesions, but also the exacerbation of acne lesions via psychosocial stress triggers.
Recently, a study of skin aging reported that CRF was up-regulated (although its binding protein, CRFR-BP, was down-regulated) in the sebaceous glands of aged vs young skin, as was the expression of CRFR-1 (but not CRFR-2) in the hair follicle and epidermis. The authors interpreted these results from the view that an up-regulation of the CRF system in aging skin may lead to an exaggerated stress response reaction in this skin (245).
In a case series of AA purportedly induced by acute stress, there was up-regulation of affected skin CRFR expression (242). It is also suspected that chronic or intermittent stress can impair hair growth (55, 209, 568–570). Moreover, mice that were engineered to overexpress CRF, as a model of chronic stress, were reported to develop bilateral symmetric hair loss in adulthood (571). Recently, Wang et al (238) investigated the ability of CRFR antagonists to influence hair growth in CRF-overexpressing mice with the long-acting CRFR-1/CRFR-2 receptor antagonist, astressin-B. Astressin-B injected peripherally into CRF-overexpressing alopecic mice induced long-term hair follicle-associated pigmentation and hair regrowth. Moreover, astressin-B prevented the development of alopecia in young CRF-overexpressing mice. Specifically, it appears that this action occurs via transformation of atrophic telogen hair follicles to normalized anagen phase follicles, and this effect was determined to be a local targeting of the skin and hair follicles because there was no change to the elevated plasma corticosterone levels. Interestingly, the selective CRFR-2 antagonist, astressin2-B, did not affect hair regrowth, implicating a CRFR-1 response in hair follicle growth in mice.
AA remains the second most common hair loss disorder after androgenetic alopecia in adult males. It has been very difficult to associate episodes of hair loss in this presumptive autoimmune disease with psychoemotional stress, given the “chicken-and-egg”-associated dilemma (572, 573) of these conditions. Still some go as far as to consider AA as a psychosomatic disorder (574, 575), although this has been actively challenged by others (576, 577). Although this research field is complex (recently reviewed in Refs. 156, 343, and 578), several highly suggestive observations have been reported implicating a role for stress. For example, abnormalities in the peptidergic innervation of lesional AA hair follicles have been reported (579). Both CRFRs and ACTH are up-regulated in lesional hair follicles (242, 580). Moreover, both nerve growth factor and SP can inhibit human hair growth in ex vivo culture, whereas the latter can collapse the immune privilege of the lower transient portion of the anagen hair follicle (581)—an occurrence that is proposed as a trigger for AA in humans (582).
Using the most robust experimental model currently available for AA, the C3H/HeJ AA mouse, Zhang et al (230) examined the impact of psychoemotional stress on stress-response pathways in the murine system and concluded that these mice had a blunted systemic HPA axis response to acute physiological stress. Both physiological (via light ether anesthesia) and psychological (via restraint stress) stresses were applied. This was followed by plasma measurements of corticosterone, ACTH, and estradiol; of CRF, CRFRs, and POMC gene expression (among others) in the brain and lymphoid organs; as well as the skin. Affected AA mice (before stress application) exhibited a dramatic increase in HPA axis tone and activity both centrally and peripherally in the skin and lymph nodes, whereas stress further exacerbated changes in their HPA axis activity both centrally and peripherally. AA mice had significantly blunted corticosterone and ACTH responses to the acute ether stress and deficient habituation to repeated restraint stress. Moreover, HPA axis hormone levels were positively correlated with skin Th1 cytokine levels. The authors interpreted this as suggesting that the altered HPA axis activity observed was a consequence of the AA-associated immune response.
The chicken-and-egg dilemma remains, however, because it is still unproven whether psychoemotional stress actually triggers AA. It could perhaps be a condition that reflects a wider, non-hair follicle-specific perturbation of cutaneous or indeed systemic immunity. Is it also not yet clear the extent to which stress mediation at the periphery (ie, within the skin itself) may affect, via systemic release, the central HPA stress axis, with the potential for amplification (49). Finally, the mouse model may simply not reflect what happens in humans in this context.
D. Skin cancers including melanoma
Because epidermal keratinocytes, melanocytes, and their malignant counterparts, such as melanoma, express exclusively the CRFR-1 receptor, targeting of this receptor represents a rational approach in hyperproliferative, premalignant, or malignant states. Because activation of CRFR-1 receptors in these cells can inhibit keratinocyte, melanocyte, and melanoma proliferation, selective CRFR-1 agonists (preferably peptides to reduce side effects) should serve as a rational choice in the treatment of melanoma or squamous or basal cell carcinomas, as well as solar keratosis. The in vivo anti-melanoma effect of CRFR-1-specific agonists has already been reported (483), and it is consistent with in vivo effects in other tumors (439, 480, 481, 583). Of note, the high antiproliferative potency of selective CRFR-1 agonists toward keratinocytes and melanoma cells has been demonstrated (80, 117, 256). Because activation of CRFR-1 stimulates keratinocyte differentiation, CRFR-1 ligands can also be used to stimulate the formation or restoration of the epidermal barrier, the function of which is distorted in many pathological states (154, 584, 585).
In addition, activation of CRFR-1 in keratinocytes induces increased p16 (Ink4a) expression and G0/1 arrest with a resultant antiproliferative effect (249, 256) that is associated with differentiation induction, and therefore, its activation may alleviate psoriasis presentation. This situation is complex, however, because serum CRF levels are reported to be higher in psoriasis patients than in controls, and PASI scores do not appear to be correlated with either CRFR-1 or serum CRF levels (239). Moreover, a role for CRF in psoriasis could be dependent on its ability to modulate keratinocyte immune function via altered expression of adhesion molecules and changes in cytokine production (199, 232, 261), as well as altered CRFR-1 signaling (90, 218, 239). The authors of the latter studies propose to use specific CRFR-1 antagonists instead of agonists in the treatment of psoriasis.
Melanoma is a deadly skin cancer for which there is no effective treatment once metastatic disease develops. Various modalities have been attempted, and whereas they have induced temporary remission and improved disease-free survival time, melanoma recurs and the effect on survival of the patients is unsatisfactory (586–589). Human melanomas do express preferentially CRFR-1 (rodent melanomas express both CRFR-1 and CRFR-2), and they produce CRF and Urc-1 (111, 112, 204, 208, 211, 231). In fact, all melanoma types we tested so far have expressed CRF and CRFR-1. Funasaka et al (205) and others also reported the presence of CRF in melanocytic cells, with the greatest levels of expression in melanoma cells (205, 590). This expression correlated with a concomitant increased expression of POMC-derived antigens (424, 590). We also performed immunocytochemistry studies on several human melanoma biopsies or excision and detected CRF and CRFR-1 in all specimens. However, we could not find a difference in their expression level in comparison to epidermis or adnexal structure and also could not correlate such expression with melanoma progression (Figure 11). However, in accordance with the above studies, we found increased expression of POMC in more advanced melanomas (376) and in other skin cancers (379, 381). To reconcile these observations, we believe that melanoma and skin cancer progression is associated with increased POMC-peptide expression that may generate a tumor-favorable environment, whereas CRF expression itself is a marker of the deregulated upper arm of the local HPA axis (CRF→CRFR-1 or CRFR-2). The latter possibility can be suggested by the heterogeneity in the CRFR-1 isoform pattern, where in each melanoma tested by us several additional CRFR-1 isoforms were observed, whereas normal melanocytes expressed only CRFR-1α (111). In accordance with our interpretation, in situ analyses in breast cancer show neither a correlation between CRF and CRFRs and tumor progression nor significant differences in their expression in the surrounding normal tissue (583). Similarly, a recent review concluded that CRFR expression loss may contribute to malignant transformation and tumor growth in prostate, colon, and lung cancer. However, CRF and CRFR expression was without value as a tumor marker in lung and breast cancer, respectively. By contrast, in endometrial cancer, CRFR-1 expression correlated with less aggressive tumors, whereas CRFR-2 correlated with advanced stage tumors (439, 583).
Figure 11.
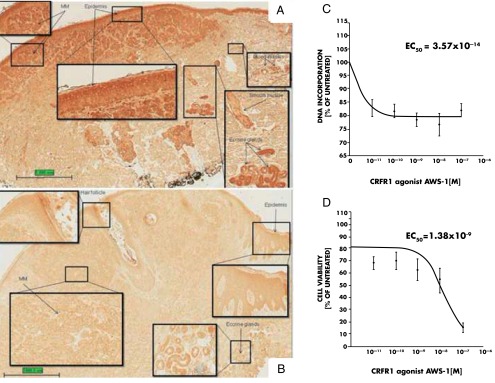
Expression of CRF and CRFR-1 in the normal skin, melanoma cells, and effects of CRF1 agonist on proliferation of melanoma cells. A, CRF1 is expressed in normal structures of human skin such as epidermis, blood vessels, eccrine glands, and smooth muscle as well as in malignant melanoma cells (MM). B, CRF is expressed in normal structures of human skin such as epidermis, eccrine glands, and hair follicle as well as in malignant melanoma cells (MM). The slides in A and B were stained with antibodies as described previously (209). Magnification, ×20; insets, ×200. C, CRFR-1 agonist AWS-1 inhibits proliferation of AbC1 hamster melanoma cells. Cells were incubated with the peptide for 48 hours in the 154 medium (Cascade Biologics, Inc) containing growth factors. The DNA synthesis was measured with titrated thymidine incorporation, and data were analyzed as described previously (117). D, CRFR-1 selective agonist AWS-1 inhibits proliferation of Melan A mouse immortalized melanocytes. Cells were incubated with the peptide for 48 hours in the F10 medium containing fetal calf serum (Invitrogen, Inc). The cell viability was measured with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and data were analyzed as described previously (117). The difference between control and treatments was analyzed with one-way ANOVA (P < .005) as described previously (117). Panels A and B were prepared by Dr Diane Kovacic, a dermatopathology fellow at the University of Tennessee Health Science Center.
Attempts were also made to assess whether CRF itself can directly influence the behavior of melanoma cells. Inhibition of melanoma proliferation in vitro and in vivo was reported using S91 and B16 melanoma models, respectively (483). We have confirmed these antiproliferative effects using a human melanoma line in vitro (80, 117). Furthermore, we were able to show an inhibitory effect of the CRFR-1 selective agonist ASW-1 on the proliferation of hamster melanoma and a mouse immortalized melanocyte line (Figure 11, C and D). Interestingly, CRF inhibited the in vivo growth of gliomas (480), human breast cancer (481), and endometrial adenocarcinoma cells (482) via the activation of CRFR-1.
In conclusion, selective CRFR-1 agonists can serve as good candidates for further preclinical testing of their utility in adjuvant therapy of melanoma or neoplastic skin disorder because of their antiproliferative properties in melanoma and their immunostimulatory activity. Nevertheless, local dysregulation of the HPA axis or defects at the receptor levels (for example, generation of alternatively spliced isoforms) may also contribute to tumor progression.
E. Proposed unified mechanism of skin pathology secondary to dysregulation of local CRF signaling
CRF and related peptides also exhibit nonendocrine activities, defining these peptides as novel and important growth factors/pleiotropic cytokines (80, 117). The most instructive cases are inflammatory and autoimmune disorders, where CRF/Urc signaling is deregulated. Thus, uncoupling of CRFR-1 from the cutaneous HPA axis or its variants (see above) will lead to sustained proinflammatory activity that can self-amplify, leading to cutaneous inflammatory states including psoriasis, acne, AA, or allergic reactions, and perhaps vitiligo. An additional instructive model here is the context-dependent coupling of CRF signaling to NF-κB (the master regulator of inflammation and context-dependent regulator of differentiation and cell survival) (Figure 8) (80, 176, 199, 254, 255, 257).
We also believe that similar mechanisms contribute to the development of some systemic autoimmune diseases such as RA (426, 547, 551), and we expect possible similar contributions in lupus erythematosus and scleroderma. Thus, dysregulation of the cutaneous CRF system can have systemic consequences. This can be extended to other organs in proposing that inefficient local attenuation of the CRF signaling system and/or defective coupling to the downstream immunosuppressive regulatory mechanisms can exacerbate or induce local proinflammatory responses leading to inflammatory and/or autoimmune disease processes.
As relates to carcinogenesis, overstimulation of POMC with downstream production of corresponding ACTH, α-MSH, and β-endorphin can lead to tumorigenic activity, because of their immunosuppressive actions together with the associated stimulation of proliferation and inhibition of apoptosis, eg, by α-MSH. The support for this hypothesis comes from clinical findings where overexpression of POMC was seen in melanoma and non-melanoma skin cancers (376, 378, 419, 424, 590). This effect can be amplified by release of corticosteroids from malignant cells, generating an immunosuppressive environment (180, 198, 391).
The detailed role of alternative splicing in the development of skin diseases and carcinogenesis remains to be investigated. However, such a role is highly possible, taking into consideration information from other organs, and so implicating an important role for alternative splicing in their physiology and pathology (67, 73, 82, 116, 118, 119, 265, 591). Similarly, alternative splicing in the skin was observed in pathological states or after repeated UVR exposure, and alternatively spliced forms have limited coupling to second messengers to modulate the activity of CRFR-1α (55, 80, 81, 109, 112, 113, 117, 252, 264).
In summary, dysregulation of CRF signaling either at the CRFR-1 receptor level or at downstream effectors and feedback mechanism can lead to skin disorders with additional systemic implications.
VIII. Quest for Novel Therapy of Cutaneous Disorders Based on Interventions to the Local CRF Signaling System
Extensive studies have identified large families of specific peptides or nonpeptide small molecules that act as selective agonists or antagonist of CRFR-1 and CRFR-2 and accordingly regulate their activity with phenotypic effects in the brain, eg, for the treatment of migraines (592), and in other organs (20, 21, 593–602). Significant clinical effort has been devoted to establishing novel pharmacological strategies for the treatment of stress-related disorders using CRFR-1 antagonists (74). Such a strategy has its own limitation on the central level. However, the same drugs (specific antagonists or agonists) can be used in the treatment of skin disorders through targeting the CRFR-1. Signaling of the latter is the predominant one in the human skin, and it is almost exclusive in the epidermis and so could be useful for the treatment of stress-induced skin diseases (49, 603). Targeting the CRFR-2 may be useful in the treatment of hair cycling disorders or malfunction of the sebaceous gland. Agonists at the CRFR-2 (eg, Urcs) can stimulate ex vivo organ cultures of intact anagen scalp hair growth by maintaining the growth-associated anagen phase longer, during which time more hair fiber can be produced. In contrast, our preliminary data also suggest that agonists selective for CRFR-1 (eg, D-Glu20-CRF) retard hair fiber elongation in this assay system, whereas CRF itself, which signals via both CRFR-1 and CRFR-2 showed an intermediate response in this ex vivo model (80) (Figure 10). This model may be suitable for the assessment of different CRFR-1 and CRFR-2 agonists and antagonists (20, 21, 596, 600, 601) for hair growth and hair pigmentary (graying) disorders. In fact, this hypothesis has been tested in part in a preclinical model of alopecia (238). Their utility as targets for pharmacological modifications or activation in therapy of skin disorders remains to be investigated in appropriate preclinical models (80, 81, 109, 111, 252, 264). In addition, there are several drugs with proven influence on alternative splicing, which can be applied topically to change the CRFR-1 splicing pattern and accordingly to regulate the sensitivity of certain skin cells to particular CRFR-1 agonists or antagonists (reviewed in Ref. 81). Note that CRFR-1 SNPs, which potentially affect CRFR-1 splicing, have been associated with many systemic disorders. Finally, the most promising candidates are soluble CRFR-1 isoforms that through binding with CRF or Urcs would decrease the availability of the ligands to the receptor, protect the ligands from degradation, or secure their delivery to the proper compartment (80, 81, 109, 111, 252, 264). Their role, which could be similar but not identical to CRF binding protein, waits testing using synthetic proteins. We also do not exclude the possibility that soluble CRFR-1 isoforms could interact with membrane-bound receptors.
IX. Theory on the Origin of CRF-Led Stress Response System
A. Differences and similarities between the central and cutaneous HPA axis organization
The main differences were discussed in Sections V.C. to V.E., and so the main points will only briefly be mentioned here. The crucial regulatory elements of the central HPA axis represent separate anatomical/functional entities of different embryonic origin that are separated anatomically, histologically, and functionally (Figure 12). Furthermore, the flow of information is unidirectional and linear starting with CRF as the upper regulator and including circular feedback inhibition of activating molecules by final effector molecule cortisol/corticosterone. The beauty of this axis lies in its simplicity, autoregulation, and functional precision.
Figure 12.
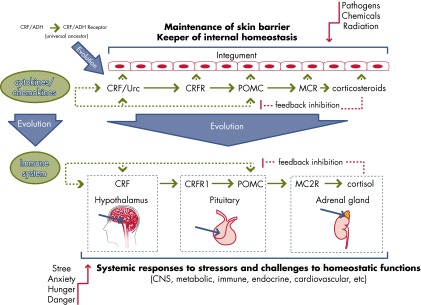
Proposed evolution of the HPA axis organization.
In the skin, all of the elements of an HPA-like axis are primarily found in the same organ, in the same or adjacent histological structures, and in neighboring cells or even within the same cells. This generates multidirectional communications that are nonlinear but with possible feedback and feedforward mechanisms operating at different levels of coordinating points and so are not restricted to a glucocorticoid effector. At its center is the CRFR-1, because in this cellular environment it can be activated by both CRF and Urc-1. Furthermore, there will be a continuous feedback and feedforward activity from other cytokines (skin is an immune organ). Finally, whereas the final effectors (ie, glucocorticoids) can overpower the system by shutting it off (via down-regulation of CRF and POMC), the intermediary molecules (POMC-derived peptides) can on their own have significant metabolic and homeostatic activities.
B. Hypothesis on the integumental origin of CRF-led HPA-like organization
Given the common ectodermal origin shared by the brain and epidermis, and the extensive discussion above, it is proposed that the central HPA axis was first developed in the integument and was later selected from its variants under evolutionary pressure, adapted, and perfected by the CNS and the endocrine system to promote species survival (Figure 12) (304). This primordial organization in the integument was designated to coordinate and cooperate with the innate and adaptive immune systems or barrier-forming systems (including melanin pigmentation) to create the optimal responses against pathogens and other physicochemical stressors, including solar radiation, to maintain or restore internal homeostasis. The fine-tuning of such responses may have been secured by the “primordial HPA” axis composed of CRF/Urc-1-signaling and POMC-signaling systems with glucocorticoids and cytokine input because of the intimately close association of all of these elements in this tissue. Throughout evolution, this axis could have undergone specialization and separation of its functional components in space and time (ie, CNS, endocrine glands, immune system), processes that allowed its hierarchical organization and predominantly linear flow of information, and became detached from its point of origin in the skin (40, 304). The retained system could serve as an evolutionary record of the parental “primordial HPA” system, but it retains important functions, at least in skin pathophysiology. Systemic implications include bidirectional communication between the skin and brain to activate the central HPA axis at the level of the hypothalamus or to modify the central HPA axis by humoral factors accessing the pituitary or adrenals.
X. Final Comments and Future Directions
The exciting experimental studies, conceptual developments, and underlying clinical findings described in this review focus on the skin-stress response. Although complementary, the role of the CRF family members is not limited to the skin, and they play a vital role in additional systems including the GI, cardiovascular, immune, and CNS.
Advances in these fields were made possible through the isolation, characterization, synthesis, and biological evaluation of ovine CRF by Catherine Rivier, Joachim Spiess, and Jean Rivier under the leadership of Wylie Vale (Figure 13). This landmark achievement completed the search for the upper regulatory element of the HPA axis. Through collaborations at the Salk Institute (La Jolla, California) and around the world, other elements of the CRF-led responses to stress, such as CRFR-1 and related Urcs, were discovered. These original and additional contributions from Wylie's team led to the avalanche of studies on the role of HPA axis elements in central and peripheral organs. One example is defining the CRF-led cutaneous skin stress response system, with its unique position determined by the location at the interface between external and internal environments. Other organs that may follow a similar organization of CRF-led loops include the GI and respiratory systems, because of their interaction with the external environment, as well as the placenta, which serves as an intermediate between maternal and fetal environments.
Figure 13.
Members of the team that discovered CRF, left to right: Joachim Spiess, Catherine Rivier, Jean Rivier, and Wylie Vale.
Further investigation of the functions and dysfunctions of the CRF system will undoubtedly lead to novel treatments of many skin diseases and other diseases, especially those worsened with stress, coupled with the design of potent and long-acting receptor-selective agonists and antagonists. The reported differences in the expression and reactivity of CRF-led systems may be due to age, gender, and species differences, as well as the use of pharmacological vs physiological levels of the ligands. Also, different local mechanisms may underlie acute and chronic responses.
Despite an extensive amount of information concerning CRF and its receptors, as well as the development of related peptide and neuropeptide CRFR agonists/antagonists (604), no drugs have been developed to date to address this regulatory system. CRFR-1 antagonists were developed for the treatment of depression but largely failed (605). Similar was the fate of such molecules for the treatment of anxiety (606), whereas they have also been proposed for IBS (85). Given the information reviewed above, it would seem reasonable to regulate the skin CRF system for the treatment of a variety of skin diseases (49, 80, 200, 607), many of which worsen with stress (Figure 14). In this respect, the suggestion has been made to modulate mast cells (346), especially because they can also synthesize and release CRF (45). For instance, the US patent no. 6,020,305 covers the use of CRFR-1 antagonists, alone or with mast cell blockers, for the treatment of atopic dermatitis and psoriasis. To the extent that skin derives from neuroectoderm and meningeal mast cells have been implicated in migraines (608) and are activated by stress (609), US patent no. 5,855,844 is also relevant because it covers the use of CRFR-1 antagonists for the prophylaxis/treatment of migraines.
Figure 14.
CRF and skin diseases. Several skin diseases are associated with CRF dysfunction. Dermal inflammatory infiltrate, illustrated in the background, is frequently seen in autoimmune diseases including lupus erythematosus. Please note that lupus erythematosus can be present as a systemic or cutaneous form (discoid). RA is a systemic autoimmune disease affecting the joints. Psoriasis is predominantly a cutaneous disease, although it can often include the joints (psoriatic arthritis). There are many forms of alopecia, including inflammatory/autoimmune alopecia such as AA, lichen planopilaris, and lupus alopecia. The category of dermatitis (inflammatory skin disorders) is represented by allergic contact dermatitis, atopic dermatitis, and nummular dermatitis. The other entities include acne, chronic urticarial, melanoma, squamous cell carcinoma, and basal cell carcinoma.
With the expectation that translational applications of the accumulated basic knowledge will soon bear clinical fruit, one owes Wylie Vale a debt of gratitude and regrets his untimely passing. His work has had and will have a continuous impact on the understanding of the CRF pathophysiology and the development of appropriate effective treatments. Thus, his ideas will continue to live through our work.
Acknowledgments
This review is dedicated to Dr Wylie Vale.
The projects described were supported by Grants R01AR052190, R01AR047079, and R01AR056666 from the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and Grants IBN-9405242, IBN-9604364, and IOS-0918934 from the National Science Foundation (to A.S.); Grants R01DK065244, R01NS038326, and R01AR047652 from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Neurological Disorders and Stroke, and NIANSD (to T.T.); NIDDK Grant PO1 26741 (to J.R.); Johnson & Johnson Skin Research Center Training Grant from Johnson & Johnson Consumer Products Worldwide, Skillman, New Jersey (to B.Z.); and grants from the Polish Ministry of Science and Higher Education for projects N405 623238 (to M.Z. and A.S.) and N402 662840 (to M.A.Z.).
J.R. is the Dr Frederik Paulsen Chair Professor in Neurosciences and President of Sentia Medical Sciences, Inc (La Jolla, California). T.T. is President of Theta Biomedical Consulting and Development, Co, Inc (Brookline, Massachusetts), to which he has assigned US Patents no. 5,855,844 and 6,020,305 covering CRF blockers for the treatment of migraines and skin diseases. A.S., M.Z., B.Z., and D.T. have nothing to disclose.
Disclosure Summary: All authors state that they have no conflict of interest.
Footnotes
- AA
- alopecia areata
- AC
- adenylate cyclase
- AP-1
- adaptor protein 1
- ATF
- activating transcription factor
- BP
- binding protein
- CNS
- central nervous system
- CRE
- cAMP responsive element
- CREB
- CRE binding protein
- CRF
- corticotropin releasing factor
- CRF-BP
- CRF binding protein
- CRFR
- corticotropin releasing factor receptor
- DCT
- dopachrome tautomerase
- DF
- dermal fibroblast
- DHEA
- dehydroepiandrosterone
- DS
- dermal sheath
- ECD
- extracellular domain
- GFP
- green fluorescent protein
- GI
- gastrointestinal
- GPCR
- G protein-coupled receptor family
- HPA
- hypothalamic-pituitary-adrenal
- HSD
- hydroxysteroid dehydrogenase
- IBS
- irritable bowel syndrome
- IP
- inositol phosphate
- β-LPH
- β-lipotropic hormone
- LPS
- lipopolysaccharide
- LRF
- luteinizing releasing factor
- MC
- melanocortin receptor
- MC2R
- melanocortin 2 receptor
- NF-κB
- nuclear factor κ-light-chain-enhancer of activated B cells
- NT
- neurotensin
- PC
- proconvertase
- PKA
- protein kinase A
- PKC
- protein kinase C
- POMC
- pro-opiomelanocortin
- POU3F2
- POU class 3 homeobox 2
- RA
- rheumatoid arthritis
- SNP
- single nucleotide polymorphism
- SP
- substance P
- TLR
- toll-like receptor
- 7-TM
- 7-transmembrane domains
- TRP
- tyrosinase-related protein
- Urc
- urocortin
- UVR
- UV radiation
- VEGF
- vascular endothelial growth factor.
References
- 1. Selye H. A syndrome produced by diverse noxious agents. Nature. 1936;138:32–33 [Google Scholar]
- 2. Selye H. The Stress of Life, revised ed New York, NY: McGraw-Hill Book Company; 1976 [Google Scholar]
- 3. Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 1981;78:6517–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397 [DOI] [PubMed] [Google Scholar]
- 5. Harris GW. Neural control of the pituitary gland. Physiol Rev. 1948;28:139–179 [DOI] [PubMed] [Google Scholar]
- 6. R, Rosenberg B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955;57:599–607 [DOI] [PubMed] [Google Scholar]
- 7. Saffran M, Schally AV. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955;33:408–415 [PubMed] [Google Scholar]
- 8. Vale W, Grant G, Amoss M, Blackwell R, Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972;91:562–572 [DOI] [PubMed] [Google Scholar]
- 9. Burgus R, Dunn TF, Desiderio D, Ward DN, Vale W, Guillemin R. Characterization of ovine hypothalamic hypophysiotropic TSH-releasing factor. Nature. 1970;226:321–325 [DOI] [PubMed] [Google Scholar]
- 10. Schally AV, Redding TW, Bowers CY, Barrett JF. Isolation and properties of porcine thyrotropin-releasing hormone. J Biol Chem. 1969;244:4077–4088 [PubMed] [Google Scholar]
- 11. Baba Y, Matsuo H, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. II. Confirmation of the proposed structure by conventional sequential analyses. Biochem Biophys Res Commun. 1971;44:459–463 [DOI] [PubMed] [Google Scholar]
- 12. Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79 [DOI] [PubMed] [Google Scholar]
- 13. Burgus R, Rivier J. Use of high pressure liquid chromatography in the purification of peptides. In: Loffet A, ed. Peptides 1976. Bruxelles, Belgium: Editions de l'Universite de Bruxelles; 1976:85–94 [Google Scholar]
- 14. Rivier J. Use of trialkyl ammonium phosphate (TAAP) buffers in reverse phase HPLC for high resolution and high recovery of peptides and proteins. J Liq Chromatog. 1978;1:343–366 [Google Scholar]
- 15. Bennett HPJ, Browne CA, Solomon S. The use of perfluorinated carboxylic acids in the reversed-phase HPLC of peptides. J Liq Chromatog. 1980;3:1353–1365 [Google Scholar]
- 16. Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci USA. 1983;80:4851–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furutani Y, Morimoto Y, Shibahara S, et al. Cloning and sequence analysis of cDNA for ovine corticotorpin-releasing factor precursor. Nature. 1983;301:537–540 [DOI] [PubMed] [Google Scholar]
- 18. Montecucchi PC, Anastasi A, de Castiglione R, Erspamer V. Isolation and amino acid composition of sauvagine. An active polypeptide from methanol extracts of the skin of the South American frog Phyllomedusa sauvagei. Int J Pept Protein Res. 1980;16:191–199 [PubMed] [Google Scholar]
- 19. Lederis K, Letter A, McMaster D, Moore G, Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982;218:162–165 [DOI] [PubMed] [Google Scholar]
- 20. Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CP, Pearse RV, II, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195 [DOI] [PubMed] [Google Scholar]
- 23. Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vita N, Laurent P, Lefort S, et al. Primary structure and functional expression of mouse pituitary and human brain corticotropin releasing factor receptors. FEBS Lett. 1993;335:1–5 [DOI] [PubMed] [Google Scholar]
- 25. Kishimoto T, Pearse RV, II, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA. 1995;92:1108–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lovenberg TW, Liaw CW, Grigoriadis DE, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrin M, Donaldson C, Chen R, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA. 1995;92:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol. 1995;9:637–645 [DOI] [PubMed] [Google Scholar]
- 29. Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2γ receptor. Mol Endocrinol. 1998;12:1077–1085 [DOI] [PubMed] [Google Scholar]
- 30. Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. New York, NY: Appleton-Century-Crofts; 1929 [Google Scholar]
- 31. Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1–5 [DOI] [PubMed] [Google Scholar]
- 32. O'Connor TM, O'Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2000;93:323–333 [DOI] [PubMed] [Google Scholar]
- 33. Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-κB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299 [DOI] [PubMed] [Google Scholar]
- 34. Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. 2000;343:37–49 [DOI] [PubMed] [Google Scholar]
- 35. Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav Immun. 2007;21:23–33 [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381 [DOI] [PubMed] [Google Scholar]
- 38. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228 [DOI] [PubMed] [Google Scholar]
- 39. Mazon AF, Verburg-van Kemenade BM, Flik G, Huising MO. Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. J Exp Biol. 2006;209:510–517 [DOI] [PubMed] [Google Scholar]
- 40. Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008;5:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alderman SL, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol. 2009;164:61–69 [DOI] [PubMed] [Google Scholar]
- 42. Roulin A, Emaresi G, Bize P, Gasparini J, Piault R, Ducrest AL. Pale and dark reddish melanic tawny owls differentially regulate the level of blood circulating POMC prohormone in relation to environmental conditions. Oecologia. 2011;166:913–921 [DOI] [PubMed] [Google Scholar]
- 43. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362 [DOI] [PubMed] [Google Scholar]
- 44. Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136 [DOI] [PubMed] [Google Scholar]
- 45. Kempuraj D, Papadopoulou NG, Lytinas M, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48 [DOI] [PubMed] [Google Scholar]
- 46. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. New York, NY: Wiley; 1988:629–649 [Google Scholar]
- 47. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904 [DOI] [PubMed] [Google Scholar]
- 48. McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(suppl 2):17180–17185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii,, 1–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uniprot Web site. http://www.uniprot.org 2012
- 51. SABiosciences Web site. EpiTect ChIP qPCR Primers. http://www.sabiosciences.com/chipqpcrsearch.php?species_id=0&nfactor=n&ninfo=n&ngene=n&B2=Search&src=genecard&factor=Over+200+TF&gene= CRH 2012
- 52. GeneCards Web site. http://www.genecards.org/cgi-bin/carddisp.pl?gene=UCN3&search=Urocortin 2012
- 53. NCBI Web site. http://www.ncbi.nlm.nih.gov/homologene/ 2012
- 54. Karteris E, Grammatopoulos D, Dai Y, et al. The human placenta and fetal membranes express the corticotropin-releasing hormone receptor 1α (CRH-1α) and the CRH-C variant receptor. J Clin Endocrinol Metab. 1998;83:1376–1379 [DOI] [PubMed] [Google Scholar]
- 55. Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693 [DOI] [PubMed] [Google Scholar]
- 56. Jin D, He P, You X, et al. Expression of corticotropin-releasing hormone receptor type 1 and type 2 in human pregnant myometrium. Reprod Sci. 2007;14:568–577 [DOI] [PubMed] [Google Scholar]
- 57. Gay J, Kokkotou E, O'Brien M, Pothoulakis C, Karalis KP. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology. 2008;149:3403–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. JensenLab Web site. http://diseases.jensenlab.org/Search 2012
- 59. Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39 [DOI] [PubMed] [Google Scholar]
- 60. Cao J, Boucher W, Kempuraj D, Donelan JM, Theoharides TC. Acute stress and intravesical corticotropin-releasing hormone induces mast cell dependent vascular endothelial growth factor release from mouse bladder explants. J Urol. 2006;176:1208–1213 [DOI] [PubMed] [Google Scholar]
- 61. Theoharides TC, Whitmore K, Stanford E, Moldwin R, O'Leary MP. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979–2994 [DOI] [PubMed] [Google Scholar]
- 62. Angelidou A, Asadi S, Alysandratos KD, Karagkouni A, Kourembanas S, Theoharides TC. Perinatal stress, brain inflammation and risk of autism-review and proposal. BMC Pediatr. 2012;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22 [DOI] [PubMed] [Google Scholar]
- 64. Lovejoy DA, Rotzinger S, Barsyte-Lovejoy D. Evolution of complementary peptide systems: teneurin C-terminal-associated peptides and corticotropin-releasing factor superfamilies. Ann NY Acad Sci. 2009;1163:215–220 [DOI] [PubMed] [Google Scholar]
- 65. Chand D, Lovejoy DA. Stress and reproduction: controversies and challenges. Gen Comp Endocrinol. 2011;171:253–257 [DOI] [PubMed] [Google Scholar]
- 66. Miller LJ, Dong M, Harikumar KG, Gao F. Structural basis of natural ligand binding and activation of the Class II G-protein-coupled secretin receptor. Biochem Soc Trans. 2007;35:709–712 [DOI] [PubMed] [Google Scholar]
- 67. Markovic D, Grammatopoulos DK. Focus on the splicing of secretin GPCRs transmembrane-domain 7. Trends Biochem Sci. 2009;34:443–452 [DOI] [PubMed] [Google Scholar]
- 68. Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357 [DOI] [PubMed] [Google Scholar]
- 69. Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–328 [DOI] [PubMed] [Google Scholar]
- 70. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286 [DOI] [PubMed] [Google Scholar]
- 71. Hillhouse EW, Randeva H, Ladds G, Grammatopoulos D. Corticotropin-releasing hormone receptors. Biochem Soc Trans. 2002;30:428–432 [DOI] [PubMed] [Google Scholar]
- 72. Hemley CF, McCluskey A, Keller PA. Corticotropin releasing hormone—a GPCR drug target. Curr Drug Targets. 2007;8:105–115 [DOI] [PubMed] [Google Scholar]
- 73. Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Arzt E, Holsboer F. CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol Sci. 2006;27:531–538 [DOI] [PubMed] [Google Scholar]
- 75. Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kuhne C, Puk O, Graw J, et al. Visualizing corticotropin-releasing hormone receptor type 1 expression and neuronal connectivities in the mouse using a novel multifunctional allele. J Comp Neurol. 2012;520:3150–3180 [DOI] [PubMed] [Google Scholar]
- 77. Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212 [DOI] [PubMed] [Google Scholar]
- 78. Graham CE, Vetter DE. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci. 2011;31:1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kiapekou E, Zapanti E, Mastorakos G, Loutradis D. Update on the role of ovarian corticotropin-releasing hormone. Ann NY Acad Sci. 2010;1205:225–229 [DOI] [PubMed] [Google Scholar]
- 80. Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57:1–13 [PMC free article] [PubMed] [Google Scholar]
- 82. Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20:432–438 [DOI] [PubMed] [Google Scholar]
- 83. Kokkotou E, Torres D, Moss AC, et al. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006;177:3355–3361 [DOI] [PubMed] [Google Scholar]
- 84. Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(suppl 7):33–46 [PMC free article] [PubMed] [Google Scholar]
- 85. Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann NY Acad Sci. 2009;1165:206–210 [DOI] [PubMed] [Google Scholar]
- 87. Yang LZ, Tovote P, Rayner M, Kockskamper J, Pieske B, Spiess J. Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol. 2010;632:1–6 [DOI] [PubMed] [Google Scholar]
- 88. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26 [DOI] [PubMed] [Google Scholar]
- 89. Jonassen AK, Wergeland A, Helgeland E, Mjos OD, Brar BK. Activation of corticotropin releasing factor receptor type 2 in the heart by corticotropin releasing factor offers cytoprotection against ischemic injury via PKA and PKC dependent signaling. Regul Pept. 2012;174:90–97 [DOI] [PubMed] [Google Scholar]
- 90. Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675 [DOI] [PubMed] [Google Scholar]
- 91. Takahashi K. Distribution of urocortins and corticotropin-releasing factor receptors in the cardiovascular system. Int J Endocrinol. 2012;2012:395284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schöneberg T, Hofreiter M, Schulz A, Römpler H. Learning from the past: evolution of GPCR functions. Trends Pharmacol Sci. 2007;28:117–121 [DOI] [PubMed] [Google Scholar]
- 93. Lovejoy DA, Jahan S. Phylogeny of the corticotropin-releasing factor family of peptides in the metazoa. Gen Comp Endocrinol. 2006;146:1–8 [DOI] [PubMed] [Google Scholar]
- 94. Denver RJ. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann NY Acad Sci. 2009;1163:1–16 [DOI] [PubMed] [Google Scholar]
- 95. Campbell RK, Satoh N, Degnan BM. Piecing together evolution of the vertebrate endocrine system. Trends Genet. 2004;20:359–366 [DOI] [PubMed] [Google Scholar]
- 96. Polymeropoulos MH, Torres R, Yanovski JA, Chandrasekharappa SC, Ledbetter DH. The human corticotropin-releasing factor receptor (CRHR) gene maps to chromosome 17q12–q22. Genomics. 1995;28:123–124 [DOI] [PubMed] [Google Scholar]
- 97. Tsai-Morris CH, Buczko E, Geng Y, Gamboa-Pinto A, Dufau ML. The genomic structure of the rat corticotropin releasing factor receptor. A member of the class II G protein-coupled receptors. J Biol Chem. 1996;271:14519–14525 [DOI] [PubMed] [Google Scholar]
- 98. Sakai K, Yamada M, Horiba N, Wakui M, Demura H, Suda T. The genomic organization of the human corticotropin-releasing factor type-1 receptor. Gene. 1998;219:125–130 [DOI] [PubMed] [Google Scholar]
- 99. Parham KL, Zervou S, Karteris E, Catalano RD, Old RW, Hillhouse EW. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145:3971–3983 [DOI] [PubMed] [Google Scholar]
- 100. Perrin MH, Fischer WH, Kunitake KS, et al. Expression, purification, and characterization of a soluble form of the first extracellular domain of the human type 1 corticotropin releasing factor receptor. J Biol Chem. 2001;276:31528–31534 [DOI] [PubMed] [Google Scholar]
- 101. Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem. 2008;283:32900–32912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grace CR, Perrin MH, Gulyas J, Rivier JE, Vale WW, Riek R. NMR structure of the first extracellular domain of corticotropin-releasing factor receptor 1 (ECD1-CRF-R1) complexed with a high affinity agonist. J Biol Chem. 2010;285:38580–38589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Perrin MH, Grace CR, Riek R, Vale WW. The three-dimensional structure of the N-terminal domain of corticotropin-releasing factor receptors: sushi domains and the B1 family of G protein-coupled receptors. Ann NY Acad Sci. 2006;1070:105–119 [DOI] [PubMed] [Google Scholar]
- 104. Arai M, Assil IQ, Abou-Samra AB. Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology. 2001;142:446–454 [DOI] [PubMed] [Google Scholar]
- 105. Hofmann BA, Sydow S, Jahn O, et al. Functional and protein chemical characterization of the N-terminal domain of the rat corticotropin-releasing factor receptor 1. Protein Sci. 2001;10:2050–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sydow S, Radulovic J, Dautzenberg FM, Spiess J. Structure-function relationship of different domains of the rat corticotropin-releasing factor receptor. Brain Res Mol Brain Res. 1997;52:182–193 [DOI] [PubMed] [Google Scholar]
- 107. Assil IQ, Abou-Samra AB. N-glycosylation of CRF receptor type 1 is important for its ligand-specific interaction. Am J Physiol Endocrinol Metab. 2001;281:E1015–E1021 [DOI] [PubMed] [Google Scholar]
- 108. Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465 [DOI] [PubMed] [Google Scholar]
- 109. Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol. 2009;218:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pal K, Swaminathan K, Xu HE, Pioszak AA. Structural basis for hormone recognition by the human CRFR2α G protein-coupled receptor. J Biol Chem. 2010;285:40351–40361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756 [DOI] [PubMed] [Google Scholar]
- 112. Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–1072 [DOI] [PubMed] [Google Scholar]
- 113. Slominski AT, Zmijewski MA, Pisarchik A, Wortsman J. Molecular cloning and initial characterization of African green monkey (Cercopithecus aethiops) corticotropin releasing factor receptor type 1 (CRF1) from COS-7 cells. Gene. 2007;389:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Grammatopoulos DK, Dai Y, Randeva HS, et al. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–2202 [DOI] [PubMed] [Google Scholar]
- 115. Seck T, Pellegrini M, Florea AM, et al. The Δe13 isoform of the calcitonin receptor forms a six-transmembrane domain receptor with dominant-negative effects on receptor surface expression and signaling. Mol Endocrinol. 2005;19:2132–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Markovic D, Lehnert H, Levine MA, Grammatopoulos DK. Structural determinants critical for localization and signaling within the seventh transmembrane domain of the type 1 corticotropin releasing hormone receptor: lessons from the receptor variant R1d. Mol Endocrinol. 2008;22:2505–2519 [DOI] [PubMed] [Google Scholar]
- 117. Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Karteris E, Markovic D, Chen J, Hillhouse EW, Grammatopoulos DK. Identification of a novel corticotropin-releasing hormone type 1β-like receptor variant lacking exon 13 in human pregnant myometrium regulated by estradiol-17β and progesterone. Endocrinology. 2011;151:4959–4968 [DOI] [PubMed] [Google Scholar]
- 119. Wu SV, Yuan PQ, Lai J, et al. Activation of type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: an enterochromaffin cell model. Endocrinology. 2011;152:126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444 [DOI] [PubMed] [Google Scholar]
- 121. Perrin MH, DiGruccio MR, Koerber SC, et al. A soluble form of the first extracellular domain of mouse type 2β corticotropin-releasing factor receptor reveals differential ligand specificity. J Biol Chem. 2003;278:15595–15600 [DOI] [PubMed] [Google Scholar]
- 122. Theoharides TC, Konstantinidou AD. Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci. 2007;12:1615–1628 [DOI] [PubMed] [Google Scholar]
- 123. Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci. 2010;31:318–325 [DOI] [PubMed] [Google Scholar]
- 124. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284 [DOI] [PubMed] [Google Scholar]
- 125. Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kino T. Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications. Sci Signal. 2012;5:pt4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J Biol Chem. 2010;285:35386–35392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kino T, Chrousos GP. Acetylation-mediated epigenetic regulation of glucocorticoid receptor activity: circadian rhythm-associated alterations of glucocorticoid actions in target tissues. Mol Cell Endocrinol. 2011;336:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487 [DOI] [PubMed] [Google Scholar]
- 130. Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J Endocrinol. 2007;193:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed St Louis, MO: Mosby Elsevier; 2008 [Google Scholar]
- 134. Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719–726 [DOI] [PubMed] [Google Scholar]
- 135. Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14 [DOI] [PubMed] [Google Scholar]
- 136. Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–1576 [DOI] [PubMed] [Google Scholar]
- 137. Elias PM, Menon G, Wetzel BK, Williams J. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am J Hum Biol. 2010;22:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494 [DOI] [PubMed] [Google Scholar]
- 139. Zouboulis CC, Baron JM, Böhm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551 [DOI] [PubMed] [Google Scholar]
- 140. Siemionow M, Gharb BB, Rampazzo A. The face as a sensory organ. Plast Reconstr Surg. 2011;127:652–662 [DOI] [PubMed] [Google Scholar]
- 141. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379 [DOI] [PubMed] [Google Scholar]
- 142. Legat FJ, Wolf P. Cutaneous sensory nerves: mediators of phototherapeutic effects? Front Biosci (Landmark Ed). 2009;14:4921–4931 [DOI] [PubMed] [Google Scholar]
- 143. Ahmad N, Mukhtar H. Cytochrome p450: a target for drug development for skin diseases. J Invest Dermatol. 2004;123:417–425 [DOI] [PubMed] [Google Scholar]
- 144. Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–1965 [DOI] [PubMed] [Google Scholar]
- 145. Botchkarev VA, Yaar M, Peters EM, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126:1719–1727 [DOI] [PubMed] [Google Scholar]
- 146. Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95 [DOI] [PubMed] [Google Scholar]
- 147. Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509 [DOI] [PubMed] [Google Scholar]
- 148. Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194 [DOI] [PubMed] [Google Scholar]
- 149. Slominski A, Zmijewski MA, Pawelek J. L-Tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Slominski A, Zmijewski M, Semak I, et al. Cytochromes P450 and skin cancer: role of local endocrine pathways [published online ahead of print July 16, 2013]. Anticancer Agents Med Chem. doi:10.2174/18715206113139990308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Slominski AT, Kim TK, Zmijewski MA, et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013;5:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions [published online ahead of print February 19, 2013]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424 [DOI] [PubMed] [Google Scholar]
- 154. Elias PM, Williams ML, Feingold KR. Abnormal barrier function in the pathogenesis of ichthyosis: therapeutic implications for lipid metabolic disorders. Clin Dermatol. 2012;30:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chen WC, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol. 2009;1:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–1525 [DOI] [PubMed] [Google Scholar]
- 157. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869 [DOI] [PubMed] [Google Scholar]
- 159. Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Klein J, Permana PA, Owecki M, et al. What are subcutaneous adipocytes really good for? Exp Dermatol. 2007;16:45–70 [DOI] [PubMed] [Google Scholar]
- 161. Zmijewski MA, Slominski AT. Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinol. 2011;3:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–120 [DOI] [PubMed] [Google Scholar]
- 163. Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Schallreuter KU, Wood JM, Lemke R, et al. Production of catecholamines in the human epidermis. Biochem Biophys Res Commun. 1992;189:72–78 [DOI] [PubMed] [Google Scholar]
- 165. Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the β-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol. 2004;123:346–353 [DOI] [PubMed] [Google Scholar]
- 166. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12 [DOI] [PubMed] [Google Scholar]
- 167. Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–36 [DOI] [PubMed] [Google Scholar]
- 168. Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15:265–282 [DOI] [PubMed] [Google Scholar]
- 169. Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44:1–15 [DOI] [PubMed] [Google Scholar]
- 171. Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–730 [DOI] [PubMed] [Google Scholar]
- 172. Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24 [DOI] [PubMed] [Google Scholar]
- 173. Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020 [DOI] [PubMed] [Google Scholar]
- 175. Tobin DJ. Biochemistry of human skin—our brain on the outside. Chem Soc Rev. 2006;35:52–67 [DOI] [PubMed] [Google Scholar]
- 176. Slominski A. Beta-endorphin/μ-opiate receptor system in the skin. J Invest Dermatol. 2003;120:xii-xiii [DOI] [PubMed] [Google Scholar]
- 177. O'Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–153 [DOI] [PubMed] [Google Scholar]
- 178. Krause K, Schnitger A, Fimmel S, Glass E, Zouboulis CC. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39:166–170 [DOI] [PubMed] [Google Scholar]
- 179. Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898 [DOI] [PubMed] [Google Scholar]
- 180. Slominski A, Zjawiony J, Wortsman J, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Slominski A, Wortsman J, Kohn L, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381–4388 [DOI] [PubMed] [Google Scholar]
- 183. Gáspár E, Hardenbicker C, Bodó E, et al. Thyrotropin releasing hormone (TRH): a new player in human hair-growth control. FASEB J. 2010;24:393–403 [DOI] [PubMed] [Google Scholar]
- 184. Gaspar E, Nguyen-Thi KT, Hardenbicker C, et al. Thyrotropin-releasing hormone selectively stimulates human hair follicle pigmentation. J Invest Dermatol. 2011;131:2368–2377 [DOI] [PubMed] [Google Scholar]
- 185. Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424–430 [DOI] [PubMed] [Google Scholar]
- 186. Slominski AT, Zmijewski MA, Zbytek B, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131:613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Slominski AT, Zmijewski MA, Semak I, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Slominski A, Kim TK, Chen J, et al. 2012 Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Slominski AT, Kim TK, Shehabi HZ, et al. 2012 In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 192. Zmijewski MA, Li W, Zjawiony JK, et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3β, 17α, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20:7–13 [DOI] [PubMed] [Google Scholar]
- 194. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bodó E, Kany B, Gaspar E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633–1642 [DOI] [PubMed] [Google Scholar]
- 196. Schallreuter KU, Lemke KR, Pittelkow MR, Wood JM, Korner C, Malik R. Catecholamines in human keratinocyte differentiation. J Invest Dermatol. 1995;104:953–957 [DOI] [PubMed] [Google Scholar]
- 197. Grando SA. Biological functions of keratinocyte cholinergic receptors. J Investig Dermatol Symp Proc. 1997;2:41–48 [DOI] [PubMed] [Google Scholar]
- 198. Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions [published online ahead of print February 19, 2013]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Slominski A, Wortsman J, Linton E, Pisarchik A, Zbytek B. The skin as a model for the immunomodulatory effects of corticotropin-releasing hormone. In: Schaefer M, Stein C, eds. Mind Over Matter—Regulation of Peripheral Inflammation by the CNS. Basel, Boston, Berlin: Birkhaeuser Verlag; 2003:149–176 [Google Scholar]
- 200. Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160:229–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99 [DOI] [PubMed] [Google Scholar]
- 202. Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–116 [DOI] [PubMed] [Google Scholar]
- 203. Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–176 [DOI] [PubMed] [Google Scholar]
- 204. Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024 [DOI] [PubMed] [Google Scholar]
- 205. Funasaka Y, Sato H, Chakraborty AK, Ohashi A, Chrousos GP, Ichihashi M. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc. 1999;4:105–109 [DOI] [PubMed] [Google Scholar]
- 206. Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001;15:2297–2299 [DOI] [PubMed] [Google Scholar]
- 207. Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA. 2002;99:7148–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000;85:815–823 [DOI] [PubMed] [Google Scholar]
- 209. Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J Clin Endocrinol Metab. 2000;85:3582–3588 [DOI] [PubMed] [Google Scholar]
- 211. Slominski AT, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?.” Ann NY Acad Sci. 1999;885:287–311 [DOI] [PubMed] [Google Scholar]
- 212. Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122:235–237 [DOI] [PubMed] [Google Scholar]
- 213. Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J. 2006;20:882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kim JE, Cho DH, Kim HS, et al. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp Dermatol. 2007;16:104–109 [DOI] [PubMed] [Google Scholar]
- 216. Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345–352 [DOI] [PubMed] [Google Scholar]
- 217. Zhou C, Yu X, Cai D, Liu C, Li C. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med Hypotheses. 2009;73:513–515 [DOI] [PubMed] [Google Scholar]
- 218. Vasiadi M, Therianou A, Sideri K, et al. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2012;129:1410–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol. 2007;127:730–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Isard O, Knol AC, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Cutaneous induction of corticotropin releasing hormone by Propionibacterium acnes extracts. Dermatoendocrinol. 2009;1:96–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–E493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Roloff B, Fechner K, Slominski A, et al. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J. 1998;12:287–297 [DOI] [PubMed] [Google Scholar]
- 223. Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996;1289:247–251 [DOI] [PubMed] [Google Scholar]
- 224. Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–2457 [DOI] [PubMed] [Google Scholar]
- 225. Yamauchi N, Otagiri A, Nemoto T, et al. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol. 2005;17:656–663 [DOI] [PubMed] [Google Scholar]
- 226. Chae JI, Ju SK, Lee MK, et al. cDNA cloning and analysis of tissue-specific gene expression of rat urocortin II [in Russian]. Mol Biol (Mosk). 2009;43:91–96 [PubMed] [Google Scholar]
- 227. Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Huang M, Berry J, Kandere K, Lytinas M, Karalis K, Theoharides TC. Mast cell deficient W/W(v) mice lack stress-induced increase in serum IL-6 levels, as well as in peripheral CRH and vascular permeability, a model of rheumatoid arthritis. Int J Immunopathol Pharmacol. 2002;15:249–254 [DOI] [PubMed] [Google Scholar]
- 229. Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–231 [DOI] [PubMed] [Google Scholar]
- 230. Zhang X, Yu M, Yu W, Weinberg J, Shapiro J, McElwee KJ. Development of alopecia areata is associated with higher central and peripheral hypothalamic-pituitary-adrenal tone in the skin graft induced C3H/HeJ mouse model. J Invest Dermatol. 2009;129:1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Slominski AT, Roloff B, Zbytek B, et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim. 2000;36:211–216 [DOI] [PubMed] [Google Scholar]
- 232. Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–54 [DOI] [PubMed] [Google Scholar]
- 233. Asadi S, Alysandratos KD, Angelidou A, et al. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J Invest Dermatol. 2012;132:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Flint MS, Morgan JB, Shreve SN, Tinkle SS. Restraint stress and corticotropin releasing hormone modulation of murine cutaneous POMC mRNA. Stress. 2003;6:59–62 [DOI] [PubMed] [Google Scholar]
- 235. Kaneko K, Kawana S, Arai K, Shibasaki T. Corticotropin-releasing factor receptor type 1 is involved in the stress-induced exacerbation of chronic contact dermatitis in rats. Exp Dermatol. 2003;12:47–52 [DOI] [PubMed] [Google Scholar]
- 236. Donelan J, Boucher W, Papadopoulou N, et al. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rassouli O, Liapakis G, Lazaridis I, et al. A novel role of peripheral corticotropin-releasing hormone (CRH) on dermal fibroblasts. PLoS One. 2011;6:e21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wang L, Million M, Rivier J, et al. CRF receptor antagonist astressin-B reverses and prevents alopecia in CRF over-expressing mice. PLoS One. 2011;6:e16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Tagen M, Stiles L, Kalogeromitros D, et al. Skin corticotropin-releasing hormone receptor expression in psoriasis. J Invest Dermatol. 2007;127:1789–1791 [DOI] [PubMed] [Google Scholar]
- 240. Ito N, Sugawara K, Bodó E, et al. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol. 2010;130:995–1004 [DOI] [PubMed] [Google Scholar]
- 241. Donelan J, Marchand JE, Kempuraj D, Papadopoulou N, Papaliodis D, Theoharides TC. Perifollicular and perivascular mouse skin mast cells express corticotropin-releasing hormone receptor. J Invest Dermatol. 2006;126:929–932 [DOI] [PubMed] [Google Scholar]
- 242. Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–161 [DOI] [PubMed] [Google Scholar]
- 243. Liu SJ, Xie YF, Dai LB, Du GW. Expression of secretions of hypothalamus-pituitary-adrenal axis in human hypertrophic scar [in Chinese]. Zhonghua Shao Shang Za Zhi. 2011;27:432–435 [PubMed] [Google Scholar]
- 244. Cemil BC, Canpolat F, Yilmazer D, Eskioglu F, Alper M. The association of PASI scores with CRH-R1 expression in patients with psoriasis. Arch Dermatol Res. 2012;304:127–132 [DOI] [PubMed] [Google Scholar]
- 245. Elewa RM, Abdallah M, Youssef N, Zouboulis CC. Aging-related changes in cutaneous corticotropin-releasing hormone system reflect a defective neuroendocrine-stress response in aging. Rejuvenation Res. 2012;15:366–373 [DOI] [PubMed] [Google Scholar]
- 246. Alysandratos KD, Asadi S, Angelidou A, et al. Neurotensin and CRH interactions augment human mast cell activation. PLoS One. 2012;7:e48934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Pisarchik A, Wortsman J, Slominski A. A novel microarray to evaluate stress-related genes in skin: effect of ultraviolet light radiation. Gene. 2004;341:199–207 [DOI] [PubMed] [Google Scholar]
- 248. Ermak G, Slominski A. Production of POMC, CRH-R1, MC1, and MC2 receptor mRNA and expression of tyrosinase gene in relation to hair cycle and dexamethasone treatment in the C57BL/6 mouse skin. J Invest Dermatol. 1997;108:160–165 [DOI] [PubMed] [Google Scholar]
- 249. Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–126 [DOI] [PubMed] [Google Scholar]
- 250. Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–190 [DOI] [PubMed] [Google Scholar]
- 251. Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–1268 [DOI] [PubMed] [Google Scholar]
- 252. Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Mitsuma T, Matsumoto Y, Tomita Y. Corticotropin releasing hormone stimulates proliferation of keratinocytes. Life Sci. 2001;69:1991–1998 [DOI] [PubMed] [Google Scholar]
- 254. Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone inhibits nuclear factor-κB pathway in human HaCaT keratinocytes. J Invest Dermatol. 2003;121:1496–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Zbytek B, Pfeffer LM, Slominski AT. Corticotropin releasing hormone stimulates NF-κB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–R7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Zbytek B, Pikula M, Slominski RM, et al. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol. 2005;152:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-κB signaling in human melanocytes. Peptides. 2006;27:3276–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wei E, Slominski A, inventors; The Regents of the University Of California, Loyola University of Chicago, assignees. Inhibition of abnormal cell growth with corticotropin-releasing hormone analogs. US patent 6803359 B2 October 12, 2004
- 259. Zhou CL, Yu XJ, Chen LM, Jiang H, Li CY. Corticotropin-releasing hormone attenuates vascular endothelial growth factor release from human HaCaT keratinocytes. Regul Pept. 2010;160:115–120 [DOI] [PubMed] [Google Scholar]
- 260. Park HJ, Kim HJ, Lee JH, et al. Corticotropin-releasing hormone (CRH) downregulates interleukin-18 expression in human HaCaT keratinocytes by activation of p38 mitogen-activated protein kinase (MAPK) pathway. J Invest Dermatol. 2005;124:751–755 [DOI] [PubMed] [Google Scholar]
- 261. Zbytek B, Mysliwski A, Slominski A, Wortsman J, Wei ET, Mysliwska J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002;70:1013–1021 [DOI] [PubMed] [Google Scholar]
- 262. Zhao J, Karalis KP. Regulation of nuclear factor-κB by corticotropin-releasing hormone in mouse thymocytes. Mol Endocrinol. 2002;16:2561–2570 [DOI] [PubMed] [Google Scholar]
- 263. Ross PC, Kostas CM, Ramabhadran TV. A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun. 1994;205:1836–1842 [DOI] [PubMed] [Google Scholar]
- 264. Zmijewski MA, Slominski AT. Modulation of corticotropin releasing factor (CRF) signaling through receptor splicing in mouse pituitary cell line AtT-20–emerging role of soluble isoforms. J Physiol Pharmacol. 2009;60(suppl 4):39–46 [PMC free article] [PubMed] [Google Scholar]
- 265. Teli T, Markovic D, Hewitt ME, Levine MA, Hillhouse EW, Grammatopoulos DK. Structural domains determining signalling characteristics of the CRH-receptor type 1 variant R1β and response to PKC phosphorylation. Cell Signal. 2008;20:40–49 [DOI] [PubMed] [Google Scholar]
- 266. Yan G, Zhang G, Fang X, et al. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol. 2011;29:1019–1023 [DOI] [PubMed] [Google Scholar]
- 267. Kishore S, Khanna A, Zhang Z, et al. The snoRNA MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Hum Mol Genet. 2010;19:1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Liu Z, Zhu F, Wang G, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404:358–362 [DOI] [PubMed] [Google Scholar]
- 269. Papiol S, Arias B, Gastó C, Gutiérrez B, Catalán R, Fañanás L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90 [DOI] [PubMed] [Google Scholar]
- 270. Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ishitobi Y, Nakayama S, Yamaguchi K, et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:429–436 [DOI] [PubMed] [Google Scholar]
- 273. Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151 [DOI] [PubMed] [Google Scholar]
- 274. Schmid B, Blomeyer D, Treutlein J, et al. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714 [DOI] [PubMed] [Google Scholar]
- 275. Skipper L, Wilkes K, Toft M, et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet. 2004;75:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Licinio J, O'Kirwan F, Irizarry K, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004;9:1075–1082 [DOI] [PubMed] [Google Scholar]
- 277. Liu Z, Zhu F, Wang G, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414:155–158 [DOI] [PubMed] [Google Scholar]
- 278. Kamdem LK, Hamilton L, Cheng C, et al. Genetic predictors of glucocorticoid-induced hypertension in children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2008;18:507–514 [DOI] [PubMed] [Google Scholar]
- 279. Lima JJ, Blake KV, Tantisira KG, Weiss ST. Pharmacogenetics of asthma. Curr Opin Pulm Med. 2009;15:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Jones TS, Kaste SC, Liu W, et al. CRHR1 polymorphisms predict bone density in survivors of acute lymphoblastic leukemia. J Clin Oncol. 2008;26:3031–3037 [DOI] [PubMed] [Google Scholar]
- 281. ElSharawy A, Manaster C, Teuber M, et al. SNPSplicer: systematic analysis of SNP-dependent splicing in genotyped cDNAs. Hum Mutat. 2006;27:1129–1134 [DOI] [PubMed] [Google Scholar]
- 282. Hull J, Campino S, Rowlands K, et al. Identification of common genetic variation that modulates alternative splicing. PLoS Genet. 2007;3:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. ElSharawy A, Hundrieser B, Brosch M, et al. Systematic evaluation of the effect of common SNPs on pre-mRNA splicing. Hum Mutat. 2009;30:625–632 [DOI] [PubMed] [Google Scholar]
- 284. Amrani N, Sachs MS, Jacobson A. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol. 2006;7:415–425 [DOI] [PubMed] [Google Scholar]
- 285. Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–761 [DOI] [PubMed] [Google Scholar]
- 286. Tantisira KG, Lake S, Silverman ES, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–1359 [DOI] [PubMed] [Google Scholar]
- 287. Keck ME, Kern N, Erhardt A, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1196–1204 [DOI] [PubMed] [Google Scholar]
- 288. Tantisira KG, Lazarus R, Litonjua AA, Klanderman B, Weiss ST. Chromosome 17: association of a large inversion polymorphism with corticosteroid response in asthma. Pharmacogenet Genomics. 2008;18:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008;7:14–19 [DOI] [PubMed] [Google Scholar]
- 290. Cruchaga C, Vidal-Taboada JM, Ezquerra M, et al. 5′-Upstream variants of CRHR1 and MAPT genes associated with age at onset in progressive supranuclear palsy and cortical basal degeneration. Neurobiol Dis. 2009;33:164–170 [DOI] [PubMed] [Google Scholar]
- 291. Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wasserman D, Wasserman J, Rozanov V, Sokolowski M. Depression in suicidal males: genetic risk variants in the CRHR1 gene. Genes Brain Behav. 2009;8:72–79 [DOI] [PubMed] [Google Scholar]
- 293. Schmidt ED, Aguilera G, Binnekade R, Tilders FJ. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience. 2003;116:275–283 [DOI] [PubMed] [Google Scholar]
- 294. Markovic D, Vatish M, Gu M, et al. The onset of labor alters corticotropin-releasing hormone type 1 receptor variant expression in human myometrium: putative role of interleukin-1β. Endocrinology. 2007;148:3205–3213 [DOI] [PubMed] [Google Scholar]
- 295. Coste SC, Heldwein KA, Stevens SL, Tobar-Dupres E, Stenzel-Poore MP. IL-1α and TNFα down-regulate CRH receptor-2 mRNA expression in the mouse heart. Endocrinology. 2001;142:3537–3545 [DOI] [PubMed] [Google Scholar]
- 296. Aubry JM, Turnbull AV, Pozzoli G, Rivier C, Vale W. Endotoxin decreases corticotropin-releasing factor receptor 1 messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 1997;138:1621–1626 [DOI] [PubMed] [Google Scholar]
- 297. Parsadaniantz SM, Batsche E, Gegout-Pottie P, et al. Effects of continuous infusion of interleukin 1 β on corticotropin-releasing hormone (CRH), CRH receptors, proopiomelanocortin gene expression and secretion of corticotropin, β-endorphin and corticosterone. Neuroendocrinology. 1997;65:53–63 [DOI] [PubMed] [Google Scholar]
- 298. O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180 [DOI] [PubMed] [Google Scholar]
- 299. Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1–13 [DOI] [PubMed] [Google Scholar]
- 300. Hamke M, Herpfer I, Lieb K, Wandelt C, Fiebich BL. Substance P induces expression of the corticotropin-releasing factor receptor 1 by activation of the neurokinin-1 receptor. Brain Res. 2006;1102:135–144 [DOI] [PubMed] [Google Scholar]
- 301. Papadopoulou NG, Oleson L, Kempuraj D, Donelan J, Cetrulo CL, Theoharides TC. Regulation of corticotropin-releasing hormone receptor-2 expression in human cord blood-derived cultured mast cells. J Mol Endocrinol. 2005;35:R1–R8 [DOI] [PubMed] [Google Scholar]
- 302. Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–1718 [DOI] [PubMed] [Google Scholar]
- 303. Reich A, Wojcik-Maciejewicz A, Slominski AT. Stress and the skin. G Ital Dermatol Venereol. 2010;145:213–219 [PubMed] [Google Scholar]
- 304. Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78 [DOI] [PubMed] [Google Scholar]
- 306. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Chakraborty AK, Funasaka Y, Slominski A, et al. UV light and MSH receptors. Ann NY Acad Sci. 1999;885:100–116 [DOI] [PubMed] [Google Scholar]
- 308. Bolognia J, Murray M, Pawelek J. UVB-induced melanogenesis may be mediated through the MSH-receptor system. J Invest Dermatol. 1989;92:651–656 [DOI] [PubMed] [Google Scholar]
- 309. Pawelek JM, Chakraborty AK, Osber MP, et al. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5:348–356 [DOI] [PubMed] [Google Scholar]
- 310. Chakraborty A, Slominski A, Ermak G, Hwang J, Pawelek J. Ultraviolet B and melanocyte-stimulating hormone (MSH) stimulate mRNA production for α MSH receptors and proopiomelanocortin-derived peptides in mouse melanoma cells and transformed keratinocytes. J Invest Dermatol. 1995;105:655–659 [DOI] [PubMed] [Google Scholar]
- 311. Chakraborty AK, Funasaka Y, Slominski A, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138 [DOI] [PubMed] [Google Scholar]
- 312. Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16:503–515 [DOI] [PubMed] [Google Scholar]
- 313. Pawelek J, Bolognia J, McLane J, Murray M, Osber M, Slominski A. A possible role for melanin precursors in regulating both pigmentation and proliferation of melanocytes. Prog Clin Biol Res. 1988;256:143–154 [PubMed] [Google Scholar]
- 314. Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10 [DOI] [PubMed] [Google Scholar]
- 315. Funasaka Y, Chakraborty AK, Hayashi Y, et al. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1α, interleukin-1β, endothelin-1 and tumour necrosis factor-α. Br J Dermatol. 1998;139:216–224 [DOI] [PubMed] [Google Scholar]
- 316. Böhm M, Wolff I, Scholzen TE, et al. α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–5802 [DOI] [PubMed] [Google Scholar]
- 317. Böhm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126:1966–1975 [DOI] [PubMed] [Google Scholar]
- 318. Abdel-Malek ZA, Knittel J, Kadekaro AL, Swope VB, Starner R. The melanocortin 1 receptor and the UV response of human melanocytes—a shift in paradigm. Photochem Photobiol. 2008;84:501–508 [DOI] [PubMed] [Google Scholar]
- 319. Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186 [DOI] [PubMed] [Google Scholar]
- 320. Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334 [DOI] [PubMed] [Google Scholar]
- 321. Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102 [DOI] [PubMed] [Google Scholar]
- 322. Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–E706 [DOI] [PubMed] [Google Scholar]
- 323. Rousseau K, Kauser S, Pritchard LE, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Sharpley CF, Kauter KG, McFarlane JR. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: latency, localization and independence effects. Physiol Res. 2009;58:757–761 [DOI] [PubMed] [Google Scholar]
- 325. Sharpley CF, Kauter KG, McFarlane JR. Hair cortisol concentration differs across site and person: localization and consistency of responses to a brief pain stressor. Physiol Res. 2010;59:979–983 [DOI] [PubMed] [Google Scholar]
- 326. Sharpley CF, Kauter KG, McFarlane JR. An investigation of hair cortisol concentration across body sites and within hair shaft. Clin Med Insights Endocrinol Diabetes. 2010;3:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Kimura A, Kanazawa N, Li HJ, Yonei N, Yamamoto Y, Furukawa F. Influence of chemical peeling on the skin stress response system. Exp Dermatol. 2012;21(suppl 1):8–10 [DOI] [PubMed] [Google Scholar]
- 328. Arck P, Handjiski B, Hagen E, et al. Is there a ‘gut-brain-skin axis’? Exp Dermatol. 2010;19:401–405 [DOI] [PubMed] [Google Scholar]
- 329. Alexacos N, Pang X, Boucher W, Cochrane DE, Sant GR, Theoharides TC. Neurotensin mediates rat bladder mast cell degranulation triggered by acute psychological stress. Urology. 1999;53:1035–1040 [DOI] [PubMed] [Google Scholar]
- 330. Boucher W, Kempuraj D, Michaelian M, Theoharides TC. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010;106:1394–1399 [DOI] [PubMed] [Google Scholar]
- 331. Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Bonaz BL, Bernstein CN. 2012 Brain-gut interactions in inflammatory bowel diseases. Gastroenterology. 2013;144:36–49 [DOI] [PubMed] [Google Scholar]
- 333. Taché Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(suppl 1):137–142 [DOI] [PubMed] [Google Scholar]
- 334. Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood). 2010;235:1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Kresse AE, Million M, Saperas E, Taché Y. Colitis induces CRF expression in hypothalamic magnocellular neurons and blunts CRF gene response to stress in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1203–G1213 [DOI] [PubMed] [Google Scholar]
- 336. Wlk M, Wang CC, Venihaki M, et al. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology. 2002;123:505–515 [DOI] [PubMed] [Google Scholar]
- 337. Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277:G391–G399 [DOI] [PubMed] [Google Scholar]
- 338. Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol. 2003;553:959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Im E, Rhee SH, Park YS, Fiocchi C, Taché Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138:2457–2467.e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Jones HP. Immune cells listen to what stress is saying: neuroendocrine receptors orchestrate immune function. Methods Mol Biol. 2012;934:77–87 [DOI] [PubMed] [Google Scholar]
- 341. Theoharides TC, Enakuaa S, Sismanopoulos N, et al. Contribution of stress to asthma worsening through mast cell activation. Ann Allergy Asthma Immunol. 2012;109:14–19 [DOI] [PubMed] [Google Scholar]
- 342. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Katsarou-Katsari A, Filippou A, Theoharides TC. Effect of stress and other psychological factors on the pathophysiology and treatment of dermatoses. Int J Immunopathol Pharmacol. 1999;12:7–11 [PubMed] [Google Scholar]
- 344. Tsagarakis S, Grossman A. Corticotropin-releasing hormone: interactions with the immune system. Neuroimmunomodulation. 1994;1:329–334 [DOI] [PubMed] [Google Scholar]
- 345. Kalantaridou S, Makrigiannakis A, Zoumakis E, Chrousos GP. Peripheral corticotropin-releasing hormone is produced in the immune and reproductive systems: actions, potential roles and clinical implications. Front Biosci. 2007;12:572–580 [DOI] [PubMed] [Google Scholar]
- 346. Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568 [DOI] [PubMed] [Google Scholar]
- 347. Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–820 [DOI] [PubMed] [Google Scholar]
- 348. Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413 [DOI] [PubMed] [Google Scholar]
- 349. Singh LK, Boucher W, Pang X, et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356 [PubMed] [Google Scholar]
- 350. Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Wolfe CD, Patel SP, Linton EA, et al. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95:1003–1006 [DOI] [PubMed] [Google Scholar]
- 352. Jeske W, Soszynski P, Lukaszewicz E, et al. Enhancement of plasma corticotropin-releasing hormone in pregnancy-induced hypertension. Acta Endocrinol (Copenh). 1990;122:711–714 [DOI] [PubMed] [Google Scholar]
- 353. Warren WB, Patrick SL, Goland RS. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992;166:1198–1204; discussion 1204–1207 [DOI] [PubMed] [Google Scholar]
- 354. McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463 [DOI] [PubMed] [Google Scholar]
- 355. Leung TN, Chung TK, Madsen G, McLean M, Chang AM, Smith R. Elevated mid-trimester maternal corticotrophin-releasing hormone levels in pregnancies that delivered before 34 weeks. Br J Obstet Gynaecol. 1999;106:1041–1046 [DOI] [PubMed] [Google Scholar]
- 356. Vrachnis N, Malamas FM, Sifakis S, Tsikouras P, Iliodromiti Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clin Dev Immunol. 2012;2012:937618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52:267–275 [DOI] [PubMed] [Google Scholar]
- 358. Tariverdian N, Theoharides TC, Siedentopf F, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29:193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Overton JM, Fisher LA. Modulation of central nervous system actions of corticotropin-releasing factor by dynorphin-related peptides. Brain Res. 1989;488:233–240 [DOI] [PubMed] [Google Scholar]
- 360. Weisinger RS, Blair-West JR, Burns P, et al. Cardiovascular effects of long-term central and peripheral administration of urocortin, corticotropin-releasing factor, and adrenocorticotropin in sheep. Endocrinology. 2004;145:5598–5604 [DOI] [PubMed] [Google Scholar]
- 361. Abdelrahman AM, Lin Lim S, Pang CC. Influence of urocortin and corticotropin releasing factor on venous tone in conscious rats. Eur J Pharmacol. 2005;510:107–111 [DOI] [PubMed] [Google Scholar]
- 362. Grossini E, Molinari C, Mary DA, Marino P, Vacca G. The effect of urocortin II administration on the coronary circulation and cardiac function in the anaesthetized pig is nitric-oxide-dependent. Eur J Pharmacol. 2008;578:242–248 [DOI] [PubMed] [Google Scholar]
- 363. Grossini E, Molinari C, Mary DA, et al. Urocortin II induces nitric oxide production through cAMP and Ca2+ related pathways in endothelial cells. Cell Physiol Biochem. 2009;23:87–96 [DOI] [PubMed] [Google Scholar]
- 364. Overton JM, Fisher LA. Differentiated hemodynamic responses to central versus peripheral administration of corticotropin-releasing factor in conscious rats. J Auton Nerv Syst. 1991;35:43–51 [DOI] [PubMed] [Google Scholar]
- 365. Davis ME, Pemberton CJ, Yandle TG, et al. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28:2589–2597 [DOI] [PubMed] [Google Scholar]
- 366. Yang LZ, Kockskamper J, Heinzel FR, et al. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A. Cardiovasc Res. 2006;69:402–411 [DOI] [PubMed] [Google Scholar]
- 367. Calderon-Sanchez E, Delgado C, Ruiz-Hurtado G, et al. Urocortin induces positive inotropic effect in rat heart. Cardiovasc Res. 2009;83:717–725 [DOI] [PubMed] [Google Scholar]
- 368. Huang M, Pang X, Letourneau R, Boucher W, Theoharides TC. Acute stress induces cardiac mast cell activation and histamine release, effects that are increased in apolipoprotein E knockout mice. Cardiovasc Res. 2002;55:150–160 [DOI] [PubMed] [Google Scholar]
- 369. Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in apolipoprotein E knockout mice. Cardiovasc Res. 2003;59:241–249 [DOI] [PubMed] [Google Scholar]
- 370. Huang M, Kempuraj D, Papadopoulou N, et al. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-κB activation. J Mol Endocrinol. 2009;42:397–405 [DOI] [PubMed] [Google Scholar]
- 371. Deliargyris EN, Raymond RJ, Theoharides TC, Boucher WS, Tate DA, Dehmer GJ. Sites of interleukin-6 release in patients with acute coronary syndromes and in patients with congestive heart failure. Am J Cardiol. 2000;86:913–918 [DOI] [PubMed] [Google Scholar]
- 372. Theoharides TC, Sismanopoulos N, Delivanis DA, Zhang B, Hatziagelaki EE, Kalogeromitros D. Mast cells squeeze the heart and stretch the gird: their role in atherosclerosis and obesity. Trends Pharmacol Sci. 2011;32:534–542 [DOI] [PubMed] [Google Scholar]
- 373. Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–54 [DOI] [PubMed] [Google Scholar]
- 374. Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:89–106 [DOI] [PubMed] [Google Scholar]
- 375. Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–C6 [DOI] [PubMed] [Google Scholar]
- 376. Slominski A, Wortsman J, Mazurkiewicz JE, et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122:658–666 [PubMed] [Google Scholar]
- 377. Wintzen M, Gilchrest BA. Proopiomelanocortin, its derived peptides, and the skin. J Invest Dermatol. 1996;106:3–10 [DOI] [PubMed] [Google Scholar]
- 378. Nagahama M, Funasaka Y, Fernandez-Frez ML, et al. Immunoreactivity of α-melanocyte-stimulating hormone, adrenocorticotrophic hormone and β-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Br J Dermatol. 1998;138:981–985 [DOI] [PubMed] [Google Scholar]
- 379. Slominski A. Identification of β-endorphin, α-MSH and ACTH peptides in cultured human melanocytes, melanoma and squamous cell carcinoma cells by RP-HPLC. Exp Dermatol. 1998;7:213–216 [DOI] [PubMed] [Google Scholar]
- 380. Luger T, Paus R, Lipton J, Slominski A. Cutaneous Neuromodulation: the Proopiomelanocortin System. Ann NY Acad Sci. 1999;885:1–479 [DOI] [PubMed] [Google Scholar]
- 381. Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol. 1999;30:208–215 [DOI] [PubMed] [Google Scholar]
- 382. Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem. 2000;48:905–914 [DOI] [PubMed] [Google Scholar]
- 383. Peters EM, Tobin DJ, Seidah NG, Schallreuter KU. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol. 2000;114:430–437 [DOI] [PubMed] [Google Scholar]
- 384. Pritchard LE, White A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology. 2007;148:4201–4207 [DOI] [PubMed] [Google Scholar]
- 385. Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. β-Endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol. 2004;123:184–195 [DOI] [PubMed] [Google Scholar]
- 386. Tobin DJ, Kauser S. Hair melanocytes as neuro-endocrine sensors—pigments for our imagination. Mol Cell Endocrinol. 2005;243:1–11 [DOI] [PubMed] [Google Scholar]
- 387. Tobin DJ, Kauser S. β-Endorphin: the forgotten hair follicle melanotropin. J Investig Dermatol Symp Proc. 2005;10:212–216 [DOI] [PubMed] [Google Scholar]
- 388. Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Zeller S, Lazovich D, Forster J, Widome R. Do adolescent indoor tanners exhibit dependency? J Am Acad Dermatol. 2006;54:589–596 [DOI] [PubMed] [Google Scholar]
- 390. Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749 [DOI] [PubMed] [Google Scholar]
- 391. Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455:364–366 [DOI] [PubMed] [Google Scholar]
- 392. Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000;1474:1–4 [DOI] [PubMed] [Google Scholar]
- 393. Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78:77–81 [DOI] [PubMed] [Google Scholar]
- 394. Milewich L, Shaw CB, Sontheimer RD. Steroid metabolism by epidermal keratinocytes. Ann NY Acad Sci. 1988;548:66–89 [DOI] [PubMed] [Google Scholar]
- 395. Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Horm Res. 2000;54:218–229 [DOI] [PubMed] [Google Scholar]
- 396. Labrie F, Luu-The V, Labrie C, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182 [DOI] [PubMed] [Google Scholar]
- 397. Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006;126:1177–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–1505 [DOI] [PubMed] [Google Scholar]
- 399. Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–67 [DOI] [PubMed] [Google Scholar]
- 400. Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11β-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131:30–36 [DOI] [PubMed] [Google Scholar]
- 403. Zouboulis CC, Degitz K. Androgen action on human skin— from basic research to clinical significance. Exp Dermatol. 2004;13(suppl 4):5–10 [DOI] [PubMed] [Google Scholar]
- 404. Milewich L, Shaw CE, Doody KM, Rainey WE, Mason JI, Carr BR. 3β-Hydroxysteroid dehydrogenase activity in glandular and extraglandular human fetal tissues. J Clin Endocrinol Metab. 1991;73:1134–1140 [DOI] [PubMed] [Google Scholar]
- 405. Simard J, Couet J, Durocher F, et al. Structure and tissue-specific expression of a novel member of the rat 3 β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) family. The exclusive 3β-HSD gene expression in the skin. J Biol Chem. 1993;268:19659–19668 [PubMed] [Google Scholar]
- 406. Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366 [DOI] [PubMed] [Google Scholar]
- 407. Zmijewski MA, Li W, Zjawiony JK, et al. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Zmijewski MA, Li W, Chen J, et al. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Slominski AT, Kim TK, Janjetovic Z, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–C541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Slominski AT, Li W, Bhattacharya SK, et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Slominski AT, Janjetovic Z, Fuller BE, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Zbytek B, Janjetovic Z, Tuckey RC, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Janjetovic Z, Zmijewski MA, Tuckey RC, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκB α levels in human keratinocytes. PLoS One. 2009;4:e5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Wang J, Slominski A, Tuckey RC, et al. 20-Hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746 [PMC free article] [PubMed] [Google Scholar]
- 416. Slominski AT, Janjetovic Z, Kim TK, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742 [PMC free article] [PubMed] [Google Scholar]
- 417. Janjetovic Z, Brozyna AA, Tuckey RC, et al. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Slominski A, Janjetovic Z, Tuckey RC, et al. 20S-Hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–E303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Slominski A. POMC gene expression in mouse and hamster melanoma cells. FEBS Lett. 1991;291:165–168 [DOI] [PubMed] [Google Scholar]
- 420. Schauer E, Trautinger F, Kock A, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Dumont M, Luu-The V, Dupont E, Pelletier G, Labrie F. Characterization, expression, and immunohistochemical localization of 3 β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in human skin. J Invest Dermatol. 1992;99:415–421 [DOI] [PubMed] [Google Scholar]
- 422. Moustafa M, Szabo M, Ghanem GE, et al. Inhibition of tumor necrosis factor-α stimulated NFκB/p65 in human keratinocytes by α-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol. 2002;119:1244–1253 [DOI] [PubMed] [Google Scholar]
- 423. Park HJ, Kim HJ, Lee JY, Cho BK, Gallo RL, Cho DH. Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J Invest Dermatol. 2007;127:1210–1216 [DOI] [PubMed] [Google Scholar]
- 424. Sato H, Nagashima Y, Chrousos GP, Ichihashi M, Funasak Y. The expression of corticotropin-releasing hormone in melanoma. Pigment Cell Res. 2002;15:98–103 [DOI] [PubMed] [Google Scholar]
- 425. Sharpley CF, McFarlane JR, Slominski A. Stress-linked cortisol concentrations in hair: what we know and what we need to know. Rev Neurosci. 2011;23:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Crofford LJ, Sano H, Karalis K, et al. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151:1587–1596 [PubMed] [Google Scholar]
- 427. Malysheva O, Wagner U, Wahle M, Wagner U, Stalla GK, Baerwald CG. Corticotropin releasing hormone (CRH) response in patients with early rheumatoid arthritis due to polymorphisms in the CRH gene. Clin Exp Rheumatol. 2012;30:421–423 [PubMed] [Google Scholar]
- 428. Oh SH, Park CO, Wu WH, et al. Corticotropin-releasing hormone downregulates IL-10 production by adaptive forkhead box protein 3-negative regulatory T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:151–159.e1–e6 [DOI] [PubMed] [Google Scholar]
- 429. Blalock JE. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989;69:1–32 [DOI] [PubMed] [Google Scholar]
- 430. Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511 [DOI] [PubMed] [Google Scholar]
- 431. Tatro JB. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996;3:259–284 [DOI] [PubMed] [Google Scholar]
- 432. Brzoska T, Luger TA, Maaser C, Abels C, Böhm M. α-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602 [DOI] [PubMed] [Google Scholar]
- 433. Mountjoy KG. Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochem J. 2010;428:305–324 [DOI] [PubMed] [Google Scholar]
- 434. Böhm M, Grässel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr Rev. 2012;33:623–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Tzioufas AG, Tsonis J, Moutsopoulos HM. Neuroendocrine dysfunction in Sjogren's syndrome. Neuroimmunomodulation. 2008;15:37–45 [DOI] [PubMed] [Google Scholar]
- 436. Wei ET, Gao GC, Thomas HA. Peripheral anti-inflammatory actions of corticotropin-releasing factor. Ciba Found Symp. 1993;172:258–268; discussion 268–276 [DOI] [PubMed] [Google Scholar]
- 437. Inada Y, Ikeda K, Tojo K, Sakamoto M, Takada Y, Tajima N. Possible involvement of corticotropin-releasing factor receptor signaling on vascular inflammation. Peptides. 2009;30:365–372 [DOI] [PubMed] [Google Scholar]
- 438. Zhu H, Wang J, Li J, Li S. Corticotropin-releasing factor family and its receptors: pro-inflammatory or anti-inflammatory targets in the periphery? Inflamm Res. 2011;60:715–721 [DOI] [PubMed] [Google Scholar]
- 439. Kaprara A, Pazaitou-Panayiotou K, Kortsaris A, Chatzaki E. The corticotropin releasing factor system in cancer: expression and pathophysiological implications. Cell Mol Life Sci. 2010;67:1293–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H. The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast Cancer Res Treat. 2001;70:47–54 [DOI] [PubMed] [Google Scholar]
- 442. Locke JA, Fazli L, Adomat H, et al. A novel communication role for CYP17A1 in the progression of castration-resistant prostate cancer. Prostate. 2009;69:928–937 [DOI] [PubMed] [Google Scholar]
- 443. Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr Relat Cancer. 2011;18:R175–R182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta. 2011;1812:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Sidler D, Renzulli P, Schnoz C, et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–2419 [DOI] [PubMed] [Google Scholar]
- 447. Bennett NC, Hooper JD, Lambie D, et al. Evidence for steroidogenic potential in human prostate cell lines and tissues. Am J Pathol. 2012;181:1078–1087 [DOI] [PubMed] [Google Scholar]
- 448. Courtney KD, Taplin ME. The evolving paradigm of second-line hormonal therapy options for castration-resistant prostate cancer. Curr Opin Oncol. 2012;24:272–277 [DOI] [PubMed] [Google Scholar]
- 449. Levina E, Chen M, Carkner R, Shtutman M, Buttyan R. Paracrine Hedgehog increases the steroidogenic potential of prostate stromal cells in a Gli-dependent manner. Prostate. 2012;72:817–824 [DOI] [PubMed] [Google Scholar]
- 450. Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7:e30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Watzka M, Bidlingmaier F, Schramm J, Klingmuller D, Stoffel-Wagner B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J Neuroendocrinol. 1999;11:901–905 [DOI] [PubMed] [Google Scholar]
- 452. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Noti M, Corazza N, Tuffin G, Schoonjans K, Brunner T. Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFα-dependent manner. FASEB J. 2010;24:1340–1346 [DOI] [PubMed] [Google Scholar]
- 454. Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Holtzmann H, Altmeyer P, Schultz-Amling W. Der einfluss ultravioletter strtahlen auf die hypothalamus-hypophysenachse des menschen. Acta Dermatol. 1982;8:119–123 [Google Scholar]
- 456. Holtzmann H, Altmeyer P, Stohr L, Chilf GN. Die beemfussung des α-MSH durch UVA-bestrahlunger der hautein funktionstest. Hautarzt. 1983;34:294–297 [PubMed] [Google Scholar]
- 457. Levins PC, Carr DB, Fisher JE, Momtaz K, Parrish JA. Plasma β-endorphin and β-lipoprotein response to ultraviolet radiation. Lancet. 1983;2:166. [DOI] [PubMed] [Google Scholar]
- 458. Belon PE. UVA exposure and pituitary secretion. Variations of human lipotropin concentrations (β LPH) after UVA exposure. Photochem Photobiol. 1985;42:327–329 [DOI] [PubMed] [Google Scholar]
- 459. Paz ML, Ferrari A, Weill FS, Leoni J, Maglio DH. Time-course evaluation and treatment of skin inflammatory immune response after ultraviolet B irradiation. Cytokine. 2008;44:70–77 [DOI] [PubMed] [Google Scholar]
- 460. Bernard JJ, Cowing-Zitron C, Nakatsuji T, et al. 2012 Ultraviolet radiation damages self noncoding RNA and is detected by TLR3 [published online ahead of print July 8, 2012]. Nat Med. doi:10.1038/nm.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Urbanski A, Schwarz T, Neuner P, et al. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990;94:808–811 [DOI] [PubMed] [Google Scholar]
- 462. Kripke ML. Ultraviolet radiation and immunology: something new under the sun—presidential address. Cancer Res. 1994;54:6102–6105 [PubMed] [Google Scholar]
- 463. Beissert S, Schwarz T. Mechanisms involved in ultraviolet light-induced immunosuppression. J Investig Dermatol Symp Proc. 1999;4:61–64 [DOI] [PubMed] [Google Scholar]
- 464. Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–141 [DOI] [PubMed] [Google Scholar]
- 465. Bornstein SR, Zacharowski P, Schumann RR, et al. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc Natl Acad Sci USA. 2004;101:16695–16700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Zacharowski K, Zacharowski PA, Koch A, et al. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci USA. 2006;103:6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Tran N, Koch A, Berkels R, et al. Toll-like receptor 9 expression in murine and human adrenal glands and possible implications during inflammation. J Clin Endocrinol Metab. 2007;92:2773–2783 [DOI] [PubMed] [Google Scholar]
- 468. Kanczkowski W, Zacharowski K, Wirth MP, Ehrhart-Bornstein M, Bornstein SR. Differential expression and action of Toll-like receptors in human adrenocortical cells. Mol Cell Endocrinol. 2009;300:57–65 [DOI] [PubMed] [Google Scholar]
- 469. Kanczkowski W, Zacharowski K, Bornstein SR. Role of toll-like receptors and inflammation in adrenal gland insufficiency. Neuroimmunomodulation. 2010;17:180–183 [DOI] [PubMed] [Google Scholar]
- 470. Manna PR, Chandrala SP, Jo Y, Stocco DM. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol. 2006;37:81–95 [DOI] [PubMed] [Google Scholar]
- 471. Deak T. Immune cells and cytokine circuits: toward a working model for understanding direct immune-to-adrenal communication pathways. Endocrinology. 2008;149:1433–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Engstrom L, Rosen K, Angel A, et al. Systemic immune challenge activates an intrinsically regulated local inflammatory circuit in the adrenal gland. Endocrinology. 2008;149:1436–1450 [DOI] [PubMed] [Google Scholar]
- 473. Mikhaylova IV, Jääskeläinen T, Jääskeläinen J, Palvimo JJ, Voutilainen R. Leukemia inhibitory factor as a regulator of steroidogenesis in human NCI-H295R adrenocortical cells. J Endocrinol. 2008;199:435–444 [DOI] [PubMed] [Google Scholar]
- 474. Martinez Calejman C, Astort F, Di Gruccio JM, et al. Lipopolysaccharide stimulates adrenal steroidogenesis in rodent cells by a NFκB-dependent mechanism involving COX-2 activation. Mol Cell Endocrinol. 2011;337:1–6 [DOI] [PubMed] [Google Scholar]
- 475. Tkachenko IV, Jaaskelainen T, Jaaskelainen J, Palvimo JJ, Voutilainen R. Interleukins 1α and 1β as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids. 2011;76:1103–1115 [DOI] [PubMed] [Google Scholar]
- 476. Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA. 2010;107:6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Nolan BV, Taylor SL, Liguori A, Feldman SR. Tanning as an addictive behavior: a literature review. Photodermatol Photoimmunol Photomed. 2009;25:12–19 [DOI] [PubMed] [Google Scholar]
- 478. Kourosh AS, Harrington CR, Adinoff B. Tanning as a behavioral addiction. Am J Drug Alcohol Abuse. 2010;36:284–290 [DOI] [PubMed] [Google Scholar]
- 479. Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423 [DOI] [PubMed] [Google Scholar]
- 480. Moroz MA, Huang R, Kochetkov T, et al. Comparison of corticotropin-releasing factor, dexamethasone, and temozolomide: treatment efficacy and toxicity in U87 and C6 intracranial gliomas. Clin Cancer Res. 2011;17:3282–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481. Graziani G, Tentori L, Muzi A, et al. Evidence that corticotropin-releasing hormone inhibits cell growth of human breast cancer cells via the activation of CRH-R1 receptor subtype. Mol Cell Endocrinol. 2007;264:44–49 [DOI] [PubMed] [Google Scholar]
- 482. Graziani G, Tentori L, Portarena I, et al. CRH inhibits cell growth of human endometrial adenocarcinoma cells via CRH-receptor 1-mediated activation of cAMP-PKA pathway. Endocrinology. 2002;143:807–813 [DOI] [PubMed] [Google Scholar]
- 483. Carlson KW, Nawy SS, Wei ET, et al. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21:1173–1179 [PubMed] [Google Scholar]
- 484. Choi EH, Demerjian M, Crumrine D, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1657–1662 [DOI] [PubMed] [Google Scholar]
- 485. Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224 [DOI] [PubMed] [Google Scholar]
- 486. Catania A, Colombo G, Rossi C, et al. Antimicrobial properties of α-MSH and related synthetic melanocortins. ScientificWorldJournal. 2006;6:1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Keita AV, Soderholm JD, Ericson AC. Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol Motil. 2010;22:770–778:e221–e222 [DOI] [PubMed] [Google Scholar]
- 488. Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58 [DOI] [PubMed] [Google Scholar]
- 489. Theoharides TC, Spanos C, Pang X, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750 [DOI] [PubMed] [Google Scholar]
- 490. Esposito P, Chandler N, Kandere K, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066 [DOI] [PubMed] [Google Scholar]
- 491. Theoharides TC, Rozniecki JJ, Sahagian G, et al. Impact of stress and mast cells on brain metastases. J Neuroimmunol. 2008;205:1–7 [DOI] [PubMed] [Google Scholar]
- 492. Tobin DJ. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:75–88 [DOI] [PubMed] [Google Scholar]
- 493. Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology. 2005;146:532–543 [DOI] [PubMed] [Google Scholar]
- 494. Lerner AB, McGuire JS. Effect of α- and βmelanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179 [DOI] [PubMed] [Google Scholar]
- 495. Levine N, Sheftel SN, Eytan T, et al. Induction of skin tanning by subcutaneous administration of a potent synthetic melanotropin. JAMA. 1991;266:2730–2736 [PubMed] [Google Scholar]
- 496. Hunt G, Donatien PD, Lunec J, Todd C, Kyne S, Thody AJ. Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Res. 1994;7:217–221 [DOI] [PubMed] [Google Scholar]
- 497. Hunt G, Todd C, Cresswell JE, Thody AJ. α-Melanocyte stimulating hormone and its analogue Nle4DPhe7 α-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–211 [DOI] [PubMed] [Google Scholar]
- 498. Hunt G, Todd C, Kyne S, Thody AJ. ACTH stimulates melanogenesis in cultured human melanocytes. J Endocrinol. 1994;140:R1–R3 [DOI] [PubMed] [Google Scholar]
- 499. Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633 [DOI] [PubMed] [Google Scholar]
- 500. Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res. 1997;10:288–297 [DOI] [PubMed] [Google Scholar]
- 501. Tsatmalia M, Wakamatsu K, Graham AJ, Thody AJ. Skin POMC peptides. Their binding affinities and activation of the human MC1 receptor. Ann NY Acad Sci. 1999;885:466–469 [DOI] [PubMed] [Google Scholar]
- 502. Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem. 2002;50:125–133 [DOI] [PubMed] [Google Scholar]
- 503. Kauser S, Westgate GE, Green MR, Tobin DJ. Human hair follicle and epidermal melanocytes exhibit striking differences in their aging profile which involves catalase. J Invest Dermatol. 2011;131:979–982 [DOI] [PubMed] [Google Scholar]
- 504. Tobin DJ. Ex vivo organ culture of human hair follicles: a model epithelial-neuroectodermal-mesenchymal interaction system. Methods Mol Biol. 2011;695:213–227 [DOI] [PubMed] [Google Scholar]
- 505. Slominski A, Costantino R, Wortsman J, Paus R, Ling N. Melanotropic activity of γ MSH peptides in melanoma cells. Life Sci. 1992;50:1103–1108 [DOI] [PubMed] [Google Scholar]
- 506. Slominski A, Plonka PM, Pisarchik A, et al. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Kadekaro AL, Kavanagh R, Kanto H, et al. α-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299 [DOI] [PubMed] [Google Scholar]
- 509. Watanuki Y, Takayasu S, Kageyama K, et al. 2013 Involvement of Nurr-1/Nur77 in corticotropin-releasing factor/urocortin1-induced tyrosinase-related protein 1 gene transcription in human melanoma HMV-II cells. Mol Cell Endocrinol. 2013;370:42–51 [DOI] [PubMed] [Google Scholar]
- 510. Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9:304–310 [DOI] [PubMed] [Google Scholar]
- 511. Nordlund JJ, Ortonne JP. The normal color of human skin. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, eds. The Pigmentary System: Physiology and Pathophysiology. Oxford, UK: Oxford University Press; 1998:475–587 [Google Scholar]
- 512. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S–97S [DOI] [PubMed] [Google Scholar]
- 513. Shafton AD, Oldfield BJ, McAllen RM. CRF-like immunoreactivity selectively labels preganglionic sudomotor neurons in cat. Brain Res. 1992;599:253–260 [DOI] [PubMed] [Google Scholar]
- 514. Janson DG, Saintigny G, van Adrichem A, Mahe C, El Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol. 2012;132:2565–2572 [DOI] [PubMed] [Google Scholar]
- 515. Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904 [DOI] [PubMed] [Google Scholar]
- 516. Collins CA, Jensen KB, MacRae EJ, Mansfield W, Watt FM. Polyclonal origin and hair induction ability of dermal papillae in neonatal and adult mouse back skin. Dev Biol. 2012;366:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Böhm M, Luger TA. Melanocortins in fibroblast biology—current update and future perspective for dermatology. Exp Dermatol. 2004;13(suppl 4):16–21 [DOI] [PubMed] [Google Scholar]
- 518. Böhm M, Raghunath M, Sunderkötter C, et al. Collagen metabolism is a novel target of the neuropeptide α-melanocyte-stimulating hormone. J Biol Chem. 2004;279:6959–6966 [DOI] [PubMed] [Google Scholar]
- 519. Huq S, Sharpe DT, Asaad K, Stevenson S, Tobin DJ. The hair follicle contains fibroblast subpopulations that exhibit preferential wound-healing characteristics, which can be modulated by proopiomelanocortin peptides and corticotropin-releasing factor. J Invest Dermatol. 2008;128:S45 [Google Scholar]
- 520. Dubicke A, Akerud A, Sennstrom M, et al. Different secretion patterns of matrix metalloproteinases and IL-8 and effect of corticotropin-releasing hormone in preterm and term cervical fibroblasts. Mol Hum Reprod. 2008;14:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Slominski AT. Proopiomelanocortin signaling system is operating in mast cells. J Invest Dermatol. 2006;126:1934–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Kawayama T, Okamoto M, Imaoka H, Kato S, Young HA, Hoshino T. Interleukin-18 in Pulmonary Inflammatory Diseases. J Interferon Cytokine Res. 2012;32:443–449 [DOI] [PubMed] [Google Scholar]
- 523. Zbytek B, Slominski AT. Role of corticotropin-releasing hormone and its receptor in the development of dermatofibroma [conference abstract]. J Cutan Pathol. 2012;39:101 [Google Scholar]
- 524. Selye H. The Mast Cells. Washington, DC: Butterworths; 1965 [Google Scholar]
- 525. Miniati A, Weng Z, Zhang B, Stratigos AJ, Nicolaidou E, Theoharides TC. Neuro-immuno-endocrine processes in vitiligo pathogenesis. Int J Immunopathol Pharmacol. 2012;25:1–7 [DOI] [PubMed] [Google Scholar]
- 526. van den Wijngaard RM, Stanisor OI, van Diest SA, et al. Peripheral α-helical CRF (9–41) does not reverse stress-induced mast cell dependent visceral hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2012;24:274–282.e111 [DOI] [PubMed] [Google Scholar]
- 527. Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239 [DOI] [PubMed] [Google Scholar]
- 528. Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432 [DOI] [PubMed] [Google Scholar]
- 529. Zhang B, Alysandratos KD, Angelidou A, et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: relevance to atopic dermatitis. J Allergy Clin Immunol. 2011;127:1522–1531.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Asadi S, Theoharides TC. Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J Neuroinflammation. 2012;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS One. 2012;7:e49767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Radulovic M, Dautzenberg FM, Sydow S, Radulovic J, Spiess J. Corticotropin-releasing factor receptor 1 in mouse spleen: expression after immune stimulation and identification of receptor-bearing cells. J Immunol. 1999;162:3013–3021 [PubMed] [Google Scholar]
- 533. Venihaki M, Zhao J, Karalis KP. Corticotropin-releasing hormone deficiency results in impaired splenocyte response to lipopolysaccharide. J Neuroimmunol. 2003;141:3–9 [DOI] [PubMed] [Google Scholar]
- 534. Kiang JG, Poree L, Wei ET. Anti-inflammatory activity of corticotropin releasing factor. II. Mechanisms of action. Proc West Pharmacol Soc. 1987;30:63–65 [PubMed] [Google Scholar]
- 535. Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Silverman ES, Breault DT, Vallone J, et al. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:747–754 [DOI] [PubMed] [Google Scholar]
- 537. Shams K, Burden AD. Updates from the Sixth International Congress “Psoriasis: from Gene to Clinic,” the Queen Elizabeth II Conference Centre, London, U.K., 1–3 December 2011 Br J Dermatol. 2012;167:757–761 [DOI] [PubMed] [Google Scholar]
- 538. Tokura Y, Mori T, Hino R. Psoriasis and other Th17-mediated skin diseases. J UOEH. 2010;32:317–328 [DOI] [PubMed] [Google Scholar]
- 539. Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22:160–168 [DOI] [PubMed] [Google Scholar]
- 540. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18 [DOI] [PubMed] [Google Scholar]
- 541. Vasiadi M, Therianou A, Alysandratos KD, et al. Serum neurotensin (NT) is increased in psoriasis and NT induces vascular endothelial growth factor release from human mast cells. Br J Dermatol. 2012;166:1349–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Cochrane DE, Carraway RE, Harrington K, Laudano M, Rawlings S, Feldberg RS. HMC-1 human mast cells synthesize neurotensin (NT) precursor, secrete bioactive NT-like peptide(s) and express NT receptor NTS1. Inflamm Res. 2011;60:1139–1151 [DOI] [PubMed] [Google Scholar]
- 543. Harvima IT, Nilsson G. Stress, the neuroendocrine system and mast cells: current understanding of their role in psoriasis. Expert Rev Clin Immunol. 2012;8:235–241 [DOI] [PubMed] [Google Scholar]
- 544. Nishioka T, Kurokawa H, Takao T, Kumon Y, Nishiya K, Hashimoto K. Differential changes of corticotropin releasing hormone (CRH) concentrations in plasma and synovial fluids of patients with rheumatoid arthritis (RA). Endocr J. 1996;43:241–247 [DOI] [PubMed] [Google Scholar]
- 545. Murphy EP, McEvoy A, Conneely OM, Bresnihan B, FitzGerald O. Involvement of the nuclear orphan receptor NURR1 in the regulation of corticotropin-releasing hormone expression and actions in human inflammatory arthritis. Arthritis Rheum. 2001;44:782–793 [DOI] [PubMed] [Google Scholar]
- 546. McEvoy AN, Bresnihan B, Fitzgerald O, Murphy EP. Corticotropin-releasing hormone signaling in synovial tissue vascular endothelium is mediated through the cAMP/CREB pathway. Ann NY Acad Sci. 2002;966:119–130 [DOI] [PubMed] [Google Scholar]
- 547. McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Corticotropin-releasing hormone signaling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1 α corticotropin-releasing hormone receptor. Arthritis Rheum. 2001;44:1761–1767 [DOI] [PubMed] [Google Scholar]
- 548. McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Cyclooxygenase 2-derived prostaglandin E2 production by corticotropin-releasing hormone contributes to the activated cAMP response element binding protein content in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2004;50:1132–1145 [DOI] [PubMed] [Google Scholar]
- 549. Baerwald CG, Panayi GS, Lanchbury JS. Corticotropin releasing hormone promoter region polymorphisms in rheumatoid arthritis. J Rheumatol. 1997;24:215–216 [PubMed] [Google Scholar]
- 550. Baerwald CG, Mok CC, Tickly M, et al. Corticotropin releasing hormone (CRH) promoter polymorphisms in various ethnic groups of patients with rheumatoid arthritis. Z Rheumatol. 2000;59:29–34 [DOI] [PubMed] [Google Scholar]
- 551. Gonzalez-Gay MA, Hajeer AH, Garcia-Porrua C, et al. Corticotropin-releasing hormone promoter polymorphisms in patients with rheumatoid arthritis from northwest Spain. J Rheumatol. 2003;30:913–917 [PubMed] [Google Scholar]
- 552. Wagner U, Wahle M, Malysheva O, Wagner U, Hantzschel H, Baerwald C. Sequence variants of the CRH 5′-flanking region: effects on DNA-protein interactions studied by EMSA in PC12 cells. Ann NY Acad Sci. 2006;1069:20–33 [DOI] [PubMed] [Google Scholar]
- 553. Fife MS, Fisher SA, John S, et al. Multipoint linkage analysis of a candidate gene locus in rheumatoid arthritis demonstrates significant evidence of linkage and association with the corticotropin-releasing hormone genomic region. Arthritis Rheum. 2000;43:1673–1678 [DOI] [PubMed] [Google Scholar]
- 554. Fife M, Steer S, Fisher S, et al. Association of familial and sporadic rheumatoid arthritis with a single corticotropin-releasing hormone genomic region (8q12.3) haplotype. Arthritis Rheum. 2002;46:75–82 [DOI] [PubMed] [Google Scholar]
- 555. Schallreuter KU, Bahadoran P, Picardo M, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–140; discussion 141–160 [DOI] [PubMed] [Google Scholar]
- 556. Kruger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–1212 [DOI] [PubMed] [Google Scholar]
- 557. Reimann E, Kingo K, Karelson M, et al. The mRNA expression profile of cytokines connected to the regulation of melanocyte functioning in vitiligo skin biopsy samples and peripheral blood mononuclear cells. Hum Immunol. 2012;73:393–398 [DOI] [PubMed] [Google Scholar]
- 558. Yu R, Huang Y, Zhang X, Zhou Y. Potential role of neurogenic inflammatory factors in the pathogenesis of vitiligo. J Cutan Med Surg. 2012;16:230–244 [DOI] [PubMed] [Google Scholar]
- 559. Graham A, Westerhof W, Thody AJ. The expression of α-MSH by melanocytes is reduced in vitiligo. Ann NY Acad Sci. 1999;885:470–473 [DOI] [PubMed] [Google Scholar]
- 560. Pichler R, Sfetsos K, Badics B, Gutenbrunner S, Aubock J. Vitiligo patients present lower plasma levels of α-melanotropin immunoreactivities. Neuropeptides. 2006;40:177–183 [DOI] [PubMed] [Google Scholar]
- 561. Kingo K, Aunin E, Karelson M, et al. Gene expression analysis of melanocortin system in vitiligo. J Dermatol Sci. 2007;48:113–122 [DOI] [PubMed] [Google Scholar]
- 562. Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Spencer JD, Gibbons NC, Böhm M, Schallreuter KU. The Ca2+-binding capacity of epidermal furin is disrupted by H2O2-mediated oxidation in vitiligo. Endocrinology. 2008;149:1638–1645 [DOI] [PubMed] [Google Scholar]
- 564. Spencer JD, Gibbons NC, Rokos H, Peters EM, Wood JM, Schallreuter KU. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J Invest Dermatol. 2007;127:411–420 [DOI] [PubMed] [Google Scholar]
- 565. Zouboulis CC, Eady A, Philpott M, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–152 [DOI] [PubMed] [Google Scholar]
- 566. Lotti T, Bianchi B, Panconesi E. Neuropeptides and skin disorders. The new frontiers of neuro-endocrine-cutaneous immunology. Int J Dermatol. 1999;38:673–675 [DOI] [PubMed] [Google Scholar]
- 567. Zouboulis CC, Böhm M. Neuroendocrine regulation of sebocytes—a pathogenetic link between stress and acne. Exp Dermatol. 2004;13(suppl 4):31–35 [DOI] [PubMed] [Google Scholar]
- 568. Aoki E, Shibasaki T, Kawana S. Intermittent foot shock stress prolongs the telogen stage in the hair cycle of mice. Exp Dermatol. 2003;12:371–377 [DOI] [PubMed] [Google Scholar]
- 569. Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Katayama M, Aoki E, Suzuki H, Kawana S. Foot shock stress prolongs the telogen stage of the spontaneous hair cycle in a non-depilated mouse model. Exp Dermatol. 2007;16:553–560 [DOI] [PubMed] [Google Scholar]
- 571. Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386 [DOI] [PubMed] [Google Scholar]
- 572. Reinhold M. Relationship of stress to the development of symptoms in alopecia areata and chronic urticaria. Br Med J. 1960;1:846–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Whitlock FA. Psychophysiological aspects of skin disease. In: Rook A, ed. Major Problems in Dermatology. London, UK: WB Saunders; 1976:110–210 [Google Scholar]
- 574. Misery L, Rousset H. Is alopecia areata a psychosomatic disease? [in French]. Rev Med Interne. 2001;22:274–279 [DOI] [PubMed] [Google Scholar]
- 575. Willemsen R, Vanderlinden J, Roseeuw D, Haentjens P. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388–393 [DOI] [PubMed] [Google Scholar]
- 576. van der Steen P, Boezeman J, Duller P, Happle R. Can alopecia areata be triggered by emotional stress? An uncontrolled evaluation of 178 patients with extensive hair loss. Acta Derm Venereol. 1992;72:279–280 [PubMed] [Google Scholar]
- 577. Brajac I, Tkalcic M, Dragojevic DM, Gruber F. Roles of stress, stress perception and trait-anxiety in the onset and course of alopecia areata. J Dermatol. 2003;30:871–878 [DOI] [PubMed] [Google Scholar]
- 578. Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol. 2009;129:1324–1326 [DOI] [PubMed] [Google Scholar]
- 579. Hordinsky MK, Ericson ME. Relationship between follicular nerve supply and alopecia. Dermatol Clin. 1996;14:651–660 [DOI] [PubMed] [Google Scholar]
- 580. Kim HS, Cho DH, Kim HJ, Lee JY, Cho BK, Park HJ. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and α-melanocyte-stimulating hormone in alopecia areata. Exp Dermatol. 2006;15:515–522 [DOI] [PubMed] [Google Scholar]
- 581. Peters EM, Liotiri S, Bodó E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582. Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Kaprara A, Pazaitou-Panayiotou K, Chemonidou MC, et al. Distinct distribution of corticotropin releasing factor receptors in human breast cancer. Neuropeptides. 2010;44:355–361 [DOI] [PubMed] [Google Scholar]
- 584. Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008;8:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Liu J, Man WY, Lv CZ, et al. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol Physiol. 2010;23:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586. Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2000;2:CD001215. [DOI] [PubMed] [Google Scholar]
- 587. Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Jr, Slominski A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008;3:569–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365 [DOI] [PubMed] [Google Scholar]
- 589. Livingstone E, Zimmer L, Vaubel J, Schadendorf D. Current advances and perspectives in the treatment of advanced melanoma. J Dtsch Dermatol Ges. 2012;10:319–325 [DOI] [PubMed] [Google Scholar]
- 590. Kim MH, Cho D, Kim HJ, et al. Investigation of the corticotropin-releasing hormone-proopiomelanocortin axis in various skin tumours. Br J Dermatol. 2006;155:910–915 [DOI] [PubMed] [Google Scholar]
- 591. Yuan PQ, Wu SV, Taché Y. Urocortins and CRF type 2 receptor isoforms expression in the rat stomach are regulated by endotoxin: role in the modulation of delayed gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2012;303:G20–G31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592. Theoharides A, inventor; Kos Pharmaceuticals, Inc, assignee. Treatment of stress-induced migraine headache with a corticotropin releasing hormone blocker. US patent 5855884 A January 5, 1999
- 593. Thomas HA, Ling N, Wei ET, Berree F, Cobas A, Rapoport H. Novel anti-inflammatory undecapeptides that contain anisolyated glutamic acid derivatives. J Pharmacol Exp Ther. 1993;267:1321–1326 [PubMed] [Google Scholar]
- 594. Wei ET, Thomas HA. Correlation of neuroendocrine and anti-edema activities of alanine-corticotropin-releasing factor analogs. Eur J Pharmacol. 1994;263:319–321 [DOI] [PubMed] [Google Scholar]
- 595. Wei ET, Thomas HA, Price JS, Kishimoto T. [D-Pro5]Corticotropin-releasing factor analogs as selective agonists at corticotropin-releasing factor receptors. Eur J Pharmacol. 1996;306:161–164 [DOI] [PubMed] [Google Scholar]
- 596. Wei ET, Thomas HA, Christian HC, Buckingham JC, Kishimoto T. D-amino acid-substituted analogs of corticotropin-releasing hormone (CRH) and urocortin with selective agonist activity at CRH1 and CRH2β receptors. Peptides. 1998;19:1183–1190 [DOI] [PubMed] [Google Scholar]
- 597. Gulyas J, Rivier C, Perrin M, et al. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci USA. 1995;92:10575–10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598. Iavicoli S, Lopez-Perez E, Buehring GC, Thomas HA, Wei ET, Kishimoto T. Bipolar-shape response of human neutrophils to corticotropin-releasing factor. Eur J Pharmacol. 1998;349:301–306 [DOI] [PubMed] [Google Scholar]
- 599. Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34–G40 [DOI] [PubMed] [Google Scholar]
- 600. Rivier J, Gulyas J, Kirby D, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747 [DOI] [PubMed] [Google Scholar]
- 601. Rivier J, Gulyas J, Kunitake K, et al. Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Chen CY, Doong ML, Rivier JE, Taché Y. Intravenous urocortin II decreases blood pressure through CRF(2) receptor in rats. Regul Pept. 2003;113:125–130 [DOI] [PubMed] [Google Scholar]
- 603. Theoharides A, inventor; Kos Pharmaceuticals, Inc, assignee. 2000 Treatment of stress-induced skin disease by corticotropin releasing hormone antagonists and skin mast cell degranulation inhibitors. US patent 6020305 A February 1, 2000
- 604. Liapakis G, Venihaki M, Margioris A, Grigoriadis D, Gkountelias K. Members of CRF family and their receptors: from past to future. Curr Med Chem. 2011;18:2583–2600 [DOI] [PubMed] [Google Scholar]
- 605. Paez-Pereda M, Hausch F, Holsboer F. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert Opin Investig Drugs. 2011;20:519–535 [DOI] [PubMed] [Google Scholar]
- 606. Steckler T. Developing small molecule nonpeptidergic drugs for the treatment of anxiety disorders: is the challenge still ahead? Curr Top Behav Neurosci. 2010;2:415–428 [DOI] [PubMed] [Google Scholar]
- 607. Ziegler CG, Krug AW, Zouboulis CC, Bornstein SR. Corticotropin releasing hormone and its function in the skin. Horm Metab Res. 2007;39:106–109 [DOI] [PubMed] [Google Scholar]
- 608. Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76 [DOI] [PubMed] [Google Scholar]
- 609. Kandere-Grzybowska K, Gheorghe D, Priller J, et al. Stress-induced dura vascular permeability does not develop in mast cell-deficient and neurokinin-1 receptor knockout mice. Brain Res. 2003;980:213–220 [DOI] [PubMed] [Google Scholar]