Abstract
Random microseed matrix screening (rMMS) is a protein crystallization technique in which seed crystals are added to random screens. By increasing the likelihood that crystals will grow in the metastable zone of a protein's phase diagram, extra crystallization leads are often obtained, the quality of crystals produced may be increased, and a good supply of crystals for data collection and soaking experiments is provided. Here we describe a general method for rMMS that may be applied to either sitting drop or hanging drop vapor diffusion experiments, established either by hand or using liquid handling robotics, in 96-well or 24-well tray format.
Keywords: Structural Biology, Issue 78, Crystallography, X-Ray, Biochemical Phenomena, Molecular Structure, Molecular Conformation, protein crystallization, seeding, protein structure
Introduction
From its initial application by Perutz, Kendrew and co-workers in determining the structures of hemoglobin and myoglobin, to the modern high throughput automated pipelines of the structural genomics consortia, macromolecular X-ray crystallography has afforded us an unprecedented structural glimpse into the protein world. This technique remains the most widely applicable experimental method that permits the direct visualization of protein structure at atomic, or near atomic resolution (i.e. in the 1-3 Å range). A prerequisite for X-ray diffraction to be applied to a protein is that it must first be crystallized, and it is this stage of the process that remains the single greatest rate-limiting step in structure determination by diffraction methods 1, 2. Despite significant advances in our understanding of the process of protein crystallization, and major improvements in the quality and availability of crystallization screens, trays, and related technologies, it remains impossible to reliably predict the likelihood of crystallization success 3. Biochemical and biophysical methods can be applied to assess whether a protein of interest displays favorable characteristics for crystal nucleation and growth, i.e. is it well-folded, homogeneous, monodisperse, etc., however, these insights in no way provide a definitive predictor of crystallization propensity.
Seeding has long been purported to be a viable method for improving the number, size, and quality of existing crystals or crystalline material 4-7. This approach is based on the premise that a condition that supports crystal nucleation may not be optimal for subsequent crystal growth and vice versa. By transferring nucleated material from one condition to another, one may attempt to effectively decouple these processes, thus giving access to new, as yet unexplored crystallization space, and as a result increasing the overall success rate of a screening experiment. Established methods have been documented for (i) macroseeding, the transfer of a single crystal in its entirety from one condition to another 8, (ii) streak seeding, the transfer of nucleated material, generally obtained by the application of directional pressure using for example a cat's whisker to the surface of an existing crystal, followed by subsequent passage of the whisker through a new crystallization drop 9, and (iii) "classical" microseeding, the transfer of crystal "seeds", generated by harvesting crushed crystals (or crystalline material), into conditions similar to those that yielded the seeds 10. Notably all three of these methods are time consuming and poorly scalable, certainly in comparison to what is achievable with modern liquid handling crystallization robotics. These factors have contributed, on some level at least, to the perception that seeding is a method to be visited only when other approaches have failed to bear fruit.
Random matrix microseeding (rMMS) is a recent methodological innovation that combines the benefits of traditional microseeding with those of high throughput screening and scalability 11-13. This approach relies on the generation of a seed stock, produced from nucleated crystalline material, which may be aliquoted into/onto each sub-well/coverslip within a standard 96-condition crystallization screen. This method is applicable to both sitting or hanging drop vapor diffusion experiments, established either by hand or using liquid handling robotics, in 24-well or 96-well tray format. rMMS has been demonstrated experimentally to significantly increase crystallization success rate, and produce crystals of greater diffraction quality and quantity 11, 13, 14, and represents an innovative tool in the crystallographers' arsenal of approaches in the ongoing effort towards crystallization success. Here we describe a general method for rMMS and provide sample data illustrating the effectiveness of this technique.
Protocol
1. Strategic Considerations
The choice of seed-crystals used for microseeding experiments will vary depending on the objective of the experiment. At the beginning of a project it is helpful to find several crystallization hits that can provide alternative starting points for crystal optimization. rMMS greatly reduces the need for crystal optimization because good-quality crystals are more likely to grow in the metastable zone of the phase diagram, i.e. in the region where following disturbance or nucleation the system returns to equilibrium. We therefore suggest using rMMS routinely as soon as the first crystals are obtained (or, more accurately, as soon as the first crystals stop growing).
For the initial round of rMMS, a seed stock should be made with as much crystalline material as possible. If only one well is available that contains crystals, or if the crystals are small, it may be helpful to set up multiple repetitions of the original hit (without seeding) to increase the supply of crystals. If, however, several different hits are obtained, seed crystals can be harvested from several conditions and mixed together.
To avoid phase separation, crystals grown in high-salt conditions should be harvested separately from crystals grown in high-PEG conditions. If crystals from several wells are mixed, a reservoir solution that is less likely to give salt crystals should be selected for seed suspension. For example, high concentrations of phosphate, sulfate, calcium and magnesium should be avoided.
Later in a project it may be important to look for crystals with different unit cells in order to improve diffraction, avoid twinned crystals, and obtain crystals that are suitable for binding ligands. At this stage only the most suitable crystals (e.g. those that diffract best) should be used to make the seed stock. Sometimes repeated rounds of microseeding are required, where only the "best" crystals are used to make the seed stock for the subsequent round. If possible, a "neutral" precipitant such as PEG 3000 should be used to suspend the seed crystals in to encourage novel crystal contacts and to crystallize complexes that may be unstable in high-salt solutions 12.
Classical microseeding experiments, where a single crystallization condition is used, are often helpful following the identification of a promising condition and during subsequent optimization. It is often necessary to dilute the seed stock in order to get the desired number of crystals per drop. A "combinatorial" microseeding experiment (where a series of seed stocks of increasing dilution are added to a crystallization condition) is a quick method for finding the optimal dilution of the seed stock. This approach is described below.
2. Preparation of the Seed Stock
- Make a rounded glass probe from a glass Pasteur pipette using a Bunsen flame.
- Heat the pipette near the middle until it becomes soft, then quickly remove it from the flame and draw it out by pulling apart the ends. Aim to pull the glass down to a diameter below 0.25 mm.
- Break the glass at the point where it is around 0.25 mm and briefly plunge the broken end into the flame. Repeat this until a hemisphere of glass with a diameter of ~0.75 mm is formed on the end. This probe is useful for crushing crystalline material as it is appropriately sized for striking solid objects on the 0.01-1.0 mm scale and it does not damage the plastic surface of a crystallization tray sub-well or coverslip. Larger probes (~1.0 mm) may crush small crystals more effectively because the crystals are trapped between the glass and plastic.
Place a 1.5 ml microcentrifuge tube containing a Seed Bead on ice.
Inspect a pre-established crystallization tray using a binocular microscope or crystal imaging system and select one or more appropriate wells from the tray from which to harvest crystalline material for seed stock generation. Any material can be used including fine needles, spherulites, microcrystals and irregular, poorly formed crystals. The seed stock should be made as soon as possible after the crystals stop growing, as older material is more likely to be partially cross-linked, thus reducing its suitability for use in seeding experiments. If any doubt exists as to whether the crystalline material to be harvested is salt or protein the well may be visualized using a UV fluorescence microscope or imaging system prior to opening, or the crystalline material may be subjected to in-situ X-ray diffraction analysis in the tray. When using the former approach protein crystals will fluoresce under UV irradiation, salt crystals will appear colorless.
Open the selected crystallization tray well. For 96-well sitting drop trays cut through the upper plastic sealing sheet around the selected well using a scalpel blade. For 24-well hanging drop trays remove the coverslip using a pair of tweezers. Invert the coverslip and place it on a stable clean surface. Remove 50 μl of the reservoir solution and transfer this liquid to the microcentrifuge tube containing the Seed Bead ensuring that the tube remains on ice throughout.
Thoroughly crush the crystals in the subwell (96-well tray) or coverslip (24-well tray) drops, using the glass probe prepared in section 2.1, viewing the results under a microscope. Small crystals may take several minutes to crush thoroughly. This step allows the experimenter to check that the crystals are easy to crush and are therefore not cross-linked, and also that they are not salt crystals. Salt crystals produce a distinctive click that can be heard and felt when they are crushed. If the crystals are tough and difficult to crush try to obtain and use fresher material.
Remove 5 μl of the reservoir solution from the Seed Bead tube and transfer to the equivalent sub-well (sitting drop experiment), or coverslip (hanging drop experiment) containing the crystalline material to be harvested. Pipette this liquid up and down 5-6 times to resuspend as much of the sub-well or coverslip contents as possible. Return the suspension to the Seed Bead tube and repeat this step twice more, ensuring that as much crystalline material is harvested as possible.
Vortex the Seed Bead for two minutes, stopping every 30 sec to cool the tube on ice.
Make a dilution series, diluting the seed stock in reservoir solution by a factor of 4 to 10 at each stage. Typically, too many crystals will be obtained when using an undiluted seed stock, so use the diluted seed stocks to control the number of crystals per well. In the first round of rMMS, it is important not to dilute the seed stock solution, since the greater the concentration of seeds the more crystallization hits will be obtained.
If it is not to be used immediately, transfer the seed stock suspension and the diluted seed stock to a -20 °C, or -80 °C freezer for storage. To prevent repeated freeze-thaw cycles, which may reduce the efficacy of the seed stock, store this material in small volume aliquots, i.e. 10-20 μl. Once frozen, seed stocks may be kept indefinitely until needed.
3. Establishment of Crystallization Trays
Depending on the availability of crystallization facilities and the preferences of the experimenter, rMMS crystallization screening can be performed using either 24-well trays employing the hanging drop vapor diffusion method, or 96-well trays using the sitting drop vapor diffusion method. Crystallization trays for rMMS can be established either by hand, using a liquid handling robotic dispenser, or a combination of the two.
A typical rMMS experiment set up by hand will comprise, 1.0 μl protein solution, 1.0 μl crystallization condition, and 0.5 μl seed stock. A typical experiment set up using a crystallization robot will comprise, 0.3 μl protein solution, 0.2 μl crystallization condition, and 0.1 μl seed stock.
- To perform rMMS screening in 24-well hanging drop trays, firstly, using a manual pipette, transfer 300 μl of each condition from a 96-well condition crystallization screen in 10 ml single tube format into each well of 4 x 24-well pregreased crystallization trays.
- Transfer 1 μl of each crystallization condition from each 300 μl reservoir onto the surface of a corresponding plastic coverslip from which both front and rear protective backing strips have been removed.
- To the 1 μl of each crystallization condition add 1 μl of protein solution followed by 0.5 μl of crystal seed stock. Invert each coverslip such that the drop of liquid is downward facing and position above the appropriate well.
- Press downwards on the coverslip, compressing the sealing grease and forming a secure seal. Once all 96 drops are established, the tray should be transferred to an incubator or constant temperature room, usually in the range 4-18 °C for storage.
- To perform rMMS screening in 96-well sitting drop trays firstly transfer 20-50 μl of each condition from a 96-well condition crystallization screen in deep well block format into each corresponding well of the crystallization tray. This transfer step may be performed using either a crystallization robot, or by manual transfer using an 8-channel pipette.
- Transfer reservoir, protein and seed stock solutions to the drops by hand or with a robot using the volumes detailed in 3.1. Some commercially available crystallization robots do not contact dispense, and performing seed stock transfer using such a system may result in blockage of dispensing tips or associated tubing. The order of dispensing is variable: some robots dispense all solutions simultaneously. If this is not possible, dispense protein first, then reservoir, then seed stock.
- If a contact dispensing robot is not available transfer the seeds using a low volume glass syringe fitted with a blunt needle. Rinse the needle by passing it through the reservoir solution then dispense the seed stock into each drop.
- Seal the tray using a transparent sealing sheet and transfer to an incubator or constant temperature room for storage.
4. Inspection of Crystallization Trays
Visualize all crystallization experiments using either a binocular microscope or a crystal imaging system. The former generally affords the user greater control over view depth and the degree of magnification than the latter, but is more time consuming.
Inspect each sub-well/coverslip in sequence and record any evidence of crystal formation. Experiments should be inspected once every 24 hr for 5 days following establishment and then subsequently once every 7 days for up to 4 weeks.
Multiple cycles of rMMS are often required before optimal diffraction quality crystals are produced.
Representative Results
(A) Example of an rMMS experiment
To demonstrate the effectiveness of rMMS screening we applied this method to the crystallization of hen egg white lysozyme (HEWL) and bovine liver catalase (BLC). Both these enzymes are eminently crystallizable and are structurally well characterized targets 15, 16. As such both provide excellent test subjects with which to illustrate the enhanced crystallization success rate achievable with rMMS. Crystallization experiments were established in 96-well sitting drop tray format using liquid handling robotics. Solutions of HEWL and BLC were produced by dissolving lyophilized powders of each in 20 mM Tris-HCl, 150 mM NaCl, pH 7.5, to final concentrations of 100 mg/ml and 20 mg/ml respectively. For each protein three 96-condition crystallization screens were used; JCSG, PACT and Morpheus. Crystallization screening without microseeding was performed using reservoir volumes of 50 μl, and 2 μl sitting drops comprising 1 μl of protein solution and 1 μl of reservoir solution. The six trays were stored in a constant temperature room at 18 °C and inspected daily using a binocular microscope. Any evidence of crystal growth was recorded. After five days a single condition from each tray that was found to support the growth of crystalline material was selected and used for seed stock generation. rMMS screening was performed as for non-rMMS screening, with the exception that 2.5 μl sitting drops comprising 1 μl of protein solution, 1 μl of reservoir solution and 0.5 μl of seed stock were used. All six rMMS trays were transferred to a constant temperature room at 18 °C and inspected daily using a binocular microscope. After five days the rMMS trays of both proteins were inspected and the number of drops with crystals was recorded. Figure 1 summarizes the results from this experiment and provides quantitative analysis. For HEWL a 4 to 10-fold increase in crystallization success rate, as compared to non-seeded trays, was observed when rMMS was applied to this protein. Notably for BLC only a single condition in the Morpheus screen was found to yield crystals as compared to 55 conditions identified following the use of rMMS. Further, there was a 3 and 7-fold increase in the success rate in BLC crystallization using JCSG and PACT screens respectively. In all cases and irrespective of the crystallization screen used rMMS screening yielded a significantly greater total number of crystals than non-rMMS screening. Repetition of the rMMS screening experiments described using a 1:100 diluted seed yielded significantly fewer hits than when the undiluted seed stock was used, reinforcing the requirement to use undiluted stocks when performing the initial round of rMMS experiments. For both proteins there was little apparent variation in crystal quality, as judged by size, morphology, and birefringence. Comparative assessment of the diffraction quality of the crystals generated using rMMS as opposed to conventional screening methods goes beyond the scope of this study, however, initial preliminary diffraction analysis using synchrotron radiation of a subset of crystals of both proteins isolated from rMMS and non-rMMS screens indicated there to be little variation in diffraction quality, as judged based on resolution limit and spot profile.
(B) Example of optimizing the number of crystals per drop
Microseeding techniques regularly give showers of small crystals. It is therefore often necessary to dilute the seed stock to reduce the level of nucleation. Moreover, it may sometimes be impossible to grow crystals in hit conditions that were found by rMMS when seeding is not used 5. It such cases it is often helpful to use "classical" microseeding, where several drops are set up using the same reservoir solution with different dilutions of a seed stock. This can be done systematically for several lead conditions on a single plate using a "combinatorial" approach (Paul Reichert, Merck Research Laboratories, personal communication), as shown in Figure 2. Here one or more hit solutions (H1 - H6) are dispensed into the columns of a crystallization plate, while a different dilution of a seed stock (S1 - S9) is added to the drops of each row. Therefore every combination of hit solution and diluted seed stock are tested on a single plate, and the appropriate level of dilution and any trends present can easily be identified.
We applied this combinatorial microseeding approach to three model proteins, xylanase, thaumatin and thermolysin. Each protein was tested with a crystallization condition that had previously been shown to give no crystals in the absence of seeding, but consistently gave crystals when seeding was used. The crystallization conditions used for xylanase, thaumatin and thermolysin are listed in Table 2. Four to six conditions were established for each protein with either two or three repetitions of each protein/condition/seed stock combination as shown. Drops comprised 0.3 μl protein, 0.29 μl reservoir solution, and 10 nl seed stock. All trays were established using a liquid handling crystallization robot. Neat seed stock and six dilutions thereof were set up for each protein. Other experimental details have been reported previously 12. The average number of crystals obtained per drop is plotted against the dilution of the seed stock in Figure 3. These data show that the number of crystals follows a roughly linear relationship with the number of seeds added. For the three proteins tested, 1:10-3 to 1:10-6 dilutions were required to obtain 1-10 crystals per drop.
Table 1. List of materials.
| Protein | Crystallization cocktail | Number of drops established per tray |
| Xylanase (36 mg/ml) | 2 M ammonium sulfate, 0.1 M Tris-HCl pH 8.5 | 3 |
| Xylanase (36 mg/ml) | 30% (w/v) PEG 4000, 0.2 M sodium acetate, 0.1 M Tris-HCl pH 8.5 | 3 |
| Xylanase (36 mg/ml) | 4 M sodium formate | 3 |
| Xylanase (36 mg/ml) | 3.5 M ammonium sulfate, 250 mM sodium chloride, 50 mM sodium/potassium phosphate pH 7.5 | 3 |
| Xylanase (36 mg/ml) | 1.5 M potassium phosphate pH 7.0 | 3 |
| Thaumatin (30 mg/ml) | 30% (w/v) PEG 4000, 0.2 M ammonium acetate, 0.1 M sodium citrate pH 5.6 | 2 |
| Thaumatin (30 mg/ml) | 20% (w/v) PEG 4000, 20 % (v/v) 2-propanol, 0.1 M sodium citrate pH 5.6 | 2 |
| Thaumatin (30 mg/ml) | 30 (% w/v) PEG 8000, 0.2 M ammonium sulfate, 0.1 M sodium cacodylate pH 6.5 | 2 |
| Thaumatin (30 mg/ml) | 20 (% w/v) PEG 8000, 0.2 M magnesium acetate, 0.1 M sodium cacodylate pH 6.5 | 2 |
| Thaumatin (30 mg/ml) | 2 M ammonium sulfate, 0.1 M Tris-HCl pH 8.5 | 2 |
| Thaumatin (30 mg/ml) | 1.5 M lithium sulfate, 100 mM HEPES pH 7.5 | 2 |
| Thermolysin (15 mg/ml) | 20.0 % (w/v) PEG 6000, 100 mM citric acid pH 5.0 | 3 |
| Thermolysin (15 mg/ml) | 1.26 M ammonium sulfate, 200 mM lithium sulfate, 100 mM Tris-HCl pH 8.5 | 3 |
| Thermolysin (15 mg/ml) | 10 % (v/v) MPD, 100 mM bicine pH 9.0 | 3 |
| Thermolysin (15 mg/ml) | 800 mM succinic acid pH 7.0 | 3 |
Table 2. Protein solutions and crystallization conditions used in the combinatorial microseeding experiment.
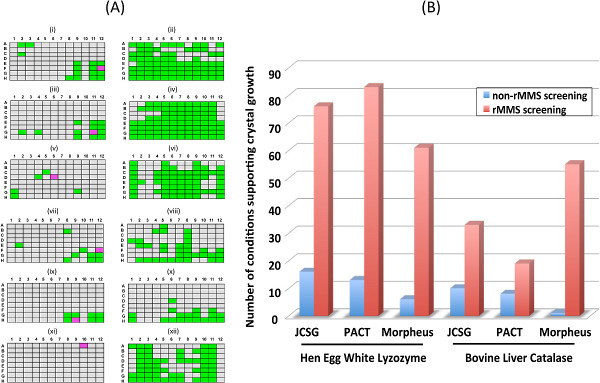 Figure 1. (A) Comparison of rMMS and non-rMMS crystallization screening experiments. Conditions supporting crystal growth are in green, conditions not supporting crystal growth are in grey. Conditions selected for use in seed stock generation are in pink. Crystallization screens are labeled as follows (i) hen egg white lysozyme, JCSG, non-rMMS, (ii) hen egg white lysozyme, JCSG, rMMS, (iii) hen egg white lysozyme, PACT, non-rMMS, (iv) hen egg white lysozyme, PACT, rMMS, (v) hen egg white lysozyme, Morpheus, non-rMMS, (vi) hen egg white lysozyme, Morpheus, rMMS, (vii) bovine liver catalase, JCSG, non-rMMS, (viii) bovine liver catalase, JCSG, rMMS, (ix) bovine liver catalase, PACT, non-rMMS, (x) bovine liver catalase, PACT, rMMS, (xi) bovine liver catalase, Morpheus, non-rMMS, (xii) bovine liver catalase, Morpheus, rMMS. (B) Comparative analysis of crystallization success rate using rMMS and non-rMMS crystallization screening. For non-rMMS screening the number of crystallization conditions identified as supporting crystal growth of either HEWL or BLC are shown in blue, for rMMS screening the number of crystallization conditions identified as supporting crystal growth of either HEWL or BLC are shown in red. Data are based on inspection of crystallization trays 5 days post establishment.
Figure 1. (A) Comparison of rMMS and non-rMMS crystallization screening experiments. Conditions supporting crystal growth are in green, conditions not supporting crystal growth are in grey. Conditions selected for use in seed stock generation are in pink. Crystallization screens are labeled as follows (i) hen egg white lysozyme, JCSG, non-rMMS, (ii) hen egg white lysozyme, JCSG, rMMS, (iii) hen egg white lysozyme, PACT, non-rMMS, (iv) hen egg white lysozyme, PACT, rMMS, (v) hen egg white lysozyme, Morpheus, non-rMMS, (vi) hen egg white lysozyme, Morpheus, rMMS, (vii) bovine liver catalase, JCSG, non-rMMS, (viii) bovine liver catalase, JCSG, rMMS, (ix) bovine liver catalase, PACT, non-rMMS, (x) bovine liver catalase, PACT, rMMS, (xi) bovine liver catalase, Morpheus, non-rMMS, (xii) bovine liver catalase, Morpheus, rMMS. (B) Comparative analysis of crystallization success rate using rMMS and non-rMMS crystallization screening. For non-rMMS screening the number of crystallization conditions identified as supporting crystal growth of either HEWL or BLC are shown in blue, for rMMS screening the number of crystallization conditions identified as supporting crystal growth of either HEWL or BLC are shown in red. Data are based on inspection of crystallization trays 5 days post establishment.
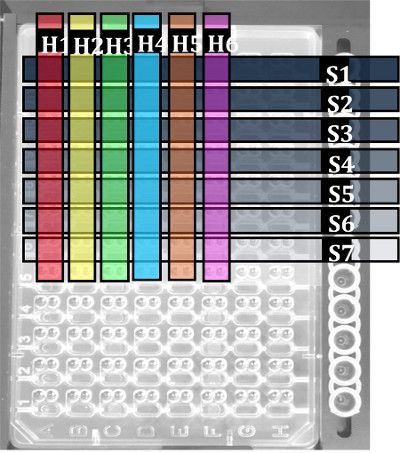 Figure 2. Distribution of crystallization conditions and diluted seed stock suspensions for a single protein in a example combinatorial microseeding experiment. Highlighted columns H1-H6 contain different crystallization conditions supporting target protein crystal growth. Highlighted rows S1-S7 contain different dilutions of a target protein seed stock suspension.
Figure 2. Distribution of crystallization conditions and diluted seed stock suspensions for a single protein in a example combinatorial microseeding experiment. Highlighted columns H1-H6 contain different crystallization conditions supporting target protein crystal growth. Highlighted rows S1-S7 contain different dilutions of a target protein seed stock suspension.
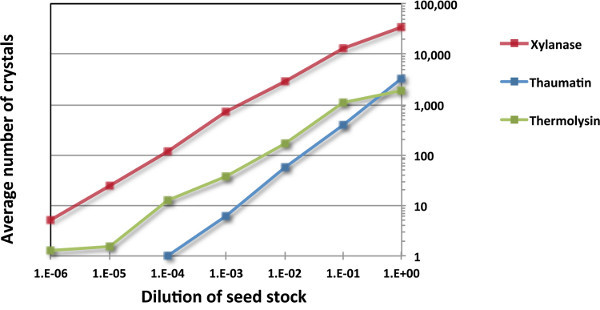 Figure 3. Correlation between seed stock dilution and average number of crystals obtained per drop in a combinatorial microseeding experiment. The three proteins used were xylanase, thaumatin and thermolysin. Drops were established in a 96-well MRC plate using a liquid handling robot, and comprise 0.3 μl protein, 0.29 μl reservoir solution, and 10 nl of fresh seed stock. The data presented are based on inspection of the crystallization trays 5 days post establishment.
Figure 3. Correlation between seed stock dilution and average number of crystals obtained per drop in a combinatorial microseeding experiment. The three proteins used were xylanase, thaumatin and thermolysin. Drops were established in a 96-well MRC plate using a liquid handling robot, and comprise 0.3 μl protein, 0.29 μl reservoir solution, and 10 nl of fresh seed stock. The data presented are based on inspection of the crystallization trays 5 days post establishment.
Discussion
In this paper we have described a general method for rMMS protein crystallization screening. We have demonstrated using two test proteins a significant enhancement in crystallization success rate using this method. Diffraction analysis using synchrotron radiation of a subset of crystals generated using rMMS and non-rMMS methods revealed little variation in diffraction quality between crystals grown using either method, although previous authors have reported that good quality crystals are more likely to grow in rMMS experiments 11, 13. HEWL and BLC may have given atypical results because they are unusually easy to crystallize. We also illustrate the importance of optimizing the concentration of crystal seed stocks used in rMMS experiments, and demonstrate how this may be adjusted by dilution to ensure that an optimal number of diffraction quality crystals are produced.
rMMS is a simple, high throughput, highly scalable method that can be used to complement traditional screening methods. Experiments can be set up by hand or by robot, in 96-well or 24-well trays, and in hanging or sitting drop formats. The major limitation of rMMS is that it is reliant on obtaining some form of crystalline material of a target protein (or of a homologous protein) in order to generate a seed stock in the first instance. It should be noted however, that high-quality crystalline material is not needed; indeed the authors have successfully used microcrystals and amorphous precipitate to make seed stocks. The use of rMMS screening is not restricted to the growth of crystals of native proteins. For example, rMMS is well suited for growing crystals of selenomethione or selenocysteine substituted variants. In such cases, crystals of the native protein can be used to make the seed stock. Although alternative crystal nucleation protocols have been documented 1,4, none are as applicable to as wide a range of proteins as rMMS.
After rMMS a combinatorial microseeding approach can be used to optimize the level of seeding in order to produce crystals for data collection. This is particularly helpful for ligand-soaking experiments where a large number of crystals are frequently required. Using this approach an entire crystallization screen can be quickly established using microseeding in combination with a diluted seed stock, with each well yielding a relatively small number of crystals.
In conclusion we encourage anyone engaged in macromolecular crystallization to make rMMS a part of their routine workflow in all cases where diffracting crystals are not obtained directly from their initial screens.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was funded in part by the BBSRC (BB/1006478/1). PRR is the recipient of a Royal Society University Research Fellowship.
References
- Bergfors T. Protein Crystallization. IUL Biotechnology Series. 2nd 2009.
- Rupp B. Biomolecular Crystallography: Priciples, Practice and Application to Structural Biology. Garland Science; 2010. [Google Scholar]
- Babnigg G, Joachimiak A. Predicting protein crystallization propensity from protein sequence. J. Struct. Funct. Genomics. 2010;11(1):71–80. doi: 10.1007/s10969-010-9080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfors T. Seeds to crystals. J. Struct. Biol. 2003;142(1):66–76. doi: 10.1016/s1047-8477(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Ireton GC, Stoddard BL. Microseed matrix screening to improve crystals of yeast cytosine deaminase. Acta. Crystallogr. D. Biol. Crystallogr. 2004;60:601–605. doi: 10.1107/S0907444903029664. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Zhu YQ, et al. Optimizing protein crystal growth through dynamic seeding. Acta. Crystallogr. D. Biol. Crystallogr. 2005;61(Pt 6):772–775. doi: 10.1107/S0907444904028768. [DOI] [PubMed] [Google Scholar]
- Bergfors T. Screening and optimization methods for nonautomated crystallization laboratories. Methods Mol. Biol. 2007;363:131–151. doi: 10.1007/978-1-59745-209-0_7. [DOI] [PubMed] [Google Scholar]
- Xu L, Butcher SJ, et al. Crystallization and preliminary X-ray analysis of receptor-binding protein P2 of bacteriophage PRD1. J. Struct. Biol. 2000;131(2):159–1563. doi: 10.1006/jsbi.2000.4275. [DOI] [PubMed] [Google Scholar]
- Rangarajan ES, Izard T. Improving the diffraction of full-length human selenomethionyl metavinculin crystals by streak-seeding. Acta. Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 2010;66(Pt 12):1617–1620. doi: 10.1107/S1744309110041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadirvelraj R, Harris P, et al. A stepwise optimization of crystals of rhamnogalacturonan lyase from Aspergillus aculeatus. Acta. Crystallogr. D. Biol . Crystallogr. 2002;58(Pt 8):1346–1349. doi: 10.1107/s0907444902009137. [DOI] [PubMed] [Google Scholar]
- D'Arcy A, Villard F, et al. An automated microseed matrix-screening method for protein crystallization. Acta. Crystallogr. D. Biol. Crystallogr. 2007;63(Pt 4):550–554. doi: 10.1107/S0907444907007652. [DOI] [PubMed] [Google Scholar]
- Shaw Stewart PD, Kolek SA, et al. Random Microseeding: A Theoretical and Practical Exploration of Seed Stability and Seeding Techniques for Successful Protein Crystallization. Crystal Growth & Design. 2011;11(8):3432–3441. [Google Scholar]
- Obmolova G, Malia TJ, et al. Promoting crystallization of antibody-antigen complexes via microseed matrix screening. Acta. Crystallogr. D. Biol. Crystallogr. 2010;66:927–933. doi: 10.1107/S0907444910026041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor AG, Wong A, et al. Acoustic matrix microseeding: improving protein crystal growth with minimal chemical bias. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 5):568–5676. doi: 10.1107/S0907444910005512. [DOI] [PubMed] [Google Scholar]
- Strynadka NC, James MN. Lysozyme: a model enzyme in protein crystallography. EXS. 1996;75:185–222. doi: 10.1007/978-3-0348-9225-4_11. [DOI] [PubMed] [Google Scholar]
- Diaz A, Loewen PC, et al. Thirty years of heme catalases structural biology. Arch. Biochem. 2012;525(2):102–110. doi: 10.1016/j.abb.2011.12.011. [DOI] [PubMed] [Google Scholar]


