Abstract
Retinoids, including all-trans-retinoic acid (RA), are considered to have anti-inflammatory properties and are used therapeutically for diseases of the skin and certain cancers. However, few studies have addressed the effects of disease states on RA metabolism. The present study was conducted to better understand the effects of exogenous RA, both in the absence and presence of inflammation, on the distribution and metabolism of a dose of [3H]RA. Female Sprague-Dawley rats fed a low vitamin A diet were pretreated with RA (po), a low dose of lipopolysaccharide (LPS, ip), or their combination. Twelve hours later, albumin-bound [3H]RA was injected intravenously, and tissue organic- and aqueous-phase 3H was determined after 10 and 30 min. In liver and plasma, 3H-labeled organic metabolites (e.g., 4-oxo- and 4-hydroxy-RA) were isolated by solid-phase extraction. LPS-induced inflammation significantly reduced plasma retinol by 47%, increased total 3H in plasma at 10 min, and reduced total 3H in liver at both times. In contrast, RA pretreatment did not affect plasma retinol, significantly increased total 3H in plasma at both times, and did not affect liver total 3H. However, by 30 min, RA significantly increased [3H]RA metabolism in plasma, liver, lung, and small intestine, as indicated by greater 3H-labeled aqueous-phase and 3H-labeled organic-phase metabolites. The results presented here demonstrate that, although LPS-induced inflammation affects the organ distribution of RA, the ability of RA to induce its own catabolism is maintained during inflammation. Thus we conclude that RA and LPS act independently to alter RA metabolism in vitamin A-marginal rats.
Keywords: inflammation, liver, retinoid metabolites
Vitamin A and its active metabolites are essential for normal immune competence, and vitamin A deficiency is well-known to increase the severity of infectious diseases (59, 60). Conversely, retinoids [all-trans-retinoic acid (RA), cis isomers, metabolites, and synthetic analogs] have been studied extensively for their ability to treat or prevent numerous disease conditions. Cis and trans isomers of RA are used therapeutically for diseases of the skin, such as cystic acne and hyperkeratotic disorders (43), and all-trans-RA has proved successful in the treatment of acute promyelocytic leukemia (23, 41). Similarly, retinoids may reduce the risk of other cancers (40). Moreover, RA may possess immune adjuvant properties, suggesting that it could be administered in conjunction with vaccines to prevent or reduce the severity of various infections (3, 31, 32). Surprisingly, however, few studies have addressed the effects of disease states on the metabolism of RA.
Under normal physiological and dietary conditions, RA is present in the circulation at very low levels, in the range of ~4–14 nM in humans (8, 12) and rats (62), which is ~0.2–0.7% of plasma retinol levels. Even when RA is administered in pharmacological doses, plasma RA remains quite low (42, 46) and turns over rapidly with a turnover time of ~2 min in vitamin A-adequate rats (25) and a first-order terminal half-life of ~45 min in rhesus monkeys given large doses of either all-trans-RA or 9-cis-RA and in humans (1, 37, 58). Despite the low concentration and rapid turnover of RA, plasma RA contributes significantly to tissue retinoid pools in a manner that is organ specific. In rats studied under steady-state conditions, the liver derived more than 75% of its RA pool from plasma, suggesting that the delivery of RA from plasma to liver is an important process for maintaining RA concentrations in the normal, homeostatically regulated range (25). The liver and biliary system also play central roles in the catabolism and excretion of retinoids (57, 68). RA is capable of inducing its own metabolism (65–67), and therefore RA turnover is likely to increase during RA therapy.
The acute-phase response to inflammation results in marked alterations in hepatic protein synthesis and energy metabolism, which in turn, widely affect other organ systems. The levels of albumin, retinol-binding protein (RBP), and other nutrient transport proteins are reduced (4), whereas other plasma proteins are elevated during inflammation (44). Previous studies of vitamin A homeostasis during inflammation have established that inflammation induces a state of low plasma retinol (hyporetinolemia), similar to that observed during natural infections, including measles and diarrhea (22, 39, 55). In rats with LPS-induced inflammation, the level of RBP mRNA in the liver was reduced prior to the reduction in the concentration of the RBP-retinol complex in plasma (50). In studies of similar design, hepatic retinol was increased (19), suggesting that inflammation impairs the transport of retinol from the liver to plasma. Moreover, in rats with marginal vitamin A status the reduction in plasma due to marginal vitamin A status was further compounded by inflammation (51). However, studies have not yet addressed the effects of acute inflammation on the metabolism of plasma RA, the active metabolite of retinol that mediates the majority of physiological functions of vitamin A.
In the present study, we have used LPS-induced inflammation in rats with marginal vitamin A deficiency, a model of subclinical vitamin A deficiency that affects many children in the developing world where infection and inflammation are also widespread (61), to study the effects of acute, nonseptic inflammation on the distribution and catabolism of plasma RA. We hypothesized that LPS would alter the distribution of RA between plasma and tissues, especially the liver, which is centrally involved in retinoid metabolism (53, 54). Because RA itself can offset the effects of vitamin A deficiency (10, 26), significantly alter the metabolism of retinol (33, 69), and induce its own catabolism in vitro and in vivo (11, 48, 63), we designed a 2 × 2 factorial study to examine the effects of RA alone, LPS-induced inflammation alone, and both in combination on RA homeostasis. The results of these studies demonstrate that RA and LPS independently affect the distribution and the metabolism of RA in a tissue-specific manner and that RA and LPS are, for the most part, independent factors in the regulation of RA homeostasis.
MATERIALS AND METHODS
Animals and diet
All animal procedures were approved by the Institutional Animal Use and Care Committee of the Pennsylvania State University. Female Sprague-Dawley (Charles River Laboratories, Boston, MA) rats with litters of 12 female pups were fed a vitamin A-deficient diet from the time of arrival to reduce the accumulation of vitamin A from milk by their nursing offspring (18). The rats were housed in a room maintained at 22°C with a 12:12-h light-dark cycle, and food and water were available ad libitum. When the pups were 21 days old, they were weaned in pairs and fed a nutritionally complete AIN-93G diet (45) modified to contain 0.412 mg of retinyl palmitate per kilogram of diet (Research Diets, New Brunswick, NJ). This amount of vitamin A was shown previously to be adequate for normal growth but results in plasma retinol levels about half that of control-fed rats (51).
Experimental design
When the rats were between 54 and 60 days of age, they were randomly assigned to one of four treatment groups (n = 9/group): control, RA alone, LPS alone, or RA + LPS. Rats treated with RA were given an oral dose of 500 µg all-trans-RA (Sigma-Aldrich, St. Louis, MO) prepared in ~30 µl of vegetable oil (65), and all other rats received an equal amount of oil only. Rats treated with LPS received an intraperitoneal injection of 50 µg/100 g of body wt (BW) of Pseudomonas aeruginosa-LPS (List Biological Laboratories, Campbell, CA) (50, 51), whereas all other rats received an equal volume of sterile phosphate-buffered saline (PBS). Food was removed immediately after treatment until the end of the 12-h experimental period (50). Twelve hours posttreatment, the rats were lightly anesthetized by isoflurane-oxygen inhalation, and 0.15 ml/100 g BW of the albumin-bound [3H]RA, prepared as described below, was injected into the exposed left common iliac vein. The incision was closed with a sterile surgical staple, and the rats were allowed to recover from the anesthesia. Rats were killed either 10 min (n = 5/group) or 30 min (n = 4/group) postinjection by carbon dioxide asphyxiation. Blood was drawn from the inferior vena cava in heparinized syringes, and the liver, lung, spleen, and small intestine were quickly removed and weighed, and aliquots were frozen in liquid nitrogen for storage at −80°C before subsequent analysis. Because RA is rapidly metabolized, it is important to collect tissues quickly (11, 47); thus each rat was treated on a defined schedule so that all tissues could be collected and frozen within 5 min or less after euthanasia. The 10- and 30- min time periods chosen for this experiment were based upon previous reports (25) and pilot work (data not shown) which showed that >95% of the [3H]RA dose was cleared from plasma within 10 min and that metabolism of [3H]RA to polar metabolites could be readily detected by 30 min.
Dose preparation
All procedures involving retinoids were carried out in either dim or yellow light. The doses of [3H]RA for intravenous injection were prepared fresh immediately prior to injection. To prepare a 1-ml aliquot, 10 µCi of [3H]RA {[11,12(N)-3H]retinoic acid, specific activity 1.96 TBq/nmol; from PerkinElmer, Boston, MA} was combined with 0.23 µmol of all-trans-RA, and the solvent was evaporated under argon until <10 µl remained. Next, 5 µl of Tween 20 (Sigma-Aldrich) and 0.9 ml of 0.1% rat serum albumin (diluted in sterile PBS; Calbiochem, San Diego, CA) were added, and the mixture was vortexed. Then, 0.10 ml of whole rat plasma was added to the sample and immediately vortexed. The final concentration of RA in the dose was 0.23 mM, and the dose delivered to each rat was standardized at 0.15 ml (0.33 nmol RA) per 100 g BW. Following the preparation and administration of each dose, aliquots were analyzed by HPLC (9), which showed them to be >97% [3H]RA (data not shown).
Determination of tissue radioactivity
Aliquots of individual plasma, liver, lung, and small intestine samples were extracted using a modification of the method of Folch et al. (15, 16). One gram of minced tissue, or 1 ml of plasma, was placed in 20 ml of chloroform: methanol (2:1, vol/vol) overnight and filtered the next day, and the filtrate was washed four times with 4 ml of the appropriate Folch wash solution. Following each wash step, the resulting aqueous phases were removed and combined. After the final wash, methanol (100%) was added to the aqueous phase until the total volume was 25 ml per sample. Next, the washes were back extracted with 10 ml of hexane, mixed, and centrifuged at 850 g for 5 min. The hexane was removed and added to the Folch organic phase. This backwash was performed to remove any nonpolar compounds present in the aqueous phase. The organic phase from each sample was dried under argon in an analytical evaporator (Organomation Associates, Berlin, MA) to completeness, and the lipophilic compounds were reconstituted in 5 ml of chloroform. Aliquots of the injected dose along with the organic and aqueous phases from each tissue were analyzed by liquid scintillation spectrometry using a Beckman LS-3801 counter (Beckman Coulter, Fullerton, CA).
Separation of RA from polar organic metabolites (4-oxo- and 4-hydroxy-retinoic acid)
Reverse-phase solid-phase extraction (SPE) was employed to separate the parent compound ([3H]RA) from its 3H-labeled oxidation products (mainly 4-oxo- and 4-hydroxy-RA) (17, 48) in the Folch organic phases of liver and pooled plasma samples. All-trans-RA and 4-oxo-RA standards (1.34 nmol each) were added to 500 µl (equal to ~100 mg tissue) of Folch-washed organic phase and dried to completeness under argon at ~37°C. The residue was reconstituted in 750 µl of HPLC-grade acetonitrile:water (65:35, vol/vol) containing 10 mM acetic acid. Supelclean LC-18 SPE columns (Supelco, Bellefonte, PA) were conditioned with 8 ml of 100% methanol followed by 2 ml of water. After conditioning the columns, the samples were loaded on to the column. Using the all-trans-RA and 4-oxo-RA as standards to monitor separation, polar metabolites were eluted first in 3 × 4 ml of acetonitrile:water (65:35, vol/vol), whereas all-trans-RA was eluted with 3 × 4 ml of acetonitrile:water (80:20, vol/vol). The elution rate was maintained at ~1 ml/min with negative pressure using an elution vacuum apparatus (Supelco). After collection, the samples were dried to completeness and analyzed by liquid scintillation spectrometry as described above.
In this paper, the various fractions are described as follows: [3H]RA, material in the organic phase that eluted from the SPE column with the all-trans-RA standard; [3H]RA organic metabolites, material into the organic phase that eluted from the SPE column with 4-oxo-RA; 3H-labeled organic-phase metabolites, material that partitioned into the organic phase during the Folch extraction; 3H-labeled aqueous-phase metabolites, material that partitioned into the aqueous phase during the Folch extraction; total 3H-labeled polar metabolites, the sum of the [3H]RA organic metabolites and 3H-labeled aqueous-phase metaboites; and total 3H-labeled retinoids, the sum of 3H-labeled organic-phase metabolites and 3H-labeled aqueous-phase metabolites.
Statistics
All results are means ± SE. Two-way ANOVA was used to test for main effects and interaction of RA treatment and LPS treatment on [3H]RA metabolism. Significant differences found by ANOVA were then tested using the Fisher protected least-squares difference test to determine which groups differed significantly (P < 0.05) from one another.
RESULTS
Inflammation- and RA-induced changes in organ weight and plasma retinol
Rats grew normally and appeared in good health, and groups did not differ in body weight at the time of treatment (Table 1). Twelve hours later, after treatment and an overnight fast, all groups lost ≤8.2 g BW (Table 1). There were no differences due to treatment in the relative weights of the lungs and small intestine. The relative weight of the spleen was higher in LPS-treated rats, indicative of mild inflammation, and the relative weight of the liver of rats treated with RA + LPS was slightly increased (Table 1). There was no difference in liver vitamin A, which averaged from 17 to 21 nmol/g (Table 1). Plasma retinol (Table 1) was measured as an indicator of vitamin A status and to confirm the effect of LPS-induced inflammation on plasma retinol. Plasma retinol averaged 0.54 µM in the control group, which was similar to previous studies of rats with marginal vitamin A deficiency (51), but it was only 42% of that in vitamin A-sufficient rats (50). Plasma retinol was further reduced by 47% in rats with LPS-induced inflammation compared with the marginally vitamin A-deficient control group, whereas RA treatment did not affect plasma retinol in this 12-h study (Table 1). There was no difference due to time (10 min vs. 30 min) in the sum of the total 3H-labeled retinoids recovered in the plasma, liver, lung, and small intestine (data not shown). However, LPS decreased the sum of the total 3H-labeled retinoids recovered in the plasma, liver, lung, and small intestine compared with RA-treated rats (Table 1).
Table 1.
Body weights, relative organ weights, and plasma retinol concentrations in RA- and LPS-treated rats
| Control | RA | LPS | RA + LPS | |
|---|---|---|---|---|
| BW at dosing, g | 233.8±9.80 | 224.2±8.67 | 212.9±5.74 | 217.9±7.36 |
| Change in BW, g/12 h | −6.8±1.0 | −6.4±0.9 | −8.0±1.0 | −8.2±1.0 |
| Liver weight, % BW | 3.64±0.06 | 3.76±0.12 | 3.91±0.11 | 4.03±0.10a |
| Lung weight, % BW | 0.51±0.02 | 0.53±0.01 | 0.54±0.02 | 0.53±0.02 |
| Spleen weight, % BW | 0.22±0.01 | 0.22±0.01 | 0.27±0.01a,b | 0.29±0.01a,b |
| Small intestine weight, % BW | 2.29±0.09 | 2.41±0.09 | 2.43±0.13 | 2.43±0.14 |
| Plasma retinol, µmol/l | 0.54±0.06 | 0.44±0.06 | 0.30±0.04a,b | 0.29±0.04a,b |
| Liver total retinol, nmol/g* | 17±11 | 21±6 | 18±5 | 14±17 |
| Total 3H-labeled retinoids, % ID† | 30.0±1.23 | 33.3±1.38 | 26.5±1.16b | 28.1±1.15b |
Values are means ± SE; n = 9/group. RA, retinoic acid; LPS, lipopolysaccharide; BW, body wt.
For liver total retinol, values are means ± range of two representative rats per group.
For total 3H-labeled retinoids, values are sums of plasma, liver, lung, and small intestine total 3H-labeled retinoids, expressed as percent injected dose (ID).
Statistically different from control rats (P < 0.05).
Statistically different from RA-treated rats (P < 0.05).
RA alone and in combination with LPS increases the total radioactivity in plasma and the proportion of [3H]RA metabolites
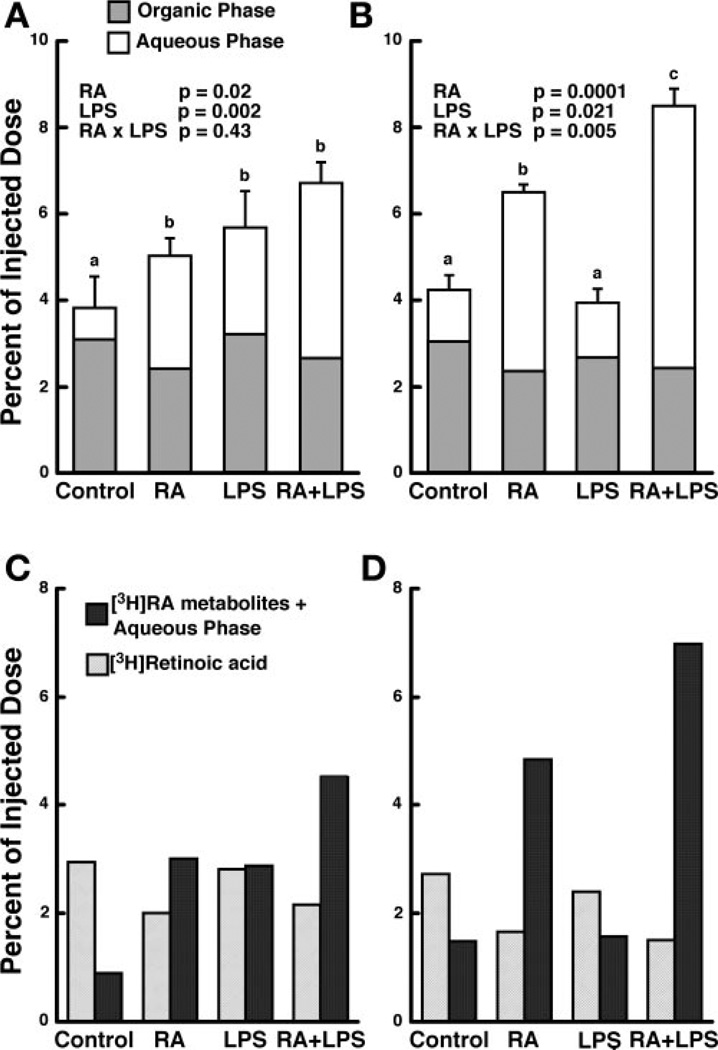
In control rats, greater than 95% of the injected dose was eliminated from plasma within 10 min (Fig. 1A). In comparison, total 3H-labeled retinoids were higher in plasma in the RA, LPS, and RA + LPS groups at this time. Using two-way ANOVA, we showed both RA and LPS to be significant factors, but there was no interaction. By 30 min, total 3H-labeled retinoids in plasma were similar in the control group and the LPS-treated group but were higher in the RA group (Fig. 1B). Furthermore, rats treated with RA + LPS had significantly more (8.5%) of the injected 3H dose in plasma, which was approximately twice the amount in the control group (P < 0.01).
Fig. 1.
Retinoic acid (RA) and lipopolysaccharide (LPS) increase both the total radioactivity and amount of 3H-labeled metabolites present in the plasma at 10 and 30 min postinjection. Female Sprague-Dawley rats were treated with RA, LPS, or the combination of RA + LPS as described in MATERIALS AND METHODS, and [3H]RA (0.23 mM) bound to rat serum albumin was injected 12 h later. Tissues were collected 10 (n = 5) or 30 (n = 4) min later, and total plasma radioactivity (A, 10 min and B, 30 min) and [3H]RA and 3H-labeled metabolites (C, 10 min and D, 30 min) were determined by liquid scintillation spectrometry. Bars are means ± SE. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c).
Total 3H-labeled polar metabolites in plasma, representing lipid-soluble metabolites of RA and its aqueous metabolites combined, were determined on a pooled sample from each treatment group at each time. 3H-labeled polar metabolites were increased by RA, compared with the control group, at both 10 and 30 min. At 10 min, about 60% of the total 3H-labeled retinoids recovered in plasma already consisted of 3H-labeled polar metabolites, which increased to 75% at 30 min (Fig. 1, C and D). In the plasma of LPS-treated rats, 3H-labeled polar metabolites were elevated 10 min postinjection but equal to the control level by 30 min. In rats treated with the combination of RA + LPS, the proportion of 3H-labeled polar metabolites was highest (Fig. 1, C and D), representing ~68% and 82% of total 3H-labeled retinoids at 10 and 30 min, respectively. Thus RA and the combination of RA + LPS resulted in a two- to threefold increase in plasma total 3H, compared with the control group, and the increase was mostly in the form of polar metabolites.
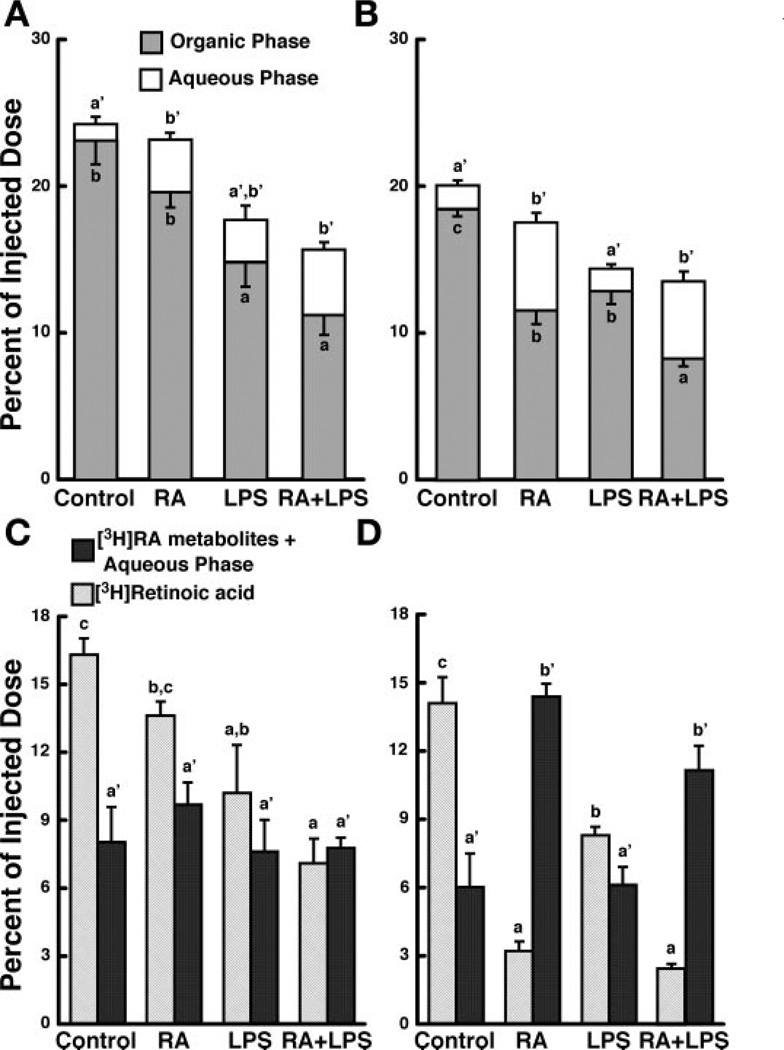
LPS and RA independently affect the total amount and distribution of 3H-labeled retinoids in liver
At 10 min postinjection, the liver of control rats contained ~24% of the injected dose as total 3H-labeled retinoids (Fig. 2A). The liver of LPS-and RA + LPS-treated rats contained less of the total dose, suggesting that LPS-induced inflammation may delay the uptake of [3H]RA by the liver. By 30 min, total 3H-labeled retinoids fell by 4.3% in the control group (Fig. 2B), and a similar reduction was observed in each treatment group (Fig. 2, A and B). Using two-way ANOVA, we showed that LPS reduced the total 3H-labeled retinoids at both 10 and 30 min (Table 2; Fig. 2, A and B).
Fig. 2.
RA and LPS independently affect both the total radioactivity and amount of 3H-labeled metabolites present in the liver at 10 and 30 min postinjection. Liver was collected 10 (n = 5) or 30 (n = 4) min after injection of [3H]RA and organic-phase and aqueous-phase radioactivity (A, 10 min; and B, 30 min), along with [3H]RA and 3H-labeled metabolites (C, 10 min; and D, 30 min) was determined by liquid scintillation spectrometry. Bars are means ± SE. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c)
Table 2.
Two-way ANOVA of liver, lung, and small intestine radioactivity 10 and 30 min postinjection
| 10 min | 30 min | |||||
|---|---|---|---|---|---|---|
| Organ | RA | LPS | RA + LPS | RA | LPS | RA + LPS |
| Liver | ||||||
| Total 3H | 0.23 | 0.002 | 0.87 | 0.93 | 0.0002 | 0.37 |
| Organic-phase 3H | 0.03 | 0.0001 | 0.99 | 0.0001 | 0.0001 | 0.18 |
| Aqueous-phase 3H | 0.004 | 0.05 | 0.42 | 0.0001 | 0.51 | 0.64 |
| [3H]RA* | 0.04 | 0.0002 | 0.89 | 0.0001 | 0.0005 | 0.004 |
| 3H-labeled polar metabolites† | 0.46 | 0.32 | 0.54 | 0.0001 | 0.16 | 0.15 |
| Lung | ||||||
| Total 3H | 0.63 | 0.01 | 0.28 | 0.02 | 0.04 | 0.85 |
| Organic-phase 3H | 0.03 | 0.23 | 0.64 | 0.0001 | 0.17 | 0.24 |
| Aqueous-phase 3H | 0.009 | 0.06 | 0.43 | 0.0001 | 0.04 | 0.06 |
| Small intestine | ||||||
| Total 3H | 0.16 | 0.0004 | 0.11 | 0.01 | 0.57 | 0.33 |
| Organic-phase 3H | 0.21 | 0.03 | 0.34 | 0.01 | 0.42 | 0.07 |
| Aqueous-phase 3H | 0.10 | 0.21 | 0.96 | 0.005 | 0.48 | 0.43 |
P value obtained via two-way ANOVA.
Amount of 3H that eluted with an all-trans-RA standard during solid-phase extraction (SPE).
Amount of 3H that eluted with a 4-oxo-RA standard during SPE.
The proportion of unmetabolized [3H]RA and 3H-labeled polar retinoids was determined on liver samples from individual rats. LPS reduced the amount of unmetabolized [3H]RA in liver at both 10 and 30 min (Fig. 2C; Table 2). Although there was no effect of RA on unmetabolized [3H]RA in liver at 10 min, unmetabolized [3H]RA was much lower in the liver of all RA-treated rats (RA alone and RA + LPS), compared with the control group, at 30 min (Fig. 2D; Table 2). The percent of total 3H-labeled polar metabolites did not differ among treatments at 10 min (Table 2), although the ratio of total 3H-labeled polar metabolites to [3H]RA was greater in all RA- and LPS-treated rats compared with the control group. By 30 min, total 3H-labeled polar metabolites increased in the liver of all RA-treated rats, regardless of inflammation (Fig. 2D; Table 2). At this time, total 3H-labeled metabolites represented ~82% of the total radioactivity present in the liver in both of these groups. Thus, although LPS significantly affected the total amount of 3H present in the liver at both times, only RA affected the amount of 3H-labeled polar metabolites in the liver (Table 2).
RA metabolism in extrahepatic tissues
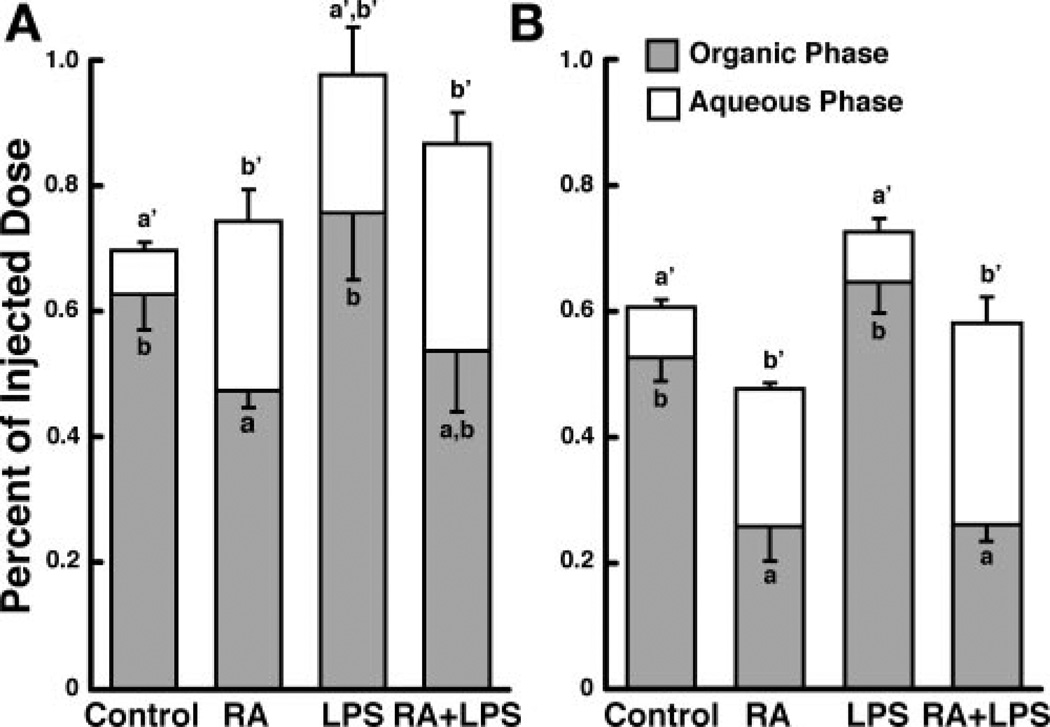
Next, we examined two extrahepatic tissues, the lungs and small intestine, both of which are intimately involved in vitamin A homeostasis (53, 54), to determine their response to RA- and LPS-induced inflammation (Fig. 3). Because to the relatively small amount of total 3H recovered in the lungs and small intestine (see below), total 3H-labeled retinoids were only partitioned into an organic phase and an aqueous phase; thus the organic phase may contain unmetabolized [3H]RA and 3H-labeled polar metabolites, whereas all 3H in the aqueous phase has undergone metabolism. Less than 1% of the injected dose of [3H]RA was recovered in the lungs of control rats at either time. However, this still represented an enrichment of 1.34 ± 0.12 and 1.25 ± 0.05 at 10 and 30 min, respectively, based on the relative weight of the lung. RA treatment did not affect total 3H-labeled. retinoids present in lung at 10 min, but it significantly decreased the amount of total 3H-labeled retinoids at 30 min (Fig. 3, A and B; Table 2). In contrast, lung total 3H-labeled retinoids were higher in LPS rats at both 10 and 30 min (Fig. 3, A and B; Table 2). Partitioning of the lipid extract showed that organic-phase radioactivity was lower and 3H-labeled aqueous-phase metabolites were higher in the lungs of RA rats at both times (Fig. 3, A and B; Table 2). LPS treatment did not affect the amount of 3H-labeled aqueous-phase metabolites in the lungs at either time (Fig. 3). Thus, although LPS affected total 3H in the lung at both times, only RA increased the amount of 3H-labeled aqueous-phase metabolites.
Fig. 3.
RA and LPS independently affect total radioactivity and 3H-labeled aqueous-phase metabolites present in the lungs. Lung was collected 10 (n = 5; A) or 30 (n = 4; B) min after injection of [3H]RA, and organic-phase and aqueous-phase radioactivity was determined. Bars are means ± SE. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c).
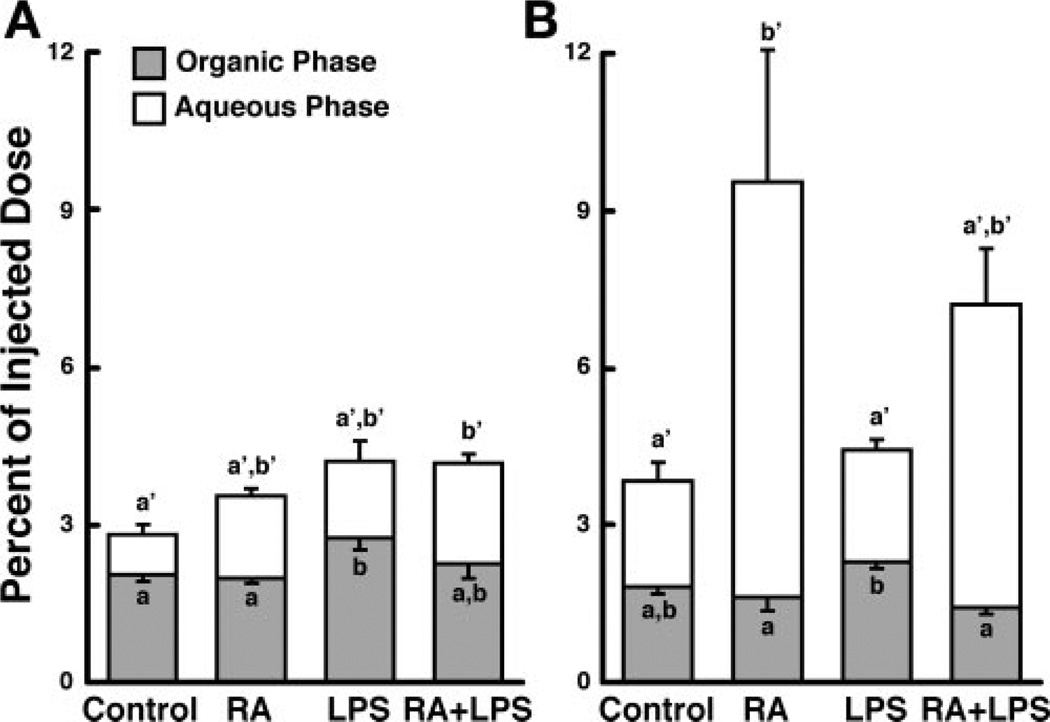
Approximately 3.5% of the injected dose of [3H]RA was recovered in the small intestine of control rats at 10 and 30 min (Fig. 4, A and B), representing an enrichment of 1.15 ± 0.07 and 1.92 ± 0.15, respectively. Treatment with RA increased the amount of 3H recovered in the small intestine at 30 min (Fig. 4, A and B; Table 2), whereas LPS increased the total amount of 3H present in the small intestine only at 10 min (Fig. 4, A and B; Table 2). The amount of 3H-labeled aqueous-phase metabolites in the small intestine was significantly increased by RA, but not by LPS, at 30 min (Fig. 4B; Table 2). In fact, 3H-labeled aqueous-phase metabolites recovered in the small intestine of RA-treated rats exceeded the total amount of radioactivity recovered from each of the other groups examined at either time (Fig. 4, A and B). Therefore, although LPS affected the total 3H in the small intestine at 10 min, only RA affected both total 3H and 3H-labeled aqueous-phase metabolites at 30 min.
Fig. 4.
RA and LPS independently affect total radioactivity and 3H-labeled aqueous-phase metabolites present in the small intestine. Small intestine was collected 10 (n = 5; A) or 30 (n = 4; B) min after injection of [3H]RA, and organic-phase and aqueous-phase radioactivity was determined. Bars are means ± SE. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c)
DISCUSSION
The present study was conducted to better understand the regulation of RA homeostasis during inflammation. To this end, we designed our study to investigate the effects of RA and LPS each alone, as well as in combination, on the distribution and metabolism of a dose of [3H]RA in vitamin A-marginal rats, a model of subclinical vitamin A deficiency relevant to human populations in which inflammation is also prominent. The amount of LPS administered (50 µg/100 g BW) was similar to that used in previous experiments to elicit a moderate inflammatory response in rats, which included a significant reduction of ~7.5% in body weight over 24 h, a moderate increase in body temperature (0.6°C), and significant hyporetinolemia (50, 51). However, the LPS treatment protocol we have used does not induce endotoxemia or septic shock, as are induced by 10- to 20-fold higher doses of LPS (24, 29). Inflammation-induced hyporetinemia was confirmed in our experiment by a 47% reduction in plasma retinol. Inflammation was also evident by a small but significant increase in relative spleen weight (Table 1), which is consistent with immune cell activation by LPS as shown in other studies (36, 64). The dose of RA used in this study was shown previously to increase the expression of several retinoid-responsive genes in both vitamin A-deficient and vitamin A-sufficient rats (65) and, therefore, would be expected to have similar effects in vitamin A-marginal rats. By examining the distribution of intravenously administered [3H]RA in the plasma, liver, and extrahepatic organs of vitamin A-marginal rats, we determined that [3H]RA is cleared from plasma and metabolized very rapidly and that each organ differs in its pattern of RA metabolism. RA and LPS each significantly modified the metabolism of [3H]RA. However, for the most part, RA and LPS were independent determinants of RA metabolism.
Plasma retinoid metabolites are predominantly regulated by RA
Reports prior to this study had established that under normal physiological conditions, the plasma pools of RA turns over rapidly in both rats and humans (1, 25, 37, 58). Several investigators have shown that following the clearance of alltrans- RA from plasma, this retinoid subsequently appears as various polar metabolites, including 4-hydroxy-RA, 4-oxo-RA, and retinoyl β-glucuronide (11, 17, 57). In the present study, greater than 95% of the injected dose of [3H]RA was cleared from plasma within 10 min, confirming the rapid clearance of RA from plasma observed in rats and other species. Although treatment with RA resulted in a higher percentage of 3H in plasma, especially at 30 min, analysis of retinoids after reverse-phase SPE showed that the increase is accounted for by the. combination of 3H-labeled organic and aqueous-phase metabolites (Fig. 1). It is evident that plasma [3H]RA was maintained within a narrow range regardless of pretreatment, whereas plasma 3H-labeled metabolites were rapidly formed and were present at a higher level in RA-treated rats. In contrast, LPS had a modest (at 10 min) or no (at 30 min) effect on total 3H and 3H-labeled organic metabolites in plasma. Thus [3H]RA delivered into the plasma compartment was rapidly metabolized by all rats, but [3H]RA metabolism was accelerated in RA-treated rats, regardless of concurrent inflammation.
Inflammation-induced changes in hepatic [3H]RA metabolism
The liver is directly involved in the maintenance of retinol homeostasis by regulating its storage, mobilization, and utilization (52). Previous work has established that hepatic retinol mobilization is impaired during acute inflammation (19, 50). Since RA mediates most of the physiological functions of vitamin A, we were interested in understanding the effects of LPS-induced inflammation on the uptake and metabolism of [3H]RA by liver. Significant amounts of RA are taken up by liver from plasma and presumably utilized or recycled (25). On average, 24% of the injected dose was recovered in the liver of control rats at 10 min (Fig. 2A). This represents an enrichment of 6.48 ± 0.50 based on the relative weight of the liver. The amount of total 3H-labeled retinoids recovered in liver was lower at 30 min than at 10 min in all four groups (Fig. 2). This reduction could be due in part to the loss from liver back into plasma of the parent compound [3H]RA or more polar metabolites such as retinoyl β-glucuronide (2). Alternatively or additionally, the decline in liver radioactivity could be caused by the formation and excretion of polar and aqueous-phase metabolites of RA, which appear in the bile of normal rats 2 h after RA administration (11, 57, 62). In our study, LPS significantly reduced the total amount of 3H present in liver at both 10 and 30 min (Fig. 2, A and B). We speculate that the observed reduction in liver radioactivity could be a consequence of three primary causes. First, LPS-induced inflammation increases vascular permeability, which could alter organ blood flow and thus influence the uptake, utilization, and recycling of RA by the liver (7, 13). Second, acute inflammation is known to diminish the expression of fatty acid transport protein (FATP) and fatty acid translocase (FAT), both of which are involved in the uptake of long-chain fatty acids from plasma (14, 34). Similarly, LPS administration reduces the expression of cellular fatty acid binding protein, L-FABP (35). While it is currently believed that RA readily diffuses across plasma membranes in a nonfacilitated manner, the effects of inflammation on different fatty acid transporters suggest that these or similar changes could affect RA homeostasis as well. Third, specific transporters involved in the uptake and secretion of chemicals by the liver are altered following LPS administration (5). Thus vascular alteration coupled with changes in membrane-bound transporters involved in fat and xenobiotic homeostasis, known to be affected by LPS, may have contributed to the LPS-induced decrease in liver 3H-labeled retinoid in our LPS-treated rats.
RA-induced changes in hepatic [3H]RA metabolism
Pharmacokinetic studies have determined that long-term administration of RA results in a decrease in plasma RA and subsequent increase in RA metabolism (42, 46, 58). Specific cytochrome P450 enzymes are induced in response to high cellular concentrations of RA, which may work in concert to regulate the degradation of RA and to return the concentration of RA to normal cellular levels (17, 28, 48, 49, 65, 66). In our studies, pretreatment of marginally vitamin A-deficient rats with oral RA significantly increased the amount of 3H-labeled metabolites present in the liver. Furthermore, this induction in RA metabolism occurred irrespective of LPS administration. The inability of LPS to affect the amount of 3H-labeled metabolites in the liver was surprising since the detoxification capacity of the liver for drugs and xenobiotics is diminished during inflammation (20, 21), evidenced by an LPS-induced reduction in both the expression and enzymatic activity of various members of the cytochrome P450 family (56). Thus the results presented here suggest that the ability of RA to induce its own catabolism is maintained during periods of physiological stress, which would serve to prevent the elevation of RA concentrations when acute or chronic RA treatment is used therapeutically as an adjuvant or to treat different disease conditions.
Inflammation- and RA-induced changes in extrahepatic [3H]RA metabolism
Similar to liver, both lung and small intestine have been implicated in retinol (6, 38, 53, 54) and RA homeostasis (27, 53). To determine whether LPS-induced inflammation affects these organs in a manner similar to its effects in liver, we examined the small intestine and lung for organic- and aqueous-phase radioactivity. Similar to liver, both lung and small intestine exhibited enrichment of the injected [3H]RA dose soon after administration, indicating these organs have the capacity to take up and concentrate RA. LPS initially increased the total amount of 3H present in the lung and small intestine, but by 30 min the amount returned to control levels in both organs (Figs. 3 and 4). In contrast, only RA administration increased the amount of aqueous-phase radioactivity at 10 and 30 min in both organs, irrespective of LPS treatment. The increased aqueous-phase radioactivity could be caused by enhanced RA oxidation because both lung and small intestine contain members of the cytochrome P450 family capable of oxidizing RA (27, 30). Interestingly, in the small intestine, the amount of aqueous-phase radioactivity increased in each treatment group over time, suggesting the presence of biliary metabolites of RA in the small intestine by 30 min (11, 57, 62).
In conclusion, the results of our study demonstrate that LPS-induced inflammation affects the organ distribution of RA, which may reflect differences in either the rate of uptake or the recycling of RA in a tissue-specific manner. The greatest effect was observed in the liver, where LPS reduced the amount of radioactivity recovered by nearly 30%. However, despite differences in the initial distribution of RA, LPS did not affect the total amount of 3H-labeled metabolites present in the liver, lung, and small intestine by 30 min. The interval from 10 to 30 min may have been sufficient for all tissues to metabolize most of the RA into metabolites. In contrast, although RA administration did not influence tissue radioactivity levels, it did cause a significant increase in the recovery of 3H-labeled metabolites, irrespective of LPS. Taken together, our results suggest that acute inflammation can alter systemic RA homeostasis by disrupting the normal physiological distribution of RA. Nevertheless, the moderate degree of inflammation induced in our rat model did not greatly compromise the metabolism of [3H]RA, whereas the conversion of [3H]RA to 3H-labeled metabolites was significantly increased after treatment with RA.
ACKNOWLEDGMENTS
We are grateful to Keigan Park for assistance with these experiments and Dr. Nan-qian Li for retinol analysis.
GRANTS
This work was supported in part by the National Cancer Institute Grant CA-90214, the Integrative Biosciences Graduate Program, and the Graduate Program in Nutrition.
Footnotes
These results were presented in part at Experimental Biology 2004, April, Washington, DC: Cifelli CJ, Park KM, and Ross AC. Retinoic acid and lipopolysaccharide independently affect the metabolism of all-trans-retinoic acid in rats. FASEB J 18: A381 (abs. 272.1) 2004.
REFERENCES
- 1.Adamson PC. All-trans-retinoic acid pharmacology and its impact on the treatment of acute promyelocytic leukemia. Oncologist. 1996;1:305–314. [PubMed] [Google Scholar]
- 2.Barua AB, Olson JA. Retinoyl β-glucuronide: and endogenous compound of human blood. Am J Clin Nutr. 1986;43:481–485. doi: 10.1093/ajcn/43.4.481. [DOI] [PubMed] [Google Scholar]
- 3.Benn CS, Blade A, George E, Kidd M, Whittle H, Lisse IM, Aaby P. Effect of vitamin A supplementation on measles-specific antibody levels in Guinea-Bissau. Lancet. 2002;359:1313–1314. doi: 10.1016/S0140-6736(02)08274-0. [DOI] [PubMed] [Google Scholar]
- 4.Birch HE, Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem. 1986;261:8077–8080. [PubMed] [Google Scholar]
- 5.Cherrington NJ, Slitt AL, Li N, Klaassen CD. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos. 2004;32:734–741. doi: 10.1124/dmd.32.7.734. [DOI] [PubMed] [Google Scholar]
- 6.Cifelli CJ, Green JB, Green MH. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J Nutr. 2005;135:746–752. doi: 10.1093/jn/135.4.746. [DOI] [PubMed] [Google Scholar]
- 7.Cousins SW, Guss RB, Howes EL, Jr, Rosenbaum JT. Endotoxin-induced uveitis in the rat: observations on altered vascular permeability, clinical findings, and histology. Exp Eye Res. 1984;39:665–676. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 8.DeLeenheer AP, Lambert WE, Claeys I. All-trans-retinoic acid: measurement of reference values in human serum by high performance liquid chromatography. J Lipid Res. 1982;23:1362–1367. [PubMed] [Google Scholar]
- 9.Disdier B, Bun H, Catalin J, Durand A. Simultaneous determination of all-trans-, 13-cis-, 9-cis-, retinoic acid and their 4-oxo-metabolites in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;683:143–154. doi: 10.1016/0378-4347(96)00112-0. [DOI] [PubMed] [Google Scholar]
- 10.Dowling JE, Wald G. The role of vitamin A acid. Vitam Horm. 1960;18:515–541. doi: 10.1016/s0083-6729(08)60878-x. [DOI] [PubMed] [Google Scholar]
- 11.Dunagin PE, Jr, Meadows EHJR, Olson JA. Retinoyl beta-glucuronic acid: a major metabolite of vitamin A in rat bile. Science. 1965;148:86–87. doi: 10.1126/science.148.3666.86. [DOI] [PubMed] [Google Scholar]
- 12.Eckhoff C, Nau H. Identification and quantitation of all-trans- and 13-cis-retinoic acid and 13-cis-4-oxo-retinoic acid in human plasma. J Lipid Res. 1990;31:1445–1454. [PubMed] [Google Scholar]
- 13.Edamitsu S, Matsukawa A, Ohkawara S, Takagi K, Nariuchi H, Yoshinaga M. Role of TNF α, IL-1, and IL-1ra in the mediation of leukocyte infiltration and increased vascular permeability in rabbits with LPS-induced pleurisy. Clin Immunol Immunopathol. 1995;75:68–74. doi: 10.1006/clin.1995.1054. [DOI] [PubMed] [Google Scholar]
- 14.Feingold K, Kim MS, Shigenaga J, Moser A, Grunfeld C. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am J Physiol Endocrinol Metab. 2004;286:E201–E207. doi: 10.1152/ajpendo.00205.2003. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Ascoli I, Lees M, Meath JA, LeBaron N. Preparation of lipid extracts from brain tissue. J Biol Chem. 1951;191:833–841. [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Frolik CA, Roberts AB, Tavela TE, Roller PP, Newton DL, Sporn MB. Isolation and identification of 4-hydroxy- and 4-oxo-retinoic acid. In vitro metabolites of all-trans-retinoic acid in hamster trachea and liver. Biochemistry. 1979;18:2092–2097. doi: 10.1021/bi00577a039. [DOI] [PubMed] [Google Scholar]
- 18.Gardner EM, Ross AC. Dietary vitamin A restriction produces marginal vitamin A status in young rats. J Nutr. 1993;123:1435–1443. doi: 10.1093/jn/123.8.1435. [DOI] [PubMed] [Google Scholar]
- 19.Geing SH, Raila J, Rosales FJ. Accumulation of retinol in the liver after prolonged hyporetinolemia in the vitamin A-sufficient rat. J Lipid Res. 2005;46:641–649. doi: 10.1194/jlr.M400415-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Ghezzi P, Saccardo B, Villa P, Rossi V, Bianchi M, Dinarello CA. Role of interleukin-1 in the depression of liver drug metabolism by endotoxin. Infect Immun. 1986;54:837–840. doi: 10.1128/iai.54.3.837-840.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorodischer F, Krasner K, McDevitt JJ, Nolan JP, Yaffe SJ. Hepatic microsomal drug metabolism after administration of endotoxin in rats. Biochem Pharmacol. 1976;25:351–353. doi: 10.1016/0006-2952(76)90226-4. [DOI] [PubMed] [Google Scholar]
- 22.Hussey GD, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med. 1990;323:160–164. doi: 10.1056/NEJM199007193230304. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez-Lara AM, Clarke N, Altucci L, Gronemeyer H. Retinoic-acid-induced apoptosis in leukemia cells. Trends Mol Med. 2004;10:508–515. doi: 10.1016/j.molmed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Kalitsky-Szirtes J, Shayeganpour A, Brocks DR, Piquette-Miller M. Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. Drug Metab Dispos. 2004;32:20–27. doi: 10.1124/dmd.32.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Kurlandsky SB, Gamble MV, Ramkrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270:17850–17857. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 26.Lamb AJ, Apiwatanaporn P, Olson JA. Induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1974;104:1140–1148. doi: 10.1093/jn/104.9.1140. [DOI] [PubMed] [Google Scholar]
- 27.Lampen A, Meyer S, Arnhold T, Nau H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther. 2000;295:979–985. [PubMed] [Google Scholar]
- 28.Leo MA, Lasker JM, Raucy JL, Kim CI, Black M, Lieber CS. Metabolism of retinol and retinoic acid by human liver cytochrome P450IIC8. Arch Biochem Biophys. 1989;269:305–312. doi: 10.1016/0003-9861(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin SL, Lee YM, Chang HY, Cheng YW, Yen MH. Effects of naltrexone on lipopolysaccharide-induced sepsis in rats. J Biomed Sci. 2005;12:431–440. doi: 10.1007/s11373-005-0647-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr. 2003;133:173–179. doi: 10.1093/jn/133.1.173. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–13561. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic: polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura T, Ross AC. Regulation of hepatic lecithin: retinol acyltransferase activity by retinoic acid. Arch Biochem Biophys. 1993;301:221–227. doi: 10.1006/abbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 34.Memon RA, Fiengold KR, Moser AH, Fuller J, Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am J Physiol Endocrinol Metab. 1998;274:E210–E217. doi: 10.1152/ajpendo.1998.274.2.E210. [DOI] [PubMed] [Google Scholar]
- 35.Memon RA, Bass NM, Moser AH, Fuller J, Appel R, Grunfeld C, Feingold KR. Down-regulation of liver and heart specific fatty acid binding proteins by endotoxin and cytokines in vivo. Biochem Biophys Acta. 1999;1440:118–126. doi: 10.1016/s1388-1981(99)00120-1. [DOI] [PubMed] [Google Scholar]
- 36.Miyake K, Ogata H, Nagai Y, Akashi S, Kimoto M. Innate recognition of lipopolysaccharide by Toll-like receptor 4/MD-2 and RP105/MD-1. J Endotoxin Res. 2000;6:389–391. [PubMed] [Google Scholar]
- 37.Muindi J, Frankel SR, Miller WH, Jr, Jakubowski A, Scheinberg DA, Young CW, Dmitrovsky E, Warrell RP., Jr Continuous treatment with all-trans-retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid “resistance” in patients with acute promyelocytic leukemia. Blood. 1992;79:299–303. [PubMed] [Google Scholar]
- 38.Nagy NE, Holven KB, Roos N, Senoo H, Kojima N, Norum KR, Blomhoff R. Storage of vitamin A in extrahepatic stellate cells in normal rats. J Lipid Res. 1997;38:645–658. [PubMed] [Google Scholar]
- 39.Neuzil KM, Gruber WC, Chytil F, Stahlman MT, Engelhardt B, Graham BS. Serum vitamin A levels in respiratory syncytial virus infection. J Pediatr. 1994;124:433–436. doi: 10.1016/s0022-3476(94)70369-8. [DOI] [PubMed] [Google Scholar]
- 40.Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, Moriwaki H. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004;4:285–298. doi: 10.2174/1568009043333023. [DOI] [PubMed] [Google Scholar]
- 41.Ortega JJ, Madero L, Martin G, Verdeguer A, Garcia P, Parody R, Fuster J, Molines A, Novo A, Deben G, Rodriguez A, Conde E, de la Serna J, Allegue MJ, Capote FJ, Gonzalez JD, Bolufer P, Gonzalez M, Sanz MA PETHEMA Group. Treatment with all-trans-retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23:7632–7640. doi: 10.1200/JCO.2005.01.3359. [DOI] [PubMed] [Google Scholar]
- 42.Ozpolat B, Lopez-Berestein G, Adamson P, Fu CJ, Williams AH. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci. 2003;6:292–301. [PubMed] [Google Scholar]
- 43.Peck GL, DiGiovanna JJ. Synthetic retinoids in dermatology. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. 2nd ed. chapt. 43. New York: Raven; 1994. pp. 631–658. [Google Scholar]
- 44.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 45.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 46.Regazzi MB, Iacona I, Gervasutti C, Lazzarino M, Toma S. Clinical pharmacokinetics of tretinoin. Clin Pharmacokinet. 1997;32:382–402. doi: 10.2165/00003088-199732050-00004. [DOI] [PubMed] [Google Scholar]
- 47.Roberts AB, DeLuca HF. Pathways of retinol and retinoic acid metabolism in the rat. Biochem J. 1967;102:600–605. doi: 10.1042/bj1020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AB, Frolik CA, Nichols MD, Sporn MB. Retinoid-dependent induction of the in vivo and in vitro metabolism of retinoic acid in tissues of the vitamin A-deficient hamster. J Biol Chem. 1979;254:6303–6309. [PubMed] [Google Scholar]
- 49.Roberts ES, Vaz AD, Coon MJ. Role of isozymes of rabbit microsomal cytochrome P-450 in the metabolism of retinoic acid, retinol, and retinal. Mol Pharmacol. 1992;41:427–433. [PubMed] [Google Scholar]
- 50.Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res. 1996;37:962–971. [PubMed] [Google Scholar]
- 51.Rosales FJ, Ross AC. Acute inflammation induces hyporetinemia and modifies the plasma and tissue response to vitamin A supplementation in marginally vitamin A-deficient rats. J Nutr. 1998;128:960–966. doi: 10.1093/jn/128.6.960. [DOI] [PubMed] [Google Scholar]
- 52.Ross AC, Zolfaghari R, Weisz J. Vitamin A: recent advances in the biotransformation, transport, and metabolism of retinoids. Curr Opin Gastroenterol. 2001;17:184–192. doi: 10.1097/00001574-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 54.Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: perspectives from studies on vitamin A status. J Nutr. 2004;134:269S–275S. doi: 10.1093/jn/134.1.269S. [DOI] [PubMed] [Google Scholar]
- 55.Salazar-Lindo E, Salazar M, Alvarez JO. Association of diarrhea and low serum retinol in Peruvian children. Am J Clin Nutr. 1993;58:100–103. doi: 10.1093/ajcn/58.1.110. [DOI] [PubMed] [Google Scholar]
- 56.Sewer MB, Koop DR, Morgan ED. Endotoxemia in rats is associated with induction of the P4504A subfamily and suppression of several other forms of cytochrome P450. Drug Metab Dispos. 1996;24:401–407. [PubMed] [Google Scholar]
- 57.Skare KL, DeLuca HF. Bilary metabolites of all-trans-retinoic acid in the rat. Arch Biochem Biophys. 1983;224:13–18. doi: 10.1016/0003-9861(83)90185-6. [DOI] [PubMed] [Google Scholar]
- 58.Smith MA, Adamson PC, Balis FM, Feusner J, Aronson L, Murphy RF, Horowitz ME, Reaman G, Hammond GD, Fenton RM. Phase I and pharmacokinetic evaluation of all-trans-retinoic acid in pediatric patients with cancer. J Clin Oncol. 1992;10:1666–1673. doi: 10.1200/JCO.1992.10.11.1666. [DOI] [PubMed] [Google Scholar]
- 59.Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983;2:585–588. doi: 10.1016/s0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- 60.Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr. 1984;40:1090–1095. doi: 10.1093/ajcn/40.5.1090. [DOI] [PubMed] [Google Scholar]
- 61.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 62.Swanson BN, Frolik CA, Zaharevitz DW, Roller PP, Sporn MB. Dose-dependent kinetics of all-trans-retinoic acid in rats. Plasma levels and excretion into bile, urine, and faeces. Biochem Pharmacol. 1981;30:107–113. doi: 10.1016/0006-2952(81)90180-5. [DOI] [PubMed] [Google Scholar]
- 63.Takatsuka J, Takahashi N, DeLuca LM. Retinoic acid metabolism and inhibition of cell proliferation: an unexpected liaison. Cancer Res. 1996;56:675–678. [PubMed] [Google Scholar]
- 64.Tokuyama H, Tokuyama Y. Endogenous cytokine expression profiles in retinoic acid-induced IgA production by LPS-stimulated murine splenocytes. Cell Immunol. 1995;66:247–253. doi: 10.1006/cimm.1995.9973. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Zolfaghari R, Ross AC. Cloning of rat cytochrome P450RAI(CYP26) cDNA and regulation of its gene expression by all-trans- retinoic acid in vivo. Arch Biochem Biophys. 2002;401:235–243. doi: 10.1016/S0003-9861(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 66.White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, Petkovich M. cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes P450. J Biol Chem. 1997;272:18538–18541. doi: 10.1074/jbc.272.30.18538. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto Y, Zolfaghari R, Ross AC. Regulation of CYP26 (cytochrome P450RAI) mRNA expression and retinoic acid metabolism by retinoids and dietary vitamin A in liver of mice and rats. FASEB J. 2000;14:2119–2127. doi: 10.1096/fj.00-0061com. [DOI] [PubMed] [Google Scholar]
- 68.Zile MH, Inhorn RC, DeLuca HF. Metabolites of all-trans-retinoic acid in bile: identification of all-trans- and 13-cis-retinoyl glucuronides. J Biol Chem. 1982;257:3537–3543. [PubMed] [Google Scholar]
- 69.Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase from mouse and rat liver: cDNA cloning and liver-specific regulation by dietary vitamin A and retinoic acid. J Lipid Res. 2000;41:2024–2034. [PubMed] [Google Scholar]