Abstract
Purpose
To quantitatively assess the superoxide dismutase 1 (SOD1), transforming growth factor, beta 1 (TGF-β1), and dual-specificity phosphatase 1 (DUSP1) messenger ribonucleic acid (mRNA) expression levels as the main intracellular reactive oxygen species neutralizers, wound healing mediators, and immunomodulators (respectively) in keratoconic (KCN) and non-KCN corneas.
Methods
Total RNA was extracted from normal and keratoconic cultured corneal stromal fibroblasts. Semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) was used to measure the relative expression levels of mRNAs of the SOD1, TGF-β1, and DUSP1 genes.
Results
The mRNA expression of TGF-β1 and DUSP1 was augmented in the KCN corneas (three- and fivefold, respectively; both p<0.05). The KCN and non-KCN samples showed no difference in comparative SOD1 mRNA levels.
Conclusions
This study demonstrated a higher level of DUSP1 and TGF-β1 expression as known molecules in the inflammatory process. These results may provide new insight into the complex molecular pathways underlying KCN for investigating other inflammatory molecules.
Introduction
Keratoconus (KCN) is the most common cause of non-inflammatory corneal thinning that leads to progressive myopia, irregular astigmatism, and cornea curvature changes [1]. KCN has an incidence rate of between 50 and 230 and a prevalence of 8.8 to 54.4 people per 100,000 [2]. KCN occurs in the second decade of life, and the rate of progression is heterogeneous. There is no significant difference in gender and race preponderance [3,4]. KCN is one of the leading causes of penetrating keratoplasty in developed countries. The etiology of KCN has not been fully clarified; however, KCN is categorized in three major classes: KCN associated with genetic disorders and syndromes (such as Down syndrome, nail-patella syndrome, neurofibromatosis, Ehlers-Danlos syndrome, Marfan syndrome, etc.); KCN in the setting of commonly reported associations (contact lens wear, eye rubbing, atopy, allergy, Leber congenital amaurosis, mitral valve prolapse, and positive family history); and isolated KCN with no associations [2].
KCN usually occurs as an isolated disease, but sometimes there is a positive family history [2]. In such cases, autosomal recessive and dominant patterns of inheritance have been reported [5,6]. Histologically, KCN is characterized by breakage in the Bowman membrane and subepithelial scarring. The affected areas have marked alterations in extracellular matrix (ECM) components because of the disturbance in enzyme activities and apoptosis, which leads to thinning of the corneal stroma [7-14].
The human eye is vulnerable to oxidative damage because of light exposure, high metabolic activity, and oxygen tension that lead to reactive oxygen species (ROS) production. As primary antioxidant systems, superoxide dismutase (SOD) isoenzymes catalyze the dismutation of the superoxide radicals to generate hydrogen peroxide. Since the superoxide anion radical exerts its effects locally and the anion poorly penetrates the membranes, different kinds of SODs (SOD1, SOD2, and SOD3) compartmentalize in the cytosol, mitochondrial matrix, and extracellular space [15]. It is suggested the predominance of a variant SOD1 transcript with exon 2-skipping or exon 2- and 3-skipping deletions may lead to a decrease in enzyme activities in the KCN cornea [16,17]; thus, in this study, SOD1 was investigated as an intracellular isoenzyme in KCN and non-KCN corneas.
Dual-specificity phosphatases (DUSPs) or mitogen-activated protein kinase phosphatases (MKPs) are a subset of proteins, many of which dephosphorylate threonine and tyrosine residues on mitogen-activated protein kinases. For immune cells, DUSPs regulate responses in positive and negative ways and are key regulators of immune responses [18,19]. One of the external stimuli that activate DUSP1 gene expression is oxidative stress [20]. To better understand the phenomenon, we characterized the expression of the DUSP1 gene in KCN and non-KCN corneas. Transforming growth factor beta 1 (TGF-β1), which is associated with various corneal dystrophies, is involved in regulating keratocyte activation, myofibroblast transformation and proliferation, chemotaxis, and wound healing [21]. In this study, we investigated the mRNA expression of DUSP1, TGF-β, and SOD1 genes in the stromal primary cell culture in KCN and non-KCN corneas.
Methods
This study was approved by the Institutional Review Board (IRB) of Tehran University of Medical Sciences, Iran, and informed consent was obtained from all family members wishing to participate in this study before corneal transplantation. Non-KCN cornea-derived fibroblasts belonging to sclerocorneal rims were obtained from healthy donor subjects within 24 h of death, and the KCN corneas were removed at the time of penetrating keratoplasty. All patients had advanced KCN (Noor Eye Hospital, Tehran, Iran). After the epithelium and the endothelium were removed, stromal explants were cultured in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen, Auckland, New Zealand) supplemented with 20% fetal bovine serum (Invitrogen), 100 units/ml penicillin (Invitrogen), and 100 mg/ml streptomycin (Invitrogen).
The medium was changed twice a week. When the cells reached the appropriate confluency (70%), the culture medium was completely removed, and the cells were washed with PBS (Invitrogen; 1.05 mM KH2PO4, 155.17 mM NaCl, 2.96 mM Na2HPO4-7H2O and a pH 7.4) and detached enzymatically with 0.25% trypsin–0.02% EDTA (Invitrogen). For genetic analysis, cultured corneal stromal fibroblasts of the third passage were harvested and maintained at −70 °C.
Total ribonucleic acid isolation
Total RNA was extracted using a High Pure RNA Isolation Kit (Roche, Mannheim, Germany), from the cultured stromal fibroblasts of KCN and non-KCN corneas according to the manufacturer’s instructions. Briefly, the lysis buffer was added to the cell pellets and homogenized by pipetting. After mixing well, the solution was applied to the filter tube and centrifuged. After DNase I treatment, total RNA was eluted in TE buffer (Tris-HCl 10 mM, EDTA 1 mM). The quality and quantity of the total RNA were determined with electrophoresis and ultraviolet-spectrophotometry with Nano-Drop ND-2000 (Thermo Scientific, Wilmington, NC), respectively.
Semiquantitative reverse transcriptase–polymerase chain reaction
Two microgram of total extracted RNA from cultured corneal stromal fibroblasts was reverse trancribed using QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany) according to protocols supplied by the manufacturers. The expression of the genes was investigated with reverse transcription–polymerase chain reaction (RT–PCR) using gene-specific primers (see Table 1 for the primer sequences). The PCR reaction was performed with a final reaction volume of 25 μl containing 50 ng of first-strand cDNA, 0.6 μl of primer F (10 μM), 0.6 μl of primer R (10 μM), and 10 μl of 2× Taq Polymerase Master Mix (Ampliqon, Copenhagen, Denmark) together with the beta 2 microglobulin (β2M) gene used as the internal control. Then, PCR was performed with a thermalcycler (ABI 2720, Applied Biosystems, Foster City, CA) under the following conditions: an initial denaturating step (94 °C for 3 min), a cycling step (denaturation at 94 °C for 30 s), annealing (58 °C for 30 s), extension (72 °C for 20 s), and final extension (72 °C for 5 min). The amplified PCR products were separated in 2% agarose gel and visualized under ultraviolet light after staining with ethidium bromide. No template and RT-minus controls were run to detect contamination, dimer formation, and presence of genomic DNA.
Table 1. RT–PCR Primers Used for B2M, TGFB1, DUSP1 and SOD1.
| Target gene | Primer sequences | PCR product size |
|---|---|---|
|
B2M
(NM_004048.2) |
F: 5′-TATCCAGCGTACTCCAAAGA-3′
R: 5′-GACAAGTCTGAATGCTCCAC-3′ |
191 |
|
TGFB1
(NM_000358) |
F: 5′-ATCACCAACAACATCCAGCA-3′
R: 5′-CCGTTACCTTCAAGCATCGT-3′ |
165 |
|
DUSP1
(NM_004417) |
F: 5′-GAGGGTCACTACCAGTACAAGAGC-3′
R: 5′-GCCTGGCAGTGGACAAACA-3′ |
137 |
| SOD1 (NM_000454) | F: 5′-CAGTGCAGGGCATCATCAAT-3′ R: 5′-CATTGCCCAAGTCTCCAACA-3′ | 222 |
All primers are from 5′ to 3′. β2M, beta-2-microglobulin; SOD1, superoxide dismutase1; DUSP1, Dual-specificity phosphatases; TGF-β1, Transforming growth factor beta1
For semiquantitative RT–PCR and exponential phase determination, PCR was performed with β2M primers for all samples. PCR products of different cycles (21, 24, 27, and 30) were loaded on 2% agarose gel. Based on band intensity, cycle 24 was the best representative of the exponential phase.
According to the β2M results, semiquantitative RT–PCR was performed to measure gene expression for SOD1, DUSP1, and TGF-β1 in ten KCN samples and non-KCN samples. The RT–PCR conditions were the same as discussed previously. Normal corneal samples were pooled for reducing tissue preparation errors. Amplicons were quantified using system image analysis performed with a GS-800 Calibrated Imaging Densitometer (BioRad Laboratories, Hercules, CA), and the bands were standardized to the β2M levels.
Statistical analysis
The independent sample Student t test was used to compare SOD1, DUSP1, and TGF-β1 expression between the KCN and non-KCN samples. We considered 0.05 the level of statistical significance.
Result
Cell culture
Seven days after tissue cultivation, the cells migrated out of the tissue pieces. When the colonies were visible (it took about 2 weeks), the tissue pieces drained out of the plates (Figure 1). After they achieved confluence (70%), they were transferred to tissue culture flasks. The second and third subcultures were used for total RNA extraction. The disease stage, gender, and concentration of the RNAs obtained from the KCN samples are depicted in Table 2.
Figure 1.
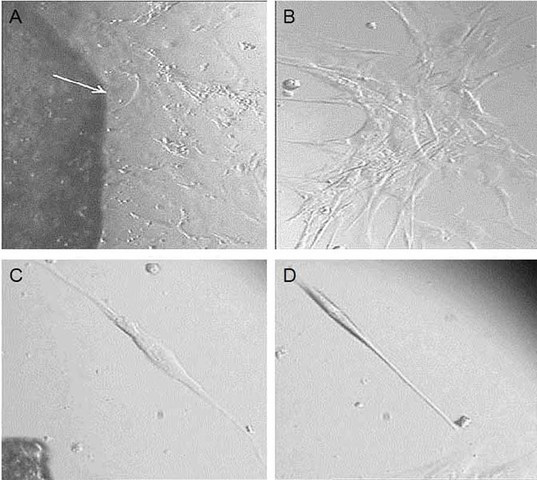
Primary corneal tissue culture. This figure represents primary tissue culture from sample no. 4 under light microscopy. A: The migration of cells out of the tissue started on day 7 after seeding (white arrow shows margin of tissue, 10X magnification). B: One of the cell colonies was performed after 15 days post-seeding (20X magnification). C, D: After cell culture expansion, the cultivated cells were starved for 24 h to compare the morphological difference between fibroblasts and keratocytes (C, D, respectively).
Table 2. Characteristics of KCN and normal corneas.
| Sample no. | Gender | Disease stage | RNA concentration (ng/ul) |
|---|---|---|---|
| 1 |
M |
Advance |
585 |
| 2 |
M |
Advance |
434 |
| 3 |
M |
Advance |
443 |
| 4 |
F |
Advance |
646 |
| 5 |
F |
Advance |
590 |
| 6 |
M |
Advance |
660 |
| 7 |
F |
Advance |
731 |
| 8 |
F |
Advance |
234 |
| 9 |
M |
Advance |
758 |
| 10 | M | Advance | 327 |
Semiquantitative reverse transcriptase–polymerase chain reaction
Based on band intensity, cycle 24 was the best representative of exponential phase (Figure 2). Total extracted RNAs were subjected to cDNA synthesis followed by semiquantitative RT–PCR. Results of gel electrophoresis in the 24th cycle are shown in Figure 3.
Figure 2.
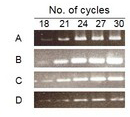
Determination of the exponential cycle of amplification. Semiquantitative reverse transcription–polymerase chain reaction (RT–PCR) mixtures were taken for five alternating cycles starting at cycles 18, 21, 24, 27, and 30 for A: beta 2 microglobulin (B2M), B: superoxide dismutase 1 (SOD1), C: dual-specificity phosphatase 1 (DUSP1), and D: transforming growth factor, beta 1 (TGF-B1). Cycle 24 was selected as an exponential cycle.
Figure 3.
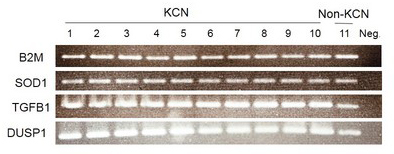
Differential messenger ribonucleic acid expression of the target genes via semiquantitative reverse transcription–polymerase chain reaction (RT-PCR; 24th cycle). This figure shows the results of the RT–PCR products in 2% agarose gel electrophoresis, stained with ethidium bromide. Lanes 1 to 10: Keratoconus (KCN) corneas; lane 11: pooled normal corneas and lane 12: negative control without RT. The fluorescent intensity of the bands was quantified with a GS-800 densitometer. The signal intensities were normalized to values obtained for beta 2 microglobulin (B2M). The messenger ribonucleic acid (mRNA) expression of transforming growth factor, beta 1 (TGF-β1), and dual-specificity phosphatase 1 (DUSP1) was increased in the KCN corneas (three- and fivefold, respectively; both p<0.05). The comparative superoxide dismutase 1 (SOD1) mRNA level showed no difference between the KCN and non-KCN samples.
Image analysis
Amplicons were quantified using system image analysis performed with a GS-800 Calibrated Imaging Densitometer (BioRad Laboratories). The results of the target genes image densitometry were normalized with the housekeeping gene (β2M) as shown in Figure 4. As demonstrated in Figure 4, the average mRNA level of SOD1 in KCN and non-KCN showed no significant difference (0.353 versus 0.36), but statistical analysis showed overexpression of TGF-β1 and DUSP1 mRNA levels in the KCN samples (three- and fivefold; both p<0.05), respectively.
Figure 4.
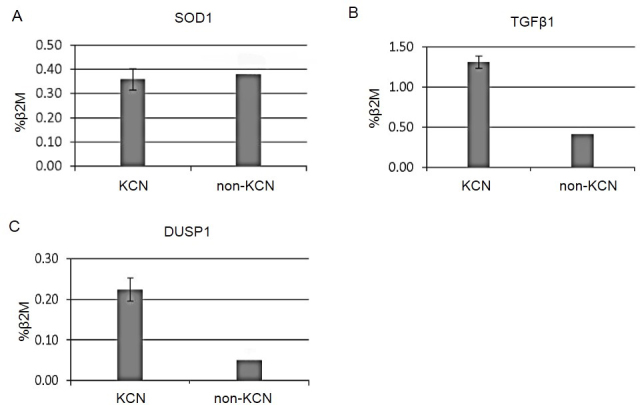
The comparative mRNA expression of target genes in ten keratoconus (KCN) and pooled normal corneal tissues. This graph indicates the comparison of transcript band intensities for genes encoding superoxide dismutase 1 (SOD1), transforming growth factor, beta 1 (TGF-β1), and dual-specificity phosphatase 1 (DUSP1) between the KCN and non-KCN samples. The values were calculated relative to the beta 2 microglobulin (B2M) levels (as normalizer) and reported as mean percentages. Note that the KCN corneas showed a three- and fivefold increase in TGF-B1 and DUSP1 when compared to the pooled normal corneas. The RNA levels for SOD1 were similar for the KCN and normal corneas (p<0.05). Normal corneal samples were pooled to reduce tissue preparation errors. The error bars represent the standard deviation.
Discussion
KCN is a corneal disorder with heterogeneity and variability in symptoms. The pathogenesis of KCN is partially known. Many hypotheses have been suggested regarding its etiology [22].
In the late 1990s, oxidative stress and damaging environmental factors were suggested as key players in changing corneal tissue integrity [23]. This view is supported by the observation that ROS such as superoxide, hydrogen peroxide, hydroxyl radicals, and nitric oxide increase in KCN samples compared with normal samples [24-26]. Although epithelial damage due to corneal surface exposure to exterior stimuli (ultraviolet radiation, trauma, atopy, allergy, pathogens, and contact lenses) predisposes the corneal tissue to KCN development [22,27], cytokines and growth factors with chemotactic effects on other inflammatory cells induce epithelial damage processes [27-29].
These observations challenge the pathophysiological concept of KCN. Today, we know that other concomitant factors such as oxidative stress and many genes play an important role; therefore, in this study, the expression of three genes in hypothesized pathways with focus on oxidative stress and the immune response mechanism was evaluated.
Dual-specificity phosphatase 1
DUSP1/MKP1, the first member of the heterogeneous family of dual-specificity phosphatases, is located on 5q34 [30]. DUSP1 dephosphorylates mitogen-activated protein kinases, which are evolutionary conserved enzymes that play an important role in orchestrating various cellular processes, including proliferation, differentiation, and apoptosis. This mediator has a regulatory role in innate and adaptive immune responses by inactivating p38 and c-Jun N-terminal kinase [31,32]. Some stimuli such as lipopolysaccharides, hypoxia, heat shock proteins, and oxidative stress can activate DUSP1 gene expression [33]. Our results showed a significant increase in the DUSP1 mRNA levels in almost all the KCN samples compared with the pooled non-KCN samples. These findings may indicate an ongoing stress- or inflammation-related reaction in KCN fibroblasts and are consistent with data that propose that environmental stresses are the main causes of KCN pathophysiology [7,26,29]. There is no evidence for investigating DUSP1 mRNA expression in corneal cells.
Superoxide dismutase 1
Superoxide dismutase gene maps to chromosome 21q22 [34,35]. This gene encodes three major mammalian enzymes (SOD1, SOD2, and SOD3) that have been identified in the cytosol, mitochondrial matrix, and its secretory form in tissue interstitium, respectively. They catalyze superoxide to hydrogen peroxide and molecular oxygen [34,35]. One of the main factors in the oxidative stress pathway is environmental stimuli such as ultraviolet exposure and epithelial trauma that damage corneal cells and may lead to destruction of the cell vital sites such as the nucleus and mitochondria. Loss of mitochondrial membrane integrity causes ROS efflux. This efflux can trigger apoptosis in corneal stromal cells as the first frontline of exposure.
Although oxidative stress has been nominated as the main cause of cell damage in KCN and a decrease in SOD1 expression has previously been demonstrated in KC corneas [16,36], the mRNA expression level of SOD1 was similar in the KCN and non-KCN samples in our study. Our results confirm other studies that have shown similar RNA levels for SOD1 in KCN and normal corneas [37]. According to our data, stromal cell ROS accumulation and mitochondrial damage might not be the only source of cellular damage and apoptosis.
Transforming growth factor, beta 1
The TGF-β1 gene, which is located on 19q13.1 [38], is a persuasive divergent growth factor and participates in embryogenesis, development, immune, and inflammatory processes. The gene’s ligands and receptors are expressed in various types of cells [39-42]. Previous studies have reported TGF-β1 as a known mediator in corneal fibrosis and wound healing [43]. TGF-β1 regulates cell cycle progression by binding to transforming growth factor, beta receptor II (TGFβR2) and activating TGF-βR1. The TGF-βR1 receptor can be inactivated in many cases of human tumor cells refractory to TGF-β-mediated cell cycle arrest [44]. In this study, we investigated the TGF-β1 mRNA level in KCN and non-KCN fibroblasts. Our results showed a significant increase in TGF-β1 expression levels in KCN samples.
Engler et al. provided evidence that TGF-β signaling pathway components were upregulated in advanced KCN [39]. This pathway is one of the main cascades that modulate ECM elements and cells through its ligands and receptors.
According to the literature, KCN is defined as the non-inflammatory thinning of the cornea, and ROS are considered the main players in the pathogenesis of KCN; however, few studies have focused on the function of the inflammatory cells and mediators in KCN. In addition, our data indicated high expression of TGF-β1 as a wound healing mediator in KCN samples. It would be useful to further investigate the role of inflammation in correlation with different components of the ECM separately and in the context of tissue. Based on our body of data and definition of KCN, these new findings would create better insight into the mechanisms of KCN to find ways to prevent disease progression or to discover new treatment options.
Acknowledgments
This work was supported by a grant number NORC900415 from Noor ophthalmology Reasearch Center, Noor Eye Hospital. The authors are grateful to Fatemeh Jafari and Mr. Olaad-daar for their kind collaboration.
References
- 1.Gillan W. The stroma and keratoconus: a review. S Afr Optom. 2007;66:87–93. [Google Scholar]
- 2.Abu-Amero KK, Kalantan H, Al-Muammar AM. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol Vis. 2011;17:667–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma R, Titiyal JS, Prakash G, Sharma N, Tandon R, Vajpayee RB. Clinical profile and risk factors for keratoplasty and development of hydrops in north Indian patients with keratoconus. Cornea. 2009;28:367–70. doi: 10.1097/ICO.0b013e31818cd077. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy RHBW, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian SA, Pye DC, Willcox MDP. Are proteinases the reason for keratoconus? Curr Eye Res. 2010;35:185–91. doi: 10.3109/02713680903477824. [DOI] [PubMed] [Google Scholar]
- 6.Saee-Rad S, Hashemi H, Miraftab M, Noori-Daloii MR, Chaleshtori MH, Raoofian R, Jafari F, Greene W, Fakhraie G, Rezvan F, Heidari M. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol Vis. 2011;17:3128–36. [PMC free article] [PubMed] [Google Scholar]
- 7.Cristina Kenney M, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. 2003;26:139–46. doi: 10.1016/S1367-0484(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 8.Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV. Abnormalities of the extracellular matrix in keratoconus corneas. 1997. p. 345. [PubMed] [Google Scholar]
- 9.Tuori A, Virtanen I, Aine E, Uusitalo H. The expression of tenascin and fibronectin in keratoconus, scarred and normal human cornea. Springer; 1997. p. 222–9. [DOI] [PubMed] [Google Scholar]
- 10.Kao WWY, Vergnes JP, Ebert J, Sundar-Raj CV, Brown SI. Increased collagenase and gelatinase activities in keratoconus. Elsevier; 1982. p. 929–36. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SEKW. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39:220–6. [PubMed] [Google Scholar]
- 12.SE W. Stimulus-specific and cell type-specific cascades:emerging principles relating to control of apoptosis in the eye. Exp Eye Res. 1999;69:255–66. doi: 10.1006/exer.1999.0698. [DOI] [PubMed] [Google Scholar]
- 13.SE W. Role of apoptosis in wound healing in the cornea. Cornea. 2000;19(Suppl 3):S7–12. doi: 10.1097/00003226-200005001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Twining SS, Sugar J, Yue BY. Involvement of Sp1elements in the promoter activity of the alpha1-proteinase inhibitor gene. J Biol Chem. 1998;273:9959–65. doi: 10.1074/jbc.273.16.9959. [DOI] [PubMed] [Google Scholar]
- 15.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 16.Arnal E, Peris-Martínez C, Menezo JL, Johnsen-Soriano S, Romero FJ. Oxidative stress in Keratoconus? Invest Ophthalmol Vis Sci. 2011;52:8592–7. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 17.Udar N, Atilano SR, Brown DJ, Holguin B, Small K, Nesburn AB, Kenney MC. SOD1: a candidate gene for keratoconus. Invest Ophthalmol Vis Sci. 2006;47:3345–51. doi: 10.1167/iovs.05-1500. [DOI] [PubMed] [Google Scholar]
- 18.Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–92. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 19.Hammer M, Echtenachter B, Weighardt H, Jozefowski K, Rose‐John S, Männel DN, Holzmann B, Lang R. Increased inflammation and lethality of Dusp1−/− mice in polymicrobial peritonitis models. Immunology. 2010;131:395–404. doi: 10.1111/j.1365-2567.2010.03313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweikl H, Hiller K-A, Eckhardt A, Bolay C, Spagnuolo G, Stempfl T, Schmalz G. Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials. 2008;29:1377–87. doi: 10.1016/j.biomaterials.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Chen Y, Han SN. Comparison of TGF-β1 in tears and corneal haze following Epi-LASIK with and without mitomycin C. Int J Ophthalmol. 2013;6:312–5. doi: 10.3980/j.issn.2222-3959.2013.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33:157–66. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Atilano SR, Coskun P, Chwa M, Jordan N, Reddy V, Le K, Wallace DC, Kenney MC. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1256–63. doi: 10.1167/iovs.04-1395. [DOI] [PubMed] [Google Scholar]
- 24.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–9. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW, Kenney MC. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1902–10. doi: 10.1167/iovs.05-0828. [DOI] [PubMed] [Google Scholar]
- 26.Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. SAGE Publications; 2002. p. 341. [DOI] [PubMed] [Google Scholar]
- 27.McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33:265–71. doi: 10.1097/ICL.0b013e31814fb64b. [DOI] [PubMed] [Google Scholar]
- 28.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45:3011–20. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 29.McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28:607–15. doi: 10.1097/ICO.0b013e318198384f. [DOI] [PubMed] [Google Scholar]
- 30.Israeli O, Goldring-Aviram A, Rienstein S, Ben-Baruch G, Korach J, Goldman B, Friedman E. In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genet Cytogenet. 2005;160:35–42. doi: 10.1016/j.cancergencyto.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–89. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 32.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato ACB, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. ARVO; 1998. p. 471. [PubMed] [Google Scholar]
- 35.Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Elsevier; 2008. p. 1047–55. [DOI] [PubMed] [Google Scholar]
- 36.Rozalen M, Peris‐Martinez C. Oxidative stress in keratoconus. Acta Ophthalmol (Copenh) 2012;•••:90. [Google Scholar]
- 37.Kenney MC, Chwa M, Atilano SR, Tran A, Carballo M, Saghizadeh M, Vasiliou V, Adachi W, Brown DJ. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46:823–32. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 38.Chang WW, Zhang L, Jin Y-l, Yao Y-s. Meta-analysis of the transforming growth factor-β1 polymorphisms and susceptibility to Alzheimer’s disease. J Neural Transm. 2013;120:353–60. doi: 10.1007/s00702-012-0850-7. [DOI] [PubMed] [Google Scholar]
- 39.Engler C, Chakravarti S, Doyle J, Eberhart CG, Meng H, Stark WJ, Kelliher C, Jun AS. Transforming Growth Factor-B Signaling Pathway Activation in Keratoconus. Am J Ophthalmol. 2011;151:752–9. doi: 10.1016/j.ajo.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li DQ, Lee SB, Tseng SCG. Differential expression and regulation of TGF-b1, TGF-b2, TGF-b3, TGF-bRI, TGF-bRII, and TGF-bRIII in cultured human corneal, limbal, and conjunctival fibroblasts. London: IRL Press,[c1981-; 1999. p. 154–61. [DOI] [PubMed] [Google Scholar]
- 41.Li D-QTS. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 42.Massagué J, Wrana JL. The TGF-b family and its composite receptors. Trends Cell Biol. 1994;4:172–8. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 43.Karamichos D, Hutcheon AE. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010;51:1382–8. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vellucci VF, Reiss M. Cloning and genomic organization of the human transforming growth factor-beta type I receptor gene. Genomics. 1997;46:278–83. doi: 10.1006/geno.1997.5023. [DOI] [PubMed] [Google Scholar]


