Abstract
Context
Although circulating glycosylphosphatidylinositol-specific phospholipase D, a minor high density lipoprotein-associated protein, is elevated in patients with insulin resistance or high triglycerides, no information is available on the effect of weight loss or changes in insulin sensitivity on circulating glycosylphosphatidylinositol-specific phospholipase D levels.
Objective
Determine the effect of weight loss and changes in insulin sensitivity on plasma glycosylphosphatidylinositol-specific phospholipase D levels.
Participants
Forty two non-diabetic obese women.
Intervention
Three month dietary intervention randomizing patients to a low fat or a low carbohydrate diet.
Main outcome measures
Plasma glycosylphosphatidylinositol-specific phospholipase D levels and insulin sensitivity as estimated by the homeostasis model assessment.
Results
The very low carbohydrate diet group lost more weight after 3 months (−7.6 ± 3.2 vs. −4.2 ± 3.5 kg, P < 0.01) although the decrease in insulin resistance was similar between groups. Weight loss with either diet did not alter plasma glycosylphosphatidylinositol-specific phospholipase D levels. However, baseline glycosylphosphatidylinositol-specific phospholipase D levels correlated with the change in insulin sensitivity in response to the low fat diet while baseline insulin sensitivity correlated the change in insulin sensitivity in response to the low carbohydrate diet.
Conclusions
Plasma GPI-PLD may serve as a clinical tool to determine the effect of a low fat diet on insulin sensitivity.
Keywords: glycosylphosphatidylinositol phospholipase D, diet, obesity, insulin sensitivity, women
Introduction
Insulin resistance and type 2 diabetes are increasing worldwide, due in part to the aging of Western Society as well as to the prolific increase in the prevalence of obesity. Perhaps the major adverse correlate of insulin resistance is increased cardiovascular mortality. The increased risk for cardiovascular disease is due to a multitude of atherogenic changes including increased thrombosis, inflammation, hypertension, and dyslipidemia. The dyslipidemia of insulin resistance is characterized by increases in serum triglycerides and decreases in high density lipoproteins (HDL).
We and others have described a unique, minor HDL-like particle in plasma containing apolipoproteins AI and AIV along with glycosylphosphatidylinositol-specific phospholipase D (GPI-PLD) [1, 2]. GPI-PLD is expressed in nearly all tissues and cells types but liver has the highest level of GPI-PLD expression and is the primary source of circulating GPI-PLD [3-7]. Similar to other minor, HDL-associated proteins, GPI-PLD is involved in triglyceride metabolism and associated with insulin resistance. For example, it has recently been demonstrated that circulating GPI-PLD is higher in human subjects with elevated triglycerides or insulin resistance [8]. Consistent with this finding, hepatic GPI-PLD mRNA and serum GPIPLD levels are increased in patients with nonalcoholic fatty liver disease [9], a condition that is associated with increased serum triglycerides, fatty acid synthesis, and insulin resistance. Although a causative role for GPI-PLD in human disease has not been proven, evidence from studies of animals and cultured cells raise this possibility. Overexpressing hepatic GPI-PLD increases serum triglycerides in mice, by reducing triglyceride-rich lipoprotein catabolism [9], and promotes the expression of genes involved in fatty acid synthesis in hepatoma cells [10]. Taken together these data implicate GPI-PLD as a component of the dysregulation of lipid metabolism associated with insulin resistance.
Weight loss associated with caloric restriction is known to improve insulin sensitivity in obese people. We hypothesized that weight loss would be associated with changes in serum levels of GPI-PLD. Furthermore, we hypothesized that the macronutrient composition of the weight loss diet would influence this effect. To evaluate this hypothesis we compared concentrations of GPI-PLD in the plasma of healthy women who participated in a randomized trial of low-carbohydrate and low-fat weight loss diets [11].
Materials and Methods
Participants
We previously reported the results of a randomized trial comparing low-fat and very low carbohydrate diets in 42 obese women [11]. The mean age was 43.73 ± 7.72 years, mean BMI was 33.63 ± 1.86 kg/m2, and the mean percent body fat was 41.36 ± 3.22%. Participants were randomized to either a reduced calorie low fat diet (mean self reported macronutrient content after 3 months was 28% fat, 18% protein, 54% carbohydrate) or to an ad libitum low carbohydrate diet (mean reported macronutrient content after 3 months was 57% fat, 28% protein, 15% carbohydrate). Both dietary groups reported a reduced caloric intake by approximately 450 calories after 3 months. The very low carbohydrate diet group lost more weight after 3 months (−7.6 ± 3.2 vs. −4.2 ± 3.5 kg, P < 0.01) [11]. The magnitude of improvement in insulin resistance did not differ between the dietary groups (Table 1) [12]. Baseline and 3 month frozen plasma samples (obtained in the fasting state) were assayed for GPI-PLD. All participants gave written, informed consent for participating in the study. The Institutional Review Board of the University of Cincinnati approved this study.
Table 1.
Effect of dietary intervention on serum GPI-PLD levels
| Low fat | Low carbohydrate | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | Delta | Baseline | 3 months | Delta | |
| GPI-PLD (μg/ml) | 189 ± 50 | 187 ± 55 | −1.4 ± 25.7 | 186 ± 37 | 193 ± 49 | 7.0 ± 30.2 |
| HOMA-IR | 5.3 ± 2.2 | 4.1 ± 2.8* | 4.5 ± 2.1 | 2.9 ± 1.9* | ||
Serum GPI-PLD was determined as described in Materials and Methods before and after dietary intervention. There was no statistically significant effect of either diet on GPI-PLD as determined by paired t-test. HOMA-IR was determined as described in Materials and Methods and were reported elsewhere [12]. HOMA-IR is shown to aid the reader.
P < 0.03 vs. baseline.
Biochemical assays
Plasma GPI-PLD mass was determined by ELISA as described previously [8]. GPI-PLD mass is stable in the frozen state for at least 3 years (M. Deeg, unpublished observation). GPI-PLD activity was not measured since serum triglycerides will alter GPI-PLD activity in vitro [8]. Other chemistries (glucose, insulin, cholesterol, triglycerides, LDL, HDL, and leptin) were determined as previously described [11]. Serum amyloid A (SAA), C reactive protein (CRP), and interleukin-6 were measured as markers of systemic inflammation and were determined as reported elsewhere for this cohort [11]. Insulin resistance was calculated using the homeostasis model assessment (HOMA-IR) as previously described [13].
Statistical analyses
Continuous variables were compared by paired t-test, unpaired t-test, or one-way ANOVA as appropriate. Pearson correlation coefficients were calculated to examine the relationship between variables. Multivariate analyses were done by Best Subset Regression and all baseline variables were included in the analysis. Results are expressed as mean ± SD and P < 0.05 was considered statistically significant. Statistical analyses were performed with SigmaStat (v3.1, SyStat Software, Inc. San Jose, CA)
Results
Effect of weight loss on plasma GPI-PLD levels
At baseline, plasma GPI-PLD levels did not differ between the two groups (Table 1). The baseline level of plasma GPI-PLD in this cohort was nearly twice a cohort with nonalcoholic fatty liver disease [10] but similar to a cohort with type 2 diabetes and low HDL [14]. Baseline plasma GPI-PLD levels did not correlate with baseline values for BMI (p = 0.637), systolic blood pressure (p = 0.34), diastolic blood pressure (p = 0.90), percent body fat (p = 0.53), percent lean body mass (p= 0.38), or percent bone mineral content (p = 0.78). There was no correlation of baseline GPI-PLD levels with any dietary parameters, including total intake of calories (p = 0.30), carbohydrates (p = 0.54), protein (p = 0.38), fat (p = 0.29), saturated fat (p = 0.31), monounsaturated fat (p= 0.67), or polyunsaturated fat (p=0.68).
In univariate analyses, baseline plasma GPI-PLD correlated with CRP (r = −0.321, p = 0.04) and SAA (r= 0.-365, p = 0.019) but not with glucose (p = 0.162), insulin (p = 0.97), HOMA-IR (p = 0.87), leptin (p = 0.099), total cholesterol (p = 0.65), triglycerides (p = 0.15), HDL (p = 0.107), low density lipoproteins (LDL) (p = 0.44), or interleukin-6 (p = 0.91). In a multivariable analysis, baseline GPI-PLD levels were predicted by a model including leptin and SAA (model 2, Table 2).
Table 2.
Multivariate analyses of baseline GPI-PLD.
| β | P | R2 | |
|---|---|---|---|
| Model #1 | |||
| Serum amyloid A | −6.053 | 0.003 | 0.315 |
| Model #2 | |||
| Serum amyloid A | −5.350 | 0.005 | 0.445 |
| Leptin | −2.204 | 0.029 |
After 3 months, the weight loss was greater in the very low carbohydrate group compared to the low fat group but the improvement in insulin resistance was similar between dietary groups (Table 1). Plasma GPI-PLD levels were not affected by either diet (Table 1). After weight loss, plasma GPI-PLD did not correlate with any of the dietary variables (total Kcal, macronutrient content, quantity or type of fat). In the total cohort, the change in GPI-PLD did not correlate with the change in any other variable examined. However, the change in GPI-PLD at 3 months did correlate with the change in SAA in participants on the low fat diet (r = 0.505, p = 0.0326), but not in those on the low carbohydrate diet (r = −0.0167, p = 0.941).
Effect of diet on insulin resistance
At baseline, both cohorts had mild insulin resistance as estimated by HOMA-IR (Table 1). Previous analyses showed that both diets improved insulin resistance as demonstrated by a reduction in HOMA-IR with most patients having normal insulin sensitivity after 3 months [12]. The change in HOMA correlated with the change in SAA but not with changes in CRP or weight [12]. Since GPI-PLD has been shown in other studies to correlate with insulin resistance [8, 10], we examined the effect of macronutrient composition on the relationship between plasma GPI-PLD and insulin resistance. Unlike previous cohorts [8, 10], baseline plasma GPI-PLD did not correlate with baseline HOMA-IR (p=0.87). The change in HOMA-IR did not correlate with the changes in either BMI or dietary fat for the total cohort or each diet group. The change in HOMA-IR did not correlate with any of the changes in serum chemistries although did approach statistical significance for HDL (−0.301, p=0.0553). However, the change in HOMA-IR was predicted by two baseline variables: GPI-PLD (r = −0.348, p = 0.0259) and baseline HOMA (r = −0.419, p=0.00646).
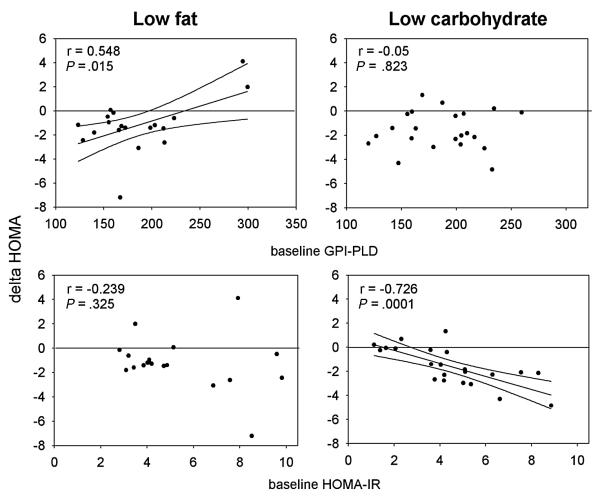
When these associations were examined within each dietary cohort, GPI-PLD predicted the change in insulin resistance in subjects randomized to the low fat diet (r = 0.548, p = 0.0151) but not in response to those randomized to the very low carbohydrate diet (r = 0.05, p = 0.823) (Figure 1). After adjusting for weight loss, baseline GPI-PLD was still an independent predictor of the change in insulin sensitivity. In contrast, baseline HOMA-IR predicted the change in HOMA-IR on the low carbohydrate diet (r = −0.726, p=0.000132) but not the low fat diet (r = −0.239, p = 0.325) (Figure 1).
Figure 1. Correlation between baseline GPI-PLD or HOMA-IR with changes in insulin resistance.
The correlation between baseline GPI-PLD or HOMA-IR with the changes in HOMA-IR was determined as described in Materials and Method. For each comparison the regression line is shown (solid line) along with its 95% confidence intervals (dotted line). An increase in HOMA-IR is consistent with a worsening of insulin resistance.
Discussion
Weight loss is associated with improvements in various metabolic parameters including reductions in lipids, inflammatory markers, and insulin resistance. There are two primary findings in this study: 1) weight loss did not produce a change in plasma GPI-PLD despite improvements in many parameters including a reduction in insulin resistance. This suggests that in the simplest model, plasma GPI-PLD levels are not regulated by insulin resistance per se although this conclusion is limited by the relatively small improvement in insulin sensitivity; the only variable associated with the change in GPI-PLD was the change in SAA, and 2) Improvements in HOMA-IR in response to the low fat diet correlated with baseline GPI-PLD.
There are two broad means of affecting plasma GPI-PLD levels: regulation of GPI-PLD expression and synthesis in the liver and alterations in metabolism of the HDL-like particle containing GPI-PLD in the plasma compartment. A few regulators of GPI-PLD expression in the liver have been identified including insulin [15]. Hepatic GPI-PLD expression is increased in mouse models of type 1 and type 2 diabetes [15, 16]. Plasma GPI-PLD levels also may be affected by metabolism of the GPI-PLD-containing particle within the plasma compartment [9, 14]. Interestingly, low fat and low carbohydrate diets have different effects on minor HDL particles in humans [17]. Hence, it is conceivable that the different diets do affect either GPIPLD expression or plasma compartment metabolism but the effects oppose each other resulting in no or little change in plasma levels. More detailed studies are needed to examine this hypothesis.
Another interesting observation of this study is the correlation of GPI-PLD with SAA: both baseline levels and changes in response to the diets. Like GPI-PLD, SAA is carried primarily on HDL in the plasma [18], and its levels are raised in the setting of obesity [12, 19-21] and insulin resistance [19, 22]. While both GPI-PLD and SAA are produced primarily by the liver [15, 18], both are also produced in macrophages [23, 24]. The contribution of macrophages to circulating GPI-PLD and SAA is unknown. Thus, GPI-PLD and SAA share many similarities and may be physically or physiologically linked via HDL metabolism. For these reasons, it is perhaps not surprising that GPI-PLD and SAA levels should correlate strongly in humans.
Interestingly, baseline plasma GPI-PLD predicted the change in insulin sensitivity in response to the low fat diet and baseline HOMA predicted the change in insulin sensitivity in response to the very low carbohydrate diet. The latter observation has been observed in other studies [25]. Few biomarkers have been identified that predict the change in insulin sensitivity. These include adiponectin [26] and tissue fat content, particularly skeletal and hepatic fat content [27-29].
What are the differences between losing weight with a low fat diet vs. a low carbohydrate? Both result in reductions in 1) weight loss and 2) insulin resistance. In the short term (<3 months) studies, the very low carbohydrate diet generally results in greater weight loss and improvements in insulin resistance although we did not see the latter effect in this cohort. Another difference is the effect on hepatic fat content.
Hepatic fat content closely correlates to hepatic and whole body insulin sensitivity [27]. Hypocaloric, low fat diets result in the reduction in hepatic fat while low carbohydrate diets have no effect or increase hepatic fat content [28, 30, 31]. We have also noted that serum GPI-PLD and liver GPI-PLD mRNA are increased in patients with fatty liver [10]. Hence, it is possible that circulating GPI-PLD may be a marker for hepatosteatosis. However, the fact that we did not see plasma GPI-PLD decrease on the low fat diet may reflect 1) hepatic fat did not change significantly in this cohort or 2) that plasma GPI-PLD levels are regulated in a complicated fashion as discussed above. Further work is needed to test this hypothesis directly.
Another obvious difference between the diets is the absolute and relative types of fat in the diet. The total and type of fat in the diet influences the effect on insulin sensitivity. In eucaloric but not hypocaloric diets, high fat diets induce hepatic steatosis and worsen insulin sensitivity [32]. Diets high in saturated fat worsen insulin resistance but only when total fat is less than 37% of total calories [33]. Hence, there is a complicated interaction between dietary fat and insulin sensitivity. Although we did not see a relationship between GPI-PLD and total fat or type fat in this study, we have noted in animal studies that dietary fat influences hepatic GPIPLD expression and serum levels (M. Deeg, unpublished observation). One of the limitations of our study is that dietary intake was only assessed by self-report. Other studies have documented that people under-report dietary intake and that obese persons under-report more than nonobese [34-36].
Based on its association with insulin resistance, one would have expected GPI-PLD levels to fall with weight loss in the very low carbohydrate diet group, but they did not. One possible explanation for this is that the high cholesterol content of the very low carbohydrate diet induced inflammation that upregulated GPI-PLD expression, thereby counteracting the GPIPLD-lowering effect of improved insulin resistance. In addition to the liver, GPI-PLD also can be produced by macrophages [37]. One recent study has demonstrated that addition of cholesterol to a diabetogenic diet dramatically increases abdominal adipose tissue macrophage content in LDL-receptor null mice [38] though the addition of dietary cholesterol does not result in weight gain greater than that seen with a diabetogenic diet alone. Thus, at least in that animal model, dietary cholesterol induced abdominal inflammation that was dissociated from the degree of weight gain.
This study has limitations. HOMA is an indirect marker of insulin resistance and may also reflect changes in insulin secretion. The length of the study (3 months) may also result in numerous compensatory changes that were not measured and therefore not included in our analyses.
In summary, plasma GPI-PLD was not altered by weight loss regardless of diet type but did predict the change in insulin sensitivity in response to a low fat but not low carbohydrate diet. Hence, measuring GPI-PLD may serve as a clinical tool to determine the effect of a low fat diet on insulin sensitivity. Additional studies are needed to confirm this hypothesis.
Acknowledgements
This work was supported by a Veterans Affairs Merit Award (MAD), the American Heart Association (BJB), University of Cincinnati Obesity Research Center (BJB), University of Cincinnati Research Council (BJB), Children’s Hospital Medical Center Clinical Research Center (supported in part by USPHS Grant M01-RR-08084 from the General Clinical Research Center Program, National Center for Research Resources, NIH) and NIH Grants DK-54263, DK-56863, and DK57900 (DAD). We thank Dr. Kieren Mather for insightful comments.
Footnotes
Disclosure: DLG, KDO, DAD, and BJB have nothing to declare. MAD is currently employed by and has an equity interest in Eli Lilly & Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Deeg MA, Bierman EL, Cheung MC. GPI-specific phospholipase D associates with an apoA-I- and apoA-IV-containing complex. J Lipid Res. 2001;42(3):442–51. [PubMed] [Google Scholar]
- [2].Hoener MC, Brodbeck U. Phosphatidylinositol-glycan-specific phospholipase D is an amphiphilic glycoprotein that in serum is associated with high-density lipoproteins. European Journal of Biochemistry. 1992;206:747–57. doi: 10.1111/j.1432-1033.1992.tb16981.x. [DOI] [PubMed] [Google Scholar]
- [3].Xie M, Sesko AM, Low MG. Glycosylphosphatidylinositol-specific phospholipase D is localized in keratinocytes. American journal of physiology. 1993;265:C1156–C68. doi: 10.1152/ajpcell.1993.265.4.C1156. [DOI] [PubMed] [Google Scholar]
- [4].Deeg MA, Verchere CB. Regulation of GPI-specific phospholipase D secretion from ßTC3 cells. Endocrinology. 1997;138:819–26. doi: 10.1210/endo.138.2.4940. [DOI] [PubMed] [Google Scholar]
- [5].Xie M, Low MG. Expression and secretion of glycosylphosphatidylinositol-specific phospholipase D by myeloid cell lines. Biochemistry Journal. 1994;297:547–54. doi: 10.1042/bj2970547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stadelmann B, Zurbiggen A, Brodbeck U. Distribution of glycosylphosphatidylinositol-specific phospholipase D mRNA in bovine tissue sections. Cell & Tissue Research. 1993;274:547–52. doi: 10.1007/BF00314552. [DOI] [PubMed] [Google Scholar]
- [7].LeBoeuf RC, Caldwell M, Guo Y, Metz C, Davitz MA, Olson LK, et al. Mouse glycosylphosphatidylinositol-specific phospholipase D (Gpld1) Characterization. Mammalian Genome. 1998;9(9):710–4. doi: 10.1007/s003359900851. [DOI] [PubMed] [Google Scholar]
- [8].Kurtz TA, Fineberg NS, Considine RV, Deeg MA. Insulin resistance is associated with increased serum levels of glycosylphosphatidylinositol-specific phospholipase D. Metabolism: clinical and experimental. 2004;53:138–9. doi: 10.1016/j.metabol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [9].Raikwar NS, Cho WK, Bowen RF, Deeg MA. Glycosylphosphatidylinositol-specific phospholipase D influences triglyceride-rich lipoprotein metabolism. Am J Physiol Endocrinol Metab. 2006;290:E463–70. doi: 10.1152/ajpendo.00593.2004. [DOI] [PubMed] [Google Scholar]
- [10].Chalasani N, Vuppalanchi R, Raikwar NS, Deeg MA. Glycosylphosphatidylinositol-specific phospholipase d in nonalcoholic Fatty liver disease: a preliminary study. J Clin Endocrinol Metab. 2006 Jun;91(6):2279–85. doi: 10.1210/jc.2006-0075. [DOI] [PubMed] [Google Scholar]
- [11].Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003 Apr;88(4):1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- [12].O’Brien KD, Brehm BJ, Seeley RJ, Bean J, Wener MH, Daniels S, et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005 Apr;90(4):2244–9. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- [13].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- [14].Deeg MA, Raikwar NS, Johnson C, Williams CD. Statin therapy reduces serum levels of glycosylphosphatidylinositol-specific phospholipase D. Transl Res. 2007 Sep;150(3):153–7. doi: 10.1016/j.trsl.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [15].Deeg MA, Bowen RF, Williams MD, Olson LK, Kirk EA, LeBoeuf RC. Increased expression of GPI-specific phospholipase D in mouse models of type 1 diabetes. Am J Physiol Endocrinol Metab. 2001;281(1):E147–54. doi: 10.1152/ajpendo.2001.281.1.E147. [DOI] [PubMed] [Google Scholar]
- [16].Bowen RF, Raikwar NS, Olson LK, Deeg MA. Glucose and insulin regulate GPI-specific phospholipase D expression in islet β cells. Metabolism: clinical and experimental. 2001;50:1489–92. doi: 10.1053/meta.2001.28087. [DOI] [PubMed] [Google Scholar]
- [17].Seshadri P, Iqbal N, Stern L, Williams M, Chicano KL, Daily DA, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. The American journal of medicine. 2004 Sep 15;117(6):398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- [18].Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. European journal of clinical investigation. 1996 Jun;26(6):427–35. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- [19].Tannock LR, O’Brien KD, Knopp RH, Retzlaff B, Fish B, Wener MH, et al. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005 Jun 14;111(23):3058–62. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- [20].Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003 Feb;166(2):387–94. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- [21].Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004 Feb 17;109(6):726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- [22].Ebeling P, Teppo AM, Koistinen HA, Viikari J, Ronnemaa T, Nissen M, et al. Troglitazone reduces hyperglycaemia and selectively acute-phase serum proteins in patients with Type II diabetes. Diabetologia. 1999 Dec;42(12):1433–8. doi: 10.1007/s001250051315. [DOI] [PubMed] [Google Scholar]
- [23].Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proceedings of the National Academy of Sciences of the United States of America. 1994 Apr 12;91(8):3186–90. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O’Brien KD, Pineda C, Chiu WS, Bowen R, Deeg MA. Glycosylphosphatidylinositol-specific phospholipase D is expressed by macrophages in human atherosclerosis and colocalizes with oxidation epitopes. Circulation. 1999;99(22):2876–82. doi: 10.1161/01.cir.99.22.2876. [DOI] [PubMed] [Google Scholar]
- [25].Volek JS, Sharman MJ. Cardiovascular and hormonal aspects of very-low-carbohydrate ketogenic diets. Obesity research. 2004 Nov;12(Suppl 2):115S–23S. doi: 10.1038/oby.2004.276. [DOI] [PubMed] [Google Scholar]
- [26].Thamer C, Haap M, Bachmann O, Zur Nieden T, Tschritter O, Stefan N, et al. Serum adiponectin levels predict the effect of short-term dietary interventions on insulin sensitivity in humans. Diabetologia. 2004 Jul;47(7):1303–5. doi: 10.1007/s00125-004-1430-7. [DOI] [PubMed] [Google Scholar]
- [27].Yki-Jarvinen H. Fat in the liver and insulin resistance. Annals of medicine. 2005;37(5):347–56. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]
- [28].Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005 Jun;90(6):3191–6. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- [29].Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002 Jan;51(1):144–51. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- [30].Westerbacka J, Lammi K, Hakkinen AM, Rissanen A, Salminen I, Aro A, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005 May;90(5):2804–9. doi: 10.1210/jc.2004-1983. [DOI] [PubMed] [Google Scholar]
- [31].Mostad IL, Qvigstad E, Bjerve KS, Grill VE. Effects of a 3-day low-fat diet on metabolic control, insulin sensitivity, lipids and adipocyte hormones in Norwegian subjects with hypertriacylglycerolaemia and type 2 diabetes. Scandinavian journal of clinical and laboratory investigation. 2004;64(6):565–74. doi: 10.1080/00365510410007053. [DOI] [PubMed] [Google Scholar]
- [32].Kasim-Karakas SE, Tsodikov A, Singh U, Jialal I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. The American journal of clinical nutrition. 2006 Apr;83(4):774–9. doi: 10.1093/ajcn/83.4.774. [DOI] [PubMed] [Google Scholar]
- [33].Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001 Mar;44(3):312–9. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- [34].Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. The New England journal of medicine. 1992 Dec 31;327(27):1893–8. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- [35].Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. The American journal of clinical nutrition. 1990 Sep;52(3):421–5. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- [36].Prentice AM, Black AE, Coward WA, Davies HL, Goldberg GR, Murgatroyd PR, et al. High levels of energy expenditure in obese women. British medical journal (Clinical research ed. 1986 Apr 12;292(6526):983–7. doi: 10.1136/bmj.292.6526.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Brien KD, Alpers CE, Hokanson JE, Wang S, Chait A. Oxidation-specific epitopes in human coronary atherosclerosis are not limited to oxidized low-density lipoprotein. Circulation. 1994;94:1216–25. doi: 10.1161/01.cir.94.6.1216. [DOI] [PubMed] [Google Scholar]
- [38].Subramanian S, O’Brien KD, Kirk E, Chait A. Dietary cholesterol selectively increases visceral adipose tissue macrophage accumulation in obese LDL receptor-deficient mice. Program of the ATVB 2007 Annual Conference. 2007;2007:105. [Google Scholar]