Abstract
Chronic hypoxia causes pulmonary hypertension associated with structural alterations in pulmonary vessels and sustained vasoconstriction. The transcriptional mechanisms responsible for these distinctive changes are unclear. We have previously reported that CREB1 is activated in the lung in response to alveolar hypoxia but not in other organs. To directly investigate the role of α and Δ isoforms of CREB1 in the regulation of pulmonary vascular resistance we examined the responses of mice in which these isoforms of CREB1 had been inactivated by gene mutation, leaving only the β isoform intact (CREBαΔ mice). Here we report that expression of CREB regulated genes was altered in the lungs of CREBαΔ mice. CREBαΔ mice had greater pulmonary vascular resistance than wild types, both basally in normoxia and following exposure to hypoxic conditions for three weeks. There was no difference in rho kinase mediated vasoconstriction between CREBαΔ and wild type mice. Stereological analysis of pulmonary vascular structure showed characteristic wall thickening and lumen reduction in hypoxic wild-type mice, with similar changes observed in CREBαΔ. CREBαΔ mice had larger lungs with reduced epithelial surface density suggesting increased pulmonary compliance. These findings show that α and Δ isoforms of CREB1 regulate homeostatic gene expression in the lung and that normal activity of these isoforms is essential to maintain low pulmonary vascular resistance in both normoxic and hypoxic conditions and to maintain the normal alveolar structure. Interventions that enhance the actions of α and Δ isoforms of CREB1 warrant further investigation in hypoxic lung diseases.
Introduction
Chronic alveolar hypoxia leads to the development of pulmonary hypertension (PH) which is characterized by a sustained elevation of pulmonary arterial pressure and pulmonary vascular resistance leading to the development of right ventricular hypertrophy. This direct effect of hypoxia on vascular resistance is unique to the pulmonary circulation. On moving to high altitude previously normal but susceptible lowlanders can develop progressive pulmonary hypertension leading to right ventricular failure which is fatal if not corrected by return to low altitude [1], [2]. Pulmonary hypertension frequently complicates chronic hypoxic lung diseases leading to right ventricular failure and reduced life expectancy [1].
Two important mechanisms that cause elevation in pulmonary vascular resistance in hypoxic pulmonary hypertension are vasoconstriction and structural changes in the vascular bed. First, sustained rho kinase dependent vasoconstriction contributes a major part of the total increase, 50–90% depending on the species [3]–[7]. The second major mechanism is structural change to the pulmonary vascular bed that causes an increase in pulmonary vascular resistance independent of vasoconstrictor activity [3]–[7]. The transcriptional mechanisms that control the changes in gene expression underlying these pulmonary specific responses are clearly unique to the lung but remain to be fully elucidated. We recently reported that the cAMP response element binding factor CREB1 was activated by hypoxia in the lung by phosphorylation of the regulatory serine within the kinase insert domain, but not simultaneously activated in other organs, suggesting a key role for this transcription factor in the specific pulmonary responses to hypoxia [8].
Previous work had shown that adenoviral mediated expression of a dominant-negative form of CREB (Ad-CREB-M1) attenuated systemic vascular smooth muscle cell hypertrophy induced by angiotensin-II [9]. Furthermore, transfection of Ad-CREB-M1 at the site of balloon angioplasty injury reduced subsequent neointimal formation [10]. Taken together these reports suggested that CREB activation contributed to disease progression in systemic vascular diseases. In contrast, other reports suggested that CREB could exert a protective effect in vascular disease. These included evidence that CREB expression was reduced in diseased regions of blood vessels in which there was a high rate of proliferation and that activation of CREB could inhibit vascular smooth muscle proliferation [11]. In view of these contradictory data, it was unclear whether the hypoxia-induced CREB activation in the lung in vivo that we had previously reported acted to worsen or to ameliorate hypoxic pulmonary hypertension [8].
The CREB1 gene encodes three functional isoforms of CREB α (also known as isoform B), Δ (isoform A) and β (isoform C) produced by alternative splicing. CREB Δ is ubiquitously expressed in adult tissues and is the most abundant isoform in normal adult tissues, comprising approximately 60–70% of the total CREB in a tissue [12]. The transactivation potential of CREB α and Δ are approximately equal whereas that of CREB β is significantly less [12]. Furthermore, CREB β when expressed together with CREB Δ does not significantly enhance promoter activity [12]. Thus, in normal tissues CREB β is thought to play a minor role in the control of CREB regulated gene expression [12].
Deletion of the three functional splice variants of CREB1, α, β and Δ, is not compatible with postnatal survival [12], [13]. Mice with a homozygous disruption of exon 2 of the CREB gene which leads to loss of the α and Δ isoforms with continued expression of CREB β, are viable and have been previously used to study memory formation and cognitive performance in which they show a hypomorphic phenotype [14]. To directly examine the role of the α and Δ isoforms of CREB1 in the lung in vivo, we investigated the pulmonary vascular responses of these hypomorphic, CREBαΔ−/− mice in normoxia and following sustained hypoxia and revealed a central role for these two isoforms of CREB1 in maintaining the normally low pulmonary vascular resistance.
Materials and Methods
Detailed methods are available in File S1.
Ethic statement
All procedures involving using mice were approved by the University College Dublin Animal Research Ethics Committee and carried out under license (B100/3430) issued by the Department of Health & Children in accordance with European Communities Regulations 2002 (Amendment of Cruelty to Animals Act 1876) and EU Directive 2010/63/EU. All surgery was performed under sodium pentobarbital anesthesia that induced abolition of reflex withdrawal responses.
Mice
CREBαΔ mutant mice were generated as described previously [14], leading to deletion of exon 2 of the CREB1 gene (see File S1 for details), which encodes the N-terminus shared by the CREB α and Δ isoforms. Thus these mice continue to express the CREB β isoform, which does not contain the amino acid sequence coded by exon 2 [12]. The initial CREB heterozygous breeding pairs were purchased from the Jackson Laboratory and genotyping was performed according to the provider's instructions (see File S1 for details).
Note that the exon structure and numbers used throughout the manuscript are those of the most recent update (NCBI Reference Sequence: NC_000067.6). However, the original CREB α, Δ and β nomenclature of the three functional isoforms has been used to facilitate reference to the previous literature; these correspond to isoforms B, A and C respectively (see File S1 for details).
Adult male and female mice (age, 10–12 weeks) were exposed to hypoxic conditions in an environmental chamber (FiO2 = 0.10) for 3 weeks, and age- and weight-matched controls were maintained in normoxic conditions (FiO2 = 0.21) [15]. Mice were killed by exsanguination under anesthesia for isolation of tissues, which were frozen for later extraction of protein for immunoblotting, and mRNA for real-time polymerase chain reaction.
Assessment of Pulmonary Vascular Resistance
Pulmonary vascular resistance was assessed with an isolated ventilated lung preparation perfused at constant flow [4], [16]. Afterwards, the hearts were fixed for later determination of right to left ventricular plus septum ratio.
Stereological Morphometry
After anesthesia, anticoagulation, and exsanguination, mouse lungs were perfused with horse blood at standard pressure (30 cm H2O) and fixed with intratracheal glutaraldehyde (25 cm H2O). Left lung volumes were measured, and lungs were then processed to obtain isotropic, uniform, random resin-embedded sections (1 μm) for stereological quantification of the pulmonary vasculature by a blinded reviewer [17]. This method of tissue fixation and embedding was chosen as it prevents shrinkage of the lung during processing (See File S1).
Real-time Polymerase Chain Reaction
Total RNA was extracted and reverse-transcribed (RT) to cDNA according to the manufacturer's protocol. TaqMan real-time PCR was performed using 18S rRNA as the endogenous loading control gene. Reactions were carried out on the ABI PRISM 7900 Sequence Detection System. Relative quantification of mRNA expression levels was determined using the standard curve method and normalized to 18S.
Western blotting
Whole lung lysates were prepared in radioimmuno-precipitation assay (RIPA) buffer and homogenized by mechanical disruption. Equal amounts of protein extract from each lung (15 μg per sample) were separated by SDS-PAGE, transferred onto PVDF membranes and detected using appropriate antibodies and secondary antibodies labeled with horseradish peroxidase. An identical amount of protein from a standard sample formed by pooling homogenate from a panel of normal lungs was loaded onto each gel to act as a loading and transfer control. Optical intensity of each sample was recorded by digital imaging, quantified using ImageJ software and normalized to the pooled standard sample.
Statistical Analyses
Normally distributed data are reported as means ± SEM, while non-normally distributed data are presented as medians ± interquartile range (IQR). For normally distributed data, statistical significance of differences between two group means was determined using t-tests. For four group designs, normally distributed data were analyzed using 2–factor analysis of variance to seek statistically significant effects of oxygen concentration and genotype, and interactions between these two. For non-normally distributed data, statistical significance was determined using the Mann-Whitney rank sum (unpaired); P values were computed using the exact (permutation) method. For four group designs in which the data were non-normally distributed, correction for multiple post hoc comparisons were made using the Holms-Sidak step-down procedure [18]. Values of P<0.05 were accepted as statistically significant.
Results
Although CREBαΔ−/− mice (subsequently referred to as CREBαΔ) appeared healthy and showed no developmental abnormalities, the yield of CREBαΔ mice (approximately 3%) following crossing of pairs of mice heterozygous for the deletion was substantially below the expected Mendelian frequency had the mutation been without effect, in agreement with previous reports [14].
CREBαΔ and wild type mice showed a similar loss of weight in response to sustained hypoxia (Table 1). Both wild type and CREBαΔ mice showed a characteristic increase in hematocrit following hypoxic exposure. However, CREBαΔ mice showed a significantly greater hematocrit following hypoxic exposure than wildtypes (Table 1) and a statistically significant (P<0.01) interaction between genotype and hypoxic exposure was observed.
Table 1. Hematocrit and body weight in wild-type and CREBαΔ mice under normoxic and hypoxic conditions.
| Wild-type | CREBαΔ | P values | |||||
| Normoxic | Hypoxic | Normoxic | Hypoxic | Genotype | Inspired O2 | Interaction | |
| N number (M:F) | 15 (8:7) | 14 (8:6) | 13 (8:5) | 17 (10:7) | |||
| Hematocrit (%) | 43.6 (±0.6) | 60.1 (±2.1) | 43.5 (±0.7) | 69.2 (±2.0) | 0.006 | <0.001 | 0.006 |
| Entry weight (g) | 27.0 (±0.9) | 27.9 (±0.9) | 27.2 (±1.0) | 26.4 (±0.9) | NS | NS | NS |
| Change in weight (%) | 2.7 (±1.2) | −11.3 (±1.6) | 1.4 (±1.8) | −14.7 (±3.5) | NS | <0.001 | NS |
Data are expressed as mean (±SEM). P values are determined by 2-way ANOVA for effects of changes in genotype, changes in inspired oxygen, and their interaction. M, male; F, female; NS, not significant.
Effect of CREBαΔ mutation on CREB expression
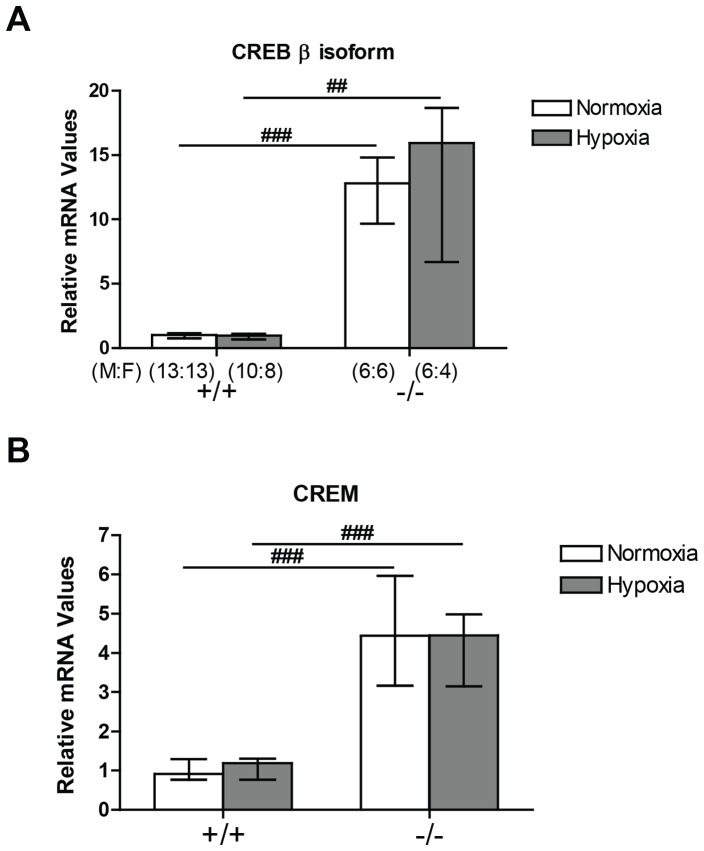
Expression of CREB β and cAMP responsive element modulator (CREM) mRNA was markedly increased in the mice with mutation of CREBαΔ although expression of these CREB family members was not further altered by hypoxia in either genotype (Figure 1).
Figure 1. CREB β and CREM mRNA expression in the mouse lung by quantitative real-time PCR.
Expression level is shown as median ± interquartile range. (A) CREB β was significantly elevated in the CREBαΔ mouse lung compared to wild-type mice in normoxia and in hypoxia. (B) CREBβ was significantly elevated in the CREBαΔ mouse lung compared to wild-type mice in normoxia and in hypoxia. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical in panel B. ## and ### indicate significant difference from corresponding wild-type mice group (P<0.01 and P<0.001, respectively).
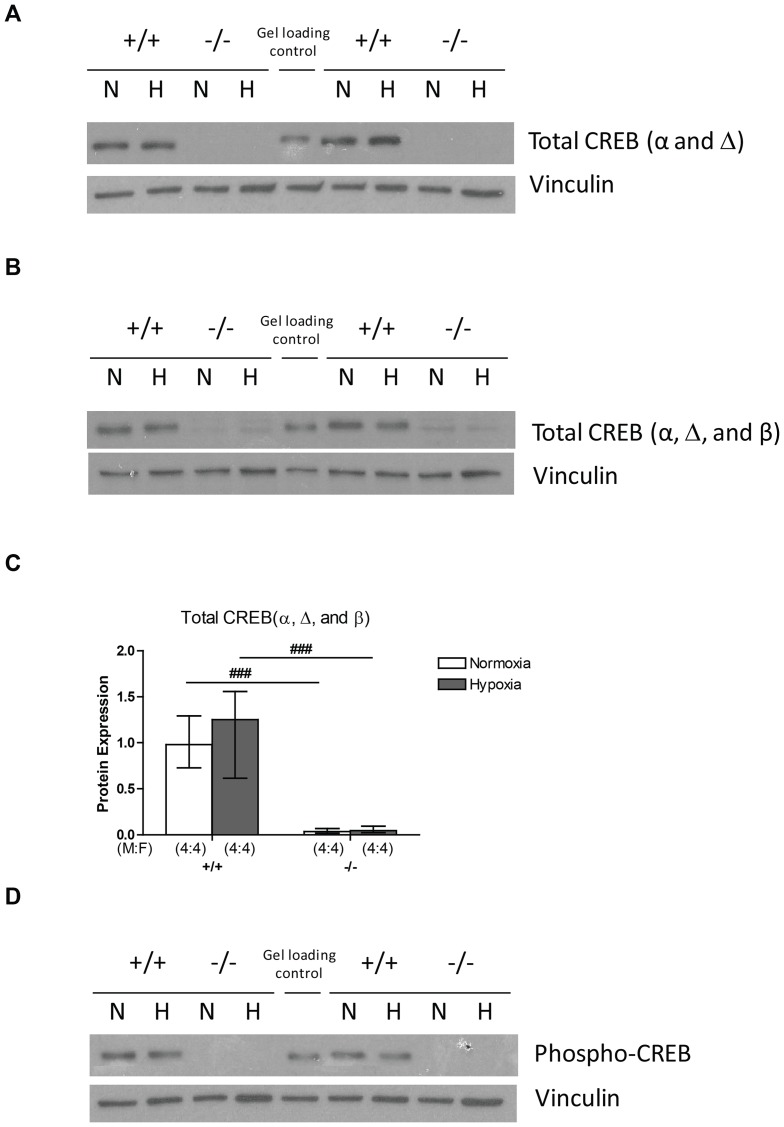
We next examined the expression of CREB protein by western blotting using three different antibodies. By using an antibody directed at an N-terminus epitope, the terminus which the α and Δ isoforms share, we showed that total level of α and Δ isoforms remain unchanged in wildtype mice following three weeks of hypoxic exposure, while CREBαΔ mice showed no expression of α and Δ isoforms due to the disruption of exon 2 (Figure 2A). A second antibody directed against an epitope in the C-terminus, the terminus shared by all three isoforms, showed that the total level of CREB protein (α, β, and Δ isoforms) also remained unchanged in wildtype mice in hypoxia (Figure 2B and 2C). In the CREBαΔ mice this antibody showed low levels of CREB1 protein in normoxic lungs compatible with CREB β isoform expression alone, which was unchanged by hypoxia (Figure 2B and 2C). This suggests that the marked upregulation of CREB β isoform mRNA in CREBαΔ mice is not reflected at the protein level. Furthermore, by using a third antibody which detects phosphorylation of the regulatory serine contained in the kinase insert domain shared by all CREB1 isoforms (Ser133 of CREBα), we showed that activated CREB protein remained at similar levels in normoxia and hypoxia in wildtype mice, while the phosphorylated CREB protein was not detectable by western blot in CREBαΔ mice (Figure 2D).
Figure 2. CREB protein in the mouse lung.
(A) Representative images of western blots of CREB protein expression (α and Δ isoforms) under normoxic and hypoxic conditions in wild-type and CREBαΔ mice. (B) Representative images of western blots of total CREB protein expression (α, β, and Δ isoforms) under normoxic and hypoxic conditions in wild-type and CREBαΔ mice. (C) Results of optical density analysis of images of western blots of total CREB protein expression (α, β, and Δ isoforms) in the lungs of wild-type and CREBαΔ mice under normoxic and hypoxic conditions. Values are expressed as median (± interquartile range) optical density values expressed relative to median normoxic value in wild type lungs. ###indicates significant difference from corresponding wild-type mice group (P<0.001). (D) Representative images of western blots of phosphorylated CREB protein level under normoxic and hypoxic conditions in wild-type and CREBαΔ mice.
Effect of CREBαΔ mutation on CREB-regulated gene expression
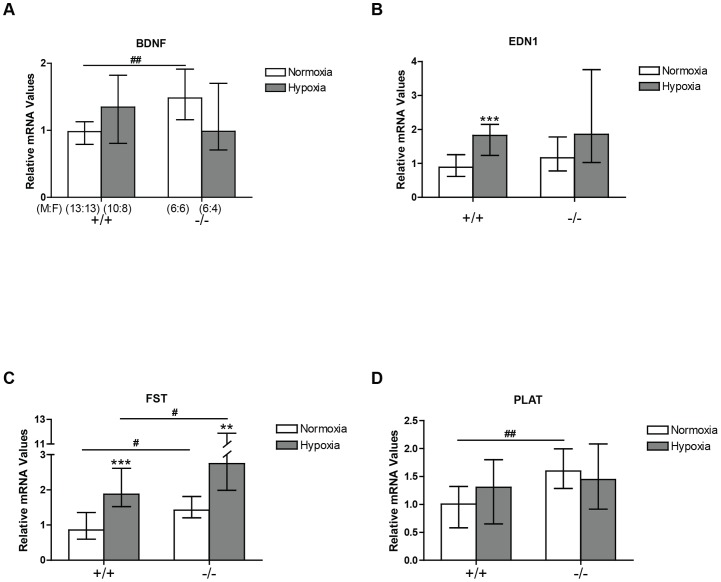
We examined expression of four genes that we had previously identified as regulated by hypoxia in the lung and whose promoters contained bioinformatically predicted CREB binding sequences, brain derived neurotrophic factor (BDNF), endothelin1 (EDN1), follistatin (FST) and tissue plasminogen activator (PLAT) [8]. All four showed statistically significant upregulation in wild type mice following 24 hours exposure to hypoxia, in agreement with our previous results (data not shown) [8].
The expression of three of these, BDNF, FST and PLAT, was significantly increased under normoxic conditions in the CREBαΔ mice when compared to normoxic wild type mice (Figure 3A, 3C, and 3D), Expression of FST was persistently elevated in the hypoxic CREBαΔ mice following 3 weeks of hypoxia when compared to similar hypoxic wild type mice. However, because normoxic basal expression of FST was higher in CREBαΔ mice than in the normoxic wildtype mice, the fold increase in FST expression produced by hypoxia in CREBαΔ mice was similar to that in wild type mice (Figure 3C).
Figure 3. BDNF, EDN1, FST, and PLAT mRNA expression in the mouse lung by real-time PCR.
Values are expressed relative to mean normoxic value in wild type lungs and shown as medians (± interquartile range). (A) BDNF expression (B) EDN1 expression (C) FST expression and (D) PLAT expression in CREBαΔ mice in normoxic and hypoxic conditions. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical for panels B, C, and D. ** and *** indicate significantly different from normoxic groups of the same genotype (P<0.01 and P<0.001, respectively). # and ## significantly different from wild-type mice (P<0.05 and P<0.01, respectively).
CREBαΔ mice show greater pulmonary vascular resistance
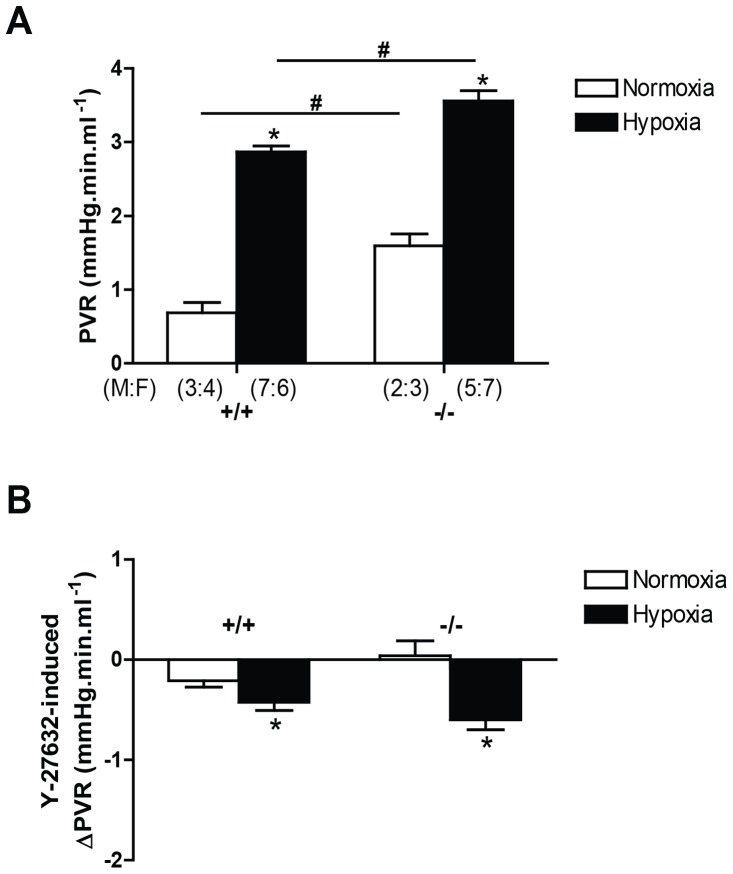
To test the hypothesis that α and Δ isoforms of CREB1 contribute significantly to the control of pulmonary vascular resistance in vivo, we examined pulmonary vascular resistance in normoxia and in response to chronic hypoxia (3 weeks) in homozygous CREBαΔ mice. We observed that pulmonary vascular resistance was significantly greater in both normoxic and hypoxic conditions in homozygous CREBαΔ mice than in wildtypes (Figure 4). Both genotypes showed a significant increase in vascular resistance in hypoxia when compared to that genotype in normoxia.
Figure 4. Pulmonary vascular resistance (PVR) in wild-type and CREBαΔ mice.
Values are shown as mean (± SEM). (A) CREBαΔ mice showed significant increase in both normoxic and hypoxic pulmonary vascular resistance when compared to wild-type mice. (B) The reduction in PVR induced by rho kinase inhibitor (Y27632; 10−5M) was similar in wild-type and CREBαΔ mice under normoxic and hypoxic conditions. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical in panel B. * indicates significantly different from normoxia (P<0.001, 2-way ANOVA). # indicates significantly different from wild-type mice (P<0.001, 2-way ANOVA).
In order to test the role of vasoconstriction on the increased pulmonary vascular resistance in the hypoxic homozygous CREBαΔ mice, we used the potent rho kinase inhibitor and vasodilator Y-27632 (10−5M) and found that chronic hypoxia caused a significantly enhanced vasodilator response (Figure 4). However, genotype did not significantly influence the response to rho kinase inhibition.
RhoA and ROCK expression in CREBαΔ and wild-type mice
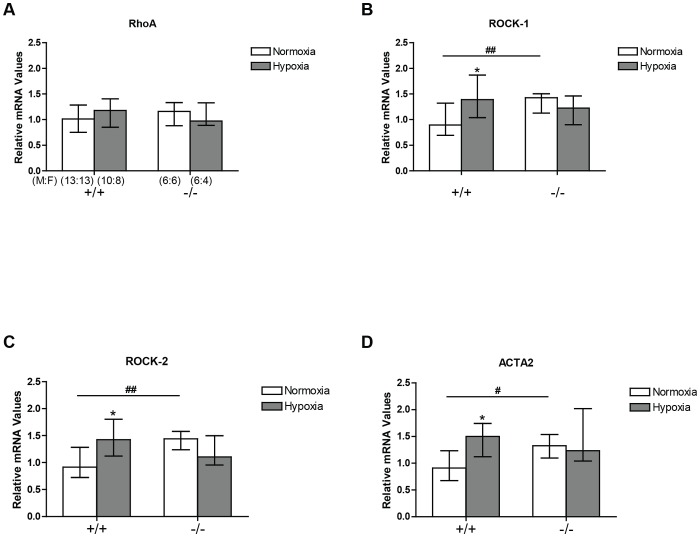
Under normoxic conditions, ROCK1, ROCK2 mRNA and smooth muscle α-actin expression (ACTA2) was significantly elevated in the CREBαΔ mice when compared to wildtypes (Figure 5). In wild type mice expression of these three genes was increased in hypoxia whereas in hypoxic CREBαΔ mice no further increase above their elevated normoxic values was observed (Figure 5).
Figure 5. RhoA, ROCK1 and ROCK2, and ACTA2 mRNA expression in the mouse lung by real-time PCR.
Values are expressed relative to mean normoxic value in wild type lungs and shown as medians (± interquartile range). (A) RhoA expression (B) ROCK–1 expression (C) ROCK–2 expression and (D) ACTA2 expression in the lungs of wild-type and CREBαΔ mice under normoxic and hypoxic conditions. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical for panel B, C, and D. *Significantly different from normoxic groups of the same genotype (P<0.05). #and ## significantly different from wild-type mice (P<0.05 and P<0.01 respectively).
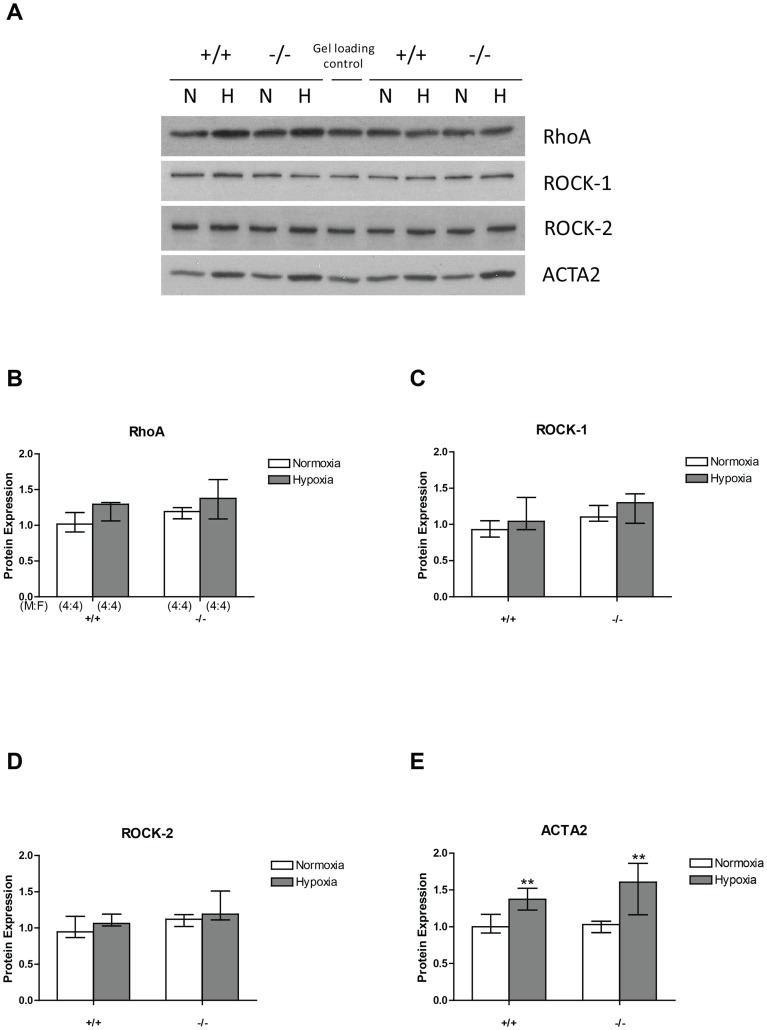
RhoA, ROCK1 and ROCK2 protein expression was unaltered either by hypoxic exposure or the CREB mutation (Figure 6). In contrast, expression of α2 smooth muscle actin (ACTA2) was similar in both wildtypes and CREBαΔ mice under normoxic conditions but increased significantly in both groups following sustained hypoxic exposure (Figure 6E). However, there was no significant difference between the two genotypes following hypoxic exposure (Figure 6E).
Figure 6. RhoA, ROCK1 and ROCK2, and ACTA2 protein in the mouse lung by western blotting.
(A) Representative images of western blots of RhoA, ROCK1, ROCK2, and ACTA2 protein expression under normoxic and hypoxic conditions in wild-type and CREBαΔ mice. Panels B, C, D and E show values of optical density analysis of images of western blots examining RhoA, ROCK–1, ROCK–2 and ACTA2 expression in normoxic and hypoxic lungs of both genotypes. Values are expressed as median (± interquartile range) relative to the mean normoxic value in wild type lungs. (B) RhoA expression (C) ROCK–1 expression (D) ROCK–2 expression and (E) ACTA2 expression in the lungs of wild-type and CREBαΔ mice under normoxic and hypoxic conditions. Number of male and female mice (M:F) in each of the four groups is shown in panel B and is identical for panel C, D, and E.
Pulmonary vascular remodeling in CREBαΔ and wild-type mice
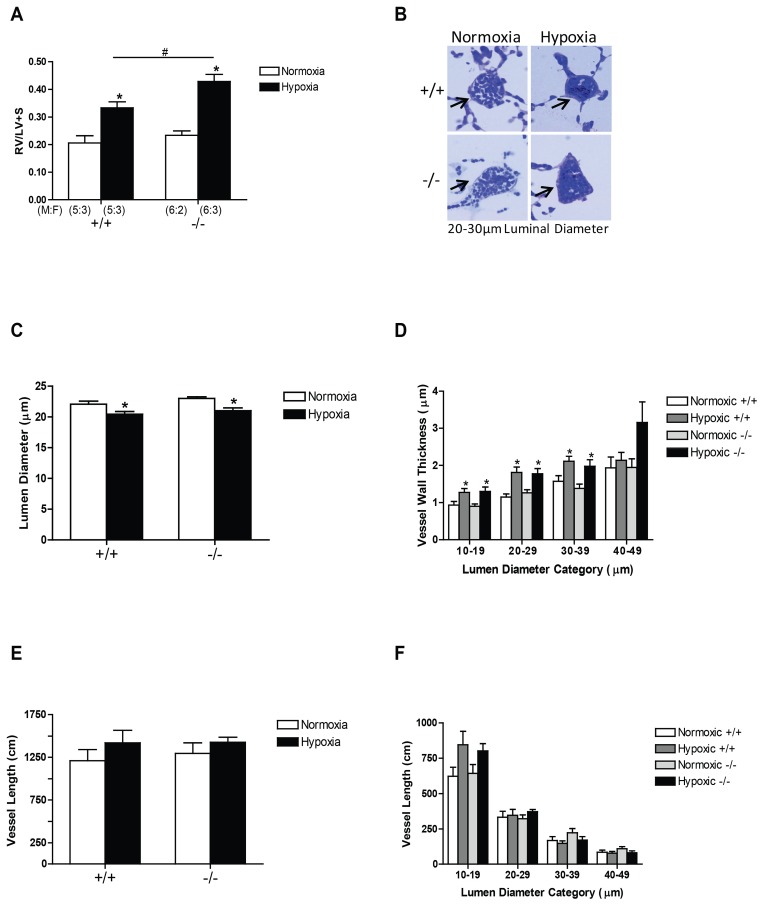
Further groups of mice were exposed to hypoxia and their lungs isolated and fixed at standard vascular and airway pressures following Y-27632-induced vascular relaxation to allow stereological analysis of changes in pulmonary vascular structure [3]. The right ventricular to left ventricular plus septum ratio (RV/LV+S) was increased significantly in both genotypes in response to hypoxia and this increase in RV/LV+S ratio in hypoxia was significantly enhanced by loss of CREB α and Δ isoforms (Figure 7A).
Figure 7. Structural changes in the right ventricle and pulmonary vasculature in wild-type and CREBαΔ mice.
Values are expressed as means ± SEM. (A) The ratio of right ventricular to left ventricular + septum weights (RV/LV+S) in response to sustained hypoxic exposure was significantly higher in the CREBαΔ mice compared to the wild-type group. (B) Representative images of small intra-acinar vessels (arrows) in wild-type and CREBαΔ mice exposed to normoxic and chronically hypoxic conditions (×40 objective). (C) The mean lumen diameter (mean±SEM) was significantly decreased in wild-type mice after 3 weeks of hypoxic exposure and a similar reduction was observed in CREBαΔ mice. (D) The increase in mean vessel wall thickness (mean±SEM) within each vessel size category (based on lumen diameter) was similar in the smaller vessels in CREBαΔ mice and wild-type mice following 3 weeks of hypoxic exposure. (E) The total length of intra-acinar vessels (mean±SEM) was not significantly different in normoxic and hypoxic wild-type and CREBαΔ groups. (F) The length of vessels (mean±SEM) within each vessel category in normoxic and hypoxic conditions in wild-type and CREBαΔ mouse lungs. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical for panels C, D, and E. * indicates significantly different from normoxia (P<0.001, 2-way ANOVA). # indicates significantly different from wild-type mice (P<0.001, 2-way ANOVA).
Microscopic examination of resin sections of lung tissue showed thickening of the walls of the small intra-acinar vessels in the hypoxic groups, which appeared to be similar in wildtypes and CREBαΔ mice (Figure 7B). Stereological analysis showed a significant reduction in mean lumen diameter in hypoxic mice of both genotypes (Figure 7C) and a similar thickening of the walls (Figure 7D) of the blood vessels within the gas exchange regions of the lung (intra-acinar vessels). The length of the intra-acinar vessels was not significantly altered by hypoxia in either of the two genotypes (Figure 7E and 7F).
Alveolar structure in CREBαΔ and wild-type mice
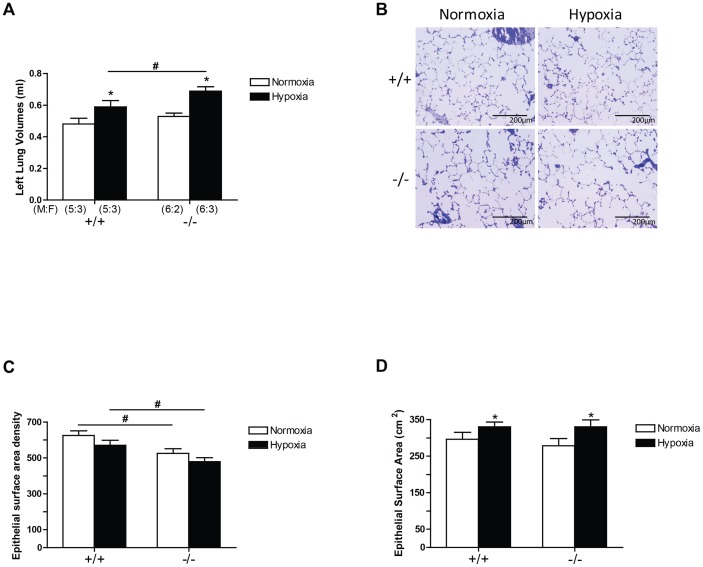
Hypoxia caused significant increases in lung volume (Figure 8A) when inflated at standard airway pressure (25 cm H20), in keeping with the well known increase in lung volume caused by sustained hypoxia [19]. The CREBαΔ mutation also caused significant lung enlargement when compared to wild type mice (Figure 8A).
Figure 8. Left lung volumes, left lung epithelial surface area, and left lung epithelial surface area density.
Values are expressed as mean ± SEM. (A) Hypoxia significantly increased lung volumes in CREBαΔ mice, but not in the wild-type mice. Under hypoxia, CREBαΔ mice had significantly larger lung volumes than the wild-type mice. (B) Representative images of gas exchange area (×20 objective). (C) Both wild-type and CREBαΔ mice had similar total epithelial surface areas in their left lungs under both normoxic and hypoxic condition. (D) Hypoxia had no effect on epithelial surface area density for both genotypes. However, CREBαΔ mice showed reduced epithelial surface area density when compared to wild-type mice under both normoxia and hypoxia. Number of male and female mice (M:F) in each of the four groups is shown in panel A and is identical for panels C and D. * Significantly different from normoxia (P<0.001, 2-way ANOVA). # significantly different from wild-type mice (P<0.001, 2-way ANOVA).
Microscopic examination of the lung demonstrated that the alveoli of CREBαΔ mouse lungs appeared to have larger airspaces (Figure 8B). Quantitative stereological examination revealed that the surface area of alveolar epithelium per cubic centimeter of lung (epithelial surface area density) was significantly reduced by the CREBαΔ mutation (Figure 8C) although the total epithelial surface area per lung was not altered (Figure 8D). Taken together with the increased lung volume, these data suggest that the lungs of the CREBαΔ mice were more compliant (reduced elastic recoil) than those of wild type animals.
Discussion
We have previously reported that alveolar hypoxia, similar to that found in lung diseases and at high altitude, leads to selective phosphorylation and activation of CREB1 in the absence of similar activation in other organs, suggesting that this transcription factor has an important role to play in the pulmonary response to hypoxia [8]. Phosphorylation dependent activation of CREB by hypoxia has also previously been shown in cell studies in vitro [20]–[23]. However, others had reported reduced CREB1 expression in pulmonary vascular smooth muscle following sustained lung hypoxia and suggested that a consequent reduction in CREB1 activity played a key role in pulmonary vascular remodeling and pulmonary hypertension [24], [25]. Hypoxia induced reduction in CREB1 expression and transcriptional activity had previously been noted in gastrointestinal epithelial cells [26]. Subsequently the same group had reported that the hypoxia induced CREB1 reduction was transient and that stable, active CREB1 expression had returned to normal values when hypoxia persisted (>24 hours) for more extended periods [27]. Others, using different cells types, had not found CREB depletion in hypoxia, in agreement with our previous findings in whole lung homogenates [8], [20]–[23]. The exact reasons for these different results were not apparent and, as a result, the role of CREB1 in the hypoxic lung remained unclear.
In the present study, we further explored the role of CREB1 by examining the pulmonary response to hypoxia following partial loss of CREB1 due to homozygous, ubiquitous deletion of two of its three isoforms, α and Δ, and found an increase in pulmonary vascular resistance in these mice under both normoxic and hypoxic conditions. Right ventricular hypertrophy was observed in both hypoxic wildtype and CREBαΔ mice. Of note, the right ventricular hypertrophy was significantly greater in hypoxic CREBαΔ mice. In addition, a greater increase in lung volume in response to hypoxia was observed in CREBαΔ mice associated with a reduction in the alveolar epithelial surface density suggestive of an emphysematous phenotype.
Mice in which all three functional isoforms of CREB, α, β and Δ, have been knocked out, die shortly after birth due to respiratory distress syndrome demonstrating that CREB activity is required for normal lung development [13]. In the CREBαΔ mouse used in these studies, only the CREBβ isoform is expressed; no mRNA for CREB α or Δ can be detected [12]. CREB Δ is the predominant isoform expressed in normal mouse tissues and in these CREBαΔ mice, it has been shown that the CREBβ isoform protein is markedly upregulated in the brain [12]. Another member of the CREB family of transcription factors, CREM, was increased in brain, liver and kidney of these knock-out mice [12]–[14]. Thus, it has been suggested that upregulation of CREB and CREM compensate for the loss of the α and Δ isoforms permitting postnatal survival. However, this has not been examined previously in the lung.
We report here for the first time upregulation of CREBβ and CREM mRNA in the lung (Figure 1) in mice lacking α and Δ isoforms of CREB, similar to that previously reported in other tissues [12]–[14]. We then examined the expression of CREB protein using three different antibodies. No CREBα or CREBΔ isoforms were detected in the lungs of the CREBαΔ mice using an antibody that detects these two isoforms (Figure 2A), confirming effective deletion. Interestingly, an antibody that detects all three isoforms, α, Δ and β showed substantially reduced total CREB1 protein in both normoxic and hypoxic lungs of the CREBαΔ mouse (Figure 2B and 2C), demonstrating that any upregulation of CREBβ protein that may have occurred still left total CREB1 protein substantially reduced below normal. Clearly post-translational mechanisms account for the low CREB protein expression in the lungs of these mice given the marked increase in CREBβ mRNA (Figure 1A) that we observed. Taken together with our finding that the amount of CREB protein phosphorylated on its regulatory serine residue (serine133 in the CREBα isoform) was markedly reduced in the CREBαΔ mice, this suggests that CREB1 activity was reduced in the knockout mice (Figure 2D).
Our findings also demonstrate that in the wild type mice total CREB1 expression and regulatory serine phosphorylation were unchanged following three weeks of hypoxia, when compared to normoxoic controls (Figure 2B, 2C and 2D), a finding consistent with our previous findings in the hypoxic mouse lung and those previously reported by other groups in a variety of cell types and species [8], [20]–[23], [27]. In contrast, Klemm and colleagues have found reduction of CREB1 expression in the hypoxic rat lung [24], [25]. This difference may be due to species differences but requires further experimentation to elucidate.
In view of our finding that CREB1 protein was reduced in the CREBαΔ mice, we examined the expression of four genes that are altered in pulmonary hypoxia, have established roles in lung disease and have bioinformatically predicted CREB binding sites in their promoters [8], [28]–[33]. For three of these, BDNF, FST and PLAT, the role of CREB1 in regulating their expression has been previously directly verified by demonstration of CREB1 binding to CRE within their promoters and attenuation of their expression following mutation of the CRE or dominant negative suppression of CREB1 activity indicating that the overall effect of CREB1 family members was to act as an enhancer of gene expression in those studies of isolated cells [34]–[36]. In our study, basal expression of these genes was increased in the lungs of CREBαΔ mice (Figure 3), demonstrating that CREB α and Δ isoforms normally act to repress their expression in the lungs. Interestingly, expression of EDN1 was unaltered in the CREBαΔ mice (Figure 3). This is the one of the four genes examined in which direct confirmation of CREB binding to, and regulation of, the promoter has not been previously reported (Figure 3).
While our results show altered regulation of these three CREB1 regulated genes, it is important to note that loss of the α and Δ isoforms resulted in increased expression of these genes in the hypoxic lungs whereas it had been previously demonstrated that dominant negative suppression of CREB1 activity or mutation of the CRE site within their promoters had reduced their expression in cell culture conditions [34]–[36]. Although this may at first seem surprising, different effects of CREB activation in different cells types are well recognized. Binding of the different CREB family members to the cAMP-response element within promoter elements and regulation of gene expression is cell type specific [37]. This is well illustrated by tissue plasminogen activator (PLAT), one of the CREB regulated genes whose expression we examined (Figure 3), which is increased by CREB family activation in HeLa cells, an epithelial cell type, but suppressed by CREB family activation in HT1080 cells, a mesenchymal cell line [38]. These differing effects in different cells types arise, at least in some cases, through competition for binding at the CRE site between the members of the CREB family including CREB1, the α, Δ and β isoforms, CREM, ATF-1 and 2, and other cyclic-AMP responsive transcription factors [38]–[40]. Cell specific expression patterns of these transcription factors can contribute to the different effects of CREB activation in different cells.
Other mechanisms could also contribute to the changes in gene expression observed in the CREBαΔ mice. CREB interacts with nuclear factor of activated T-cells (NFAT), often antagonizing NFAT actions [41]; interestingly NFAT plays an important role in the development of hypoxic pulmonary hypertension [42], [43]. CREB can bind to the hypoxia response element (HRE) within promoters and thus interact with HIF regulated transcription in hypoxia [44]–[46]. CREB1 and HIF can also interactively control gene expression by binding to CRE and HRE respectively within a gene promoter [47]. Similarly, CREB and NFκB can exert an interactive effect on the regulation of gene expression [48]. Thus, loss of CREB α and Δ might have led to altered gene expression through the partial loss of a modulating effect of CREB on HIF, NFκB and NFAT actions. A further mechanism by which these transcription factors could interact is through competition for binding of CBP/P300 [47] and loss of CREB α and Δ could have altered the availability of CBP/P300gene for HIF and NFκB interaction. It is interesting to note that all of these transcription factors are known to be important in the transcriptional responses of the lung to hypoxia [42], [43], [49]. Any of these mechanisms, or several acting together, could have contributed to the altered gene expression and pulmonary phenotype that we observed in the CREBαΔ mice. Nonetheless, it is clear that CREB α and Δ are required for normal pulmonary vascular function and a normal response to hypoxia.
To determine the effect of hypoxia on the pulmonary vascular resistance, we used an isolated ventilated perfused lung preparation. This preparation eliminates any potential influences of the CREBαΔ deletion on pulmonary arterial pressure mediated indirectly by changes in cardiac output, right or left heart function, endocrine and reflex influences acting on the pulmonary circulation. Thus, the increased pulmonary vascular resistance that we observed in the CREBαΔ mice (Figure 4) must have resulted from changes that were intrinsic to the lung. Moreover, since we perfused the lungs with a standard physiological buffer solution, the greater pulmonary vascular resistance in hypoxic CREBαΔ lungs could not have been the result of the differences in hematocrit in wild-type and CREBαΔ mice (Table 1).
We next examined two mechanisms of increased pulmonary vascular resistance that have previously been shown to be important in the chronically hypoxic lung, vasoconstriction and vascular remodelling. The RhoA-ROCK pathway is particularly important in the regulation of vascular tone in the pulmonary circulation, making a greater contribution to agonist-induced vasoconstriction than in the systemic circulation under normal conditions and contributing substantially to two important specific features of the normal pulmonary vascular bed, hypoxic pulmonary vasoconstriction and the resistance of the pulmonary vessels to the vasodilator effects of hypercapnic acidosis [6], [50], [51]. Following long term alveolar hypoxia, this pathway is unregulated and makes a major contribution to the development of pulmonary hypertension by causing sustained vasoconstriction [3]–[5], [7]. Moreover, in the chronically hypoxic lung, following maximal vasodilation by rho kinase inhibition, no further reduction in resistance can be achieved by chelation of Ca++, demonstrating that maximal rho kinase inhibition can be used to assess the vasoconstrictor contribution to the increased resistance of the chronically hypoxic pulmonary vascular bed [3]. For these reasons we specifically analyzed the effects of CREB α and Δ loss on rho kinase mediated vasoconstriction, although it must be remembered that CREB may regulate the expression of other proteins that control pulmonary vascular smooth muscle contraction e.g. by modulating membrane potential or calcium entry mechanisms [52], [53].
The data presented here confirm increased ROCK mediated vasoconstriction in chronic hypoxia in normal wild type lungs, as demonstrated by an increased vasodilator effect of the ROCK inhibitor Y-27632 (Figure 4), but did not show a statistically significant difference in this response between the two genotypes (P<0.07, 2-way ANOVA, interaction). This suggests that the increased pulmonary vascular resistance observed in both normoxic and hypoxic CREBαΔ mice when compared to wild types was not caused by a difference in rho kinase activity between the two genotypes, making it unlikely that the enhanced resistance in CREBαΔ mice was the result of increased vasoconstriction.
Hypoxia has previously been reported to increase α-actin expression and RhoA dependent actin polymerization in the lung during the development of pulmonary hypertension and, in view of this, we examined the effect of the CREBαΔ mutation on the expression of smooth muscle α-actin [43], [54], [55]. In agreement with those previous reports, we observed a hypoxia-induced increase in α-actin mRNA expression in wild-type mice (Figure 5). We also observed a significant increase in α-actin mRNA expression in the normoxic CREBαΔ mice when compared to normoxic wildtypes (Figures 5D). This actin protein increase by hypoxia is compatible with hypoxia-induced smooth muscle cell hypertrophy and hyperplasia, in keeping with the vessel wall thickening that we observed in both genotypes (Figure 7D). It is also compatible with the enhanced vasoconstrictor tone we found in both hypoxic wild-type and hypoxic CREBαΔ mice (Figure 4B).
A second major mechanism that contributes to increased pulmonary vascular resistance in hypoxic lungs is structural remodeling of the pulmonary vascular bed. To analyze the contribution of structural changes in the vasculature to the elevated pulmonary vascular resistance, we examined intra-acinar vessels by quantitative stereological analysis. Our findings in the wild-type hypoxic group (Figure 7) are in good agreement with our previous reports using this technique, in that we found thickening of the walls of the vessels within the intra-acinar region and reduction in the mean lumen diameter when compared to normoxic controls [3], [4]. As the vessels were fixed following dilatation with Y-27632 and at standard maximal transmural pressures, these differences in diameter must have been a consequence of structural changes [4], [17]. However, we did not detect any structural differences between the wild-type and CREBαΔ mice in normoxia or following hypoxic exposure that could account for the augmented resistance observed in the CREBαΔ mice.
Thus, the higher resistance observed in the normoxic and hypoxic CREBαΔ mice must be due to mechanisms that are independent of hypoxia. Pulmonary vascular resistance increases with increasing lung volumes independently of changes in airway pressure [38], [56]. Furthermore, in more compliant emphysematous lungs, vascular resistance increases due to loss of tissue recoil which normally contributes to maintaining vessel patency [37], [39]. Taken together with our finding that the lungs of CREBαΔ mice were more compliant and had reduced alveolar surface area density (Figure 8), these data suggest that increased lung volume and reduced elastic recoil may have caused the increased vascular resistance that we observed. Interestingly, Martorana and colleagues demonstrated very similar findings in tight-skin mice in which emphysema and associated lung enlargement developed spontaneously. Those mice developed right ventricular hypertrophy typical of pulmonary hypertension without pulmonary vascular remodeling, providing evidence of increased vascular resistance secondary to changed lung compliance [57], [58]. Thus, while our data suggest that altered lung compliance may have increased pulmonary vascular resistance in CREBαΔ mice; further work is needed to directly confirm this.
In summary, this study represents the first direct investigation of the role of CREB α and Δ in pulmonary homeostasis in vivo. We show that a CREB1 mutation that leads to loss of these two isoforms, causes increased pulmonary vascular resistance in both normoxia and hypoxia, demonstrating that normal expression and function of these isoforms is essential to maintain the normal low pulmonary vascular resistance. Similarly enhanced lung volume with reduced alveolar epithelial surface density was observed in both normoxic and hypoxic conditions in CREBαΔ mice demonstrating normal function of all CREB1 isoforms is required to maintain the normal alveolar structure. These findings suggest that interventions that mimic or enhance CREB α and Δ isoform activation in hypoxic lung diseases should be investigated as potential therapeutic strategies.
Supporting Information
Contains the following documents: Figure S1. Bland Altman plots of differences of RV/LV+S ratios using two assessments. The X axis represents the mean of RV/LV+S and the Y axis represents RV/LV+S difference between the two assessments, Table S1. Exon structures of three CREB isoforms, Table S2. Commercially available primers and probes used in gene expression study, Table S3. Custom designed primers and probe, Table S4. Effect of fixation and embedding on lung volume. Data are expressed as mean (±SEM). None of the ratios differs significantly from 1.00.
(DOC)
Funding Statement
This research was supported by funding from the Health Research Board, Ireland (www.hrb.ie), Science Foundation Ireland (www.sfi.ie) and St Vincent's Hospital Anaesthesia Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hopkins N, McLoughlin P (2002) The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis? J Anat 201: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. West JB (2004) The physiologic basis of high-altitude diseases. Ann Intern Med 141: 789–800. [DOI] [PubMed] [Google Scholar]
- 3. Cahill E, Rowan SC, Sands M, Banahan M, Ryan D, et al. (2012) The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: vasoconstrictor and structural mechanisms contribute equally. Experimental physiology 97: 796–806. [DOI] [PubMed] [Google Scholar]
- 4. Cahill E, Costello CM, Rowan SC, Harkin S, Howell K, et al. (2012) Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 125: 920–930. [DOI] [PubMed] [Google Scholar]
- 5. Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, et al. (2005) Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191. [DOI] [PubMed] [Google Scholar]
- 6. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, et al. (2004) Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–664. [DOI] [PubMed] [Google Scholar]
- 7. Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, et al. (2004) Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–672. [DOI] [PubMed] [Google Scholar]
- 8. Leonard MO, Howell K, Madden SF, Costello CM, Higgins DG, et al. (2008) Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med 178: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Funakoshi Y, Ichiki T, Takeda K, Tokuno T, Iino N, et al. (2002) Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. The Journal of biological chemistry 277: 18710–18717. [DOI] [PubMed] [Google Scholar]
- 10. Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, et al. (2003) Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation 108: 1246–1252. [DOI] [PubMed] [Google Scholar]
- 11. Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, et al. (2001) cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. The Journal of biological chemistry 276: 46132–46141. [DOI] [PubMed] [Google Scholar]
- 12. Blendy JA, Kaestner KH, Schmid W, Gass P, Schutz G (1996) Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. EMBO J 15: 1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 13. Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, et al. (1998) Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Natl Acad Sci U S A 95: 4481–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, et al. (1994) Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci U S A 91: 5647–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howell K, Preston RJ, McLoughlin P (2003) Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. J Physiol 547: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cadogan E, Hopkins N, Giles S, Bannigan JG, Moynihan J, et al. (1999) Enhanced expression of inducible nitric oxide synthase without vasodilator effect in chronically infected lungs. Am J Physiol 277: L616–627. [DOI] [PubMed] [Google Scholar]
- 17. Howell K, Costello CM, Sands M, Dooley I, McLoughlin P (2009) L-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L1042–1050. [DOI] [PubMed] [Google Scholar]
- 18. Ludbrook J (1998) Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 25: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 19.McLoughlin P, Keane M (2011) Physiological and pathophysiolgocial angiogenesis in the adult pulmonary circulation. Comprehensive Physiology: Wiley. 1473–1508. [DOI] [PubMed]
- 20. Beitner-Johnson D, Millhorn DE (1998) Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. The Journal of biological chemistry 273: 19834–19839. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K (2013) CREB and NF-kappaB are activated during prolonged hypoxia and cooperatively regulate the induction of matrix metalloproteinase MMP1. The Journal of biological chemistry. [DOI] [PMC free article] [PubMed]
- 22. Meyuhas R, Pikarsky E, Tavor E, Klar A, Abramovitch R, et al. (2008) A Key role for cyclic AMP-responsive element binding protein in hypoxia-mediated activation of the angiogenesis factor CCN1 (CYR61) in Tumor cells. Molecular cancer research: MCR 6: 1397–1409. [DOI] [PubMed] [Google Scholar]
- 23. Signorelli S, Jennings P, Leonard MO, Pfaller W (2010) Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 25: 135–144. [DOI] [PubMed] [Google Scholar]
- 24. Klemm DJ, Majka SM, Crossno JT Jr, Psilas JC, Reusch JE, et al. (2011) Reduction of reactive oxygen species prevents hypoxia-induced CREB depletion in pulmonary artery smooth muscle cells. Journal of cardiovascular pharmacology 58: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garat CV, Crossno JT Jr, Sullivan TM, Reusch JE, Klemm DJ (2010) Thiazolidinediones prevent PDGF-BB-induced CREB depletion in pulmonary artery smooth muscle cells by preventing upregulation of casein kinase 2 alpha' catalytic subunit. Journal of cardiovascular pharmacology 55: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor CT, Furuta GT, Synnestvedt K, Colgan SP (2000) Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc Natl Acad Sci U S A 97: 12091–12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, et al. (2003) Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc Natl Acad Sci U S A 100: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fonseca C, Abraham D, Renzoni EA (2011) Endothelin in pulmonary fibrosis. Am J Respir Cell Mol Biol 44: 1–10. [DOI] [PubMed] [Google Scholar]
- 29. Rich S, McLaughlin VV (2003) Endothelin receptor blockers in cardiovascular disease. Circulation 108: 2184–2190. [DOI] [PubMed] [Google Scholar]
- 30. Aoki F, Kurabayashi M, Hasegawa Y, Kojima I (2005) Attenuation of bleomycin-induced pulmonary fibrosis by follistatin. Am J Respir Crit Care Med 172: 713–720. [DOI] [PubMed] [Google Scholar]
- 31. Hardy CL, Nguyen HA, Mohamud R, Yao J, Oh DY, et al. (2012) The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax 68: 9–18. [DOI] [PubMed] [Google Scholar]
- 32. Yndestad A, Larsen KO, Oie E, Ueland T, Smith C, et al. (2009) Elevated levels of activin A in clinical and experimental pulmonary hypertension. Journal of applied physiology 106: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 33. Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, et al. (2012) BDNF/TrkB Signaling Augments Smooth Muscle Cell Proliferation in Pulmonary Hypertension. The American journal of pathology 181: 2018–2029. [DOI] [PubMed] [Google Scholar]
- 34. Eberhardt W, Engels C, Muller R, Pfeilschifter J (2002) Mechanisms of dexamethasone-mediated inhibition of cAMP-induced tPA expression in rat mesangial cells. Kidney Int 62: 809–821. [DOI] [PubMed] [Google Scholar]
- 35. Winters SJ, Ghooray D, Fujii Y, Moore JP Jr, Nevitt JR, et al. (2007) Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol 271: 45–54. [DOI] [PubMed] [Google Scholar]
- 36. Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–726. [DOI] [PubMed] [Google Scholar]
- 37. Cha-Molstad H, Keller DM, Yochum GS, Impey S, Goodman RH (2004) Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc Natl Acad Sci U S A 101: 13572–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costa M, Medcalf RL (2001) Ectopic expression of the cAMP-responsive element binding protein inhibits phorbol ester-mediated induction of tissue-type plasminogen activator gene expression. Eur J Biochem 268: 987–996. [DOI] [PubMed] [Google Scholar]
- 39. De Cesare D, Sassone-Corsi P (2000) Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol 64: 343–369. [DOI] [PubMed] [Google Scholar]
- 40. Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, et al. (2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31: 47–54. [DOI] [PubMed] [Google Scholar]
- 41. Grossmann C, Wuttke M, Ruhs S, Seiferth A, Mildenberger S, et al. (2010) Mineralocorticoid receptor inhibits CREB signaling by calcineurin activation. FASEB J 24: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 42. Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, et al. (2011) NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. American journal of physiology Lung cellular and molecular physiology 301: L872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Frutos S, Spangler R, Alo D, Bosc LV (2007) NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. The Journal of biological chemistry 282: 15081–15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kvietikova I, Wenger RH, Marti HH, Gassmann M (1997) The hypoxia-inducible factor-1 DNA recognition site is cAMP-responsive. Kidney Int 51: 564–566. [DOI] [PubMed] [Google Scholar]
- 45. Kvietikova I, Wenger RH, Marti HH, Gassmann M (1995) The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Res 23: 4542–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dimova EY, Jakubowska MM, Kietzmann T (2007) CREB binding to the hypoxia-inducible factor-1 responsive elements in the plasminogen activator inhibitor-1 promoter mediates the glucagon effect. Thromb Haemost 98: 296–303. [PubMed] [Google Scholar]
- 47. Ebert BL, Bunn HF (1998) Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol 18: 4089–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Illi B, Puri P, Morgante L, Capogrossi MC, Gaetano C (2000) Nuclear factor-kappaB and cAMP response element binding protein mediate opposite transcriptional effects on the Flk-1/KDR gene promoter. Circ Res 86: E110–117. [PubMed] [Google Scholar]
- 49. Frohlich S, Boylan J, McLoughlin P (2013) Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am J Respir Cell Mol Biol 48: 271–279. [DOI] [PubMed] [Google Scholar]
- 50. Hyvelin JM, O'Connor C, McLoughlin P (2004) Effect of changes in pH on wall tension in isolated rat pulmonary artery: role of the RhoA/Rho-kinase pathway. American journal of physiology Lung cellular and molecular physiology 287: L673–684. [DOI] [PubMed] [Google Scholar]
- 51. Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM (2000) Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol 131: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mori Y, Matsubara H, Folco E, Siegel A, Koren G (1993) The transcription of a mammalian voltage-gated potassium channel is regulated by cAMP in a cell-specific manner. The Journal of biological chemistry 268: 26482–26493. [PubMed] [Google Scholar]
- 53. Zhang S, Remillard CV, Fantozzi I, Yuan JX (2004) ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. American journal of physiology Cell physiology 287: C1192–1201. [DOI] [PubMed] [Google Scholar]
- 54. Fediuk J, Gutsol A, Nolette N, Dakshinamurti S (2012) Thromboxane-induced actin polymerization in hypoxic pulmonary artery is independent of Rho. American journal of physiology Lung cellular and molecular physiology 302: L13–26. [DOI] [PubMed] [Google Scholar]
- 55. Hai CM, Kim HR (2005) An expanded latch-bridge model of protein kinase C-mediated smooth muscle contraction. Journal of applied physiology 98: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 56. Hakim TS, Michel RP, Chang HK (1982) Effect of lung inflation on pulmonary vascular resistance by arterial and venous occlusion. Journal of applied physiology: respiratory, environmental and exercise physiology 53: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 57. Martorana PA, van Even P, Gardi C, Lungarella G (1989) A 16-month study of the development of genetic emphysema in tight-skin mice. The American review of respiratory disease 139: 226–232. [DOI] [PubMed] [Google Scholar]
- 58. Martorana PA, Wilkinson M, van Even P, Lungarella G (1990) Tsk mice with genetic emphysema. Right ventricular hypertrophy occurs without hypertrophy of muscular pulmonary arteries or muscularization of arterioles. The American review of respiratory disease 142: 333–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains the following documents: Figure S1. Bland Altman plots of differences of RV/LV+S ratios using two assessments. The X axis represents the mean of RV/LV+S and the Y axis represents RV/LV+S difference between the two assessments, Table S1. Exon structures of three CREB isoforms, Table S2. Commercially available primers and probes used in gene expression study, Table S3. Custom designed primers and probe, Table S4. Effect of fixation and embedding on lung volume. Data are expressed as mean (±SEM). None of the ratios differs significantly from 1.00.
(DOC)