Abstract
Capsule is an important virulence factor in bacteria. A total of 78 capsular types have been identified in Klebsiella pneumoniae. However, there are limitations in current typing methods. We report here the development of a new genotyping method based on amplification of the variable regions of the wzc gene. Fragments corresponding to the variable region of wzc were amplified and sequenced from 76 documented capsular types of reference or clinical strains. The remaining two capsular types (reference strains K15 and K50) lacked amplifiable wzc genes and were proven to be acapsular. Strains with the same capsular type exhibited ≧94% DNA sequence identity across the variable region (CD1-VR2-CD2) of wzc. Strains with distinct K types exhibited <80% DNA sequence identity across this region, with the exception of three pairs of strains: K22/K37, K9/K45, and K52/K79. Strains K22 and K37 shared identical capsular polysaccharide synthesis (cps) genes except for one gene with a difference at a single base which resulted in frameshift mutation. The wzc sequences of K9 and K45 exhibited high DNA sequence similarity but possessed different genes in their cps clusters. K52 and K79 exhibited 89% wzc DNA sequence identity but were readily distinguished from each other at the DNA level; in contrast, strains with the same capsular type as K52 exhibited 100% wzc sequence identity. A total of 29 strains from patients with bacteremia were typed by the wzc system. wzc DNA sequences confirmed the documented capsular type for twenty-eight of these clinical isolates; the remaining strain likely represents a new capsular type. Thus, the wzc genotyping system is a simple and useful method for capsular typing of K. pneumoniae.
Introduction
Klebsiella pneumoniae is an important human pathogen in both hospital and community settings. This species causes nosocomial infections, such as septicemia, pneumonia, urinary tract infections, surgical site infections and catheter-related infections [1], [2], and is also associated with community-acquired infections, such as pyogenic liver abscess (PLA) complicated with meningitis and endophthalmitis, soft tissue abscesses, urinary tract infections, and pneumonia [3]–[11]. Community-acquired PLA caused by K. pneumoniae with or without meningitis and endophthalmitis metastatic complications represents an emerging infectious disease worldwide [6], [9], [10], [12]–[16]. Approximately 50% of patients with community-acquired PLA K. pneumoniae infections exhibit no apparent underlying disease, whereas the remainder of this population harbors predisposing conditions such as diabetes mellitus [13].
Capsule is a major virulence factor of K. pneumoniae, and capsular types are related to the severity of infection [17], [18]. The prevalence of capsular types in each K. pneumoniae-related disease could be crucial for disease control and prevention. However, determination of capsular types often is difficult due to the limitations of traditional serotyping [19], [20]. The results of serotyping also are inconsistent, except in patients with community-acquired PLA [8], [13], [19], [21]–[24].
Molecular methods based on the capsule polysaccharide synthesis (cps) region have been developed for K. pneumoniae capsular typing. For example, polymerase chain reaction-based genotyping of the capsular polysaccharide synthesis region, cps (wzy)-PCR genotyping, was first adopted for K. pneumoniae type K1 [6], [7], [25]–[28], and subsequently applied for other capsular types related to community-acquired PLA [19], [29], [30]. However, only capsular types with known sequences of capsule specific genes (e.g., wzy) can be typed, and a separate pair of primers is needed for each type. PCR amplification of the cps gene cluster (∼20 kb) followed by restriction enzyme digestion, i.e., cps PCR-restriction fragment length polymorphism (RFLP) analysis, is another commonly used method. Capsular types can be distinguished based on distinct RFLP profiles (C-patterns) [31]; however, amplifications of the cps region can be very difficult in some strains. In addition, different C-patterns have been observed in some strains that share same capsular type.
As described here, we have developed a new method for capsular typing of K. pneumoniae based on the sequence of the variable region of a gene, wzc, that encodes a capsule synthesis-related tyrosine kinase.
Materials and Methods
Ethics statement
The clinical strains used in this study were provided from the strain collection of National Taiwan University Hospital, En Chu Kong Hospital, Far Eastern Memorial Hospital, Chang Gung Memorial Hospital in Taiwan. The Ethics Committee confirmed that no formal ethical approval was needed to use these clinically obtained materials, because the strains were remnants from patient samples, and the data were analyzed anonymously.
Bacterial strains
A total of 77 K-serotype Klebsiella reference strains purchased from Statens Serum Institute, Copenhagen, Denmark. An additional strain (A1517) of novel type KN1 was identified in a previous study from our laboratory [19]. Another eleven K. pneumoniae clinical isolates were obtained from Taiwanese and overseas clinical laboratories, including National Taiwan University Hospital (NTUH; Taipei, Taiwan), En Chu Kong Hospital (ECKH; Sansia, Taiwan), Far Eastern Memorial Hospital (FEMH; Banciao, Taiwan), Chang Gung Memorial Hospital (CGMH; Linkou, Taiwan), Department of Medical Microbiology, University of Manitoba (Winnipeg, MB, Canada), and Department of Clinical Microbiology, Kuopio University Hospital (Finland) [19]. Together, strains representing the 78 known capsular types were included for wzc sequencing.
Between 2004 and 2006, Twenty-nine strains were collected from the blood of patients admitted to NTUH with bacteremia. To evaluate the wzc typing system in typing strains with unknown capsular types, all of the 29 K. pneumoniae clinical isolates of unknown capsular type were screened by wzc sequencing.
A K. pneumoniae clinical isolate from NTUH, NTUH-K2044 (K1), and its isogenic mutants NTUH-K2044 ΔmagA (capsule deficient) and NTUH-K2044 ΔwbbO (O-antigen deficient) [32] were used as controls for Alcian blue staining.
wzc sequencing
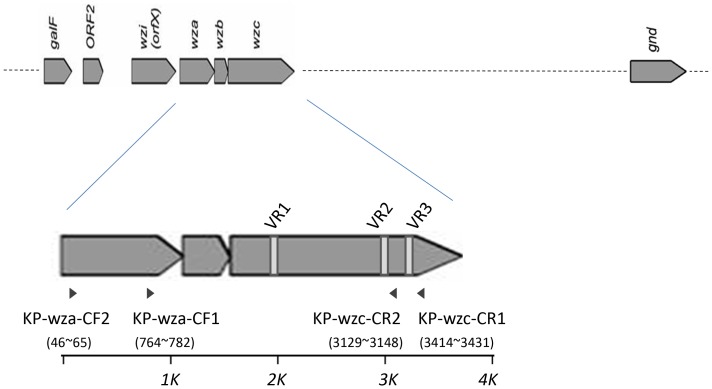
Consensus sequences were identified based on the published cps sequences of 12 capsular types (K1, K2, K5, K9, K10, K14, K20, K52, K54, K57, K62, and KN1; sequences obtained from Genbank as Accession Numbers AB198423, AY762939, D21242, AB289646, AB371292, AB289645, AB371293, AB371291, AB371294, AF118250, AB289648, AB371289, CP000647, AB289650, AB334776, AB371295, and AB334777). The reference sequences were used to design forward primers KP-wza-CF1 and KP-wza-CF2 (corresponding to wza gene sequences) and reverse primers KP-wzc-CR1 and KP-wzc-CR2 (corresponding to wzc gene sequences), combinations of which were expected to permit PCR amplification of the wza-wzb-wzc region (Table 1, Figure 1, and Figure 2). Positions of these primers were shown in Figure 1 according to the sequences of NTUH-K2044. PCR amplifications were performed with the Long and Accurate PCR system (Takara, Tokyo, Japan). The cycling program was 96°C for 3 min, followed by 30 temperature cycles of 96°C for 30 s, 46°C for 15 s, and 72°C for 3 min. The expected size of PCR amplicons was ∼2.7 kb by use of primer pair 1 (KP-wza-CF1 and KP-wzc-CR1); ∼3.4 kb by use of primer pair 2 (KP-wza-CF2 and KP-wzc-CR1); ∼2.4 kb by use of primer pair 3 (KP-wza-CF1 and KP-wzc-CR2); ∼3.1 kb by use of primer pair 4 (KP-wza-CF2 and KP-wzc-CR2). PCR products were isolated and subjected to sequencing using reverse primers. In order to establish wzc database of documented capsular types, the amplicons were sequenced by primer walking using internal primers (Table S1) (each walk read ≧600 bp and there were at least 50 bp overlaps with previous obtained sequences). The obtained sequences were aligned from 96 strains representing 76 of the documented capsular types (Accession Numbers AB719985-AB720026, AB720650-AB720698, and AB819898). Amplification products were not obtained from strains representing types K15 and K50. The database is composed of wzc sequences (∼1.5 kb) from start codon of the wzc gene to the conserved domain CD2 (GANNTNNCNNTNNA) located in the downstream of VR2 region.
Table 1. Primers used in this study.
| Primer name | Sequence | Purpose or reference |
| 1166F | GGTGCTCTTTACATCATTGC | K1 genotyping [25] |
| 936R | GCAATGGCCATTTGCGTTAG | K1 genotyping [25] |
| K2-wzyF | ATGATTCGAAGAAAGTTTTC | K2 genotyping |
| K2-wzyR | TTAGTTGATGTCATTTTCGG | K2 genotyping |
| K9-wzyF | ATGGTGATTATGAATGAAG | K9 genotyping |
| K9-wzyR | ACACAATGAAAACATTGCC | K9 genotyping |
| K14-wzyF | GACTCTGAATAAAAGAACAC | K14 genotyping |
| K14-wzyR | CTCAATAAATCTGTTCTGAAG | K14 genotyping |
| K15-wzyF | TACCCATAGCTATATGCGCC | K15 genotyping |
| K15-wzyR | GGGAAAGTTGCAGCATATTC | K15 genotyping |
| K16-wzyF | ATGGTACCGTTGGGGTTATC | K16 genotyping |
| K16-wzyR | TAATCAACAATGTCGTAGCG | K16 genotyping |
| K20-wzyF | GTGAGGACACTTTCGAAAGC | K20 genotyping |
| K20-wzyR | TCATTTACATTCCTTCTTCC | K20 genotyping |
| K23-wzyF | GTCATCTACTCTGTCCTTTTGGTCC | K23 genotyping |
| K23-wzyR | ATTACTATGTTGCGCCGAGG | K23 genotyping |
| K39-wzyF | ATGACCAATGACTTACAAAG | K39 genotyping |
| K39-wzyR | GAATTCCGTTCCAGCCCAC | K39 genotyping |
| K45-wzyF | GAACGAAGATGAACTCTGAC | K45 genotyping |
| K45-wzyR | GCTAGATTCGCATATGGAGA | K45 genotyping |
| K50-gly1F | CCAATGATAA TACTGCGCAG | K50 genotyping |
| K50-gly1R | CAACCCGATC ATATCATCTC | K50 genotyping |
| K54-F | TTACCTCAGAGCGTTGCATTG | K54 genotyping |
| K54-R | TTAGGTATGACAATTGAGCTC | K54 genotyping |
| K62-wzyF | ATGTCAGTGATTATTTCAGG | K62 genotyping |
| K62-wzyR | AGAGTATGTCATCACGCACG | K62 genotyping |
| N1-wzyF | TATGGGCTTAGGTTTCCTGG | KN1 genotyping |
| N1-wzyR | TGCAATATAAATCTCCCCAG | KN1 genotyping |
| 1461-wzyF | GCAGAATTCGATAGCTTGTC | 1461 genotyping |
| 1461-wzyR | CCGCATAAACCATGTCATTG | 1461 genotyping |
| KP-wza-CF1 | TGAAAGTGTTTGTCATGGG | wzc PCR |
| KP-wza-CF2 | GGGTTTTTATCGGGTTGTAC | wzc PCR |
| KP-wzc-CR1 | TTCAGCTGGATTTGGTGG | wzc PCR |
| KP-wzc-CR2 | GCTTCCATCATTGCAAAATG | wzc PCR |
| K22-acyl-F | TATCCATATGCTGTTTGGTC | Acetyltransferase sequencing |
| K22-acyl-R | TCGCAGCGGTATACAAATTC | Acetyltransferase sequencing |
Note: primers used for sequencing were shown in a supplementary table (Table S1).
Figure 1. Variable regions VR1, VR2 and VR3, and the primers for PCR amplification of the wza-wzb-wzc region.
The conserved genes of capsular polysaccharide synthesis (cps) region are shown as arrows. Variable regions (designated VR1, VR2, and VR3) of wzc are indicated as vertical white bars in the wzc ORF. Arrowheads indicate the positions and orientations of primers used for PCR amplification of the wza-wzb-wzc region. The positions of the primers in NTUH-K2044 were shown in brackets.
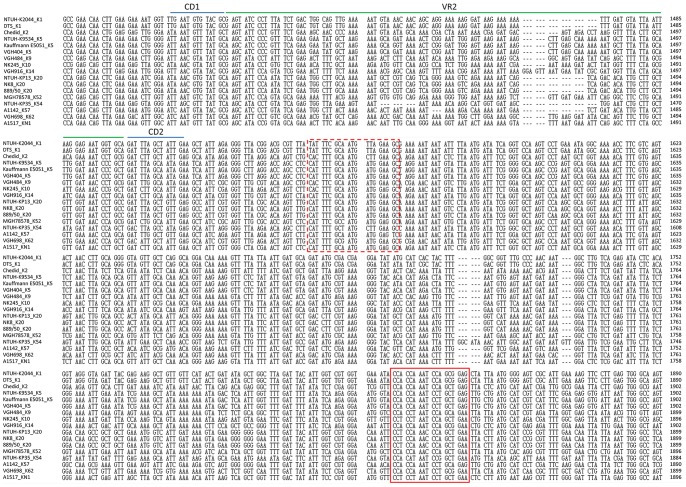
Figure 2. wzc sequence alignment across 12 capsular types.
wzc sequences of 17 strains (NTUH-K2044, DTS, Chedid, NTUH-K9534, Kauffmann E5051, VGH404, VGH484, NK245, VGH916, NTUH-KP13, NK8, 889/50, MGH 78578, NTUH-KP35, A1142, VGH698 and A1517) representing 12 capsular types (K1, K2, K5, K9, K10, K14, K20, K52, K54, K57, K62, and KN1) were aligned with amino acid sequences and then back-translated to DNA sequences using MEGA 4.0 (ClustalW) with default parameters. The alignments of nucleotides number ∼1300–1900 of wzc ORF are shown in this figure. As shown by the alignment, two variable regions of wzc (∼1420–1480 and ∼1700–1820) are flanked by conserved regions. wzc sequences used in the design of reverse primers are marked by boxed domains (KP-wzc-CR1: continuous line box; KP-wzc-CR2: dashed line box).
Sequencing of cps region
Since we failed to amplify wzc genes from reference strains K15 and K50, we instead amplified the cps region from these strains using conserved primers CPS-1 (located in the wzi gene) and rCPS (located in gnd), as previously described [19]. To permit comparison among the cps regions of selected strains, the corresponding regions were amplified from strains K22, K37, K45, K79, and novel type strain 1461 using primers CPS-1 and rCPS as well. PCR amplifications were performed with the Long and Accurate PCR system. The cycling program consisted of one denaturation step of 2 min at 94°C and 10 initial cycles of 10 s at 98°C, 30 s at 63°C, and 12 min at 68°C, followed by 20 iterative cycles of 10 s at 98°C, 30 s at 63°C, and 12 min plus 20 s for each new cycle at 72°C. A final elongation step was performed for 10 min at 72°C. To extend upstream and downstream from the conserved regions (from galF to gnd), primers pre-galF-F and yegH (located in the sequences at the upstream end of cps) and post-gnd R and ugd (located in the sequences at the downstream end of cps) were used to amplify the flanking sequences [19]. The PCR cycling program for these reactions consisted of 96°C for 3 min, followed by 30 cycles of 96°C for 30 s, 52°C for 15 s, and 72°C for 2–5 min. The products were sequenced by primer walking, providing complete sequences for the cps regions (from galF to gnd, extending approximately 20 kb). The resulting sequences were deposited to Genbank as Accession Numbers AB819892-AB819894, AB819896, AB819897, AB819895, and AB822494). Genes were annotated by NCBI-blast.
wzy-PCR genotyping
To confirm the capsular types of clinical isolates, primers located in the capsular type-specific wzy gene in variable region of cps loci were used. Along with the published wzy genes of types K1, K2, K14, K20, K54, K62, and KN1 (Accession Numbers AB198423, D21242, AB371294, AB289648, AB289650, AB371295, AB334777), we also resolved sequences for the cps regions of reference strains for types K16, K23, and K39 (Accession Numbers AB742228, AB742229, and AB742230). Specifically, we designed specific wzy primers based on the sequences for cps-PCR genotyping (Table 1). PCR was performed as previously described [19].
Primers specific for the wzy gene of strain 1461 were designed (Table 1) with the intent of confirming the presence of cps genes distinct from the 78 documented capsular types. In parallel to PCR with strain 1461, cps-PCR genotyping using the same primers was performed in 77 K-serotype reference strains (Statens Serum Institute) and KN1 (A1517). Primers pair 1461-wzyF and 1461-wzyR were used in 1461 wzy-PCRgenotyping.
Alcian blue staining
Extracellular polysaccharides, including both capsule and lipopolysaccharide, were isolated as previously reported [33]. Briefly, bacteria were cultured overnight in 1 mL Luria-Bertani (LB) medium and then harvested and resuspended in 150 µL of water. An equal volume of phenol (pH 6.6; Amresco) was added, and the mixture was vortexed. After incubation at 65°C for 20 min, samples were extracted with chloroform and centrifuged. The extracted samples were separated by 10%-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and capsule was detected with Alcian blue as previously described [34], [35]. In brief, after electrophoresis, the gel was washed three times (5 min, 10 min, and 15 min; at 50°C for each step) with fix/wash solution (25% ethanol, 10% acetic acid in water). The gel then was soaked (15 min in the dark at 50°C) in 0.125% Alcian blue dissolved in fix/wash solution, and finally destained (overnight at room temperature) with fix/wash solution. CPS was visualized as blue-stained material.
Results
wzc sequences are distinct in different capsular types
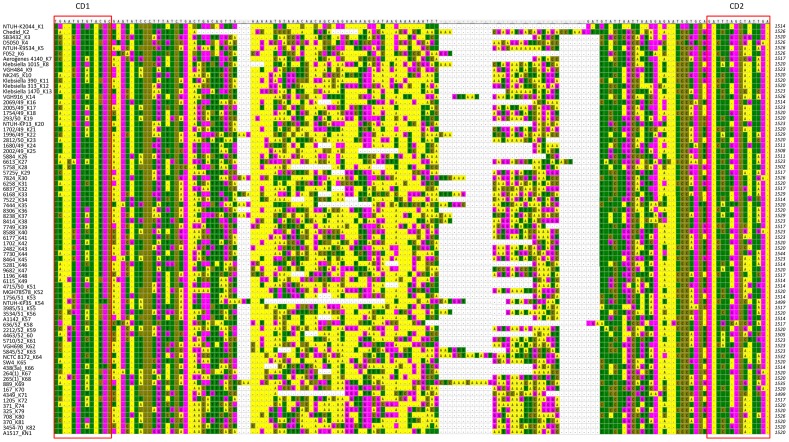
The capsular polysaccharide synthesis (cps) region of K. pneumoniae shows conserved genetic organization (synteny) extending from galF through orf2, wzi, wza,wzb, wzc, and gnd. Genes between wzc and gnd (downstream of wzc to upstream of gnd) vary among different capsular types and therefore are considered to constitute a variable region (Figure 1). We compared the conserved genes (galF, orf2, wzi, wza, wzb, wzc, and gnd) among the published cps sequences of 17 strains with 12 capsular types (K1, K2, K5, K9, K10, K14, K20, K52, K54, K57, K62, and KN1) and noted that the wzc genes (encoding a tyrosine kinase) had three variable regions flanked by more conserved regions. The sequences of variable regions were highly variable among different capsular types but were conserved in the strains with same capsular type. The wzc genes were aligned with amino acid sequences and then back-translated into codons. These variable regions of wzc (which we refer to as VR1, VR2, and VR3) mapped to nucleotides ∼420–480, ∼1420–1480, and ∼1700–1820 (respectively) of the ORF from different capsular types (The position of VR1, VR2 and VR3 in NTUH-K2044 was ∼423–460, ∼1422–1480 and ∼1713–1801 respectively). Moreover, the domains at ∼1480–1660 and∼1820–1860 bases showed DNA sequence conservation (The position of the conserved domain in NTUH-K2044 was ∼1489–1634, and ∼1828–1852 respectively). Based on these sequences, we designed consensus reverse primers KP-wzc-CR1 and KP-wzc-CR2 (Figure 1 and Figure 2) (The position of KP-wzc-CR1 and KP-wzc-CR2 in NTUH-K2044 were 1828–1845 and 1543–1562 of the wzc gene, respectively). Similar analysis of the wza genes in the 12 capsular types permitted the design of forward primers KP-wza-CF1 and KP-wza-CF2 (Figure 1) (The position of KP-wza-CF1 and KP-wza-CF2 in NTUH-K2044 were 764–782 and 46–65 of the wza gene, respectively). In order to complete the wzc sequences database of 78 documented capsular types, the primers (in each of the four primer pair combinations) were used to amplify the wza-wzb-wzc region (∼2.5–3 kb) from a total of 81 strains with known capsular types (Table 2). The PCR results revealed that primer pair 1 (KP-wza-CF1 and KP-wzc-CR1), primer pair 2 (KP-wza-CF2 and KP-wzc-CR1), primer pair 3 (KP-wza-CF1 and KP-wzc-CR2), and primer pair 4 (KP-wza-CF2 and KP-wzc-CR2) amplified fragments from 92%, 88%, 82%, and 81% of the documented capsular types, respectively. The combination of primer pair 1 and primer pair 2 yielded products from 76 of the 78 documented capsular types (97%). The exceptions were capsular type K15 and K50 strains, which did not yield product by any of the four primer pairs. PCR products amplified by primer pair 1 were sequenced by the reverse primer KP-wzc-CR1 in 75 of the 81 strains excluding reference strains of K15, K32, K50, K59, K67 and K79; PCR products amplified by primer pair 2 were sequenced by the reverse primer KP-wzc-CR1 in reference strains K32, K59, K67 and K79. The PCR amplicons were sequenced to the start codon of wzc gene by primer walking. All of the sequences deposited in Genbank were from start codon of wzc to CD2 domain (GANNTNNCNNTNNA) which is located in the downstream of VR2 (Figure 3) and exhibit conservation among 76 capsular types. Thus, the sequences obtained from published cps sequences and from our sequencing results together constitute a 96-strain database of wzc sequences (Table 2). Comparison among the amino acid and DNA sequences of wzc revealed high levels of similarity (≧99% identity both by amino acid sequences and DNA sequences) derived from strains with same capsular type. Strains belonging to distinct capsular types exhibited lower levels of similarity (40–80% identity by amino acid sequences and 60–80% identity by DNA sequences), with three exceptions. Specifically, the K22 and K37 type strains had wzc sequences that were identical to each other; the K9 and K45 type strains shared 99% amino acid or DNA sequence identity; and the K52 and K79 type strains shared 93% amino acid sequence identity (89% DNA sequence identity). In order to make the method more easily to be used for capsular type identification, conserved regions, CD1 (TNANNGTNTANNC) and CD2 (GANNTNNCNNTNNA), nearby VR2 were identified in 76 capsular types. The CD1-VR2-CD2 region (115–151 bp in length from different capsular types) was selected for comparison (Figure 3 and File S1). Therefore, only one-run sequencing using KP-wzc-CR1 (∼350 bp from CD2) or KP-wzc-CR2 (∼60 bp from CD2) can cover this region for further comparison. The CD1-VR2-CD2 region from distinct capsular types in our wzc database showed <80% DNA identity and the region derived from strains with same capsular type shared ≧97% DNA identity with the exception of K22/K37 (142/142, 100%), K9/K45 (133/136, 98%) and K52/K79 (123/136, 90%).
Table 2. Strains included in the wzc database established in this study.
| Capsular type | Strain |
| K1 | NTUH-K2044a (AB719985), DTSa (AY762939) |
| K2 | Chedida (AB719986) |
| K3 | SB3432a (AB719987) |
| K4 | D5050b, c (AB719988) |
| K5 | NTUH-K9534a (AB719989), Kauffmann E5051a (AB289645), VGH404a (AB371292), E6b (AB719990), can0525b (AB719991) |
| K6 | F052b, c (AB719992) |
| K7 | Aerogenes 4140b, c (AB719993) |
| K8 | Klebsiella 1015b, c (AB719994) |
| K9 | VGH484 a (AB719995), ATCC29013b (AB719996) |
| K10 | NK245a (AB719997), Klebsiella 919b, c (AB719998) |
| K11 | Klebsiella 390b, c (AB719999) |
| K12 | Klebsiella 313b, c (AB720000) |
| K13 | Klebsiella 1470b, c (AB720001) |
| K14 | VGH916a (AB720002), Klebsiella 1193b, c (AB720003) |
| K15 | N/A |
| K16 | 2069/49b, c (AB720004), can0416b (AB720005), N4795b (AB720006) |
| K17 | 2005/49b, c (AB720007) |
| K18 | 1754/49b, c (AB720008) |
| K19 | 293/50b, c (AB720009) |
| K20 | NTUH-KP13a (AB720010), NK8a (AB720011), 889/50 a (AF118250) |
| K21 | 1702/49b, c (AB720012) |
| K22 | 1996/49b, c (AB720013) |
| K23 | 2812/50b, c (AB720014) |
| K24 | 1680/49b, c (AB720015) |
| K25 | 2002/49b, c (AB720016) |
| K26 | 5884b, c (AB720017) |
| K27 | 6613b, c (AB720018) |
| K28 | 5758b, c (AB720019) |
| K29 | 5725yb, c (AB720020) |
| K30 | 7824b, c (AB720021) |
| K31 | 6258b, c (AB720022) |
| K32 | 6837b, c (AB720023) |
| K33 | 6168b, c (AB720024) |
| K34 | 7522b, c (AB720025) |
| K35 | 7444b, c (AB720026) |
| K36 | 8306b, c (AB720650) |
| K37 | 8238b, c (AB720651) |
| K38 | 8414b, c (AB720652) |
| K39 | 7749b, c (AB720653) |
| K40 | 8588b, c (AB720654) |
| K41 | 6177b, c (AB720655) |
| K42 | 1702b, c (AB720656) |
| K43 | 2482b, c (AB720657) |
| K44 | 7730b, c (AB720658) |
| K45 | 8464b, c (AB720659) |
a, sequences were obtained from Genbank; b, sequences were resolved in this study; c, reference strain. N/A, not available. Accession numbers were shown in brackets.
Figure 3. Variable region 2 (VR2) of wzc and conserved domain (CD1 and CD2) in 76 capsular types.
VR2 and the nearby sequences of 76 capsular types were shown. Nucleotides above the alignment indicate K1 sequences and conserved bases identical to K1 were shown as dots. Cells were in different colors according to the nucleotides (A: yellow; T: green; C: brown; G: pink). CD1 (TNANNGTNTANNC) and CD2 (GANNTNNCNNTNNA) marked by opened square were conserved among all 76 capsular types.
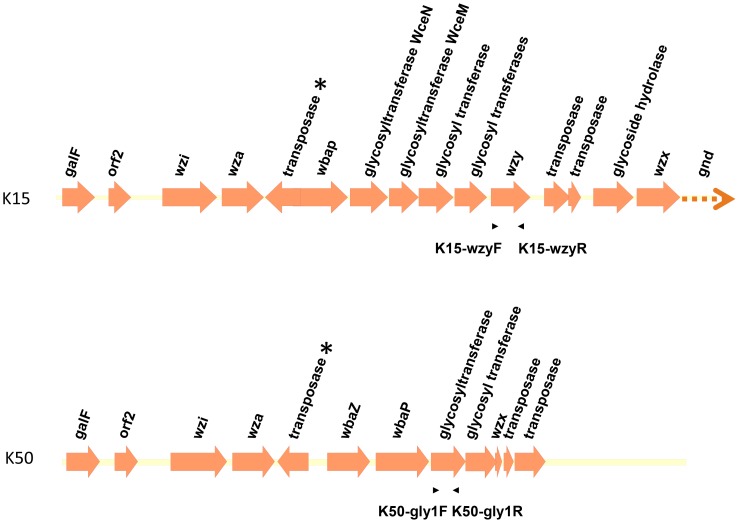
K15 and K50 were found to have transposase insertions that precludes capsule expression
As noted above, PCR amplification of the ∼2.5–3 kb wza-wzb-wzc region using the wza and wzc primers failed in reference strains K15 and K50. We therefore amplified and sequenced the full cps region by PCR. The resulting sequences (Accession Numbers AB819895 and AB822494) revealed that both the wzb and wzc genes were replaced by genes encoding transposases both in K15 and K50 (Figure 4). We further designed additional specific primer pairs based on the sequences of the wzy gene of K15 (primers K15-wzyF and K15-wzyR) and the sequences of a gene encoding a glycosyltransferase homolog in K50 (primers K50-gly1F and K50-gly1R) (Table 1 and Figure 4). PCR performed on each of the 78 capsular type strains confirmed that these primers were specific for the K15 and K50 capsular types (data not shown). Therefore, although wzc genotyping was not successful for capsular type K15 and K50, type-specific primers can be used to genotype the K15 and K50 strains.
Figure 4. Genetic alignment of K15 and K50 cps regions.
Open reading frames (ORFs) are shown as arrows. The arrows with dotted lines indicate that only partial sequences were obtained for these ORFs. Asterisks indicate the genes encoding putative transposases that replace the wzb and wzc genes in the K15 and K50 strains. The positions and orientations of primers used for cps-PCR genotyping are indicated by arrow heads. Primer pair K15-wzyF and K15-wzyR was used for K15 cps-PCR genotyping, and primer pair K50-gly1F and K50-gly1R was used for K50 cps-PCR genotyping.
Moreover, the wzb and wzc genes are thought to be essential for capsule synthesis in Klebsiella, suggesting the loss of capsule in the K15 and K50 strains. Therefore, we used Alcian blue staining to determine the capsular status of these strains. Our results revealed the absence of CPS in reference strains K15 and K50, as also seen with NTUH-K2044 ΔmagA, a known capsule-deficient mutant; in contrast, CPS (visualized as high-molecular weight Alcian blue stained material at the top of an SDS-PAGE gel) was observed in positive controls, including a K1 strain (NTUH-K2044) and an isogenic ΔwbbO (O-antigen-deficient) mutant (Figure 5). Thus, the reference strains K15 and K50 are acapsular.
Figure 5. Alcian blue staining of polysaccharide extracts from reference strains K15 and K50.
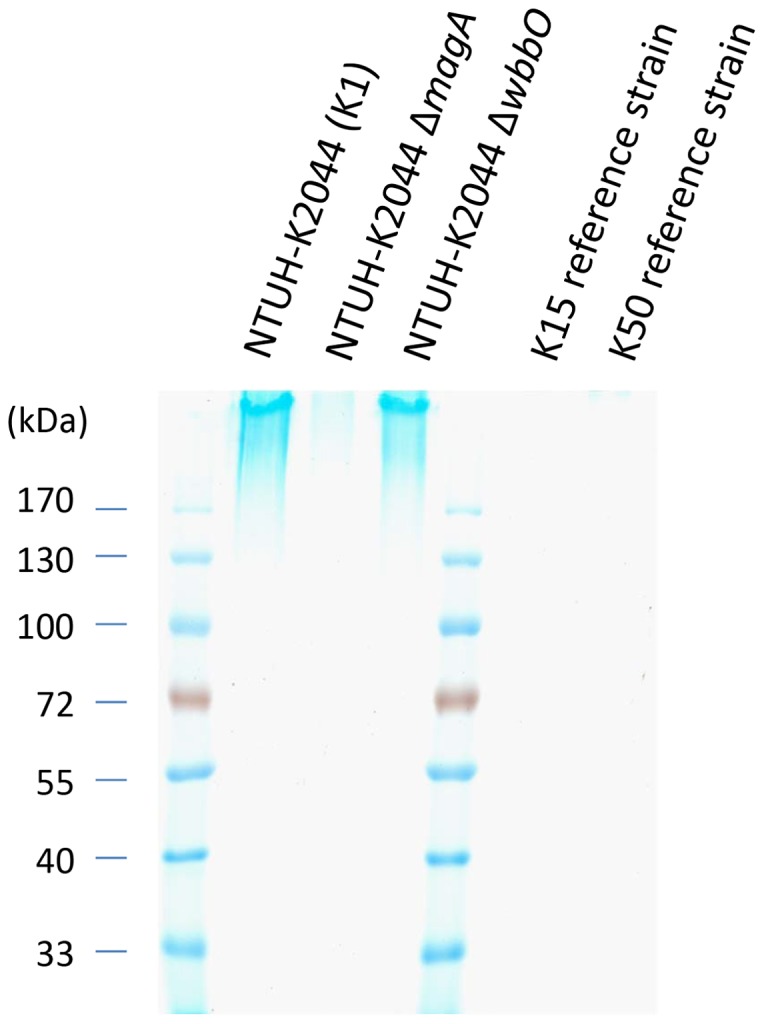
Polysaccharide extracts from NTUH-K2044 (K1), isogenic mutant NTUH-K2044 ΔmagA (capsule deficient), isogenic mutant NTUH-K2044 ΔwbbO (O-antigen deficient), and reference strains K15 and K50 were stained with Alcian blue (see Materials and Methods).
cps regions of K22/K37, K9/K45, and K52/K79
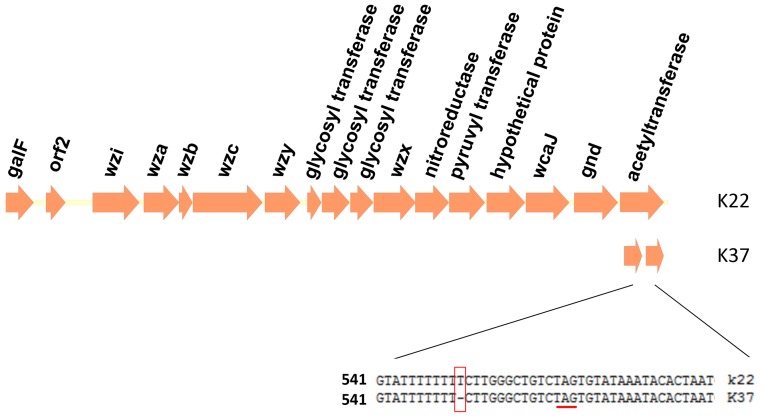
As noted above, sequencing of wzc revealed higher than expected DNA sequence similarities between the type strains for K22 and K37 (100% identity at wzc), K9 and K45 (99% identity), and K52 and K79 (89% identity). We therefore further explored the genetic structure of the cps regions in these strains. The sequences (Accession Numbers AB819893 and AB819894) showed that K22 and K37 not only have the same wzy gene which is thought to be distinct among different capsular types, but also have indistinguishable cps regions with the exception of a sequence difference in the ORF downstream of gnd. In K22, this ORF encodes a putative acetyltransferase; in K37, the ORF is truncated as a result of a frameshift mutation (single nucleotide deletion) relative to K22 (Figure 6). Interestingly, this result is consistent with the previous finding that the capsule structures of K22 and K37 differ only by the presence of acetyl group in K22 CPS [36]. We designed two primers (K22-acylF and K22-acylR) appropriate for amplification of the acetyltransferase gene (Table 1). Sequencing of the resulting amplicon is expected to reveal the status of the putative acetyltransferase-encoding gene, permitting the distinction between K22 and K37 despite identity in both wzc and wzy.
Figure 6. Genetic alignment of K22 and K37 cps regions and a frameshift mutation in K37.
Open reading frames (ORFs) are shown as arrows. In K37, a single nucleotide deletion (boxed) was observed at nucleotide position 551 of a gene encoding a putative acetyltransferase. This deletion is predicted to result in truncation (at the underlined stop codon) of the ORF in K37.
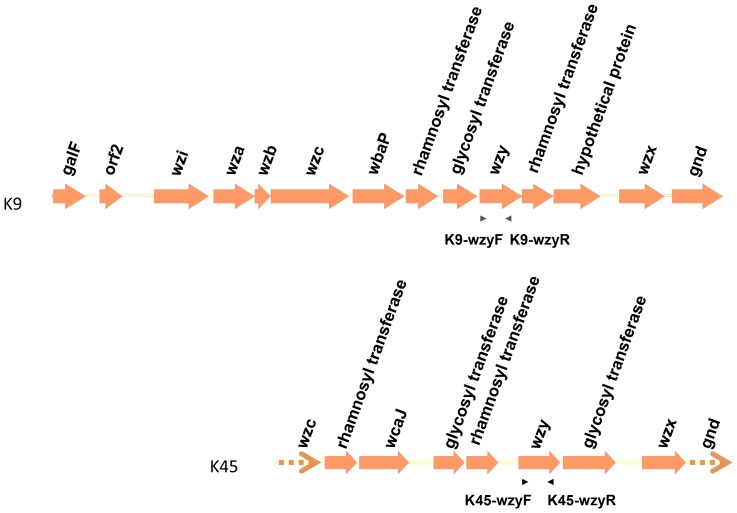
Although the wzc genes of K9 and K45 showed high DNA sequences similarity (99% identity), genes located in the cps regions differed between these two capsular types (Figure 7; Accession Numbers AB371293 and AB819892). We designed primers K9-wzyF, K9-wzyR, K45-wzyF, and K45-wzyR based on the sequences of the wzy genes of K9 and K45 (Table 1 and Figure 7), and demonstrated that PCR amplification with K9-wzyF and K9-wzyR was detected in the K9 capsular type strain but not in the other 77 capsular type strains. Likewise, K45-wzyF and K45-wzyR also showed specificity for capsular type K45 (data not shown). Therefore, these two type-specific primer pairs can be used to distinguish K9 and K45, despite highly similar wzc sequences.
Figure 7. The genetic alignment of K9 and K45 cps regions and the primers for cps-PCR genotyping.
Open reading frames (ORFs) are shown as arrows. The arrows with dotted lines indicate that only partial sequences were obtained for these ORFs. The positions and orientations of cps-PCR genotyping primers, located in the wzy genes, are indicated by arrow heads. Primer pair K9-wzyF and K9-wzyR was used for K9 cps-PCR genotyping; primer pair K45-wzyF and K45-wzyR was used for K45 cps-PCR genotyping.
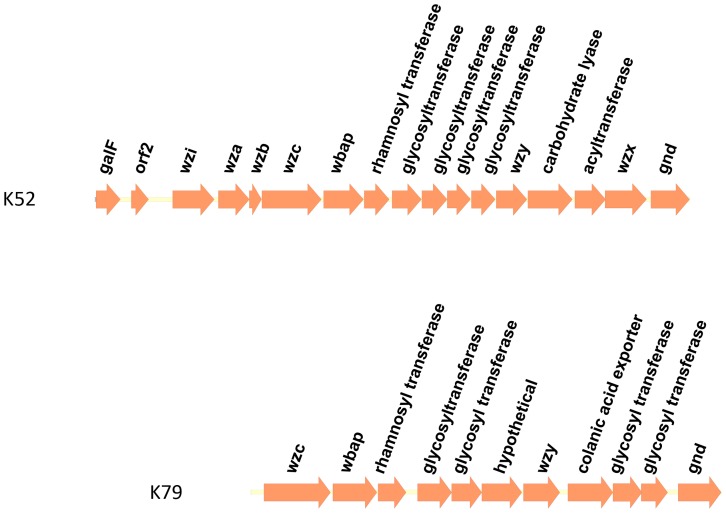
The full sequenced cps regions of K52 and K79 type strains revealed that these type strains possessed different genes in the clusters, although the strains shared 89% identity in wzc sequences (Figure 8; Accession Numbers CP000647 and AB819896). Furthermore, the wzc sequences of the two K52 strains in our panel exhibited 100% identity at the DNA level, suggesting that K52 and K79 can still be distinguished despite similarities in wzc sequences.
Figure 8. The genetic alignment of the K52 and K79 cps regions.
Open reading frames (ORFs) are shown as arrows.
wzc genotyping of clinical isolates with unknown capsular types
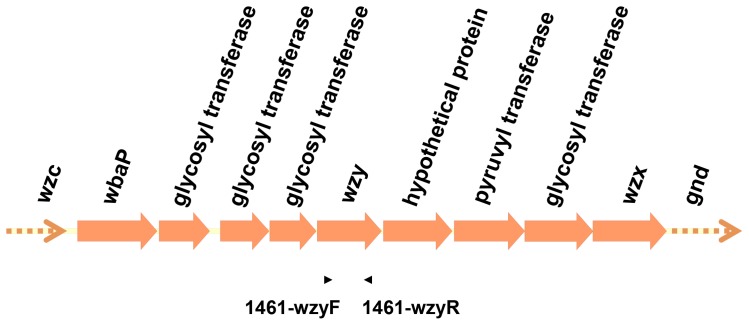
To evaluate the wzc genotyping system, capsular types of 29 K. pneumoniae blood isolates (obtained from patients admitted to NTUH) were determined by our method. The four primer pairs described above were used for PCR amplifications of the wza-wzb-wzc regions of these strains. The four primer pairs provided typing by PCR amplification in 90% (26/29) of these strains using primer pair 1 (KP-wza-CF1 and KP-wzc-CR1), 97% (28/29) using primer pair 2 (KP-wza-CF2 and KP-wzc-CR1), 59% (17/29) using primer pair 3 (KP-wza-CF1 and KP-wzc-CR2), and 62% (18/29) using primer pair 4 (KP-wza-CF2 and KP-wzc-CR2). The combination of primer pairs 1 and 2 permitted typing of all 29 of the tested strains. The amplified PCR products by use of primer pairs 1 were sequenced by the reverse primer KP-wzc-CR1 in 26 of the 29 strains, whereas the primer pair 2-amplicons were subjected to sequencing with KP-wzc-CR1 in the remaining three strains, my1684, 5872, and 5982-2. Sequences from CD1 to CD2 covered VR2 region (115-151 bp) were used for comparing with our wzc database. The results revealed that among the 29 strains, 28 strains showed high DNA sequence similarity (≧94% identity) with documented capsular types in CD1-VR2-CD2 region. Based on the DNA sequences, these 28 strains were classified as capsular type K1 (n = 6), K2 (5), K16 (3), K20 (2), K54 (2), K28 (2), K14 (1), K23 (1), K24 (1), K27 (1), K39 (1), K61 (1), K62 (1), and KN1 (1). We further confirmed the results by wzy-PCR genotyping using type-specific wzy primers for K1, K2, K14, K16, K20, K23, K39, K54, K62, and KN1 [19], [25]. The results demonstrated that wzc genotyping provided results consistent with wzy genotyping (Table 3). The one (out of 29) remaining strain showed relatively low DNA sequence similarity in CD1-VR2-CD2 region (<70% identity) with the documented capsular types in our wzc panel, suggesting that this strain represented a novel wzc sequence. Therefore, we further evaluated this strain to determine whether this strain represented a new capsular type distinct from the previously described 78 types. Specifically, the cps region of strain 1461 was amplified and the variable regions of cps gene cluster was analyzed (Figure 9) (Accession Number AB819897). Specific primers 1461-wzyF and 1461-wzyR were designed based on the novel wzy sequence (Table 1 and Figure 9); PCR genotyping with this primer pair provided detection only for strain 1461, and not for any of the 78 documented capsular types (data not shown). Based on these results, we infer that this strain likely represents a novel capsular type.
Table 3. wzc type of K. pneumoniae clinical isolates causing bacteremia.
| Strain | wzc type | DNA sequences identity (%) | wzy PCR check |
| 526 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 1024 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 229 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 217 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 9285 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 92 | K1 | 124/124(100%), NTUH-K2044 | K1 + |
| 7313 | K2 | 133/133(100%), Chedid | K2 + |
| 1529 | K2 | 133/133(100%), Chedid | K2 + |
| 1730 | K2 | 133/133(100%), Chedid | K2 + |
| 5154 | K2 | 133/133(100%), Chedid | K2 + |
| 9951-2 | K2 | 133/133(100%), Chedid | K2 + |
| 4410 | K14 | 142/142(100%), K14 ref | K14 + |
| 7476 | K16 | 124/124(100%), K16 ref | K16+ |
| 7270 | K16 | 124/124(100%), K16 ref | K16+ |
| 4001-2 | K16 | 122/124(98%), K16 ref | K16 + |
| 5262 | K20 | 127/127(100%), NTUH-KP13 | K20 + |
| 1296 | K20 | 120/127(94%), NTUH-KP13 | K20 + |
| 3329 | K23 | 133/133(100%), K23 ref | K23+ |
| 8531 | K24 | 124/124(100%), K24 ref | N/A |
| 8577 | K27 | 139/139(100%), K27 ref | N/A |
| my1684 | K28 | 115/115(100%), K28 ref | N/A |
| 5982-2 | K28 | 115/115(100%), K28 ref | N/A |
| 6737 | K39 | 133/133(100%), K39 ref | K39 + |
| 3200 | K54 | 115/115(100%), NTUH-KP35 | K54+ |
| 5872 | K54 | 114/115(99%), NTUH-KP35 | K54 + |
| 6257 | K61 | 136/136(100%), K61 ref | N/A |
| 6341 | K62 | 136/136(100%), VGH698 | K62 + |
| 8393 | KN1 | 133/136(98%), A1517 | KN1 + |
| 1461 | new | (wzc database <70%) | N/A |
Note: the ratio of identity indicates no. of matching nucleotides/total no. of nucleotides of the CD1-VR2-CD2 region from the strain with highest similarity in our wzc database.
Figure 9. The genetic alignment of cps region of a probable new type and primers for cps-PCR genotyping.
Open reading frames (ORFs) are shown as arrows. The arrows with dotted lines indicate that only partial sequences were obtained for these ORFs. The positions and orientations of cps-PCR genotyping primers, located in the wzy gene, are indicated by arrow heads. Primer pair 1461-wzyF and 1461-wzyR was used for 1461 cps-PCR genotyping.
Discussion
Serotyping has been used for determination of K. pneumoniae K-types since 1926 [37]. However, several studies have suggested that a substantial proportion (ranging from 23% to 75% in different laboratories) of strains are non-typable by serotyping. [20], [22], [23]. These observations could reflect limited assay sensitivity, or could reflect limited assay specificity (e.g., serological cross-reactivity between different capsular types). In addition, the high cost and limited sources of anti-sera and tedious experimental procedures of serotyping make the practice of serotyping difficult. Therefore, capsular genotyping methods that bypass the use of anti-sera have become more widely used in discriminating the capsular types of K. pneumoniae [6], [7], [19], [25]–[30]. PCR-based cps genotyping is a rapid and accurate method for detecting cps genotype [25]. Since the gene layout and DNA sequences of variable regions in the cps synthesis loci are distinct in different capsular types, type-specific primers (located in wzy-like genes or other genes of the cps gene cluster) can be used for distinguishing capsular types. However, this method does not permit detection of all capsular types, because classification cannot be performed unless the DNA sequences of the entire cps gene cluster are available. One study reported a novel capsular genotyping method, cps PCR-RFLP analysis, that permitted typing with high discriminatory power [31]. In this method, capsular types are determined according to the distinct RFLP profiles (C-patterns). In addition, this technique permits distinction among strains with the same K serotype, because subtle differences in DNA sequences can be detected based on variations in cps PCR-RFLP pattern. However, this increased complexity may complicate interpretation of capsular genotyping. Moreover, these two capsular genotyping methods (cps-PCR genotyping and cps PCR-RFLP) require the amplification of the entire ∼20 kb capsule synthesis region; such long PCR products can be difficult to obtain. By comparison, the wzc genotyping method (developed in the present study) requires amplification of a ∼2.5–3 kb PCR fragment and ∼350 bp of DNA sequencing can cover the variable region for comparison. As demonstrated by our PCR analysis of 78 capsular type strains, along with multiple clinical isolates, PCR amplicons were obtained in more than 90% of strains screened with primer pair 1 alone, and in up to 100% of strains screened with the combination of primer pairs 1 and 2. Therefore, our method is expected to be convenient and useful in clinical settings; most isolates will be identifiable using only one or two primer pairs, with few strains requiring testing with additional primers.
Our results indicated that wzc CD1-VR2-CD2 sequences were highly similar (≧94% DNA identity) among strains with the same capsular type. Relatively low levels of similarity (<80% identity) were observed among strains of different capsular types, with the exceptions of K22/K37 (100% wzc identity), K9/K45 (98% identity), and K52/K79 (90% identity). Since K52 and K79 can still be discriminated based on differences in wzc sequences, our proposed typing method is expected to discriminate 74 types (including type K22/K37 and K9/K45). Therefore, only the differentiation between types K22 and K37 (requiring sequencing of the putative acetyltransferase-encoding gene), between K9/K45 (requiring cps-PCR genotyping) and in wzc-deficient K15 and K50 (requiring cps-PCR genotyping) would require the use of additional assays.
After the cps regions of K22 and K37 were resolved, we found that K22 and K37 shared same cps genes for their capsule synthesis. This result was consistent with previous observation that the cps PCR-RFLP patterns of K22 and K37 were indistinguishable [38] and that K22 and K37 were usually cross-reactive by serotyping [39]. Interestingly, we also observed the truncation of a putative acetyltransferase-encoding ORF in K37 compared to K22, providing an explanation for the virtually identical (except for an acetylation modification) capsule structures of K22 and K37 [36]. This phenomenon is similar to that of pneumococcus 9V and 9A, which differ from each other only in the acetylation of capsule. Serotype 9V was found to possess an intact acetyltransferase-encoding gene, while the equivalent gene of serotype 9A was disrupted by a frameshift mutation (deletion of guanine at nucleotide 726) [40].
Although wzc sequences were almost identical in K9 and K45, the cps gene clusters differed between the two capsular types. This could be due to recombination in the region from wzc to gnd, resulting in gene replacement across this interval. Therefore, the cps sequence similarities between K9 and K45 and between K22 and K37 may provide insights into the evolution and divergence of capsular types.
The cps sequences of reference strains K15 and K50 revealed the presence in the clusters of several genes encoding transposase homologs. Notably, the typical wzb-wzc locus of the cps region was replaced by transposase-like genes in these two capsular types. The wzb and wzc genes may have been lost during chromosomal rearrangements associated with transposition events. Wzc, a tyrosine autokinase, is dephosphorylated by its cognate phosphatase, Wzb. Wza, located in the outer membrane, is known to interact with the periplasmic domain of Wzc and is believed to act as a channel [41]. These gene products (Wza, Wzb, and Wzc) are associated with the control of capsule polysaccharide polymerization and cross-membrane translocation, and are thought to be essential for capsule synthesis in E. coli and Klebsiella sp. [42]–[44]. We demonstrated that reference strains K15 and K50 were in fact acapsular. This observation is consistent with the absence of wzb and wzc in these two strains. Capsule structures of reference strains K15 and K50 from the same origin (Statens Serum Institute) have been reported in previous studies in 1992 and 1982 respectively [45], [46]. Our reference strains K15 and K50 were purchased from Statens Serum Institute in 2004 and stored at –80°C. Experiments in this study were performed using original stock in our laboratory, therefore, these two strains seemed to have lost capsule before we obtained them.
Using our proposed wzc typing method, we were able to successfully determine the capsular types of all of the clinical isolates tested, with the exception of a single strain that appears to represent a new capsular type. According to the comparison of sequences in our wzc database, strains with same capsular type shared ≧97% identity, but one strain among the clinical isolates of known types did not hit 97% identity. However, even though the only exception revealed 94% (<97%) DNA identity in CD1-VR2-CD2 region with the corresponding locus of capsular type K20 from our wzc database, wzy PCR genotyping confirmed that this strain was type K20. Therefore, our data suggest that strains harboring wzc CD1-VR2-CD2 sequences of ≧94% DNA sequence identity can be expected to share the same capsular type. Furthermore, the consistency of the results between wzc- and wzy-genotyping suggests that wzc should provide genotyping as accurate as that of wzy. Since not all of the wzy genes for the documented capsular types are currently available, wzc genotyping, a simple alternative method, may be more useful for complete capsular typing. Moreover, we infer that strains with novel wzc sequences probably represent new cps genotypes. Consistent with this hypothesis, we noted that the cps region of strain 1461 was distinct from those of previously reported capsular types. Notably, the cps gene cluster of strain 1461 was most similar to that of E. coli MS 146-1(Accession No. ADTN00000000). Previous studies had reported that Klebsiella K20 and E. coli K30 harbor identical capsule structures and highly similar cps sequences, implying that horizontal gene transfer had occurred between these strains [47]. Our results with strain 1461 provided further evidence for this phenomenon.
Wzc, an inner membrane protein with a cytosolic C-terminal tyrosine autokinase domain, is believed to interact with the outer membrane protein Wza, forming a trans-envelope capsule translocation complex. In the current study, we demonstrated capsular type-specific regions in the wzc locus. And we also found that VR2 region is rich in lysine (a basic amino acid). Therefore, the variable regions of wzc genes might encode binding domains containing positively charged amino acids. The lysine-rich domains might interact with type-specific acidic capsular polysaccharides during the process of translocation.
In conclusion, we have developed a simple and useful capsular genotyping method for K. pneumoniae based on wzc sequences. We demonstrated the use of this typing method for the detection of existing and novel capsular types of K. pneumoniae. Sequencing of cps loci suggested a molecular basis (frameshift mutation) for the difference between types K22 and K37, and revealed that reference strains K15 and K50 were acapsular.
Supporting Information
Primers used for wzc sequencing.
(DOCX)
CD1-VR2-CD2 sequences.
(TXT)
Acknowledgments
We thank Ms Yi-Li Liu and I-Ching Huang for technical support in DNA sequencing. Sequencing was supported in part by Department of Medical Research in National Taiwan University Hospital.
Funding Statement
This study was supported by grants from the National Science Council, National Taiwan University, National Taiwan University Hospital and the Liver Disease Prevention and Treatment Research Foundation in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abbot SL (2003) Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and other Enterobacteriaceae. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, eds Manual of clinical microbiology 8th ed Washington DC: American Society for Microbiology Press: 684–700.
- 2. Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clinical Microbiology Reviews 11: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin WH, Wang MC, Tseng CC, Ko WC, Wu AB, et al. (2010) Clinical and microbiological characteristics of Klebsiella pneumoniae isolates causing community-acquired urinary tract infections. Infection 38: 459–464. [DOI] [PubMed] [Google Scholar]
- 4. Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, et al. (2005) Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 40: 915–922. [DOI] [PubMed] [Google Scholar]
- 5. Chang CM, Lee HC, Lee NY, Lee IW, Wu CJ, et al. (2008) Community-acquired Klebsiella pneumoniae complicated skin and soft-tissue infections of extremities: emphasis on cirrhotic patients and gas formation. Infection 36: 328–334. [DOI] [PubMed] [Google Scholar]
- 6. Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ, et al. (2007) Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 54: 578–583. [DOI] [PubMed] [Google Scholar]
- 7. Fang FC, Sandler N, Libby SJ (2005) Liver abscess caused by magA+ Klebsiella pneumoniae in North America. Journal of Clinical Microbiology 43: 991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, et al. (2002) A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okano H, Shiraki K, Inoue H, Kawakita T, Yamamoto N, et al. (2002) Clinicopathological analysis of liver abscess in Japan. Int J Mol Med 10: 627–630. [PubMed] [Google Scholar]
- 10. Rahimian J, Wilson T, Oram V, Holzman RS (2004) Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 39: 1654–1659. [DOI] [PubMed] [Google Scholar]
- 11. Tsai FC, Huang YT, Chang LY, Wang JT (2008) Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 14: 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS (1991) Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 151: 1557–1559. [PubMed] [Google Scholar]
- 13. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT (2004) A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, et al. (2002) Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohmori S, Shiraki K, Ito K, Inoue H, Ito T, et al. (2002) Septic endophthalmitis and meningitis associated with Klebsiella pneumoniae liver abscess. Hepatol Res 22: 307–312. [DOI] [PubMed] [Google Scholar]
- 16. Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, et al. (1998) Primary liver abscess due to Klebsiella pneumoniae in Taiwan.[see comment]. Clinical Infectious Diseases 26: 1434–1438. [DOI] [PubMed] [Google Scholar]
- 17. Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, et al. (2002) Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infection & Immunity 70: 2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, et al. (1983) Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun 40: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, et al. (2008) Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. Journal of Clinical Microbiology 46: 2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenney AW, Clements A, Farn JL, Wijburg OL, McGlinchey A, et al. (2006) Seroepidemiology of Klebsiella pneumoniae in an Australian Tertiary Hospital and its implications for vaccine development. Journal of Clinical Microbiology 44: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cryz SJ Jr, Mortimer PM, Mansfield V, Germanier R (1986) Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. Journal of Clinical Microbiology 23: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, et al. (2000) A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. The Journal of infectious diseases 181: 2075–2079. [DOI] [PubMed] [Google Scholar]
- 23. Tsay RW, Siu LK, Fung CP, Chang FY (2002) Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med 162: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 24. Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, et al. (2008) Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagnostic microbiology and infectious disease 62: 1–6. [DOI] [PubMed] [Google Scholar]
- 25. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT (2006) Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. Journal of Infectious Diseases 193: 645–654. [DOI] [PubMed] [Google Scholar]
- 26. Keynan Y, Karlowsky JA, Walus T, Rubinstein E (2007) Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand J Infect Dis 39: 828–830. [DOI] [PubMed] [Google Scholar]
- 27. Nadasy KA, Domiati-Saad R, Tribble MA (2007) Invasive Klebsiella pneumoniae syndrome in North America. Clin Infect Dis 45: e25–28. [DOI] [PubMed] [Google Scholar]
- 28. Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA (2005) Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol 54: 1111–1113. [DOI] [PubMed] [Google Scholar]
- 29. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, et al. (2007) Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45: 284–293. [DOI] [PubMed] [Google Scholar]
- 30.Yu WL, Fung CP, Ko WC, Cheng KC, Lee CC, et al.. (2007) Polymerase chain reaction analysis for detecting capsule serotypes K1 and K2 of Klebsiella pneumoniae causing abscesses of the liver and other sites. J Infect Dis 195: 1235–1236; author reply 1236. [DOI] [PubMed]
- 31. Brisse S, Issenhuth-Jeanjean S, Grimont PA (2004) Molecular serotyping of Klebsiella species isolates by restriction of the amplified capsular antigen gene cluster. J Clin Microbiol 42: 3388–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsieh PF, Lin TL, Yang FL, Wu MC, Pan YJ, et al. (2012) Lipopolysaccharide O1 antigen contributes to the virulence in Klebsiella pneumoniae causing pyogenic liver abscess. PLoS One 7: e33155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT (2006) Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193: 645–654. [DOI] [PubMed] [Google Scholar]
- 34. Moller HJ, Heinegard D, Poulsen JH (1993) Combined alcian blue and silver staining of subnanogram quantities of proteoglycans and glycosaminoglycans in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem 209: 169–175. [DOI] [PubMed] [Google Scholar]
- 35. Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, et al. (2002) Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J Biol Chem 277: 41613–41623. [DOI] [PubMed] [Google Scholar]
- 36. Parolis LA, Parolis H, Niemann H, Stirm S (1988) Primary structure of Klebsiella serotype K22 capsular polysaccharide: another glycan containing 4-O-[(S)-1-carboxyethyl]-D-glucuronic acid. Carbohydr Res 179: 301–314. [DOI] [PubMed] [Google Scholar]
- 37. Julianelle LA (1926) A Biological Classification of Encapsulatus Pneumoniae (Friedlander's Bacillus). J Exp Med 44: 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brisse S, Issenhuth-Jeanjean S, Grimont PA (2004) Molecular serotyping of Klebsiella species isolates by restriction of the amplified capsular antigen gene cluster. Journal of Clinical Microbiology 42: 3388–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ørskov I, Ørskov F (1984) Serotyping of Klebsiella. Methods Microbiology 14: 143–164. [Google Scholar]
- 40. Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, et al. (2006) Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet 2: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whitfield C (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75: 39–68. [DOI] [PubMed] [Google Scholar]
- 42. Drummelsmith J, Whitfield C (2000) Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J 19: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wugeditsch T, Paiment A, Hocking J, Drummelsmith J, Forrester C, et al. (2001) Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J Biol Chem 276: 2361–2371. [DOI] [PubMed] [Google Scholar]
- 44. Lin MH, Hsu TL, Lin SY, Pan YJ, Jan JT, et al. (2009) Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol Cell Proteomics 8: 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Altman E, Dutton GG (1983) Structure of the capsular polysaccharide of Klebsiella serotype K50. Carbohydrate Research 118: 183–194. [DOI] [PubMed] [Google Scholar]
- 46. Parolis H, Parolis LA, Whittaker DV (1992) Re-investigation of the structure of the capsular polysaccharide of Klebsiella K15 using bacteriophage degradation and inverse-detected NMR experiments. Carbohydr Res 231: 93–103. [DOI] [PubMed] [Google Scholar]
- 47. Rahn A, Drummelsmith J, Whitfield C (1999) Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae. J Bacteriol 181: 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for wzc sequencing.
(DOCX)
CD1-VR2-CD2 sequences.
(TXT)