Abstract
The interaction of arthropods with the environment and the management of their populations is a focus of the ecological agenda. Spatial autocorrelation and under-sampling may generate bias and, when they are ignored, it is hard to determine if results can in any way be trusted. Arthropod communities were studied during two seasons and using two methods: window and panel traps, in an area of ancient temperate lowland woodland of Zebracka (Czech Republic). The composition of arthropod communities was studied focusing on four site level variables (canopy openness, diameter in the breast height and height of tree, and water distance) and finally analysed using two approaches: with and without effects of spatial autocorrelation. I found that the proportion of variance explained by space cannot be ignored (≈20% in both years). Potential bias in analyses of the response of arthropods to site level variables without including spatial co-variables is well illustrated by redundancy analyses. Inclusion of space led to more accurate results, as water distance and tree diameter were significant, showing approximately the same ratio of explained variance and direction in both seasons. Results without spatial co-variables were much more disordered and were difficult to explain. This study showed that neglecting the effects of spatial autocorrelation could lead to wrong conclusions in site level studies and, furthermore, that inclusion of space may lead to more accurate and unambiguous outcomes. Rarefactions showed that lower sampling intensity, when appropriately designed, can produce sufficient results without exploitation of the environment.
Introduction
Research on diversity of arthropods, management of populations and their interaction with the environment is one of the main topics of the present ecological agenda, especially with respect to potential biotical and abiotic threats [1]. However, recent research on biota suffers from many pitfalls [2], which may lead to biased conclusions [3].
Arthropods are, mostly quickly, responding to environmental changes [1] and knowledge of their response to habitat parameters continues to increase [4], [5]. Many arthropods are influenced by the fact that they are often dispersal-limited and thus not able to reach more distant habitats [6], [7]. Response of arthropods to the environmental variables may be biased by spatial structure of their distribution [8].
With respect to data analyses, spatial autocorrelation is one of the statistical problems encountered when modelling species-environmental relationships [9], [10]. Spatial heterogeneity is defined as either the variation in space in the distribution of a point pattern, or as variation in the qualitative or quantitative value of a surface pattern [11], [12], which can be caused also by site level factors [13]. Spatial dependency within geographic space leads to the spatial autocorrelation [14]. It is known that all of nature is autocorrelated that everything is related to everything else and that objects close to each other are more related than those that are further apart [15] – thus, spatial autocorrelation generates possible bias [16]. Although the existence of spatial autocorrelation does not in itself constitute real bias, it does in terms of what ecologists want to understand [9]. Spatial autocorrelation can be fundamental to building a spatial component into statistical models [17]. If spatial autocorrelation is ignored it is always hard to determine if results can in any way be trusted [3].
Present studies indicate that potential bias of spatial autocorrelation is not absolutely dependent on scale of trapping designs [8], [9], [17], [18]. Thus, mass trapping is not necessary for site level type of studies. Lower trapping intensity in appropriately designed studies gain useful results and may lead to a higher significance without exploitation of the environment [19].
The main goals of this study were to determine the response of arthropod communities to environmental variables at the site level in an area of continuous lowland woodland and to determine how their response could be influenced by sampling intensity and spatial autocorrelation, while employing commonly used trapping methods at the same trees during two seasons.
Materials and Methods
Ethics Statement
All necessary permits were obtained for the described study. As the study area was a part of the protected area, this study was undertaken with the permission of the Czech Government (no. 1473/09).
Study Area
All sample occasions were situated within approximately 50 ha situated in the national nature reserve of Zebracka (Prerov, Olomouc Region, Central Moravia, Czech Republic; Fig. 1), one of the most continuous (i.e. ancient) deciduous woodlands near the River Becva [20], known from at least the 1750s (i.e. the time of the first Austro-Hungarian Military Mapping). Zebracka is known to be one of the species rich woodlands in Moravia [21].
Figure 1. Map of the study sites in Zebracka (Czech Republic).
Sampling Methods
During the 2009 season, fifteen crossed-panel window traps [22] were used, replaced in 2010 by panel traps [23] – two commonly used methods. All traps were placed on the stems of standing veteran poplars (Populus) at 1.3 m. The sampling methods are described in Table 1. All trapped arthropods were identified to the taxon level listed in Table 2.
Table 1. Description and comparison of trapping methods used in deciduous woodland area of Zebracka.
| Character | Window trap | Panel trap |
| Season | 2009 | 2010 |
| Number | 15 | 15 |
| Intercept area (m2) | 0.800 | 0.063 |
| Colour | Blank | Yellow |
| Activity | Passive | Active |
| Medium | Water+NaCl+detergent [22] | Soveurode® Witasek [23] |
| Selection | Not known | Larger (>1.5 cm) individuals |
| Trapping activity | 20.V.–12.IX. | 9.IV.–11.IX. |
| Trapping days per trap | 115 | 155 |
| Placement | Trunk | Trunk |
| Height of the centre (m) | 1.3 | 1.3 |
| Irradiance | South | South |
Table 2. Results of trapped taxa (sorted in alphabetical order) and abundances during the study seasons 2009 and 2010 in a deciduous woodland area of Zebracka.
| Taxa | 2009 | 2010 | Total |
| Araneae | 383 | 184 | 567 |
| Coleoptera | 1,756 | 2,256 | 4,012 |
| Collembola | 15 | – | 15 |
| Dermaptera | 1,090 | 1 | 1,091 |
| Diptera | 546 | 9,485 | 10,031 |
| Ephemeroptera | 9 | 2 | 11 |
| Glomerida | 104 | – | 104 |
| Hemiptera: Heteroptera | 95 | – | 95 |
| Hemiptera: Sternorrhyncha | 106 | 19,019 | 19,125 |
| Hymenoptera | 2,413 | 1,269 | 3,682 |
| Chilopoda | 3 | – | 3 |
| Isopoda | 41 | – | 41 |
| Ixodida | 20 | – | 20 |
| Julida | 182 | 6 | 188 |
| Lepidoptera | 284 | 74 | 358 |
| Mecoptera | 10 | 12 | 22 |
| Neuroptera | – | 11 | 11 |
| Opilionida | 84 | 4 | 88 |
| Orthoptera | 37 | – | 37 |
| Prostigmata | 5 | – | 5 |
| Pseudoscorpionida | 6 | 2 | 8 |
| Pulmonata | 76 | – | 76 |
| Raphidioptera | 14 | – | 14 |
| Trichoptera | 39 | 1 | 40 |
Study Variables
I focused on four site level variables that potentially most influenced the occurrence of the studied taxa of arthropods (Table 3): (1) Canopy openness (an expression of light conditions) was measured under full foliage using a Sigma 4.5 mm F2.8 EX DC Circular FISHEYE HSM on July 15, 2010. Photographs were evaluated using a Gap Light Analyser 2.0 [24]. (2) Diameter at breast height of tree (an expression of tree diameter) was calculated from the circumference of a tree at 1.3 m from ground level. (3) Height of a tree (an expression of vertical biological frontier) was estimated and rounded in metres in the field. (4) Water distance (an expression of humidity) was calculated from the Euclidean distance of a tree to the River Becva.
Table 3. Results of descriptive statistics of study site level variables in a deciduous woodland area of Zebracka (sorted in alphabetical order).
| Variable | Mean | S.E. | Minimum | Maximum |
| Canopy openness (%) | 28.64 | 3.97 | 11.29 | 57.95 |
| Diameter (cm) | 82.29 | 6.35 | 49.68 | 135.35 |
| Height (m) | 13.73 | 0.50 | 10.00 | 15.00 |
| Water distance (m) | 104.87 | 18.47 | 3.00 | 230.00 |
Statistical Analyses
For identification of sufficient number of trapping occasions, sample-based rarefaction with 95% confidence intervals computed using a Mao Tau function [2] and the Chao estimation function [25] were used. Analyses were computed in EstimateS 8.2 [26]. The number of randomisations was set at 1,000, with strong hash encryption and randomisation of samples without replacement. The upper abundance limit for rare or infrequent species was set at 10. I used the classic formulae for Chao for bias correction [26].
Correlation of taxa between seasons was evaluated using Spearman correlation coefficient in R.
Spatial autocorrelation was preliminary tested by randomized Geary’s C test using packages spdep and RANN in R [27].
For the final analyses with and without space, I used multivariate statistical methods provided by CANOCO for Windows version 4.5 [28]. All species data were square-root transformed, as is recommended for trapping designs [29]. The length of gradient in detrended correspondence analysis (DCA) in each season was at <2, which demonstrated that the data did not reveal high heterogeneity [28]. I therefore used redundancy analysis (RDA), a constrained linear ordination method [29]. I used focused scaling on inter-species correlations, and species scores divided by S.D., Monte-Carlo permutation tests with significance of canonical axes together (9,999 permutations) under the full model were used [29].
During the process of variance partitioning [30], two sets of environmental categories were used. Each fraction was measured based on three multivariate analyses using combinations of taxa, variables and co-variables [9], [31]. The first set of explanatory variables was composed of five most commonly spatial variables [32], namely geographical coordinates of the sampling points (x, y) and their squares and cross-product terms (x2, y2, xy) [8]. The cubic terms [30] were not included, keeping a comparable number of factors for the three explanatory data sets [8]. Thus, I used firstly space (x, y, xy, x2 and y2) as variables and habitat (Canopy openness, Diameter, Height and Water distance) as co-variables, then the same in reverse order, and finally with all variables included [9]. I visualised the results of variance partitioning using a two-circle Venn diagram.
The shared explained variance and p values of habitat variables were employed with a Monte Carlo permutation test (9,999 permutations) under the full model [29]. For the resulting ordination diagrams, I used RDA environmental ordination plots created in CanoDraw 4.14 [28].
Results
The number of trapped taxonomical groups (Table 2) was 23 (7,318 individuals) in 2009 and 14 (32,326 individuals) in 2010. Total number of taxa was 24 (39,644 individuals), while 13 taxa overlapped in both seasons (Table 2; 4).
Taxa Accumulations and Correlations in Distribution between Seasons
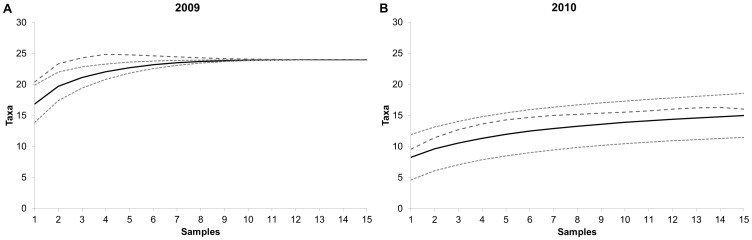
Sample-based rarefactions were made separately for each season (Fig. 2) and indicated that trapping success was higher for window traps than for panel traps. The curve reached its asymptote in 2009 (Fig. 2a), but not in 2010. However, the Chao estimate seemed to approach the total number of taxa (Fig. 2b), suggesting that the majority of the taxa in the study area were represented in the forthcoming analysis and that the number of samples was sufficient. Except of millipedes from order Julida, there was no correlation structure between 13 taxa overlapped in both seasons (Table 4).
Figure 2. Taxa rarefactions and estimates of total richness of the trapped arthropods in the deciduous woodland.
Complete data for taxa from all samples are included from a) 2009 and b) 2010. The solid black line shows a sample-based rarefaction of assemblages; the two surrounding light-grey dashed lines are Mao Tau estimates with 95% confidence intervals and the dark-grey dashed line is the Chao 1 estimate of the total number of taxa.
Table 4. Correlation of taxa distribution in traps between the study seasons 2009 and 2010 in a deciduous woodland area of Zebracka (sorted in alphabetical order).
| Taxa | r | p |
| Araneae | −0.05 | 0.87 |
| Coleoptera | 0.10 | 0.72 |
| Dermaptera | −0.17 | 0.55 |
| Diptera | 0.21 | 0.46 |
| Ephemeroptera | −0.14 | 0.64 |
| Hemiptera | 0.12 | 0.67 |
| Hymenoptera | 0.27 | 0.34 |
| Julida | 0.89 | <0.0001 |
| Lepidoptera | 0.41 | 0.13 |
| Mecoptera | 0.38 | 0.16 |
| Opilionida | −0.23 | 0.25 |
| Pseudoscorpionida | −0.15 | 0.58 |
| Trichoptera | −0.34 | 0.21 |
Preliminary Tests on Spatial Autocorrelation of Taxa Richness and Habitat Variables
Richness of study taxa was not spatially autocorrelated in either year (Geary’s C2009 = 0.78, p = 0.19; Geary’s C2010 = 0.94, p = 0.41), with the same being the case for Diameter (Geary’s C = 0.67, p = 0.10) and Height of tree (Geary’s C = 0.63, p = 0.08). On the other hand, two site level variables were spatially autocorrelated: Canopy openness (Geary’s C = 0.34, p = 0.0060) and Water distance (Geary’s C = 0.13, p = 0.0005).
Response of Arthropod Communities using Multivariate Statistics
Venn diagrams (Fig. 3) demonstrate that the total explained variance was high in both seasons. Site level variables explained a high level of variance (especially in 2010) and the percentage of shared variance did not much differ between study seasons. In contrast, the significance of space was high in both seasons. This indicated potential bias in the response of arthropods to site level variables in analyses that do not include space.
Figure 3. Venn diagrams.
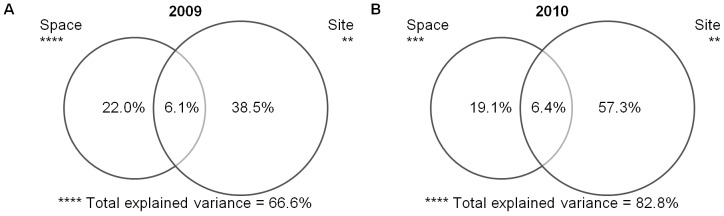
Figures are demonstrating percentage values of total, shared (values in circle overlaps) and independent explained variance of space and site using variance partitioning in a) 2009 and b) 2010 in a deciduous woodland area of Zebracka (**p<0.01; ***p<0.001; ****p = 0.0001).
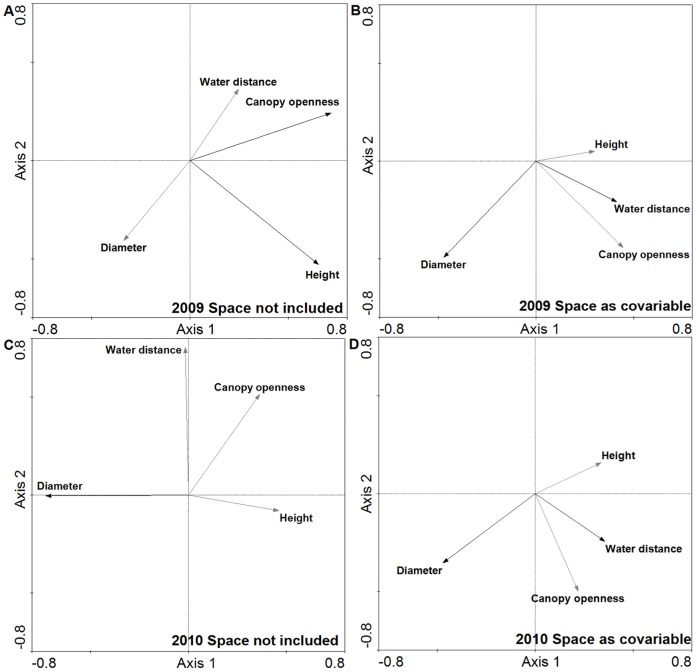
The main difference was that environmental site level variables were in all cases differently rotated along the first axis. It can be seen that Diameter is separated from other variables on the second axis, and that Height has a different influence than Canopy openness and Water distance, both of which have nearly the same effect on taxa composition (Fig. 4).
Figure 4. Relative position of environmental site level variables in RDA environmental ordination plots.
Figures are with respect to taxa composition in a) 2009 without spatial covariables, b) 2009 with spatial covariables, c) 2010 without spatial covariables, and d) 2010 with spatial covariables, all in a deciduous woodland area of Zebracka (habitat variables p<0.05, black; p = n.s., grey).
The analyses showed that the level of total explained variance was lower when the effect of space was included in the analyses. On the other hand, the significance of the results at the site level increased (Table 5). Furthermore, the values of shared variance and significance differed strikingly between analyses with and without spatial variables (Table 5) and the relative position of habitat variables in RDA was highly variable (Fig. 4). Including space led to more accurate results, because Water distance and Diameter were significant, showing approximately the same ratio of variance and direction in both seasons (Table 5). The results without spatial co-variables were much more disordered (e.g. the effect of Diameter) and were also relatively difficult to explain (Table 5; Fig. 4).
Table 5. Results of taxa composition response to study site level variables in a deciduous woodland area of Zebracka, with space included as co-variable (left) and not included (right).
| Variable | Trace | F | p | Variable | Trace | F | p |
| Space as co-variable | Space notincluded | ||||||
| 2009 | |||||||
| Total | 0.385 | 2.72 | 0.0024 | Total | 0.446 | 2.01 | 0.0118 |
| Water distance | 0.234 | 5.88 | 0.0001 | Canopyopenness | 0.180 | 2.85 | 0.0265 |
| Diameter | 0.135 | 2.72 | 0.0287 | Height | 0.169 | 2.65 | 0.0331 |
| Canopyopenness | 0.100 | 1.87 | 0.12 | Water distance | 0.061 | 0.84 | 0.46 |
| Height | 0.073 | 1.30 | 0.25 | Diameter | 0.059 | 0.82 | 0.50 |
| 2010 | |||||||
| Total | 0.573 | 6.66 | 0.0013 | Total | 0.636 | 4.38 | 0.0033 |
| Water distance | 0.310 | 7.49 | 0.0027 | Diameter | 0.323 | 6.21 | 0.0100 |
| Diameter | 0.217 | 4.27 | 0.0358 | Height | 0.138 | 2.08 | 0.15 |
| Height | 0.128 | 2.15 | 0.14 | Canopyopenness | 0.089 | 1.28 | 0.28 |
| Canopyopenness | 0.045 | 0.66 | 0.50 | Water distance | 0.018 | 0.24 | 0.76 |
Variables are sorted by value of trace (from highest to lowest).
Discussion
As arthropods dominate the landscape with respect to their abundance and richness, local trapping success may be highly dependent on habitat and landscape heterogeneity [33]. Much of the current research uses mass trapping designs, not only for eradication of potential pests, but also for estimating biodiversity [34], [35], [36]. It is known, but often overlooked, that a large number of samples does not necessarily imply more significant results [37], [38], [39]. From this point of view, appropriate statistical design [9], [29], [31], [40] may lead to a higher significance without major exploitation of the environment, which is illustrated on rarefaction curves therein.
The trapping methods used in this study are mainly used for flying arthropods [41], [42]. However, when traps are suitably designed, they are known also to trap flightless fauna [18]. This is illustrated by sedentary taxa such as snails (Gastropoda) [43] or pillbugs (Isopoda) in this study.
Økland [44] writes that the total explained variance in ordination methods using variance partitioning, even using sets of carefully selected explanatory variables, is normally 20–50%, and occasionally up to 65%. On the other hand, inclusion of the spatial autocorrelation [9], [45] into analyses often leads to lowering of the explained variance of habitat variables [46], as in this study.
Previous studies, similar to this case, have used multivariate analysis with variation partitioning to separate the confounding effects of space and environment [8], [47], [48]. The results show that including spatial autocorrelation in analyses may lead to a more accurate outcome while its exclusion may lead to wrong conclusions [10], [17]. Even if there were differences in trapped arthropods between seasons, the response to habitat variables was, surprisingly, nearly the same in both seasons.
The results showed that it is necessary, for the study of organismal distribution spatial patterns using site level variables, to take spatial autocorrelation into account [46], [47], [48]. This also makes it possible to distinguish when the spatial structure is mainly due to biotic interactions with an underlying unmeasured environmental factor, or a common spatial gradient shared by data on taxa and environmental variables [8], [30]. Results of variance partitioning showed that spatial terms gave a lesser explanation of variance than did site, although significance of space and shared variation led to a much better explanation than when spatial autocorrelation was not included [49].
In the context of this article, there is a strong need for spatial autocorrelation to be included in the analysis, as it is also in similar relatively small-scale studies.
Distance to the River Becva and Diameter of tree were significant habitat variables, when spatial autocorrelation was included. Water distance reflected the humidity of habitats, which is known to influence arthropod communities [50], [51], Diameter is a traditional habitat variable in studies, used to explain diameter and age of the tree, especially for saproxylic communities [4], [52] and thus the response of communities to Diameter was not surprising.
Conclusions
This study showed that neglecting spatial autocorrelation could possibly lead to wrong conclusions in small site level studies and, furthermore, that inclusion of spatial terms may lead to more accurate and less ambiguous outcomes. Lower sampling intensity, when appropriately designed, is able to gain sufficient results and may lead to a higher significance without major exploitation of the environment.
Acknowledgments
I would like to thank I. Neradilová for her help in the field, sorting and determinations, J. Klápště with Geary’s C and Z.F. Fric for his many good suggestions on the early version of the manuscript, two anonymous referees provided constructive comments. Thanks is also owed to P. Stloukal for his support, to A. Kohutka for help with FITs logistics, L. Hort for fish eye, Š. Vodka for fish eye and construction of FITs, the non-governmental organisation Lesák (www.lesak.eu) for logistics, and A. Scott for English correction.
Funding Statement
This study was supported by a grant of the Ministry of Agriculture of the Czech Republic (no. QH91097). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Samways M (2007) Insect conservation: a synthetic management approach. Annual Review of Entomology 52: 465–487. [DOI] [PubMed] [Google Scholar]
- 2. Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391. [Google Scholar]
- 3. Diniz-Filho JAF, Bini LM, Hawkins B (2002) Spatial autocorrelation and red herrings in geographical ecology. Global Ecology and Biogeography 12: 53–64. [Google Scholar]
- 4. Buse J, Schröder B, Assmann T (2007) Modelling habitat and spatial distribution of an endangered longhorn beetle – a case study for saproxylic insect conservation. Biological Conservation 137: 372–381. [Google Scholar]
- 5. Horak J, Chumanova E, Hilszczanski J (2012) Saproxylic beetle thrives on the openness in management: a case study on the ecological requirements of Cucujus cinnaberinus from Central Europe. Insect Conservation and Diversity 5: 403–413. [Google Scholar]
- 6. Götmark F, Von Proschwitz T, Franc N (2008) Are small sedentary species affected by habitat fragmentation? Local vs. landscape factors predicting species richness and composition of land molluscs in Swedish conservation forests. Journal of Biogeography 35: 1062–1076. [Google Scholar]
- 7. WallisDeVries MF (2004) A quantitative conservation approach for the endangered butterfly Maculinea alcon . Conservation Biology 18: 489–499. [Google Scholar]
- 8. Barbaro L, Rossi JP, Vetillard F, Nezan J, Jactel H (2007) The spatial distribution of birds and carabid beetles in pine plantation forests: the role of landscape composition and structure. Journal of Biogeography 34: 652–664. [Google Scholar]
- 9. Legendre P (1993) Spatial autocorrelation: trouble or new paradigm? Ecology 74: 1659–1673. [Google Scholar]
- 10. Mauricio Bini L, Diniz-Filho JAF, Rangel TF, Akre TS, Albaladejo RG, et al. (2009) Coefficient shifts in geographical ecology: an empirical evaluation of spatial and non-spatial regression. Ecography 32: 193–204. [Google Scholar]
- 11. Dutilleul P, Legendre P (1993) Spatial heterogeneity against heteroscedasticity: an ecological paradigm versus a statistical concept. Oikos 66: 152–171. [Google Scholar]
- 12. Vinatier F, Tixier P, Duyck P–F, Lescourret F (2011) Factors and mechanisms explaining spatial heterogeneity: a review of methods for insect populations. Methods in Ecology and Evolution 2: 11–22. [Google Scholar]
- 13. Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: a mini review. Ecological Research 17: 229–239. [Google Scholar]
- 14. Dormann CF, McPherson JM, Araujo MB, Bivand R, Bolliger J, et al. (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30: 609–628. [Google Scholar]
- 15. Tobler WR (1970) A computer movie simulating urban growth in the Detroit region. Economical Geography 46: 234–240. [Google Scholar]
- 16. Hawkins BA (2012) Eight (and a half) deadly sins of spatial analysis. Journal of Biogeography 39: 1–9. [Google Scholar]
- 17. Kühn I (2007) Incorporating spatial autocorrelation may invert observed patterns. Diversity and Distributions 13: 66–69. [Google Scholar]
- 18. Horak J, Vodka S, Pavlicek J, Boza P (2013) Unexpected visitors: flightless beetles in window traps. Journal of Insect Conservation 17: 441–449. [Google Scholar]
- 19. Horak J, Rebl K (2013) The species richness of click beetles in ancient pasture woodland benefits from a high level of sun exposure. Journal of Insect Conservation 17: 307–318. [Google Scholar]
- 20.Safar J (2003) Olomoucko. Chráněná území ČR VI. AOPK ČR Praha, EkoCentrum Brno.
- 21. Hradilek Z, Duchoslav M (2007) Flóra a vegetace Národní přírodní rezervace Žebračka u Přerova. Přírodopisný Časopis Slezského Muzea Opava (A) 56: 193–226. [Google Scholar]
- 22. Horak J (2011) Response of saproxylic beetles to tree species composition in a secondary urban forest area. Urban Forestry & Urban Greening 10: 213–222. [Google Scholar]
- 23. Francese JA, Oliver JB, Fraser I, Lance DR, Youssef N, et al. (2008) Influence of trap placement and design on capture of the emerald ash borer (Coleoptera: Buprestidae). Journal of Economic Entomology 101: 1831–1837. [DOI] [PubMed] [Google Scholar]
- 24.Frazer GW, Canham CD, Lertzman KP (1999) Gap Light Analyzer (GLA): imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York.
- 25. Chao A (1984) Non-parametric estimation of the number of classes in a population. Scandinavian Journal of Statistics 11: 265–270. [Google Scholar]
- 26.Colwell RK (2006) EstimateS: Statistical estimation of species richness and shared species from samples. Version 8. Available: http://viceroy.eeb.uconn.edu/estimates. Accessed 2010 May 5.
- 27.R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 28.ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca, NY, USA.
- 29.Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, UK.
- 30. Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055. [Google Scholar]
- 31. Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625. [DOI] [PubMed] [Google Scholar]
- 32. Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832. [Google Scholar]
- 33. Weibull AC, Ostman O, Granqvist A (2003) Species richness in agroecosystems: the effect of landscape, habitat and farm management. Biodiversity and Conservation 12: 1335–1355. [Google Scholar]
- 34. El-Sayed AM, Suckling DM, Wearing CH, Byers JA (2006) Potential of mass trapping for long-term pest management and eradication of invasive species. Journal of Economical Entomology 99: 1550–1564. [DOI] [PubMed] [Google Scholar]
- 35. Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annual Review of Entomology 53: 387–408. [DOI] [PubMed] [Google Scholar]
- 36. Økland B (1996) A comparison of three methods of trapping saproxylic beetles. European Journal of Entomology 93: 195–209. [Google Scholar]
- 37. Basset Y (1988) A composite interception trap for sampling arthropods in tree canopies. Journal of Australian Entomological Society 27: 213–219. [Google Scholar]
- 38. Erwin TL (1982) Tropical forests: their richness in Coleoptera and other arthropod species. Coleopterists Bulletin 36: 74. [Google Scholar]
- 39. Fric Z, Konvicka M (2007) Dispersal kernels of butterflies: Power-law functions are invariant to marking frequency. Basic and Applied Ecology 8: 377–386. [Google Scholar]
- 40. Collins MD, Simberloff D (2009) Rarefaction and non random spatial dispersion patterns. Environmental and Ecological Statistics 16: 89–103. [Google Scholar]
- 41. Hosking GP (1979) Trap comparison in the capture of flying coleopteran. New Zealand Entomologist 7: 87–92. [Google Scholar]
- 42. Lassau SA, Hochuli DF (2005) Wasp community responses to habitat complexity in Sidney sandstone forests. Australian Ecology 30: 179–187. [Google Scholar]
- 43. Peltanova A, Dvorak L, Horak J (2012) Land snail community of fruit trees or what can be found in tree trunk window traps. Acta Musei richnovensis 19: 12–16. [Google Scholar]
- 44. Økland RH (1999) On the variation explained by ordination and constrained ordination axes. Journal of Vegetation Science 10: 131–136. [Google Scholar]
- 45. Koenig WD (1999) Spatial autocorrelation of ecological phenomena. Trends in Ecology and Evolution 14: 22–26. [DOI] [PubMed] [Google Scholar]
- 46.Frutos A, Olea PP, Vera R (2007) Analyzing and modelling spatial distribution of summering lesser kestrel: the role of spatial autocorrelation. Ecological Modelling 200: 33–.
- 47. Heikkinen RK, Luoto M, Virkkala R, Rainio K (2004) Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural-forest mosaic. Journal of Applied Ecology 41: 824–835. [Google Scholar]
- 48. Titeux N, Dufrene M, Jacob JP, Paquay M, Defourny P (2004) Multivariate analysis of a fine-scale breeding bird atlas using a geographical information system and partial canonical correspondence analysis: environmental and spatial effects. Journal of Biogeography 31: 1841–1856. [Google Scholar]
- 49.Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam.
- 50. Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annual Review of Entomology 45: 175–201. [DOI] [PubMed] [Google Scholar]
- 51. Southwood TRE (1962) Migration of terrestrial arthropods in relation to habitat. Biological Reviews of Cambridge Philosophical Society 37: 171–214. [Google Scholar]
- 52. Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annual Review of Ecology and Systematics 33: 1–23. [Google Scholar]