Abstract
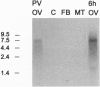
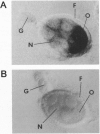
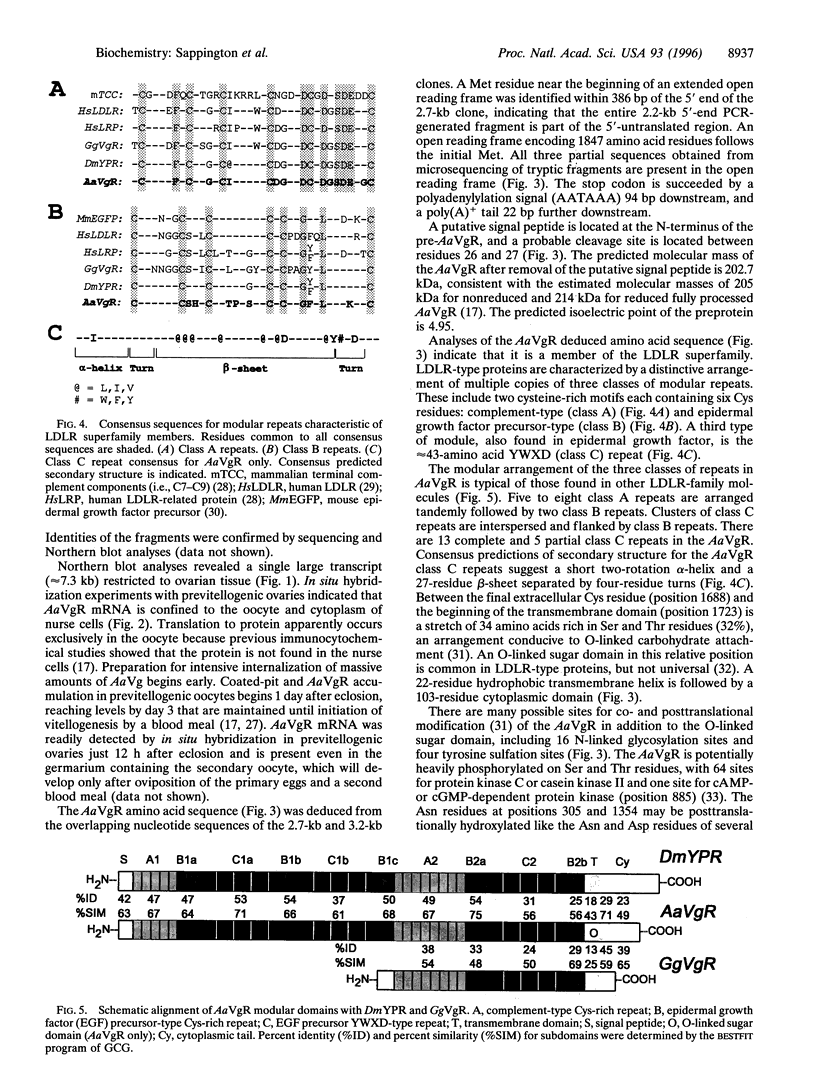
The mosquito (Aedes aegypti) vitellogenin receptor (AaVgR) is a large membrane-bound protein (214 kDa when linearized) that mediates internalization of vitellogenin, the major yolk-protein precursor, by oocytes during egg development. We have cloned and sequenced two cDNA fragments encompassing the entire coding region of AaVgR mRNA, to our knowledge the first insect VgR sequence to be reported. The 7.3-kb AaVgR mRNA is present only in female germ-line cells and is abundant in previtellogenic oocytes, suggesting that the AaVgR gene is expressed early in oocyte differentiation. The deduced amino acid sequence predicts a 202.7-kDa protein before posttranslational processing. The AaVgR is a member of the low density lipoprotein receptor superfamily, sharing significant homology with the chicken (Gallus gallus) VgR and particularly the Drosophila melanogaster yolk protein receptor, in spite of a very different ligand for the latter. Distance-based phylogenetic analyses suggest that the insect VgR/yolk protein receptor lineage and the vertebrate VgR/low density lipoprotein receptor lineage diverged before the bifurcation of nematode and deuterostome lines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. G., Raikhel A. S. Mosquito vitellogenin subunits originate from a common precursor. Biochem Biophys Res Commun. 1988 Aug 30;155(1):436–442. doi: 10.1016/s0006-291x(88)81105-7. [DOI] [PubMed] [Google Scholar]
- Bownes M., Shirras A., Blair M., Collins J., Coulson A. Evidence that insect embryogenesis is regulated by ecdysteroids released from yolk proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1554–1557. doi: 10.1073/pnas.85.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujo H., Hermann M., Kaderli M. O., Jacobsen L., Sugawara S., Nimpf J., Yamamoto T., Schneider W. J. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 1994 Nov 1;13(21):5165–5175. doi: 10.1002/j.1460-2075.1994.tb06847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujo H., Yamamoto T., Hayashi K., Hermann M., Nimpf J., Schneider W. J. Mutant oocytic low density lipoprotein receptor gene family member causes atherosclerosis and female sterility. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9905–9909. doi: 10.1073/pnas.92.21.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Cho W. L., Raikhel A. S. Analysis of mosquito vitellogenin cDNA. Similarity with vertebrate phosvitins and arthropod serum proteins. J Mol Biol. 1994 Apr 15;237(5):641–647. doi: 10.1006/jmbi.1994.1261. [DOI] [PubMed] [Google Scholar]
- Cho W. L., Deitsch K. W., Raikhel A. S. An extraovarian protein accumulated in mosquito oocytes is a carboxypeptidase activated in embryos. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10821–10824. doi: 10.1073/pnas.88.23.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W. L., Raikhel A. S. Cloning of cDNA for mosquito lysosomal aspartic protease. Sequence analysis of an insect lysosomal enzyme similar to cathepsins D and E. J Biol Chem. 1992 Oct 25;267(30):21823–21829. [PubMed] [Google Scholar]
- Dittrich E., Rose-John S., Gerhartz C., Müllberg J., Stoyan T., Yasukawa K., Heinrich P. C., Graeve L. Identification of a region within the cytoplasmic domain of the interleukin-6 (IL-6) signal transducer gp130 important for ligand-induced endocytosis of the IL-6 receptor. J Biol Chem. 1994 Jul 22;269(29):19014–19020. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988 Dec 20;7(13):4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesberger T., Hermann M., Jacobsen L., Novak S., Hodits R. A., Bujo H., Meilinger M., Hüttinger M., Schneider W. J., Nimpf J. The chicken oocyte receptor for yolk precursors as a model for studying the action of receptor-associated protein and lactoferrin. J Biol Chem. 1995 Aug 4;270(31):18219–18226. doi: 10.1074/jbc.270.31.18219. [DOI] [PubMed] [Google Scholar]
- Jacobsen L., Hermann M., Vieira P. M., Schneider W. J., Nimpf J. The chicken oocyte receptor for lipoprotein deposition recognizes alpha 2-macroglobulin. J Biol Chem. 1995 Mar 24;270(12):6468–6475. doi: 10.1074/jbc.270.12.6468. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kounnas M. Z., Stefansson S., Loukinova E., Argraves K. M., Strickland D. K., Argraves W. S. An overview of the structure and function of glycoprotein 330, a receptor related to the alpha 2-macroglobulin receptor. Ann N Y Acad Sci. 1994 Sep 10;737:114–123. doi: 10.1111/j.1749-6632.1994.tb44305.x. [DOI] [PubMed] [Google Scholar]
- Kulakosky P. C., Telfer W. H. Lipophorin as a yolk precursor in Hyalophora cecropia: uptake kinetics and competition with vitellogenin. Arch Insect Biochem Physiol. 1990;14(4):269–285. doi: 10.1002/arch.940140406. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992 Jun 26;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Nimpf J., Schneider W. J. The chicken LDL receptor-related protein/alpha 2-macroglobulin receptor family. Ann N Y Acad Sci. 1994 Sep 10;737:145–153. doi: 10.1111/j.1749-6632.1994.tb44308.x. [DOI] [PubMed] [Google Scholar]
- Nimpf J., Stifani S., Bilous P. T., Schneider W. J. The somatic cell-specific low density lipoprotein receptor-related protein of the chicken. Close kinship to mammalian low density lipoprotein receptor gene family members. J Biol Chem. 1994 Jan 7;269(1):212–219. [PubMed] [Google Scholar]
- Raikhel A. S., Dhadialla T. S. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Sappington T. W., Hays A. R., Raikhel A. S. Mosquito vitellogenin receptor: purification, developmental and biochemical characterization. Insect Biochem Mol Biol. 1995 Jul;25(7):807–817. doi: 10.1016/0965-1748(95)00016-o. [DOI] [PubMed] [Google Scholar]
- Sauer J. R., McSwain J. L., Essenberg R. C. Cell membrane receptors and regulation of cell function in ticks and blood-sucking insects. Int J Parasitol. 1994 Feb;24(1):33–52. doi: 10.1016/0020-7519(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Schneider W. J. Yolk precursor transport in the laying hen. Curr Opin Lipidol. 1995 Apr;6(2):92–96. doi: 10.1097/00041433-199504000-00006. [DOI] [PubMed] [Google Scholar]
- Schonbaum C. P., Lee S., Mahowald A. P. The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1485–1489. doi: 10.1073/pnas.92.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J., Blumenthal T. The Caenorhabditis elegans vitellogenin gene family includes a gene encoding a distantly related protein. Mol Cell Biol. 1985 Oct;5(10):2495–2501. doi: 10.1128/mcb.5.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J., Ohlin A. K., Owen W. G., Schneider W. J. beta-Hydroxyaspartic acid or beta-hydroxyasparagine in bovine low density lipoprotein receptor and in bovine thrombomodulin. J Biol Chem. 1988 Jan 5;263(1):21–24. [PubMed] [Google Scholar]
- Suter B., Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991 Nov 29;67(5):917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Van Antwerpen R., Law J. H. Lipophorin lipase from the yolk of Manduca sexta eggs: identification and partial characterization. Arch Insect Biochem Physiol. 1992;20(1):1–12. doi: 10.1002/arch.940200102. [DOI] [PubMed] [Google Scholar]
- Wahli W. Evolution and expression of vitellogenin genes. Trends Genet. 1988 Aug;4(8):227–232. doi: 10.1016/0168-9525(88)90155-2. [DOI] [PubMed] [Google Scholar]
- Williams S. E., Kounnas M. Z., Argraves K. M., Argraves W. S., Strickland D. K. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein and the receptor-associated protein. An overview. Ann N Y Acad Sci. 1994 Sep 10;737:1–13. doi: 10.1111/j.1749-6632.1994.tb44297.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yochem J., Greenwald I. A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4572–4576. doi: 10.1073/pnas.90.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden M. C., van der Horst D. J., van Doorn J. M., Beenakkers A. M. Partial purification of locust flight muscle lipoprotein lipase (LpL): apparent differences from mammalian LpL. Comp Biochem Physiol B. 1987;88(2):523–527. doi: 10.1016/0305-0491(87)90338-5. [DOI] [PubMed] [Google Scholar]