Abstract
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII; NR2F2) is an orphan nuclear receptor involved in cell-fate specification, organogenesis, angiogenesis, and metabolism. Ablation of COUP-TFII in the mouse uterus causes infertility due to defects in embryo attachment and impaired uterine stromal cell decidualization. Although the function of COUP-TFII in uterine decidualization has been described in mice, its role in the human uterus remains unknown. We observed that, as in mice, COUP-TFII is robustly expressed in the endometrial stroma of healthy women, and its expression is reduced in the ectopic lesions of women with endometriosis. To interrogate the role of COUP-TFII in human endometrial function, we used a small interfering RNA-mediated loss of function approach in primary human endometrial stromal cells. Attenuation of COUP-TFII expression did not completely block decidualization; rather it had a selective effect on gene expression. To better elucidate the role of COUP-TFII in endometrial stroma cell biology, the COUP-TFII transcriptome was defined by pairing microarray comparison with chromatin immunoprecipitation followed by deep sequencing. Gene ontology analysis demonstrates that COUP-TFII regulates a subset of genes in endometrial stroma cell decidualization such as those involved in cell adhesion, angiogenesis, and inflammation. Importantly this analysis shows that COUP-TFII plays a role in controlling the expression of inflammatory cytokines. The determination that COUP-TFII plays a role in inflammation may add insight into the role of COUP-TFII in embryo implantation and in endometrial diseases such as endometriosis.
The nuclear receptor superfamily of transcription factors regulate signaling pathways involved in development, differentiation, homeostasis, and reproduction (1). Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) is a member of the orphan receptor subfamily and has been shown to be a critical regulator of cell-fate specification, angiogenesis, and energy metabolism (2). Germ line ablation of COUP-TFII in mice results in embryonic lethality due to failures in the development of the embryonic vascular system (3). The finding that COUP-TFII heterozygote female mice have reduced fertility led to the investigation of the functions of COUP-TFII in female reproduction (4). In the mouse uterus, the expression of COUP-TFII is observed in the stromal compartment of the endometrium and is undetectable in the epithelium (5). Conditional ablation of COUP-TFII in the reproductive axis was achieved by crossing COUP-TFII floxed mice with progesterone receptor (PGR)-Cre (PGRCre) mice (6). PGRCre COUP-TFIIf/f females are infertile due to impairments in blastocyst attachment and uterine decidualization, the process by which endometrial stromal cells undergo a complex series of events involving cell proliferation, differentiation, and increased angiogenesis. PGR expression is significantly reduced in the endometrial stroma of these mice, resulting in the deregulation of PGR-mediated inhibition of epithelial estrogen receptor (ER) expression and activity, thus causing a block in blastocyst attachment (5). These studies demonstrated that COUP-TFII is critical for the coordination of the complex network of paracrine signaling in the mouse uterus governing pregnancy. Although COUP-TFII is essential for murine decidualization, little is known about its function in human female fertility. However, observations made in women with endometriosis implicated a potential role of COUP-TFII in regulating human endometrial function (7).
Endometriosis, defined as the growth of endometrial tissue outside of the uterine cavity, is an estrogen-dependent disease that affects approximately 6%–10% of women of reproductive age and has been estimated to occur in 40%–50% of infertile women (8). Recurrent pelvic pain is the predominant and most debilitating symptom affecting most patients with endometriosis (8). Emerging evidence indicates that progesterone insensitivity is an important factor involved in endometriosis (9–11). Progesterone insensitivity may result from the deregulation of the progesterone receptor, steroid receptor coactivators, or downstream effectors (11). One important function of progesterone signaling is the inhibition of the mitogenic effects of estrogen (E2) on the uterine epithelium. The down-regulation of stromal PR observed in mice lacking COUP-TFII suggests that it may play an important role in progesterone resistance in women with endometriosis. The expression of aromatase, an enzyme necessary to synthesize estrogen from androgens, was found to be significantly increased in endometriosis (12). Interestingly, COUP-TFII was reported to bind the promoter and repress expression of aromatase in the human endometrium (7). The results from these studies indicate that COUP-TFII may be involved in the pathogenesis of endometriosis.
To investigate the function of COUP-TFII, we utilized an in vitro primary human endometrial stromal cell (HESC) decidualization system. Attenuation of COUP-TFII expression using small interfering RNA (siRNA) had a selective effect on decidual genes with expression of WNT4 being reduced, PRL being unaffected, and IGF-binding protein (IGFBP)1 surprisingly being elevated. To better elucidate the COUP-TFII transcriptome, we performed microarray comparisons coupled with chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) analysis on HESCs transfected with or without COUP-TFII siRNA. The genes identified to be regulated by COUP-TFII are involved in a number of signaling pathways and biological functions including cell adhesion, inflammation, cytokine and chemokine signaling, and angiogenesis, all of which play an important role in endometriosis. Furthermore, we observed reduced expression of COUP-TFII in ectopic lesions of women with endometriosis, suggesting that loss of COUP-TFII expression is a major contributing factor to the pathogenesis of endometriosis.
Materials and Methods
Endometrial samples used for immunostaining and quantitative real-time PCR (qPCR)
The human endometrial samples used to determine the COUP-TFII expression pattern were collected from Greenville Hospital System and the Specialized Cooperative Centers Program in Reproduction and Infertility Tissue Bank and the Obstetrics and Gynecology Clinic of Baylor College of Medicine. All patients gave informed consent at their preoperative visit using approved protocols through institution review committees. For experiments examining COUP-TFII expression in endometrium throughout the menstrual cycle, full-thickness endometrium was obtained at the time of surgery from 23 regularly cycling women with no history of endometriosis between the age of 18 and 50 undergoing hysterectomy. Seven samples were collected from proliferative phase, 6 were from the early secretory phase, 7 were from the midsecretory phase, and 3 were late luteal. Samples were transported immediately upon removal to the pathology department where the samples were placed into 10% buffered formalin and then imbedded in paraffin prior to sectioning. To compare COUP-TFII expression patterns of eutopic endometrium between with and without endometriosis, endometrial biopsies were obtained at the time of surgery from 47 regularly cycling women between the age of 18 and 50. For normal eutopic endometrium, 10 samples were collected from proliferative phase, 7 were from the early secretory phase, 3 were from the midsecretory phase, and 6 were late secretory. For endometriosis eutopic endometrium, 2 samples were collected from proliferative phase, 6 were from the early secretory phase, 10 were from the midsecretory phase, and 3 were late secretory. For experiments comparing ectopic and eutopic endometrium, tissue was obtained via biopsies collected during the secretory phase of the menstrual cycle from 9 patients diagnosed with endometriosis. Ectopic tissue was collected from the peritoneal region, at which time a eutopic biopsy was also performed. Of the samples obtained, 2 were from the early secretory phase, 4 were from the midsecretory phase, and 3 were late luteal (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Histologic dating of endometrial samples was achieved using the methods of Noyes (13).
Isolation and culture of HESCs
Human endometrial biopsies were collected from the proliferative phase of normally cycling women with no history of uterine disease at the Obstetrics and Gynecology Clinic of Baylor College of Medicine. The study was approved by the Institutional Review Board of Baylor College of Medicine. Patient-written informed consent was obtained before biopsy. The samples were collected in Hank's Balanced Salt Solution containing 1% antibiotic-antimycotic (Life Technologies) and transported to the laboratory on ice. HESCs were isolated from endometrial biopsies as previously described (14). HESCs were cultured in growth media consisting of DMEM/F12 containing 10% of fetal bovine serum and 1% antibiotic-antimycotic, and all experiments were conducted prior to 5 cell passages.
siRNA transfection and in vitro decidualization
HESCs (1 × 105) were seeded in 6-well plates and allowed to reach approximately 70% confluency. Transfection of nontargeting control or COUP-TFII on-target siRNA was done using Lipofectamine 2000 (Life Technologies) according to manufacturer's protocol. Forty-eight hours after transfection, HESC decidualization was induced by treatment with decidual media (Opti-MEM media containing 2% charcoal-stripped fetal bovine serum, 1% antibiotic-antimycotic, 1 μM Medroxyprogesterone acetate [MPA], 10 nM E2, and 50 μM dibutyryl-cAMP). Media containing decidual hormones were changed every 48 hours.
Immunohistochemistry
Sections of paraffin-embedded full-thickness endometrium were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Antigen retrieval was done using a citrate unmasking solution (Vector Laboratories). Sections were blocked with 10% normal goat serum in PBS, and then incubated at 4°C overnight with 1:1000 anti-COUP-TFII antibody (PP-H7147–10, Perseus Proteomics, Inc.) in 10% normal goat serum in PBS. Sections were washed in PBS and incubated with secondary antibody for 1 hour at room temperature. The stain was detected using the Vectastain Elite ABC kit (Vector Laboratories) followed with 3,3′-diaminobenzidine substrate kit for peroxidase (SK-4100, Vector Laboratories). The slides were counterstained with hematoxylin. The semiquantitative assessment of expression was made using the HSCORE, calculated using the following equation: HSCORE =Σ Pi (i + 1), where i = intensity of staining with a value of 1, 2, or 3, (weak, moderate, or strong, respectively), and Pi is the percentage of stained cells at a given intensity, varying from 0%–100%.
Immunofluorescence
Sections from formalin-fixed, paraffin-embedded human eutopic and ectopic endometrium were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Antigen retrieval was done using a citrate unmasking solution (Vector Laboratories). Sections were immune blocked with 10% normal goat serum in PBS and then incubated at 4°C overnight with 1:500 anti-COUP-TFII antibody (PP-H7147–10, Perseus Proteomics, Inc.) in 10% normal goat serum in PBS. Sections were washed in PBS and incubated with biotinylated secondary antibody (5 μL/mL; Vector Laboratories) for 1 hour at room temperature. Immunoreactivity was detected using the TSA Kit (Life Technologies). The nuclear DNA was stained with 4′,6-diamino-2-phenylindole. The semiquantitative assessment of expression was made using the Immuno signal score, from least to greatest, of 0, 1, 2, 3, 4, and 5.
Microarray analysis
Three HESC subcultures from different patients were transfected with siRNA and treated with decidual media (see above) for 3 days. Total RNA was extracted using the Qiagen RNeasy RNA isolation kit (Qiagen). The RNA from 3 replicates (wells) was pooled for each treatment per cell line. The integrity of all RNA samples was tested with the Bioanalyzer 2100 (Agilent Technologies). The concentration of RNA was quantified on the Nanodrop Spectrophotometer (Nanodrop Technologies). The samples with 260/280 greater than 1.8 were used for microarray hybridization. Microarrays were performed by the Genomic and RNA Profiling Core of Baylor College of Medicine using Affymetrix human genome U133 Plus 2.0 arrays (Affymetrix). Array data have been deposited in the Gene Expression Omnibus (GEO, accession no. GSE47052).
Microarray CEL files were analyzed using dChip (http://www.hsph.harvard.edu/cli/complab/dchip PM-MM model, Quantile normalization). The combat software (http://www.bu.edu/jlab/wp-assets/ComBat/Abstract.html) was used to normalize patient-specific differences. Two-side t test and fold changes were used to define differentially expressed genes. Genes with P < .05 and an absolute fold change (COUP-TFII knockdown vs nontargeting) > 1.4 were considered significant.
qPCR analysis
Total RNA was isolated from endometrial biopsies or HESCs using the Qiagen RNeasy RNA isolation kit (Qiagen). Total RNA (1 μg) was used for cDNA synthesis with a random hexamer primers of a Thermoscript RT-PCR kit (Invitrogen) according to the manufacturer's protocol. Target gene expression was quantified by qPCR using the ABI QuantStudio 12K Flex system with Taqman Universal PCR Master Mix (Applied Biosystems) or SYBR Green PCR Supermix (Bio-Rad Laboratories, Inc.) (See primers information in Supplemental Table 2). The cycling conditions of qPCR amplification were: 1 cycle at 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Dissociation curves of SYBR Green amplicons were performed at the end of amplification for data quality validation. All samples were run in triplicate and the threshold cycle was used for calculating relative mRNA levels of target genes by fold-change and statistical significance (paired t test for HESCs and Two-Sample t test for endometrial samples).
Western blotting
Total protein was isolated from HESCs using protein lysis buffer (50 mM Tris HCl [pH 7.4], 50 mM KCl, 4 mM EDTA, 2 mM EGTA, 1% Nonidet P-40) containing protease and phosphatase inhibitor cocktails (Roche Diagnostics). Denatured protein (30 μg) was loaded into 4%–12% NuPAGE Novex Bis-Tris Pre-Cast Gels (Life Technologies) for electrophoresis separation. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore Corp.) in transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol) (Life Technologies). Membranes were blocked in 5% nonfat dry milk that was dissolved in Tris-buffered saline/Tween solution (20 mM Tris, 150 mM NaCl [pH 7.6], and 0.1% Tween 20) and then incubated with 1:1000 anti-PGR antibody (sc-7208, Santa Cruz Biotechnology, Inc.) or 1:500 anti-COUP-TFII antibody (PP-H7147–10; Perseus Proteomics, Inc.) overnight at 4°C with gentle rocking. The membranes were incubated with secondary antibody for 1 hour, washed with Tris-buffered saline/Tween solution, and developed with enhanced chemiluminescence prime reagents (GE Healthcare).
ELISA
The protein level of IL-6 and IL-8 in HESC culture supernatant was measured using ELISA kits according to the manufacture's protocol (R&D Systems). Forty-eight hours after control or COUP-TFII siRNA transfection, HESCs were treated with decidualizing media (mentioned above). The culture medium was collected after 3 days of decidual treatment and centrifuged at 13 000 rpm for 15 minutes to obtain the supernatant media for analysis. The mean minimum detection limit was 0.7 pg/mL and 3.5 pg/mL for IL-6 and IL-8, respectively. The intraassay CV was 3.1 and 5.8%, and the interassay CV was 2.7 and 7.7% for IL-6 and IL-8, respectively.
ChIP-seq
HESCs isolated from 6 patients were cultured separately in 150-mm culture dishes with HESC growth media and allowed to reach approximately 90% confluency before being treated with decidual media. Decidual media was changed every 48 hours. After 72 hours of treatment, cells were fixed for 15 minutes with 1/10 vol of freshly prepared formaldehyde solution (11% formaldehyde, 0.1 M NaCl, 1 mM EDTA, and 50 mM HEPES). The fixation was stopped by adding 1/20 vol 2.5 M glycine for 5 minutes. Fixed HESCs were collected and pelleted at 800 × g for 10 minutes at 4°C. Cell pellets were washed 2 times with cold PBS-Igepal (0.5% Igepal, 1 mM phenylmethylsulfonylfluoride). HESCs from 6 patients were pooled before genomic DNA isolation. COUP-TFII immunoprecipitation and DNA library generation were performed by Active Motif as previously described (15). DNA libraries were sequenced by Illumina's Hi-Seq Sequencing Service. The 50-nucleotide sequence reads were mapped to the human genome (GRCh Build 37; February 2009) using the Burrows-Wheeler Aligner algorithm with default settings. Alignment information for each read was stored in the Sequence Alignment/Map or Binary version of the Sequence Alignment/Map format. Sequence alignments were extended in silico (using Active Motif software) at their 3′-ends to a length of 150–250 bp and assigned to 32-nucleotide bins along the genome. The resulting histogram of fragment densities was stored in a binary analysis results file.
Sequence conservation, cis-regulatory Element Annotation System (CEAS), and motif enrichment were performed using the Cistrome Analysis Pipeline software (http://cistrome.org/ap/) under default settings (16). Ingenuity Systems Pathway Analysis (IPA) software (http://www.ingenuity.com) and the public Database for Annotation, Visualization, and Integrated Discovery (DAVID, http://david.abcc.ncifcrf.gov/) were used for gene functional annotations (17).
Chromatin immunoprecipitation-qPCR (ChIP-qPCR)
ChIP was performed with the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology) with slight modification. Briefly, fixed HESCs were digested with 5 μL of micrococcal nuclease for 15 minutes. The lysate was sheared by sonication to a size range of 150–900 bp using VirTis Virsonic 100 Ultrasonic Homogenizer. An aliquot of sheared chromatin to be used as input DNA was treated with RNase A and proteinase K, and cross-linking was reversed by heat. The purified input DNA was quantified on a Biotek Microplate Spectrophotometer. Chromatin/protein complexes (10 μg) were precleared with normal rabbit IgG (sc-2017, Santa Cruz Biotechnology, Inc). ChIP was performed using an antibody against COUP-TFII (Active Motif). Following immunoprecipitation, cross-linking was reversed as described above. Purified DNA was used for real-time qPCR. The primers were designed to amplify COUP-TFII binding regions identified by ChIP-seq (Supplemental Table 3). Real-time qPCR was carried out in duplicate using SYBR Green Supermix (Bio-Rad Laboratories, Inc.). Immunoprecipitation with normal rabbit IgG was performed as a negative control. Primers were designed for a region of the human genome devoid of any known genes to serve as a negative qPCR primer set (Active Motif). The resulting signals were normalized to input DNA.
Results
COUP-TFII expression in human eutopic and ectopic endometrium
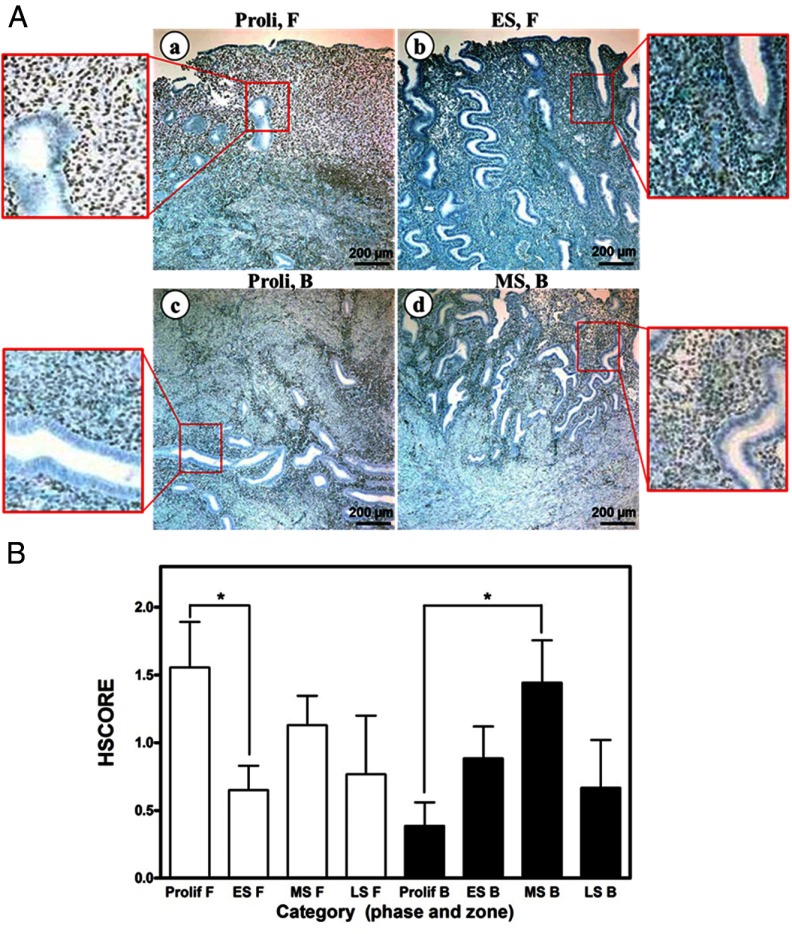
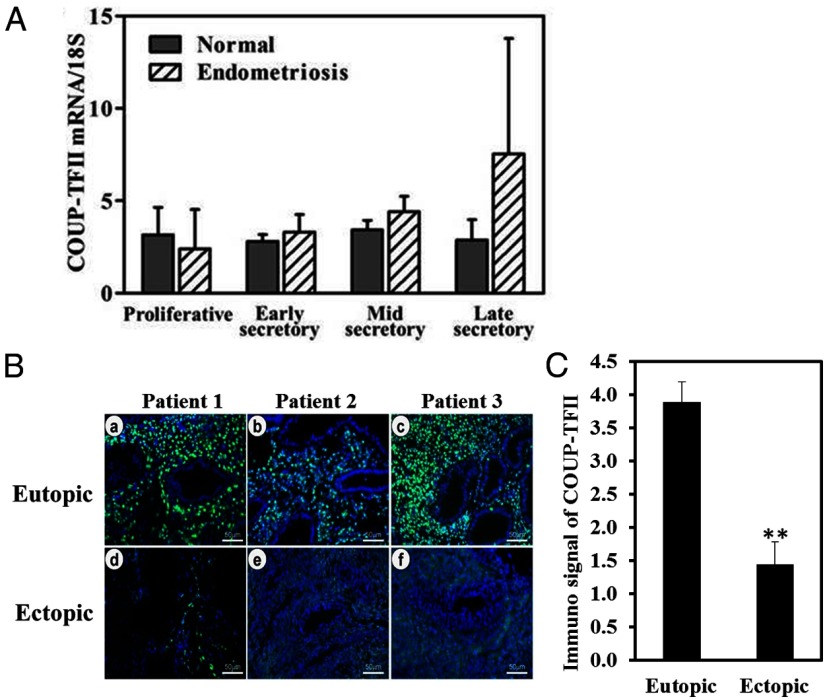
Immunohistochemical analysis was conducted on full thickness endometrial sections from hysterectomy material and endometrial biopsies to determine the cell-specific expression of COUP-TFII throughout the endometrium and in endometriosis. COUP-TFII protein is specifically expressed in the stromal compartment of the human endometrium. During the menstrual cycle, the expression of COUP-TFII is detectable in the proliferative phase but then decreases in the zona functionalis in the early secretory phase (Figure 1,A (a,b) and B). As the menstrual cycle progresses to the midsecretory phase, COUP-TFII expression is induced in the zona basalis (Figure 1, A (c,d) and B). We next used real-time quantitative PCR and compared the expression of COUP-TFII in endometrial biopsies throughout the menstrual cycle from patients with and without diagnosed endometriosis. As shown in Figure 2A, there was no significant difference in the expression of COUP-TFII mRNA in the eutopic endometrium from patients with endometriosis vs control. We also saw no difference in the expression of COUP-TFII throughout the menstrual cycle in either patient set. However, when we compared the expression of COUP-TFII by immunohistochemical analysis in paired ectopic endometrium and eutopic lesions of women with disease, the ectopic lesions exhibited a reduction of COUP-TFII expression (Figure 2, B and C).
Figure 1.
Spatiotemporal expression of COUP-TFII in the endometrium of healthy women during the menstrual cycle. A, Immunohistochemical staining of COUP-TFII in zona functionalis at the proliferative phase (a) and the early secretory phase (b), and in zona basalis at the proliferative phase (c) and the midsecretory phase (d) of the menstrual cycle. B, The HSCORE of COUP-TFII in zona functionalis (F) and zona basalis (B) during the menstrual cycle. Proli, proliferative phase; ES, early secretory phase; MS, midsecretory phase; LS, late secretory phase; F, zona functionalis; B, zona basalis. *, P < .05.
Figure 2.
Comparison of COUP-TFII expression in the endometrium between women with and without diagnosed endometriosis. A, qPCR analysis of COUP-TFII gene expression in eutopic endometrium between normal women and the patients with endometriosis during the menstrual cycle normalized using the ddCt method to the 18S gene. B, Representative immunofluorescence staining of COUP-TFII in paired (a and d from patient 1; b and e from patient 2; c and f from patient 3) ectopic and eutopic endometrium. C, Quantification of the immunofluorescence signal of COUP-TFII between eutopic and ectopic endometrium of 9 patients with endometriosis. **, P < .01.
COUP-TFII attenuation has only select effects on HESC decidualization
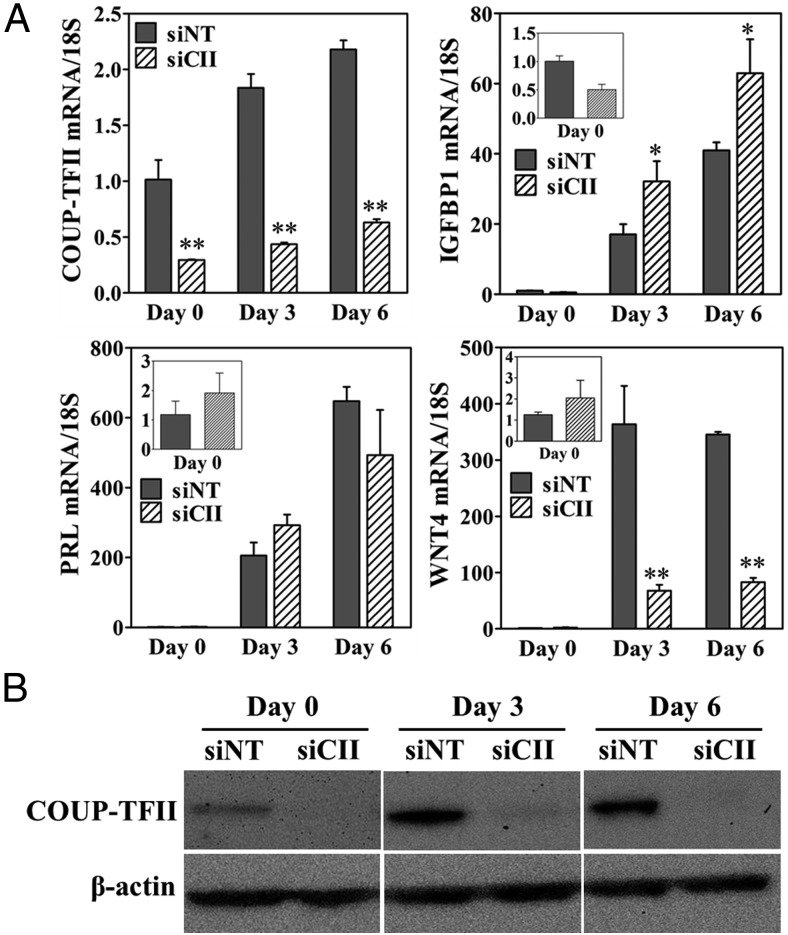
HESCs can be induced to undergo decidualization with a hormone cocktail of cAMP, E2, and MPA. Decidualization is assayed by a change in morphology and the expression of prolactin (PRL) and IGFBP1. We analyzed the role of COUP-TFII in HESC decidualization by conducting siRNA-mediated knockdown of COUP-TFII and confirmed that expression at both mRNA and protein levels was effectively attenuated (Figure 3, A and B). Reductions in the expression of COUP-TFII had no effect on the decidualization-dependent changes in cell morphology (data not shown) and had what appeared to be selective effects on decidual target gene expression. Decidual regulator WNT4 exhibited reduced expression, but expression of decidual marker PRL was unaffected. Surprisingly, the expression of decidual marker IGFBP1 was significantly elevated in HESCs transfected with COUP-TFII siRNA (Figure 3A).
Figure 3.
Gene expression of decidual genes regulated by COUP-TFII knockdown in HESCs at 0, 3, and 6 days after treatment with deciduogenic hormones. A, Gene expression levels of COUP-TFII, IGFBP1, PRL, and WNT4 between COUP-TFII siRNA (siCII) and nontargeting siRNA (siNT) as assessed by qPCR. Gene expression was normalized using the ddCt method to the 18S gene. B, Protein levels of COUP-TFII between siCII and siNT normalized to β-actin as determined by Western blotting. *, P < .05; **, P < .01.
Microarray identification of genes regulated by COUP-TFII
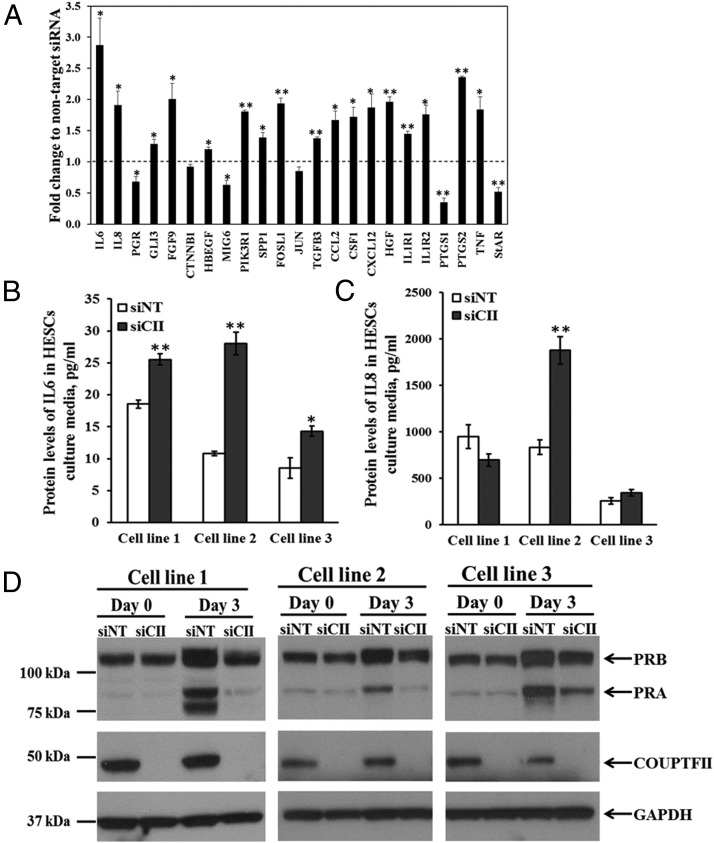
To better elucidate which genes are regulated by COUP-TFII in HESCs, microarray expression analysis was conducted on HESCs transfected with either control or COUP-TFII siRNA and then treated with deciduogenic hormones. A total of 5290 gene probes were altered by COUP-TFII knockdown giving 1591 genes, 905 of which were up-regulated whereas 686 genes were down-regulated (P < .05; fold change > 1.4) (Supplemental Excel 1). DAVID analysis revealed that the altered genes are involved in many signaling pathways such as cytokine-cytokine receptor interaction, vascular endothelial growth factor (VEGF) signaling, focal adhesion, MAPK signaling, and WNT signaling (Table 1 and Supplemental Table 4). To validate the array hybridization data, expression of candidate genes was examined by qPCR. All 23 genes analyzed were in agreement with the microarray data with regard to change in direction, although 2 were not significantly altered (Figure 4A). Of the genes that were altered with COUP-TFII knockdown during decidualization were genes associated with inflammation. IL-6, IL-8, CSF1, CXCL12 ILR1, ILR2 PTGS2, and TNF were all up-regulated. This implies that COUP-TFII is important for the repression of these inflammatory proteins during decidualization. We next measured the secretion of cytokines IL-6 and IL-8 in HESC culture media using ELISAs. Whereas cytokine levels were undetectable in fresh decidual media, the levels of secreted IL-6 protein were found to be consistently increased in the culture media of 3 HESC lines after COUP-TFII knockdown and 3 days of treatment with decidual media (Figure 4B). However, the secreted IL8 protein levels were found to be elevated in only 1 of 3 HESC lines with COUP-TFII knockdown (Figure 4C). Finally, one of the genes down-regulated in the absence of COUP-TFII in the microarray analysis was PGR. Analysis of the PGR protein levels revealed that in comparison to the observed induction of both PGR isoforms after decidualization in control cells, both PGR isoforms exhibited reduced induction. Furthermore, whereas the PGR-B isoform showed a modest reduction (∼25%), the PGR-A isoform was found to be significantly reduced (∼70%) by COUP-TFII knockdown after 3 days of treatment with decidual media (Figure 4D).
Table 1.
Signaling Pathways of Genes Affected by COUP-TFII siRNA Identified by Microarray
| Term | Genes | Count | P Value |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | IL1R2, IL1R1, TNF, CCL2, IL6ST, PDGFA, CCR1, CRLF2, CSF1, TGFB3, KITLG, CXCR2, PF4V1, CXCL12, IFNA2, TNFRSF1A, TNFRSF11B, CXCR5, IFNA6, IL1RAP, IL15RA, IFNK, CD27, LTA, IL8, IL25, TNFRSF17, HGF, IL6R, IL11RA, CCL18, KDR, CCL17, INHBB, CCR9, CCL11, INHBA, CCR7, CXCL14, CXCL13, CX3CR1, IL12B, IFNA13, EDA | 44 | <.0001 |
| Vascular smooth muscle contraction | RAMP3, KCNMB4, ROCK1, ADORA2B, CALD1, MYLK3, MRVI1, NPR1, ITPR1, PRKCB, EDNRA, PLA2G4A, MYH11, GUCY1B3, PLCB1, ADRA1D, PPP1R14A, PLA2G2F | 18 | .0071 |
| VEGF signaling pathway | PTGS2, SPHK1, PPP3R2, KDR, PRKCB, SH2D2A, PLA2G4A, PPP3CC, NOS3, NFATC2, SHC2, PIK3R1, PLA2G2F | 13 | .0147 |
| Focal adhesion | PDGFA, TNC, COL2A1, CTNNB1, IGF1R, COL6A6, ITGB7, COL6A1, PAK1, THBS1, SHC2, PIK3R1, SPP1, TNXB, ROCK1, MYLK3, ITGA4, HGF, MAPK10, BIRC3, KDR, PRKCB, LAMA2, LAMA1, ITGA6, JUN | 26 | .0164 |
| MAPK signaling pathway | FGF19, IL1R2, IL1R1, TNF, PDGFA, FGF9, FGF17, TGFB3, PPP3R2, CACNB3, FOS, TNFRSF1A, MAP3K5, MOS, PPP3CC, PAK1, NFATC2, NF1, MAPK10, TAB1, CACNA2D2, PRKCB, MAP4K4, DUSP4, PLA2G4A, RPS6KA2, JUN, MAPK8IP1, GADD45A, DUSP6, MAP3K11, PLA2G2F | 32 | .0203 |
| Calcium signaling pathway | GNA14, SLC8A2, ERBB4, ADORA2B, MYLK3, SPHK1, PPP3R2, BDKRB1, PTGFR, ITPKA, ITPR1, PRKCB, EDNRA, P2RX5, EDNRB, ADRB1, ATP2A2, PPP3CC, NOS3, NOS2, PLCB1, HTR2B, ADRA1D | 23 | .0223 |
| WNT signaling pathway | PPP2R1B, TBL1XR1, CTBP2, ROCK1, MMP7, PPP3R2, MAPK10, DKK4, WNT2B, PRKCB, CTNNB1, DKK2, WNT4, WNT3, JUN, PPP3CC, PLCB1, NFATC2, FOSL1, WNT8A | 20 | .0305 |
| TGF-β signaling pathway | BMP4, PPP2R1B, TNF, ROCK1, SMAD6, TGFB3, DCN, INHBB, INHBA, CDKN2B, ZFYVE9, ID4, THBS1 | 13 | .0423 |
| Pathways in cancer | FGF19, PTGS2, PDGFA, FGF9, FGF17, TGFB3, SPI1, KITLG, GLI3, SHH, CTNNB1, IGF1R, FOS, WNT4, WNT3, CDKN2B, NKX3–1, TGFA, NOS2, WNT8A, PIK3R1, CEBPA, BMP4, CTBP2, IL8, BIRC5, MAPK10, HGF, BIRC3, PRKCB, WNT2B, LAMA2, LAMA1, ITGA6, JUN, PTCH1 | 36 | .0439 |
| Dilated cardiomyopathy | TNF, TGFB3, CACNB3, ITGA4, TPM2, TTN, CACNA2D2, LAMA2, ADRB1, ATP2A2, ITGA6, ITGB7, SGCD | 13 | .0606 |
| Natural killer cell-mediated cytotoxicity | TNF, KLRK1, PPP3R2, KIR2DS1, GZMB, NCR3, PRKCB, IFNA2, IFNA6, PPP3CC, PAK1, IFNA13, NFATC2, KIR2DL2, SHC2, PIK3R1, KIR2DL4 | 17 | .0628 |
| Hedgehog signaling pathway | BMP4, WNT4, WNT3, PTCH1, LRP2, GLI3, SHH, WNT8A, WNT2B | 9 | .0745 |
Figure 4.
Validation of microarray results with qPCR, ELISA, and Western blotting after 3 days of treatment with deciduogenic hormones. A, Fold change in mRNA abundance in COUP-TFII siRNA relative to nontargeting siRNA in HESCs. B and C, Secreted protein levels of IL-6 and IL-8 in HESC culture media. Forty-eight hours after transfection (defined as day 0) with nontargeting siRNA (siNT) or COUP-TFII siRNA (siCII), HESCs were treated with deciduogenic hormones for 3 days. The culture media were collected on day 3, and the levels of IL-6 and IL-8 were measured by ELISA. D, Protein levels of COUP-TFII and PGR isoforms on day 0 and day 3 of treatment with deciduogenic hormones between COUP-TFII siRNA (siCII) and nontargeting siRNA (siNT) normalized to glyceraldehyde-3-phospate dehydrogenase (GAPDH). *, P < .05; **, P < .01.
ChIP-seq identification of genome-wide COUP-TFII binding sites in HESCs
To better elucidate the mechanisms with which COUP-TFII regulates target gene transcription, genome-wide COUP-TFII binding sites in HESCs treated with deciduogenic hormones were identified using ChIP-seq. A total of 16 298 intervals (binding regions) for COUP-TFII were identified compared with the input in HESC chromatin with a very low false discovery rate (0.17%) using a stringent cutoff of P = 1 × 10−10. Interval locations are provided in Browser Extensible Data format in the supplemental data (Supplemental COUP-TFII BED file of ChIP-seq), which also can be accessed on the Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) (GEO, accession number GSE52008).
Evolutionary conservation of ChIP-seq peaks compared with flanking nonpeak regions is regarded as a good indicator of data quality and proper data processing (16). A high level of conservation within the region of COUP-TFII binding compared with surrounding nonbinding regions was found by analyzing COUP-TFII binding intervals among placental mammalian genomes using the Conservation Plot program at Cistrome (Figure 5A).
Figure 5.
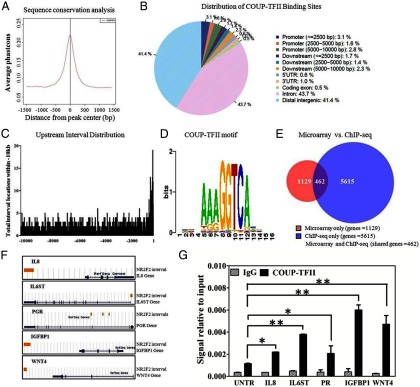
ChIP-seq analysis of COUP-TFII in decidualized HESCs. A, Sequence conservation analysis. COUP-TFII ChIP intervals were aligned at the center and expanded 3000 bp around the center. Phastcons scores were retrieved and averaged at each position. B, Distribution of COUP-TFII binding sites throughout the HESC genome. The CEAS module at Cistrome was used for analysis, and the promoter region was defined in increments of 2500 bp to 10 000 bp. The binding locations relative to gene boundaries were further defined as within the 5′-untranslated region (UTR), 3′-UTR, coding exon, or intron. Intergenic regions are defined as more than 10 kb from gene boundaries. C, Frequency of COUP-TFII binding sites located within −10 kb of the transcriptional start site. D, COUP-TFII DNA binding motif enrichment in immunoprecipitated chromatin as determined using the SeqPos tool at Cistrome. E, Venn diagram illustrating the overlap between nonredundant genes bound by COUP-TFII as determined by ChIP-seq and those genes regulated by COUP-TFII by microarray (>1.4-fold). F, UCSC Genome Browser illustrations of select genes with COUP-TFII binding sites. Yellow bars indicate the location of COUP-TFII binding intervals to genes (blue). G, Validation of COUP-TFII binding on the IL8, IL6ST, PGR, IGFBP1, and WNT4 genes by ChIP-qPCR. The putative binding regions identified by ChIP-seq were validated with the COUP-TFII antibody (Active Motif) on HESCs. Antibody against host IgG was used as an immunoprecipitation control and untranslated region (UNTR) primers designed for a region of the human genome devoid of any known genes were used as a qPCR control. Data are represented as signal relative to input. Illustrated statistical significance represents differences in signal between putative binding regions and the UNTR in COUP-TFII-immunoprecipitated chromatin samples. *, P < .05; **, P < .01.
Distribution of intervals was analyzed by the CEAS module at Cistrome and showed that more than half (58.6%) of the COUP-TFII binding sites are located within 10 kb of gene boundaries (Figure 5B); 7.5% of total intervals reside within the 10-kb promoter region, with a sharp increase in the frequency of binding sites as they approach the transcription start site (Figure 5C). A total of 6077 unique genes were identified to have COUP-TFII binding sites within 10 kb of their gene boundaries (Supplemental Excel 2). Binding data of several genes, including IL8, IL6ST, PGR, IGFBP1, and WNT4, was validated using ChIP-qPCR (Figure 5, F and G).
The SeqPos motif tool at Cistrome was used to identify the specific DNA response elements found within COUP-TFII-immunoprecipitated chromatin. A consensus AGGTCA element, which is consistent with previous reports, was identified to be the top COUP-TFII binding motif (18) (Figure 5D). Moreover, consensus sequences for many transcription factors with binding sites adjacent to COUP-TFII binding were identified, including both those previously reported such as SP1 as well as novel potential COUP-TFII interacting factors such as FOXO1 and HOXC10. Most the transcription factor binding sites identified belong to the nuclear hormone receptor family (Supplemental Table 5).
Functional analysis of genes both bound and regulated by COUP-TFII
To identify which of the genes that exhibit altered expression in the absence of COUP-TFII are due to potential direct regulation, we compared those genes identified by ChIP-seq to the genes identified by microarray, of which a total of 462 genes were found in both datasets (Figure 5E and Supplemental Excel 3). The biologic functions of genes in common were analyzed by DAVID and IPA. Many of the genes are involved in cell adhesion and the inflammatory response. A number of genes also participate in vasculature development, the response to steroid hormones and pregnancy (Table 2 and Supplemental Table 6). IPA analysis showed that enriched genes are involved in many diseases and disorders, such as cancer (184 genes), reproductive system disease (103 genes), cardiovascular disease (83 genes), inflammatory response (78 genes), and developmental disorders (43 genes). Furthermore, nearly 10% of all genes that exhibit COUP-TFII binding and altered expression in its absence have been shown to play a role in endometriosis (Supplemental Table 7).
Table 2.
Signaling Pathways of Genes With Altered Expression by Microarray That Exhibit COUP-TFII Binding as Identified by ChIP-seq
| Term | Genes | Count | P Value |
|---|---|---|---|
| Focal adhesion | TNXB, ROCK1, TNC, HGF, MAPK10, LAMA2, LAMA1, IGF1R, COL6A6, ITGA6, COL6A1, PAK1, THBS1, PIK3R1, SPP1 | 15 | .0003 |
| Cytokine-cytokine receptor interaction | IL1R2, IL1R1, CCL2, IL8, IL6ST, CSF1, TGFB3, KITLG, IL6R, HGF, PF4V1, CXCL12, IL11RA, TNFRSF1A, TNFRSF11B, CCR7, CXCL13, IL1RAP, IL12B | 19 | .0008 |
| WNT signaling pathway | TBL1XR1, WNT4, CTBP2, ROCK1, JUN, PPP3R2, MAPK10, PLCB1, NFATC2, FOSL1, WNT2B | 11 | .0055 |
| Vascular smooth muscle contraction | EDNRA, KCNMB4, PLA2G4A, ROCK1, CALD1, MRVI1, GUCY1B3, PLCB1, ITPR1, ADRA1D | 10 | .0062 |
| MAPK signaling pathway | IL1R2, IL1R1, NF1, TGFB3, PPP3R2, CACNB3, MAPK10, FOS, TNFRSF1A, MAP4K4, PLA2G4A, MAP3K5, RPS6KA2, PAK1, NFATC2 | 15 | .0138 |
| Pathways in cancer | CTBP2, IL8, SPI1, TGFB3, KITLG, HGF, MAPK10, GLI3, WNT2B, LAMA2, LAMA1, FOS, IGF1R, WNT4, ITGA6, PIK3R1 | 16 | .0187 |
| Phosphatidylinositol signaling system | SYNJ2, DGKI, PLCB1, ITPR1, INPP5A, PIK3R1 | 6 | .0710 |
| Calcium signaling pathway | EDNRA, GNA14, ATP2A2, PPP3R2, BDKRB1, PTGFR, PLCB1, HTR2B, ITPR1, ADRA1D | 10 | .0822 |
Discussion
Here we demonstrate that COUP-TFII is expressed specifically in the endometrial stroma, and the expression is spatially and temporally regulated throughout the menstrual cycle. The expression of COUP-TFII is not only regulated temporally through the menstrual cycle but also shows a spatial regulation in expression. This spatial expression of COUP-TFII likely reflects the local paracrine signaling in the different compartments of the endometrium during the menstrual cycle, given that COUP-TFII has been shown to be regulated by morphogens such as members of the hedgehog family (19, 20). The differential protein expression of COUP-TFII through the menstrual cycle is only observed by analyzing histologic sections of full-length endometrium. When mRNA expression from endometrial biopsies was measured, this differential expression was not detected. Although limited in size, the latter analysis did not identify differences in the expression of endometrial COUP-TFII between women with and without diagnosed endometriosis. However, when comparing the expression of COUP-TFII in ectopic lesions to paired eutopic biopsies taken from the same patient, COUP-TFII expression was found to be significantly reduced. Again the loss of COU-TFII in the ectopic endometrium may reflect loss of paracrine signaling present in the eutopic endometrium. The reduction in COUP-TFII expression in the ectopic endometrium in endometriosis patients would likely result in the misregulation of the same molecular pathways shown to be regulated by COUP-TFII in the eutopic endometrium. The differential expression of COUP-TFII observed in ectopic and eutopic endometrium acts to corroborate previous reports that ectopic lesions behave quite differently from their eutopic counterparts (21). Endometriosis is a prevalent reproductive disease, affecting up to 10% of reproductive-age women (8). Despite intense studies, a clear understanding of the pathogenic mechanisms contributing to the genesis of endometriosis remains elusive. Emerging evidence suggests that a lack of adequate immune surveillance in the peritoneum represents a major contribution to disease development, indicating that endometriosis is an inflammatory disorder (22, 23). The results herein shed light on the molecular mechanisms regulating this process by investigating the role of COUP-TFII in endometrial stromal cell function.
To address the role of COUP-TFII in the human endometrium, we first conducted loss of function studies in primary HESCs using COUP-TFII siRNA. Surprisingly, contrary to previous reports on murine COUP-TFII, knockdown had only a selective (in contrast to complete) effect on HESC decidualization following treatment with a hormone cocktail of cAMP, MPA, and E2. Although decidual-dependent changes in both cell morphology (data not shown) and the expression of decidual markers IGFBP1 and PRL were not impaired, the expression of WNT4 was markedly reduced by COUP-TFII knockdown. WNT4, a member of the WNT family, is critical for female sexual development (24). Conditional ablation of Wnt4 in the uterus rendered female mice subfertile due to a failure in blastocyst invasion into the stroma and defects in decidualization (25). WNT4 was also reported to regulate HESC differentiation via β-catenin signaling (26). Our results show that COUP-TFII is a regulator of WNT4 in the endometrium. The function of COUP-TFII in regulating WNT4 expression in the human endometrium warrants future study. The incomplete block of human endometrial decidualization shown here could reflect a lack of either 100% COUP-TFII knockdown or epithelial-stromal interactions in the in vitro vs the in vivo system.
We also found that the expression of PGR was decreased after knockdown of COUP-TFII in HESCs, which is consistent with the previous result that the PGR expression was reduced in the endometrial stroma of mice lacking COUP-TFII (5). PGR has been reported to negatively regulate the expression of cytokines and thus inflammation in HESCs (27). Our result show that in the absence of COUP-TFII, PGR-A protein was significantly down-regulated, whereas a modest decrease in PGR-B levels was observed. The decrease in PGR levels did not result, however, in a concomitant reduction in expression of the decidual markers PRL and IGFBP1. In fact IGFBP1, a direct target of PGR (28, 29), was increased. Interestingly, our data also show that IGFBP1 is a direct target of COUP-TFII. Elevated expression of IGFBP1 may indicate that the COUP-TFII represses this gene. Additionally, the ratio of PGR-B to PGR-A has been shown to play an important role in gene regulation because PGR-A has been proposed to be an inhibitor of PGR-B (30). The ratio of PGR-B to PGR-A was markedly elevated in the absence of COUP-TFII, and this may also be contributing to the aberrant expression of IGFBP1. Defining the specific role of PGR-B and PGR-A isoforms would be requisite to further understand the impact of COUP-TFII on the regulation of PGR target genes during decidualization and warrants future investigation.
In order to identify the human endometrial COUP-TFII transcriptome, important for understanding the mechanisms with which loss of COUP-TFII may contribute to endometriosis, a HESC COUP-TFII siRNA microarray was conducted. Differential expression of genes regulated by COUP-TFII, when analyzed using DAVID, fell into many signaling pathways such as cytokine-cytokine receptor interaction, VEGF signaling, focal adhesion, and MAPK and WNT signaling pathways. Some of the signaling pathways regulated by COUP-TFII in HESCs have been shown to play an important role in other organs and diseases. For example, COUP-TFII has been shown to regulate VEGF signaling to promote angiogenesis and tumor growth in pancreatic tumors (31). Other signaling pathways identified in this study to be regulated by COUP-TFII, such as WNT signaling and cytokine-cytokine receptor interaction, are known to play important roles in endometrial function and endometriosis, and their direct regulation by COUP-TFII is a novel observation. Previous studies have also linked COUP-TFII with endometriosis by determining that it competes for promoter occupancy with the transcription factor steroidogenic factor 1 (SF1) to inhibit the expression of aromatase. Aromatase plays an important role in the pathogenesis of endometriosis because it is the rate-limiting enzyme in the synthesis of estrogen, contributing to the mitogenic nature and sustained growth of endometrial implants (7). Although we did not see a significant alteration in aromatase expression in our study, the ChIP-seq analysis did identify COUP-TFII binding sites in the aromatase gene. The failure to observe the aberrant up-regulation in aromatase expression in the absence of repressive COUP-TFII can be explained by the fact that HESCs obtained from healthy women do not express SF1, the transcription factor that induces aromatase expression and itself is aberrantly up-regulated in endometriosis (7).
COUP-TFII is a transcription factor that, when bound directly to DNA, can act as a repressor of transcription, as is the case with aromatase. Conversely, when COUP-TFII binds to promoters via tethering to other factors, it tends to act as a coactivator. Furthermore, COUP-TFII may affect gene expression indirectly by regulating the expression of other transcription factors, each itself possessing the ability to alter which genes are expressed in the cell. To gain insight into how the absence of COUP-TFII is affecting the expression of the genes identified in the microarray, a genome-wide interrogation of COUP-TFII DNA occupancy was achieved using ChIP-seq. We observed that a total of 6077 genes exhibit COUP-TFII binding within 10 kb of their boundaries. As alluded to above, one of the benefits of ChIP-seq is the ability to gain insight into with which transcription factors COUP-TFII may be binding to DNA by analyzing the motifs found within binding intervals (16). The DNA response elements of many transcription factors, such as those in the FOX and HOX family, were identified in the regions of chromatin bound by COUP-TFII. Transcription factors binding these motifs are known to play an important role in endometrial function (reviewed in Ref. 32). The increased representation of said response elements in the COUP-TFII intervals may indicate that these factors cooperate with COUP-TFII in the regulation of endometrial function. However, future studies will be required to determine the nature of the interaction between COUP-TFII and these putative factors in the regulation of endometrial biology.
When the genes bound (ChIP-seq) and regulated (microarray) by COUP-TFII are compared, a total of 462 genes were identified as direct targets of COUP-TFII action. Although the number of genes, both bound and regulated, represents only a fraction of the total number of genes in either dataset, a number of signaling pathways and biological functions were identified by DAVID. Importantly, most the COUP-TFII-regulated genes are involved in cell adhesion, inflammation, and cytokine and chemokine signaling. These signaling pathways have been widely reported to be involved in endometriosis. Furthermore, of the genes directly regulated by COUP-TFII, nearly 10% of them have a role in endometriosis as annotated by IPA.
Successful implantation of endometrial tissue in the peritoneal cavity is the first requirement in the establishment of endometrial lesions. The ability of dislocated endometrium to implant and survive in ectopic sites of women with endometriosis is quite astounding (33). Cell-adhesion molecules are of great importance in the implantation process because they are essential to establish cell-cell or cell-extracellular matrix interactions between the endometrial cells and the peritoneal lining (23). We have identified a number of COUP-TFII-regulated genes, such as COL6A1, SPP1, and LAMA1, that have functions in cell adhesion and extracellular matrix interaction. The aberrant up-regulation of these genes involved in cell adhesion in the absence of COUP-TFII represents yet another mechanism with which loss of COUP-TFII might favorably contribute to the development of endometriosis (34).
Accumulating studies indicated that inflammation is an integral component of endometriosis (22, 23). Cytokines are a major family of genes that participate in inflammation. Cytokines in the peritoneal fluid of women with endometriosis are produced by peritoneal macrophages, lymphocytes, mesothelial cells of the peritoneum, and the ectopic endometrial implants themselves, resulting in a proinflammatory environment within the peritoneal cavity (35). Herein we found a number of cytokines that were directly up-regulated in the absence of COUP-TFII in HESCs. IL-1 is thought to play an important role in endometriosis. IL-1 triggers inflammation via forming a complex with its receptor IL1R1 and IL-1 receptor accessory protein (IL1RAP). IL1R1 expression was found to be increased in the ectopic implants compared with eutopic endometrium of either healthy women or those with endometriosis (36). Here we show that knockdown COUP-TFII in HESCs resulted in elevated expression of IL1RA and IL1RAP, which suggests that COUP-TFII may modulate inflammation via regulation of IL-1 complex formation. IL-6 is another proinflammatory cytokine that has also been shown to be increased in the peritoneal fluid of endometriosis patients (36). The expression of IL-6 signaling pathway members IL6R and IL6ST were elevated following COUP-TFII depletion in HESCs. Moreover, secreted IL-6 protein levels were found to be elevated in HESC culture media in the absence of COUP-TFII after 3 days of treatment with decidual media. IL-6 expression in HESCs with COUP-TFII knockdown was also determined to be elevated by qPCR, although this difference was not identified in the less sensitive microarray.
We identified many chemokines regulated by COUP-TFII. Chemokines are a family of small cytokines that promote the chemotaxis of effector T cells, thereby recruiting them to sites of inflammation to initiate the immune response. Recent important work has revealed that chemokines are involved in maternal fetal immune tolerance in which epigenetic silencing of chemokines prevents the accumulation of effector T cells within the decidua (37). One of the chemokines regulated directly by COUP-TFII found here is IL-8. The expression of IL-8 increased significantly in the absence of COUP-TFII. IL-8 is a member of the CXC chemokine family, which plays roles in inducing chemotaxis of neutrophils and macrophages (35). Interestingly, not only is the concentration of IL-8 higher in the peritoneal fluid of women with endometriosis (38), but its levels directly correlate with the severity of the disease (39). Moreover, the expression of IL-8 in ectopic endometriosis is higher than that in the eutopic counterpart of women with endometriosis (21, 40). Although we identified a reduction in the transcript levels of IL-8 in the absence of COUP-TFII, the level of secreted protein was found to be increased in only 1 of 3 HESC lines, indicating there may be variations between patients or that the mechanisms regulating its secretion are not fully recapitulated in our in vitro system.
The results shown here suggest that COUP-TFII can directly suppress the expression of the proinflammatory cytokines and chemokines. Thus, loss of COUP-TFII expression in ectopic lesions results in the aberrant up-regulation of proinflammatory cytokines. The elevated secretions of cytokines in the region of ectopic lesions results in the production of a proinflammatory environment within the peritoneal cavity, promoting implant growth and contributing to the predominant symptom of endometriosis and pelvic pain.
The one limitation of the primary HESC system used in this study is the absence of epithelial cells and loss of the cellular cross talk observed in vivo. It is known that COUP-TFII plays an important role in epithelial-stromal cellular cross talk, warranting future studies that use the coculture of both stromal and epithelial cells to determine the role of COUP-TFII in influencing epithelial function (41). In addition to its ability to directly repress inflammatory cytokines, cell adhesion, and ER activity, these results indicate that COUP-TFII may affect the level of inflammation.
In conclusion, our study found that attenuation of COUP-TFII expression had only select effects on HESC decidualization. However, microarray analysis revealed that COUP-TFII regulates many genes in HESCs, many of which are regulated directly by COUP-TFII binding as indicated by ChIP-seq and ChIP-qPCR results. Of the genes regulated by COUP-TFII, most are involved in cell adhesion and inflammation, hallmarks of endometriosis. Furthermore, we determined that COUP-TFII expression is reduced in ectopic lesions compared with that in eutopic endometrium of women with endometriosis. The antiinflammatory effects of COUP-TFII and its reduced expression in ectopic lesions indicate that COUP-TFII plays a vital role in the pathogenesis of endometriosis. Further investigation of the COUP-TFII targets identified in this study and the mechanisms with which they are regulated, as well as the events that cause the down-regulation of COUP-TFII in ectopic endometriosis, has broad implications in our approach to treating one of the most important afflictions of female health.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr. Paul Labhart of Active Motif for performing the ChIP-seq, Yiqun Zhang for technical assistance with the microarray analysis, Yasmin M. Vasquez for helping with HESCs isolation and culture, and Dr. William E. Gibbons and Dr. Ertug Kovanci for the biopsies of endometrium from healthy women.
This work was supported by National Institute of Health Grants R01HD042311 (to F.J.D.), 5U54HD007495 (to F.J.D.), R01 HD067721 (to S.L.Y. and B.A.L.), and P30 CA125123 (to C.J.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CEAS
- cis-regulatory element annotation system
- ChIP
- chromatin immunoprecipitation
- ChIP-seq
- ChIP followed by massively parallel sequencing
- COUP-TFII
- chicken ovalbumin upstream promoter-transcription factor II
- DAVID
- database for annotation, visualization, and integrated discovery
- ER
- estrogen receptor
- HESC
- human endometrial stromal cell
- IGFBP1
- IGF-binding protein
- IL1RAP
- IL-1 receptor accessory protein
- IPA
- ingenuity systems pathway analysis
- MPA
- medroxyprogesterone acetate
- PGR
- progesterone receptor
- PRL
- prolactin
- RT-qPCR
- real-time quantitative PCR
- VEGF
- vascular endothelial growth factor.
References
- 1. McEwan IJ. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. [DOI] [PubMed] [Google Scholar]
- 2. Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup d'Etat: an orphan takes control. Endocr Rev. 2011;32:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. [DOI] [PubMed] [Google Scholar]
- 7. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol. 1999;13:239–253. [DOI] [PubMed] [Google Scholar]
- 8. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- 9. Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208–215. [DOI] [PubMed] [Google Scholar]
- 10. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. [DOI] [PubMed] [Google Scholar]
- 11. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- 12. Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. [DOI] [PubMed] [Google Scholar]
- 13. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. [DOI] [PubMed] [Google Scholar]
- 14. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. [DOI] [PubMed] [Google Scholar]
- 15. Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol. 2012;26:1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu T, Ortiz JA, Taing L, et al. Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol. 2011;12:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 18. Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev. 1997;18:229–240. [DOI] [PubMed] [Google Scholar]
- 19. Krishnan V, Elberg G, Tsai MJ, Tsai SY. Identification of a novel sonic hedgehog response element in the chicken ovalbumin upstream promoter-transcription factor II promoter. Mol Endocrinol. 1997;11:1458–1466. [DOI] [PubMed] [Google Scholar]
- 20. Krishnan V, Pereira FA, Qiu Y, et al. Mediation of sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. [DOI] [PubMed] [Google Scholar]
- 21. Wu Y, Kajdacsy-Balla A, Strawn E, et al. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–246. [DOI] [PubMed] [Google Scholar]
- 22. Barrier BF. Immunology of endometriosis. Clin Obstet Gynecol. 2010;53:397–402. [DOI] [PubMed] [Google Scholar]
- 23. Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. 1996;2:371–384. [DOI] [PubMed] [Google Scholar]
- 24. Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. [DOI] [PubMed] [Google Scholar]
- 25. Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Kannan A, Das A, et al. WNT4 acts downstream of BMP2 and functions via β-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pabona JM, Simmen FA, Nikiforov MA, et al. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97:E376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell SC, Jackson JA, Ashmore J, Zhu HH, Tseng L. Regulation of insulin-like growth factor-binding protein-1 synthesis and secretion by progestin and relaxin in long term cultures of human endometrial stromal cells. J Clin Endocrinol Metab. 1991;72:1014–1024. [DOI] [PubMed] [Google Scholar]
- 29. Gao JG, Mazella J, Powell DR, Tseng L. Identification of a distal regulatory sequence of the human IGFBP-1 gene promoter and regulation by the progesterone receptor in a human endometrial adenocarcinoma cell line. DNA Cell Biol. 1994;13:829–837. [DOI] [PubMed] [Google Scholar]
- 30. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res. 2010;70:8812–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Somigliana E, Vigano P, Benaglia L, Busnelli A, Vercellini P, Fedele L. Adhesion prevention in endometriosis: a neglected critical challenge. J Minim Invasive Gynecol. 2012;19:415–421. [DOI] [PubMed] [Google Scholar]
- 34. Witz CA. Cell adhesion molecules and endometriosis. Semin Reprod Med. 2003;21:173–182. [DOI] [PubMed] [Google Scholar]
- 35. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. [DOI] [PubMed] [Google Scholar]
- 36. Lawson C, Bourcier N, Al-Akoum M, Maheux R, Naud F, Akoum A. Abnormal interleukin 1 receptor types I and II gene expression in eutopic and ectopic endometrial tissues of women with endometriosis. J Reprod Immunol. 2008;77:75–84. [DOI] [PubMed] [Google Scholar]
- 37. Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63:929–932. [PubMed] [Google Scholar]
- 39. Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR. Peritoneal fluid concentrations of interleukin-8 in women with endometriosis: relationship to stage of disease. Hum Reprod. 1998;13:1957–1961. [DOI] [PubMed] [Google Scholar]
- 40. Luk J, Seval Y, Kayisli UA, Ulukus M, Ulukus CE, Arici A. Regulation of interleukin-8 expression in human endometrial endothelial cells: a potential mechanism for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2005;90:1805–1811. [DOI] [PubMed] [Google Scholar]
- 41. Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.