Abstract
The type II iodothyronine deiodinase (D2) is a type I endoplasmic reticulum (ER)-resident thioredoxin fold-containing selenoprotein that activates thyroid hormone. D2 is inactivated by ER-associated ubiquitination and can be reactivated by two ubiquitin-specific peptidase-class D2-interacting deubiquitinases (DUBs). Here, we used D2-expressing cell models to define that D2 ubiquitination (UbD2) occurs via K48-linked ubiquitin chains and that exposure to its natural substrate, T4, accelerates UbD2 formation and retrotranslocation to the cytoplasm via interaction with the p97-ATPase complex. D2 retrotranslocation also includes deubiquitination by the p97-associated DUB Ataxin-3 (Atx3). Inhibiting Atx3 with eeyarestatin-I did not affect D2:p97 binding but decreased UbD2 retrotranslocation and caused ER accumulation of high-molecular weight UbD2 bands possibly by interfering with the D2-ubiquitin-specific peptidases binding. Once in the cytosol, D2 is delivered to the proteasomes as evidenced by coprecipitation with 19S proteasome subunit S5a and increased colocalization with the 20S proteasome. We conclude that interaction between UbD2 and p97/Atx3 mediates retranslocation of UbD2 to the cytoplasm for terminal degradation in the proteasomes, a pathway that is accelerated by exposure to T4.
Ubiquitination is an evolutionary conserved ATP-dependent protein modification pathway that utilizes the 8-kDa ubiquitin molecule to tag proteins for destruction in the 26S proteasomal system. This pathway has also been implicated in nonproteolytic mechanisms such as regulation of gene transcription by histone modulation, DNA repair, and vesicle trafficking (1–3). Ubiquitination follows a hierarchical 3-step enzymatic chain, in which an ubiquitin activating enzyme (E1) activates and transfers ubiquitin to a conjugating enzyme (E2) that acts in concert with ubiquitin ligase enzymes (E3) to conjugate an ubiquitin molecule via its carboxyl glycine residue at position 76 (Gly76) to an exposed lysine (K) residue on the target protein (at monoubiquitination) or to a specific K residue of another ubiquitin molecule to form a polyubiquitin chain (3).
The pattern and composition of the substrate-linked ubiquitin chain dictate the fate of the target substrate (3). For example, K63-linked ubiquitin chains link ubiquitination to nonproteolytic events (4), whereas K48- and K11-linked chains are associated with 26S proteasomal targeting and proteolysis (5). Notably, ubiquitination can be a reversible cellular event, with a small number of deubiquitinases (DUBs) preserving the ubiquitin pool and rescuing ubiquitinated proteins from proteasomal degradation (6).
The type 2 deiodinase (D2) is a dimeric thioredoxin-like fold containing selenoprotein that activates thyroid hormone and resides on the endoplasmic reticulum (ER) membrane as a type 1 integral membrane protein (7, 8). In the case of ER-resident proteins such as D2, degradation occurs through ER-associated degradation (ERAD), with ubiquitination taking place in the cytosolic side of the ER membrane and proteasomal degradation in the cytoplasm. ER proteins are “pulled out” of the membrane through retrotranslocation and delivered to the 26S proteasome. In many cases, retrotranslocation requires the participation of an ATPase complex consisting of the p97 (the mammalian orthologue of the yeast Cdc48a also known as valosin-containing protein), a dimeric cofactor Ufd1-Npl4 and the DUB, ataxin-3 (Atx3) (9).
A fundal role of ERAD is quality control. In addition, ERAD is also known for mediating basal and conditionally regulated degradation of endogenous ER proteins under normal physiologic conditions, eg, 3-hydroxy-3-methylglutaryl coenzyme A reductase, fatty acid desaturases, and transcription factors (10). D2 degradation in both yeast and mammalian cells (11, 12) involves many of the known ERAD components such as UBC-6, UBC-7, Doa10, and its mammalian homologue TEB4 (11, 13–15), a process that is accelerated by its natural substrate, T4. Notably, ubiquitinated D2 is not immediately retrotranslocated and delivered to the proteasomes. Instead, D2 can be reactivated and rescued from terminal degradation via interaction with two highly related DUBs, ie, ubiquitin-specific peptidase (USP)20 and USP33 (16). The mechanisms and the identity of the proteins regulating the fate of UbD2, ie, retrotranslocation vs deubiquitination, remain largely unknown.
D2 is a suitable ERAD substrate due to an 18-amino acid loop in its globular domain that confers metabolic instability, transferable to stable proteins (17, 18). However, specific mechanisms that regulate D2 processing by ERAD should exists given that ER stress does not increase D2 ubiquitination or its proteasomal degradation (19). Instead, ER stress greatly reduces D2 expression by inhibiting its de novo D2 protein synthesis via activation of the phospho-ERK-eukaryotic translation initiation factor 2A pathway (19). Second, given that binding and/or catalysis of T4 accelerates D2 ubiquitination and degradation, it has been proposed that interaction with its substrate alters the proper folding of the D2:D2 dimer, increasing its suitability as an ERAD substrate (12, 20).
Here we used human embryonic kidney (HEK)-293 cells expressing a doubled tagged D2 protein and an in vitro ubiquitination system to show that D2 is ubiquitinated via K48-linked ubiquitin chain even in the absence of T4 binding and/or enzymatic catalysis, indicating that D2 is intrinsically unstable. Because D2 is also deubiquitinated, at any given time there are 2 pools of active (non-Ub) and inactive D2 (UbD2) that remain in equilibrium. Exposure to its natural substrate T4 accelerates D2 ubiquitination (also via K48-linked ubiquitin chain), shifting the equilibrium toward UbD2. This increases binding of UbD2 to p97, retrotranslocation of D2 to the cytoplasm, and delivery to the proteasome system.
Materials and Methods
Cell lines and reagents
HEK-293 cells were purchased from American Type Culture Collection (Manassas, Virginia) and were cultured in 100-mm dishes with DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS). Unless otherwise noted, all cellular experiments were performed with 10% FBS-containing media. For hypothyroid experiments, cells were cultured in DMEM supplemented with 10% charcoal stripped serum (CSS) as described elsewhere (19), whereas a hyperthyroid condition was generated by addition of 20 μM T4 to CSS-containing media. MG132 was purchased from Calbiochem; eeyarestatin I (EERI) was from Santa Cruz Biotechnology, and T4 was from Sigma Aldrich. Purified wild-type, K0, and K48R ubiquitin proteins were obtained from Boston Biochem.
DNA vectors and cell transfection
The 6×His-CysD2-YFP (D2) vector was created fusing enhanced yellow fluorescent protein (EYFP) in frame to the C terminus of either a 133Cys/266Cys or 133Ala/266Cys mutant human D2. The D2-EYFP cassette was inserted in-frame between EcoRI-NotI of pcDNA™4/ HisMax C (Invitrogen). CysD2 stable HEK-293 cell line was established by transfecting 2.5 μg of His-D2-YFP vector using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer's instructions. CysD2-expressing clones were selected 48 hours after transfection by antibiotic selection (Zeocin, 300 μg/mL) for 2 weeks. The control yellow fluorescent protein (YFP)-expressing vector has been described elsewhere (20). The green fluorescent protein (GFP)-p97 (vector 23971) vector was purchased from Addgene and was described previously (21). For HEK-293-expressing K48R mutant, we generated wild-type and K48R human ubiquitin-expressing constructs in pCI-Neo vector (Promega Corp). In short, overlap-extension PCR was used to perform site-directed mutagenesis. The PCR-generated wild-type and mutant fragments were cloned into the vector followed by sequencing. ubiquitin-encoding construct (2.7 μg) was cotransfected with 200 ng wild-type D2 (previously described in Ref. 18) into HEK-293 cells using Lipofectamine (Invitrogen) in the presence of 40 ng pS2 encoding a secreted alkaline phosphatase internal control. Secreted alkaline phosphatase was assayed from the culture media according to the instructions of the manufacturer (Invitrogen).
Immunoprecipitation (IP), Western blots, and antibodies
α-YFP IP was performed by the use of the μMACS GFP-tagged protein isolation kit (Miltenyi Biotec) and according to manufacturer's instructions. Briefly, HEK-293 cells expressing the CysD2- or AlaD2-tagged vector were treated with vehicle, 1 μM MG132, 20 μM T4, and/or 10 μM EER1 for 24 hours. After incubation, cells were harvested in ice-cold PBS and pelleted by centrifugation at 1000 × g for 10 minutes at 4°C after which the cell pellet was lysed in lysis buffer containing 150 mM NaCl, 1% Triton X-100, and 50 mM Tris HCl (pH 8.0) for 30 minutes on a orbital shaker at 4°C. The resulting lysate was centrifuged for 15 minutes at 1000 × g, in which the supernatant was defined as Triton-soluble fraction and the pellet was determined as the Triton-insoluble fraction. The pellet was discarded and the supernatant fraction was used in our studies. For coimmunoprecipitation of D2 with ubiquitin chains and p97, the Triton-soluble fraction was processed as described above and included 4× column washes with wash buffer containing 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mM Tris HCl (pH 8.0). For D2 and S5a coimmunoprecipitation, columns were washed 4 times with wash buffer containing 300 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mM Tris HCl (pH 8.0). Following the column washes, the excess salt was removed by washing the column-bound immunocomplexes with 100 μL of 20 mM Tris-HCl buffer. All Western blotting and signal detection were performed as described elsewhere (19) and input was 5%–10% of pre-IP sample. Mouse α-ubiquitin (Ub; clone P4D1; 1:1000), rabbit α-K48-linkage ubiquitin (clone 4289S; 1:1000), rabbit α-p97/VCP (clone 7F3; 1:1000), rabbit α-S5a (clone PRDM4; 1:1000), and rabbit α-ERp72 (clone D70D12; 1:250) antibodies were from Cell Signaling Technology; rabbit α-20S proteasome α/β subunits (clone 22673; 1:250), and mouse α-KDEL (clone 10C3; 1:250) were from Abcam; goat α-GFP (1:1000), used to detect YFP-tagged proteins, was from Rockland.
Measurement of D2 activity
D2 enzymatic activity assay was performed as described previously (19, 23).
Cellular fractionation and in vitro ubiquitination
Cellular fractions of D2-expressing HEK-293 cells were prepared as described elsewhere (24, 25). In short, cells were plated in five 150-mm dishes and grown until they reached 90% confluence. Cells were washed, harvested in ice-cold PBS, and collected by centrifugation at 1000 × g for 10 minutes at 4°C. Cells were then lysed in a cell homogenizer as described elsewhere (25) using a 10-μm bead in 50 mL of lysis buffer that contains 0.25 M sucrose, 100 mM KCl, and 100 μM phenylmethylsulfonyl fluoride. The lysate was first centrifuged at 9000 × g for 30 minutes at 4°C after which the supernatant was spun at 113 000 × g for 30 minutes at 4°C to collect a microsome (pellet) and cytosolic (supernatant)-enriched fractions. For in vitro ubiquitination, the pellet was resuspended in sample buffer containing 100 mM KCl, pH 7. For the in vitro ubiquitination reaction, an ubiquitin conjugation kit (K-960; Boston Biochem) was used with 300 ng of microsomal-enriched fraction and performed according to manufacturer's instructions with purified wild-type, K48R, or K0 ubiquitin molecules. For Western blotting, samples were resuspended in loading buffer.
Immunofluorescent confocal microscopy
Immunocytochemistry was performed as previously described (25). Briefly, 1 day before treatment, 12 × 103 D2-expressing HEK-293 cells were plated on poly-D-lysine-coated chambered slides and cultured in DMEM supplemented with 10% FBS. On the day of treatment, cells were incubated with DMEM with 10% CSS and supplemented with vehicle or 20 μM T4 and with dimethylsulfoxide or 1 μM MG132 for up to 3 hours. After treatment, cells were either fixed with fresh-made 4%-paraformaldehyde/PBS solution at room temperature for 20 minutes (20S α/β subunits staining) or with ice-cold methanol (MeOH) solution at −20°C for 5 minutes (for endoplasmic reticulum protein 72 staining (26)). Fixation was followed by 3 washes with 1×PBS of 5 minutes each. Sample permeabilization and blocking were performed for 1 hour at room temperature with a PBS-based blocking buffer containing 5% BSA, 0.5% FBS, and 0.3% Triton X-100. After blocking, samples were probed with a goat α-GFP antibody (Rockland) and with either rabbit α-ERp72, α-KDEL, or α-20S proteasome for an overnight period at 4°C. The next day samples were washed 3 times with 1×PBS, for 5 minutes each. Secondary antibody incubation was performed at room temperature for 40 minutes in PBS-based blocking buffer containing 1% BSA and with Alexa Fluor-conjugated antibodies (Invitrogen) donkey α-goat 568 (1:400), donkey α-rabbit 488 (1:400), and 4′,6-diamidino-2-phenylindole (Invitrogen) as described elsewhere (25). After incubation, cells were washed 4 times with 1×PBS, mounted with VectaShield (Vector Laboratories), imaged, and analyzed for colocalization as described elsewhere (25).
Fluorescent resonance energy transfer (FRET)
The used D2-enhanced CFP (ECFP), D2-EYFP (ECFP or EYFP were fused to the C portion of D2), and ECFP-USP33 (ECFP was fused to the N portion of USP33), and ECFP-EYFP tandem DNA constructs have been previously described (20). HEK-293T cells were plated in 35-mm glass-bottom dishes (MatTek Co) and transfected using Lipofectamine 2000 reagents (Invitrogen). Twenty hours before FRET measurement, transfection medium was changed to 10% charcoal serum-supplemented medium containing 10 μM eeyarestatin I or 1 μM MG132 or equal volume of dimethylsulfoxide combined with 4 hours of 10 μM T4 treatment before FRET measurement. FRET detection was taken on the second day after transfection using confocal microscopy (Nikon A1R system) equipped with CFI Super Plan Fluor ELWD 20× objective. wavelengths of 457 nm and 514 nm were used for excitation of cyan fluorescent protein (CFP) and YFP, respectively, and fluorescence signal was detected using a spectral detector with the following gating intervals: 460–500 nm for CFP and 520–540 nm for YFP. FRET calculation was based on the increase of CFP signal by the photobleaching of acceptor in cells with at least 80% bleach efficiency and normalized by the average increase of the ECFP-EYFP tandem construct (27), in which the following equation was used to calculate FRET efficiency: FRET = (CFP[postbleach] − CFP[prebleach])/CFP(postbleach)
Results
Modeling D2 ubiquitination in HEK-293 cells stably expressing a double-tagged D2 protein
D2 is normally expressed at relatively low levels. In a mesothelioma cell line (MSTO-211H) expressing unusually high levels of D2, visualization of endogenously expressed [75]Se-D2 requires a minimum of 3 weeks exposure to a film (28). Thus, to study UbD2 complexes and characterize their interactions with other cell proteins, we developed a HEK-293-based cell system stably expressing a His/YFP-doubled tagged Sec133Cys-D2 protein (D2HY). Replacing Sec for Cys increases the translation efficiency of deiodinase mRNA (29), bringing expression levels to approximately 100-fold of endogenously expressed D2 in MSTO-211H cells (28).
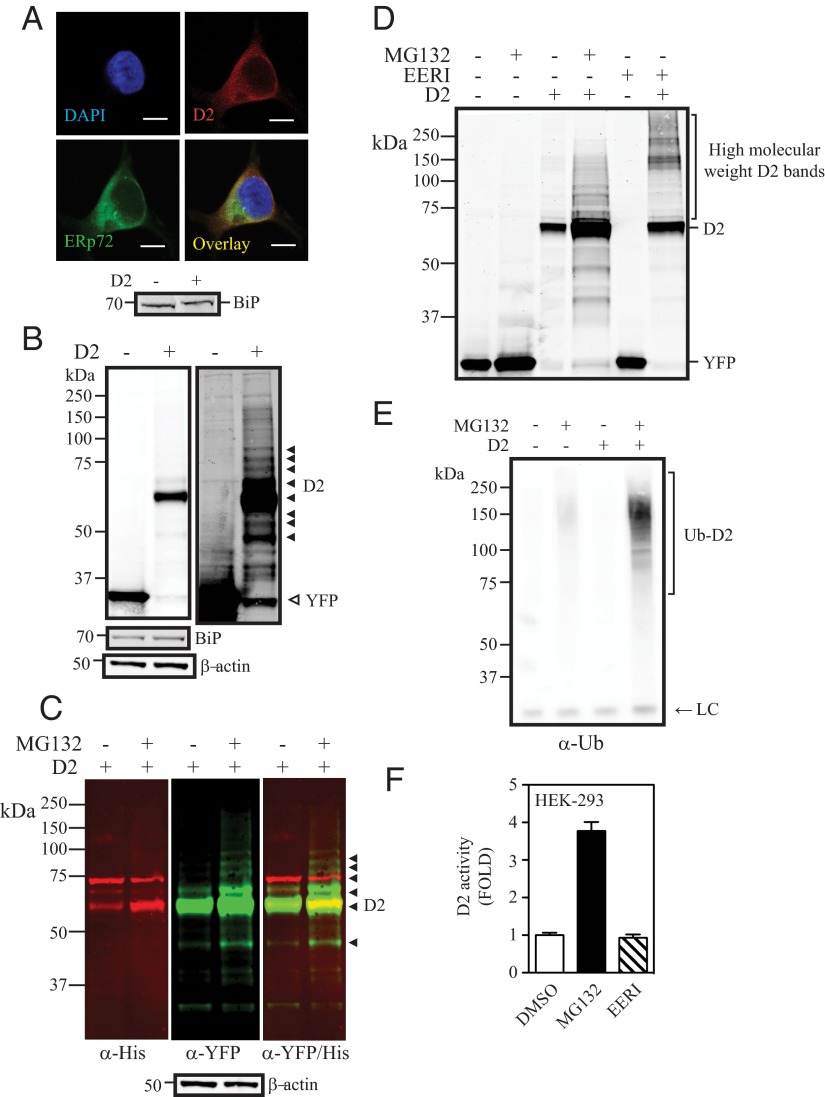
The utility of this system was validated by studies demonstrating that 1) the host cells are not adversely affected by the stable overexpression of D2HY, and that 2) D2HY exhibits similar cellular properties as native D2 in this setting. First, we looked at cell cycle and rate of cell replication comparing HEK-293 cells stably expressing YFP vs D2HY, not finding any significant differences (Supplemental Figure 1, A and B, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Second, a microarray analysis comparing these 2 cell lines identified 10 up-regulated (>2-fold) and 4 down-regulated (>2-fold) genes, none of which are related to ERAD or the ubiquitin proteasome system (Supplemental Figure 1C). The absence of ER stress was confirmed by stable levels of the ER-stress marker BiP (Figure 1A, lower panel). Third, the cellular properties of D2HY were found to be indistinguishable from those of endogenous D2 (8, 28), including subcellular localization [colocalization with the ER-resident protein ERp72 (Figure 1A)], turnover rate (∼20-minute half-life [Supplemental Figure 1D]), and inactivation when exposed to its natural substrate T4 (loss of ∼50% activity in the presence of T4 [Supplemental Figure 1D]).
Figure 1.
D2 is an ERAD pathway substrate. A, HEK-293 cells transiently expressing the double-tagged YFP/His D2 protein (D2HY) were fixed in 4% paraformaldehyde (PFA) and stained with a α-YFP for D2HY (red) and α-ERp72 for the ER marker ERp72 (green). Nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 7.5 μm. Lower panel, BiP protein levels in control or D2-expressing cells. B, Western blot analysis of HEK-293 cells transiently expressing D2HY or control YFP with an α-YFP antibody. D2HY migrates at an approximate molecular weight of 62 kDa and YFP migrates at 30 kDa. Overexposure of same blot reveals high-molecular weight D2HY bands (solid arrows). BiP (70 kDa; middle panel) and β-actin (50 kDa; lower panel) are shown. C, D2HY protein levels in HEK-293 transiently expressing D2HY and treated with either vehicle or 1 μM MG132 for 24 hours. In red, D2HY bands are observed with an α-His antibody. In green, D2HY bands are observed with an α-YFP antibody. The overlay of both images and high-molecular weight D2HY bands is seen on the right panel, with overlaying bands appearing in yellow. D, Western blot analysis with an α-YFP antibody of lysates of control or D2HY-expressing HEK-293 cell treated with vehicle, 1 μM MG132, or 10 μM EERI for 24 hours. E, Western blot analysis of α-YFP immunoprecipitates of HEK-293 cells expressing either control YFP or D2HY and treated with vehicle or 1 μM MG132 for 24 hours. Blot was probed with α-ubiquitin antibody. LC, IgG light chain. F, D2 activity of HEK-293 cells stably expressing the D2HY protein and treated with vehicle, 1 μM MG132, or 10 μM EERI for 24 hours. DMSO, dimethylsulfoxide.
The molecular weight of D2HY is approximately 62 kDa (Figure 1B). Additional high-molecular weight D2HY-specific bands (UbD2) can be observed in overexposed blots (Figure 1B, right panel). Exposing cells to the proteasome inhibitor MG132 for 24 hours increases the abundance of UbD2 bands in the 70- to 150-kDa range (Figure 1, C and D). That these higher molecular weight D2HY-specific bands contain UbD2 complexes was confirmed by IP with α-YFP and Western analysis with α-ubiquitin (Figure 1E).
UbD2 complexes contain predominantly K48-linked ubiquitin chains
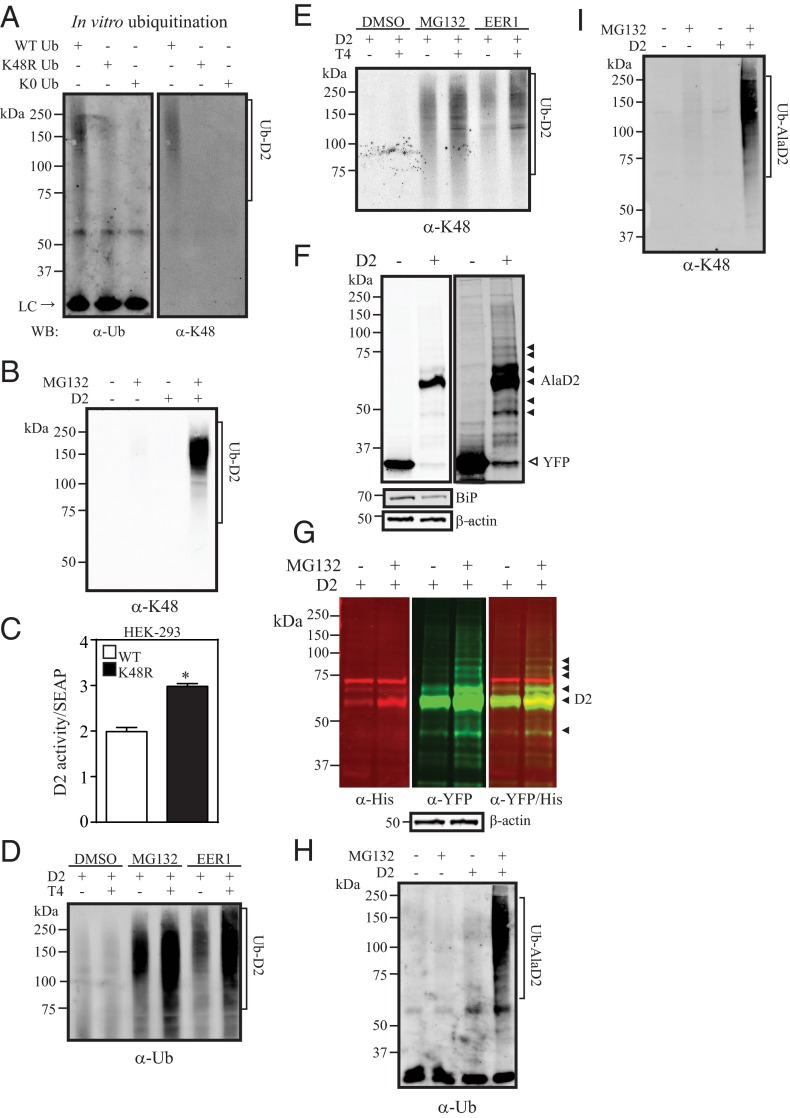
To define the type of ubiquitin linkage in UbD2 complexes we used ubiquitin chain-specific antibodies (31) and set up an in vitro ubiquitination assay in which D2HY-containing microsomes were incubated in the presence of either purified wild-type or mutant ubiquitin molecules, ie, K48R or K0 (all K residues mutated to R) (Figure 2A). At the end of the 4-hour ubiquitination period, D2HY was pulled down and processed for Western analysis using α-ubiquitin or α-K48-linked ubiquitin-chain. Only in the presence of wild-type ubiquitin were there high molecular weight UbD2 bands (Figure 2A). The fact that most of these bands were eliminated in the presence of K0 or K48R indicates that UbD2 contains mainly K48-linkage (Figure 2A). K48-linked D2 ubiquitination was also observed in the cell system stably expressing D2HY after pull down with α-YFP and Western analysis with α-K48-linked ubiquitin chain (Figure 2B). Furthermore, transient coexpression of D2HY with K48R ubiquitin, a mutant that interferes with K48-linked ubiquitin chain formation, elevated D2HY activity by approximately 50% in HEK-293 cells. This confirms that D2 is inactivated via K48-mediated ubiquitination (Figure 2C).
Figure 2.
UbD2 is composed of substrate-induced K48-ubiquitin chains. A, Isolated microsomes (300 ng) of vehicle-treated D2HY-expressing HEK-293 cells subject to a 4-hour in vitro ubiquitination reaction followed by IP and analyzed by Western blot with α-ubiquitin (Ub; left panel) and α-K48-linked chain antibodies (K48; right panel). B, α-K48-linked ubiquitin chain Western blot analysis of α-YFP immunoprecipitates of control YFP or D2HY-expressing HEK-293 cells treated with vehicle or 1 μM MG132 for 24 hours. C, D2 activity of HEK-293 cells transiently transfected with wild-type (WT) D2 and wild-type or K48R ubiquitin. Transfection efficiency was monitored by the cotransfection of secreted alkaline phosphatase (SEAP). D2 activity was expressed as fmol/min/mg/a.u serum alkaline phosphatase (SEAP). D, α-Ubiquitin and α-K48-linked ubiquitin chain (panel E) Western blot analysis of α-YFP immunoprecipitates of D2HY-expressing HEK-293 cells cultivated in stripped serum and supplemented with vehicle or 20 μM T4 in the presence of dimethylsulfoxide (DMSO), 1 μM MG132, or 10 μM EERI for 24 hours. F, α-YFP Western blot of lysates from HEK-293 cells transiently expressing either a YFP or a Cys133AlaD2 (AlaD2HY) isoform. AlaD2HY-specific bands are indicated by solid arrowheads. In lower panels, BiP and β-actin protein levels of same samples from upper panel. G, α-His (red) and α-YFP (green) Western blots of AlaD2HY-expressing HEK-293 cells treated with DMSO or 1 μM MG132 for 24 hours. AlaD2HY-specific bands are indicated by solid arrows. Overlay of α-His and α-YFP Western blots is shown on the panel on the right. On the lower panel, β-actin protein levels are shown. H, Western blot analysis with either an α-ubiquitin or K48-linkage (panel I) of α-YFP immunoprecipitates of HEK-293 cells expressing either control YFP or AlaD2HY and treated with vehicle or 1 μM MG132 for 24 hours. C, Data are shown as mean ± SD; n = 3; *, P < .001 by t test. LC, IgG light chain; WB, Western blot.
D2 is intrinsically unstable
To find out whether D2 is a targeted for ubiquitination due to its intrinsic instability or due to conformational changes occurring as a result of substrate occupancy of its catalytic pocket, D2HY-expressing cells were cultured in serum depleted of T4. Strikingly, also in this case there is an abundance of UbD2 bands, indicating that D2 is ubiquitinated even when its catalytic pocket is empty (Figure 2, D and E). Of course, exposure to T4 increases the intensity of the UbD2 bands (Figure 2D), which also contain K48-linked ubiquitin (Figure 2E). This issue was further studied using cells expressing the catalytically inactive Ala-D2HY protein, which is refractory to substrate (32). The pattern of Ala-D2HY expression is similar to that observed for D2HY (catalytically active) (Figure 1, B–E), with high-molecular weight bands (Figure 2F) that accumulate during proteasome blockade with MG132 (Figure 2G). Such bands contain UbD2 (Figure 2H), in which K48 linkage was identified (Figure 2I). Notably, nonubiquitinated Ala-D2HY also accumulates during proteasome blockade (Figure 2G), indicating that Ub-AlaD2 also undergoes deubiquitination.
UbD2 exits the ER via p97-mediated retrotranslocation to the cytoplasm
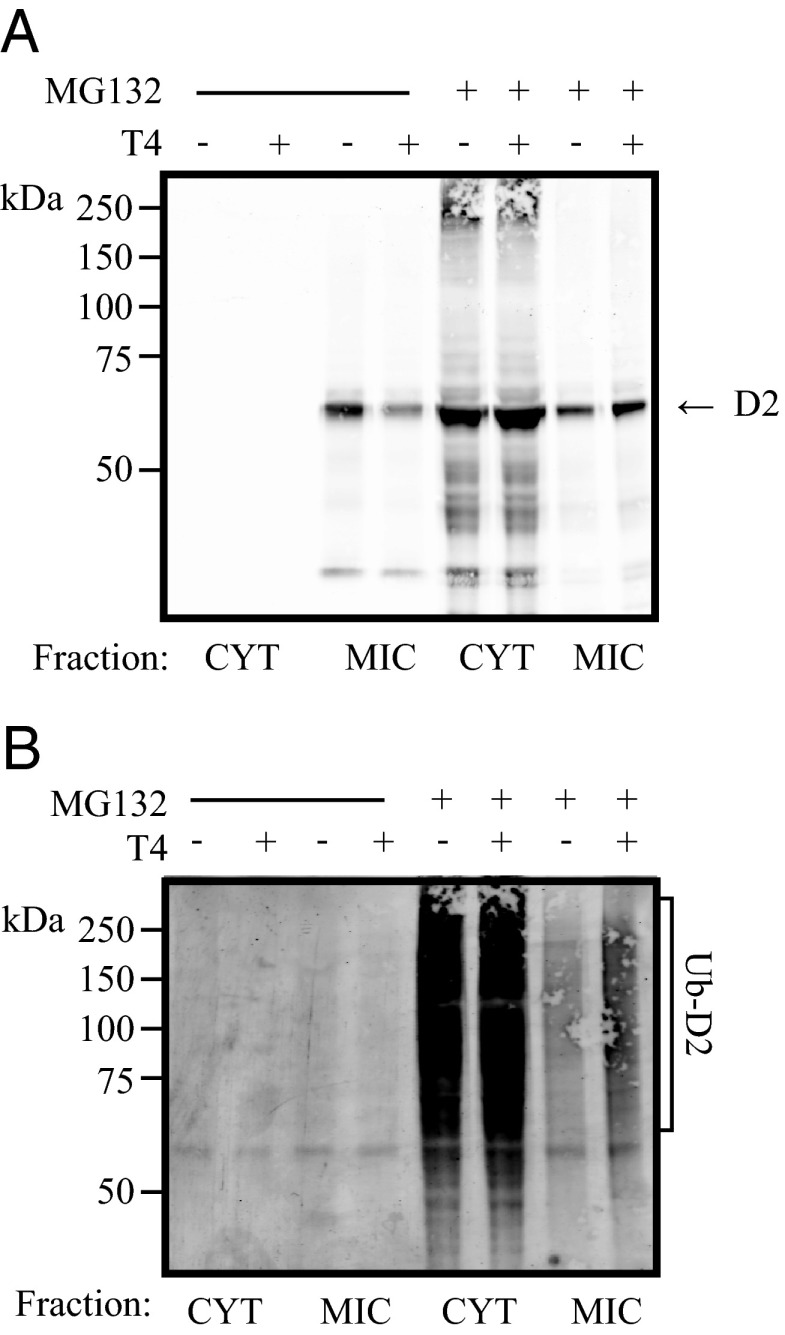
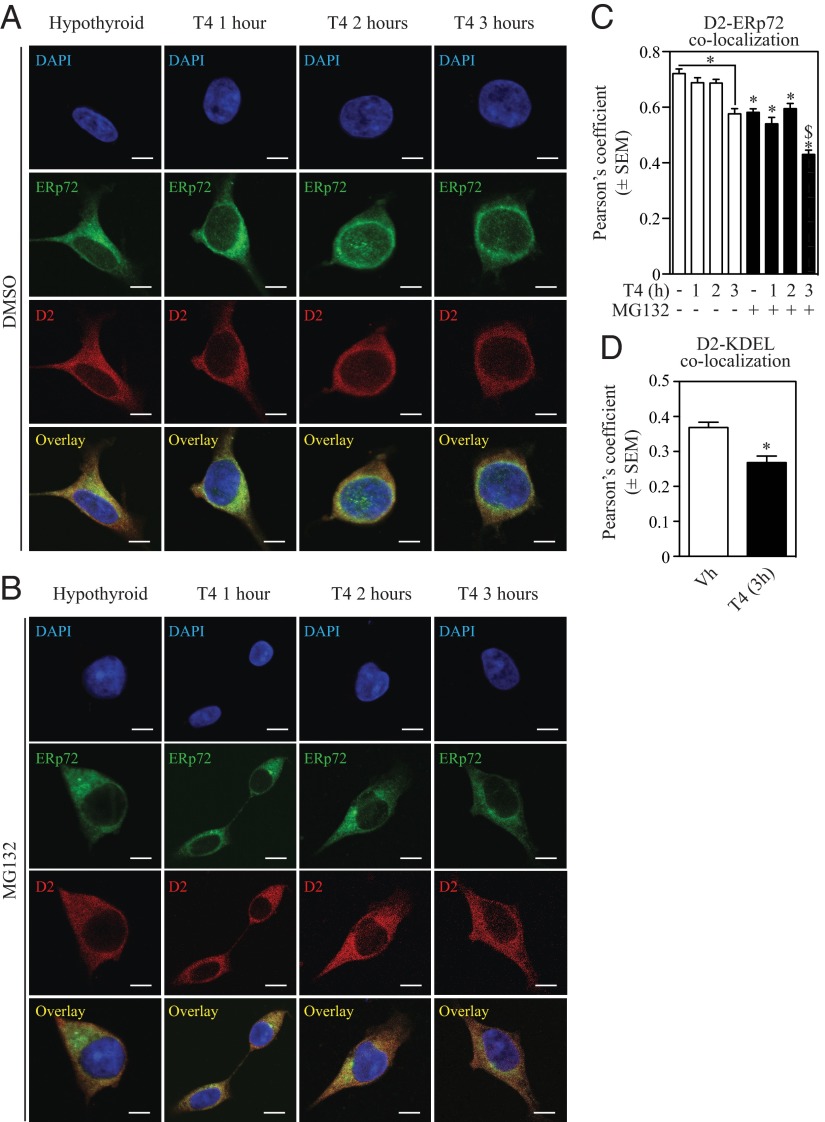
Subcellular fractionation studies show that D2HY is predominantly found in the microsomal pellet (Figure 3A) and that blocking the proteasomes leads to marked accumulation of D2HY and its high-molecular weight UbD2 forms in the cytosol (Figure 3, A and B). This suggests that UbD2 is pulled out of the ER membrane and deubiquitinated before it is delivered to the proteasome system. Retrotranslocation of UbD2 was also monitored by assessing D2HY colocalization with 2 ER markers, ERp72 and KDEL (Figure 4). Blocking the proteasomes resulted in loss of D2 colocalization with the ER markers, confirming the buildup of UbD2 outside the ER (Figure 4, B–D). At the same time, exposure to T4 for 0–3 hours, which we know accelerates D2 ubiquitination (Figure 2D), also accelerated UbD2 retrotranslocation as documented by the approximately 20% decrease in the colocalization of D2HY and both ER markers (Figure 4, B and C).
Figure 3.
D2 is ubiquinated at the ER level. α-YFP (panel A) and α-ubiquitin (panel B) Western blot of cytosolic (CYT) and microsomal (MIC) cellular fractions of cells expressing D2HY protein, grown in CSS, and treated with either control or 1 μM MG132 and in combination with vehicle or 20 μM T4 for 24 hours.
Figure 4.
Upon ubiquitination D2 exits the ER. HEK-293 cells stably expressing D2HY were maintained with charcoal-stripped media and treated with dimethylsulfoxide (DMSO) (A) or 1 μM MG132 (B) in combination with 20 μM T4 for the indicated times. Following the treatment window, cells were methanol fixed and stained for D2HY (red) and the ER marker ERp72 (green) with α-YFP and α-Erp72 antibodies, respectively. Nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 7.5 μm. C, Quantification of Pearson's coefficient between D2HY and ERp72 colocalization from panels A and B and quantification of Pearson's coefficient between D2 and KDEL colocalization after treatment with 20 μM T4 for 3 hours (panel D). *, P < .01 vs DMSO-treated group at time 0; $, P < .01 vs. MG132-treated group at time 0.
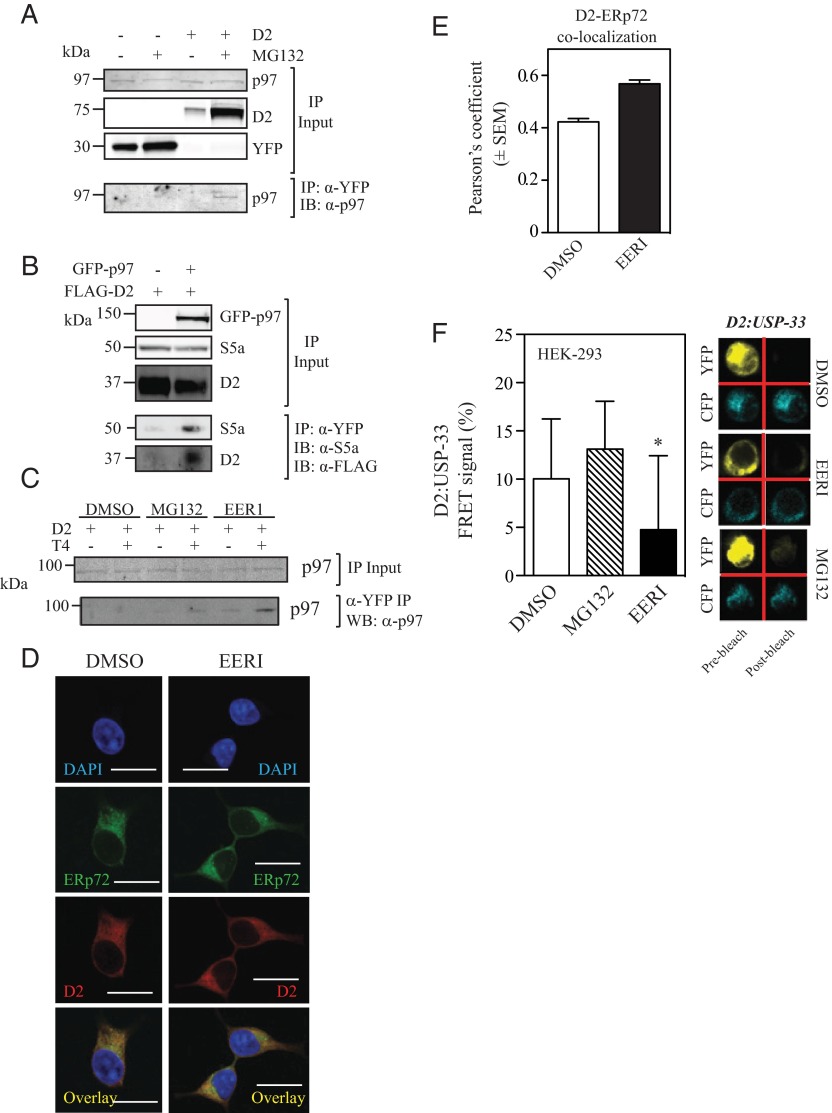
ER proteins containing K48-ubiquitin chains are typically retrotranslocated to the cytoplasm via the p97 (9, 33, 34). To test whether this was the case with D2, D2HY was pulled down, and p97 was identified in the D2 immunoprecipitates (Figure 5A). This was further confirmed with the reverse strategy, which led to finding D2 in p97 immunoprecipitates (Figure 5B). Notably, exposure to T4 also enhances D2-p97 coprecipitation, indicating that exposure to its substrate accelerates entry of UbD2 into the p97 pathway (Figure 5C).
Figure 5.
UbD2 interaction with the ATPase p97 blunts D2-USP33 binding. A, An aliquot of the same D2HY- or control-expressing samples treated with either vehicle or MG132 from Figure 1D were immunoprecipitated with an α-YFP and processed for Western blot analysis with an α-p97 antibody. B, HEK-293 cells transiently expressing a GFP-tagged p97 protein (GFP-p97) in combination with a FLAG-tagged D2 were subject to α-GFP immuprecipitation as in Figures 1 and 2 and analyzed by Western blot with α-S5a and α-FLAG antibodies. C, α-p97 Western blot of α-YFP immuprecipitates of HEK-293 cells expressing D2HY treated with vehicle or T4, and in combination with dimethylsulfoxide (DMSO), 1 μM MG132, or 10 μM EERI for 24 hours. D, HEK-293 cells stably expressing D2HY were treated with 10 μM EERI for 3 hours and methanol fixed and immunostained with α-ERp72 and α-YFP antibodies to detect the ER marker ERp72 (fluorescein isothiocyanate [FITC]) and D2 (Texas Red), respectively. Overlay is shown in yellow. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 13 μm. E, Pearson's coefficient of D2HY and ERp72 treated with DMSO or 10 μM EERI for 3 hours. Data are displayed as ±SEM. F, D2-USP-33 interaction in cells treated with either vehicle or 10 μM EERI for 24 hours as measured by FRET. Panel on the right shows representative scan images of YFP and CFP FRET signal of D2-USP-33 in cells treated with DMSO, EERI, or MG132. F, Data were acquired with at least 40 measurements and displayed as ±SD of % of control FRET signal, where * represents P < .05 by one-way ANOVA with Dunnett's post-test. IB, immunoblotting; WB, Western blot.
ERAD-specific DUBs such as Atx3 are physically associated with the retrotranslocation-driving ATPase p97, possibly facilitating substrate targeting to the proteasome. By shortening the Ub-chain of type I membrane proteins, Atx3 might allow the transfer of ubiquitinated proteins from the p97 complex to the proteasome system (35–37). Accordingly, exposure to the Atx3 inhibitor EERI did not affect D2-p97 interaction (Figure 5C) but resulted in the accumulation of much higher molecular weight forms of UbD2, within the 150- to 300-kDa-range (Figure 1D and Figure 2, D and E). Furthermore, in EER1-treated cells D2HY accumulated in the ER as evidenced by increased colocalization between D2HY and ERp72 (Figure 5, G and H), suggesting that Atx-3-mediated deubiquitination of UbD2 is key for p97-mediated retrotranslocation of UbD2.
Blocking the proteasomes leads to accumulation of UbD2 but also increases the level of nonubiquitinated D2HY protein (Figure 1, C and D) and D2HY catalytic activity (Figure 1F) as a result of D2-binding USPs that reactivate UbD2 (16). However, blocking p97-mediated retrotranslocation of UbD2 with EER1 led to accumulation of UbD2 without affecting the level of nonubiquitinated D2HY protein (Figure 1D) or D2HY catalytic activity (Figure 1F). This would suggest that deubiquitination of UbD2 by D2-binding USP is also impaired in EER1-treated cells. However, given that EER1 does not inhibit members of the USP family (37) or binding to p97 (Figure 5C), we hypothesized that the p97-bound UbD2 is not accessible to reactivation by USPs. To test this hypothesis, we used FRET to evaluate D2-USP-33 interaction (20) in cells expressing YFP-D2 and CFP-USP-33. Whereas MG132 does not affect D2-USP-33 interaction, treatment with EERI led to a significant loss in D2-USP-33 signal (Figure 5F).
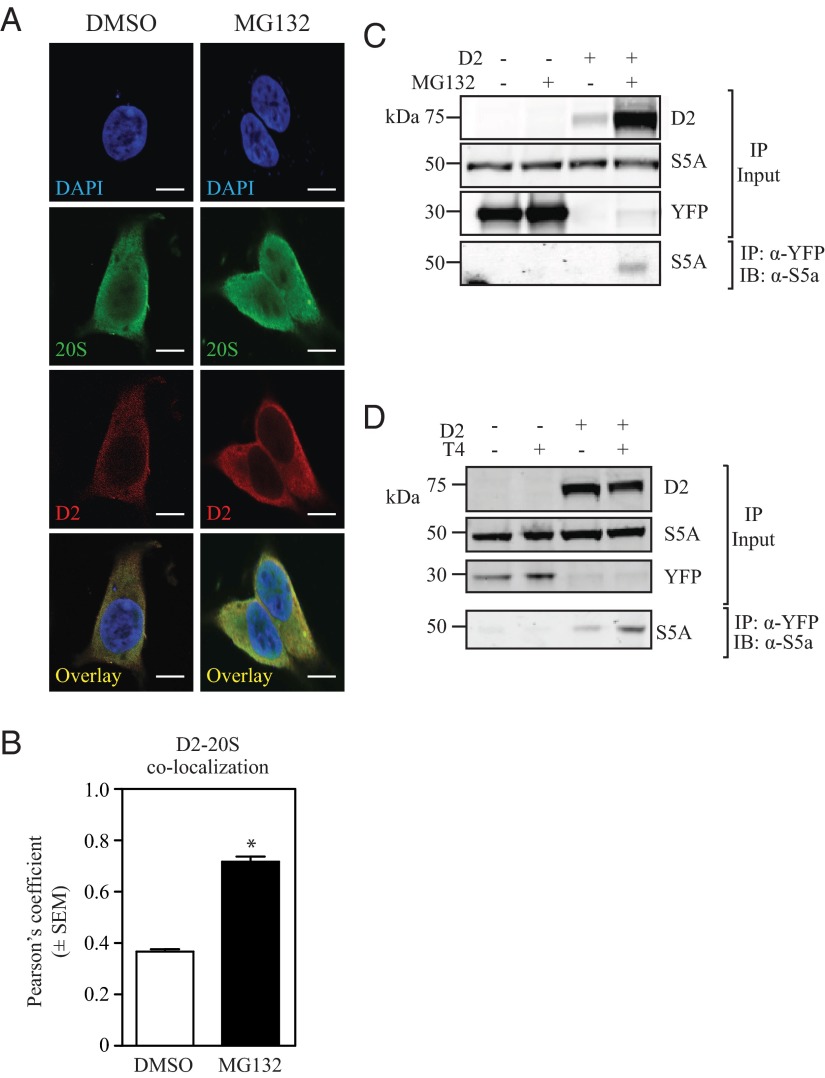
Retrotranslocated UbD2 is delivered to proteasomes in the cytoplasm
The buildup of D2HY outside the ER that follows proteasome blockade with MG132 (Figure 4, B–D) is associated with a marked increase in D2HY-20S colocalization (Figure 6, A and B). That D2 is eventually delivered to the proteasomes is supported by finding the 19S subunit S5a in p97 pull downs (Figure 5B) and in D2HY pull downs (Figure 6C). Most importantly, exposure to T4 increased the presence of S5a in the D2HY pull down (Figure 6D).
Figure 6.
UbD2 complexes colocalize with the 20S proteasome after exiting the ER. A, HEK-293 cells stably expressing D2HY were maintained with charcoal-stripped media and treated with dimethylsulfoxide (DMSO) or 1 μM MG132 for 24 hours and were paraformaldehyde (PFA)-fixed and stained for D2HY (Texas Red), the α- and β-subunit of the 20S proteasome chamber (fluorescein isothiocyanate [FITC]) with an α-YFP and α-20S (with α/β-subunits) antibodies, respectively. Nucleus was stained with DAPI. Scale bar, 7.5 μm. B, Quantification of Pearson's coefficient of conditions from panels A and B, where * represents P < .01 vs DMSO-treated group by Student's t test. C, HEK-293 cells transiently expressing either D2HY or YFP were treated with vehicle or 1 μM MG132 for 24 hours, immunoprecipitated with α-YFP antibody, and analyzed by Western blot with an α-S5a antibody. D, D2HY- or YFP-expressing HEK-293 cells were grown in charcoal-stripped medium and supplemented with vehicle or 20 μM T4, treated with 1 μM MG132 for 24 hours and processed by α-YFP IP followed by Western blot analysis with an α-S5a antibody. IB, immunoblotting.
Discussion
D2 plays critical roles in mammalian development (17, 38, 39), energy homeostasis (40–42), and hypothalamic-pituitary-thyroid axis feedback mechanism (43, 44). Here we used D2HY-expressing cells to show that UbD2 complexes are formed whereas D2 is naturally inserted in the ER (Figures 2A and 3A) and contain mainly K48-linked chains (Figure 2, B–E). UbD2 interacts with p97 (Figure 5, A and C) and is retrotranslocated to the cytoplasm (Figures 3A and 4A) and delivered to the proteasome system (Figure 5B) via a mechanism that involves Atx3-mediated deubiquitination of UbD2 (Figure 1, D and E, Figure 5D,and Figure 6). Retrotranslocation is greatly accelerated by T4 (Figure 2, D and E; Figure 4, A–D; and Figure 6D), indicating that, in addition to the intrinsic metabolic instability, interaction with its substrate and/or T4 catalysis makes D2 a better substrate for ubiquitination and proteasomal degradation.
The observation that D2 half-life is greatly extended in cells grown in media containing stripped serum suggests that D2 ubiquitination is minimal in the absence of its substrate, T4 (11, 12). In fact, D2 ubiquitination was thought to occur exclusively as a result of T4 catalysis, with binding of T4 to D2's active site changing D2's conformation and exposing K237 and K244 within the D2 molecule that could then be ubiquitinated (20). Thus, the intensity with which D2 is ubiquitinated independently of T4 is unexpected (Figure 2, D and E). That the catalytically inactive AlaD2 is K48-linked ubiquitinated as much as active D2 (Figure 2, H and I) and also accumulates in MG132-treated cells (Figure 2G), while not responding to T4-induced loss of D2 protein (32), is also unexpected. It is therefore clear that there is ongoing T4-independent D2 ubiquitination and deubiquitination. However, it is also clear that interaction with T4 accelerates D2 ubiquitination and retrotranslocation via the p97/Atx3 complex. This is indeed supported by 2 findings: 1) the pattern of K48-linked D2 ubiquitination is the same during exposure to T4 but the intensity of the bands is much greater (Figure 2, D and E), and 2) there is enhanced D2:p97 coimmunoprecipitation after exposure to T4 (Figure 5C).
In general, ER proteins containing K48-ubiquitin chains are pulled out of the ER via interaction with the ATPase p97, deubiquitinated, and delivered to the 26S proteasome (9, 33, 34). The present data indicate that D2 also follows this route given the observation that p97 coprecipitates with D2 when either one is used as bait (Figure 5, A and B). Notably, this interaction is also enhanced by exposure to T4 (Figure 5C). In turn, inhibition of Atx3 with EERI, which participates in the p97-mediated translocation of Ub-proteins, led to a massive accumulation of UbD2 and no increase in D2 activity, suggesting that UbD2 is at least partially deubiquitinated by Atx3 even as it remains bound to p97 and is delivered to the proteasomes. This is indeed supported by the finding of large amounts of nonubiquitinated D2 alongside UbD2 in the cytosol of MG132-treated cells (Figure 3A). Alternatively, it is also conceivable that when complexed with p97, UbD2 can no longer undergo DUB-mediated (USPs) deubiquitination and reactivation. The latter is supported by FRET studies, in which treatment with EERI caused a significant loss in D2-USP-33 interaction (Figure 5F). It is unlikely that these results stem from EERI-mediated inhibition of USP33 activity given that EERI inhibits members of the Machado-Josephin domain-class of DUBs such as Atx3, but not members of the USP class of DUBs (37).
After retrotranslocation, ubiquitinated substrates can be either targeted to cytosolic proteasomes or to proteasomes bound to the ER via interaction with the protein translocation channel Sec61 (22, 30). UbD2 seems to be retrotranslocated to the cytoplasm within 3 hours of exposure to T4, as suggested by confocal microscopy (Figure 4). This is also observed when the proteasomes are inhibited with MG132, with a relative decrease in the D2 colocalizing with ER markers and a relative increase in the amount of D2 that colocalizes with the proteasomes outside the ER (Figure 6, A and B). That D2's destination is the proteasomes was confirmed by 19S-resident ubiquitin receptor S5a (mammalian orthologue of yeast Rpn10) coprecipitation with D2 (Figure 6C), an interaction that was increased by exposure to T4 (Figure 6D). Furthermore, these observations were supported by confocal studies in which colocalization of D2 with the 20S proteasome subunit was markedly increase in cells treated with MG132 (Figure 6, A and B).
In conclusion, our studies have established that the ER-resident D2 is intrinsically unstable as it is continuously ubiquitinated via K48-linked ubiquitin chains and degraded in the proteasomes. UbD2 retrotranslocation to the cytoplasm and delivery to the proteasome is mediated by p97 and Atx3. Exposure of D2 to its substrate T4 accelerates D2 ubiquitination and consequently all downstream steps in this process up until proteasomal degradation. This mechanism illustrates how ubiquitination controls thyroid hormone signaling during development and ensures steady production of the active form of thyroid hormone T3 when T4 secretion is reduced such as during iodine deficiency.
Acknowledgments
We thank Nico Dantuma for the p97 vector, Barry Hudson for the pcDNA™4/ HisMax C vector, and László Barna (Nikon Microscopy Center) for his excellent technical help. We also thank the Nikon Microscopy Center at the Institute of Experimental Medicine Budapest, Nikon Austria GmbH, and Auro-Science Consulting Ltd for kindly providing microscopy support.
Current address for R.A.D.: Nanyang Technological University, Lee Kong Chian School of Medicine, Singapore.
This work was supported by The National Institutes of Health, the National Science Foundation of Hungary (OTKA K81226), and the European Community's Seventh Framework Programme (FP7/2007–2013; Grant 259772). Peter Egri is supported by the TÁMOP 4.2.4. A/2–11–1–2012–0001 “National Excellence Program-Elaborating and Operating an Inland Student and Researcher Personal Support System Convergence Program,” subsidized by the European Union and cofinanced by the European Social Fund.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Atx3
- ataxin-3
- CFP
- cyan fluorescent protein
- CSS
- charcoal-stripped serum
- D2
- type II iodothyronine deiodinase
- DUB
- D2-interacting deubiquitinase
- ECFP
- enhanced CFP
- EERI
- eeyarestatin I
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- EYFP
- enhanced YFP
- FBS
- fetal bovine serum
- FRET
- fluorescent resonance energy transfer
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- IP
- immunoprecipitation
- UbD2
- D2 ubiquitination
- USP
- ubiquitin-specific peptidase.
References
- 1. Ciehanover A, Hod Y, Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978;81:1100–1105 [DOI] [PubMed] [Google Scholar]
- 2. Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci USA. 1980;77:1783–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229 [DOI] [PubMed] [Google Scholar]
- 4. Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205 [DOI] [PubMed] [Google Scholar]
- 5. Chau V, Tobias JW, Bachmair A, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583 [DOI] [PubMed] [Google Scholar]
- 6. Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563 [DOI] [PubMed] [Google Scholar]
- 7. Curcio-Morelli C, Gereben B, Zavacki AM, et al. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology. 2003;144:937–946 [DOI] [PubMed] [Google Scholar]
- 8. Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–4312 [DOI] [PubMed] [Google Scholar]
- 9. Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim Biophys Acta. 2012;1823:117–124 [DOI] [PubMed] [Google Scholar]
- 10. Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012;151:1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Botero D, Gereben B, Goncalves C, De Jesus LA, Harney JW, Bianco AC. Ubc6p and ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol Endocrinol. 2002;16:1999–2007 [DOI] [PubMed] [Google Scholar]
- 12. Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol. 2000;14:1697–1708 [DOI] [PubMed] [Google Scholar]
- 13. Kim BW, Zavacki AM, Curcio-Morelli C, et al. Endoplasmic reticulum-associated degradation of the human type 2 iodothyronine deiodinase (D2) is mediated via an association between mammalian UBC7 and the carboxyl region of D2. Mol Endocrinol. 2003;17:2603–2612 [DOI] [PubMed] [Google Scholar]
- 14. Zavacki AM, Arrojo E Drigo R, Freitas BC, et al. The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol Cell Biol. 2009;29:5339–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravid T, Kreft SG, Hochstrasser M. Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J. 2006;25:533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curcio-Morelli C, Zavacki AM, Christofollete M, et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest. 2003;112:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dentice M, Bandyopadhyay A, Gereben B, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeöld A, Pormüller L, Dentice M, et al. Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J Biol Chem. 2006;281:31538–31543 [DOI] [PubMed] [Google Scholar]
- 19. Arrojo E Drigo R, Fonseca TL, Castillo M, et al. Endoplasmic reticulum stress decreases intracellular thyroid hormone activation via an eIF2a-mediated decrease in type 2 deiodinase synthesis. Mol Endocrinol. 2011;25:2065–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sagar GD, Gereben B, Callebaut I, et al. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol. 2007;27:4774–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tresse E, Salomons FA, Vesa J, et al. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system [published online September 1, 2013]. Cold Spring Harb Perspect Biol. doi:10.1101/chperspect.a013185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gereben B, Bartha T, Tu HM, Harney JW, Rudas P, Larsen PR. Cloning and expression of the chicken type 2 iodothyronine 5′-deiodinase. J Biol Chem. 1999;274:13768–13776 [DOI] [PubMed] [Google Scholar]
- 24. Nunes MT, Bianco AC, Migala A, Agostini B, Hasselbach W. Thyroxine induced transformation in sarcoplasmic reticulum of rabbit soleus and psoas muscles. Z Naturforsch C. 1985;40:726–734 [DOI] [PubMed] [Google Scholar]
- 25. Jo S, Kalló I, Bardóczi Z, et al. Neuronal hypoxia induces hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J Neurosci. 2012;32:8491–8500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzarella RA, Srinivasan M, Haugejorden SM, Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990;265:1094–1101 [PubMed] [Google Scholar]
- 27. Cicchetti G, Biernacki M, Farquharson J, Allen PG. A ratiometric expressible FRET sensor for phosphoinositides displays a signal change in highly dynamic membrane structures in fibroblasts. Biochemistry. 2004;43:1939–1949 [DOI] [PubMed] [Google Scholar]
- 28. Curcio C, Baqui MM, Salvatore D, et al. The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J Biol Chem. 2001;276:30183–30187 [DOI] [PubMed] [Google Scholar]
- 29. Berry MJ, Maia AL, Kieffer JD, Harney JW, Larsen PR. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992;131:1848–1852 [DOI] [PubMed] [Google Scholar]
- 30. Kalies KU, Allan S, Sergeyenko T, Kröger H, Römisch K. The protein translocation channel binds proteasomes to the endoplasmic reticulum membrane. EMBO J. 2005;24:2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newton K, Matsumoto ML, Wertz IE, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678 [DOI] [PubMed] [Google Scholar]
- 32. Steinsapir J, Bianco AC, Buettner C, Harney J, Larsen PR. Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme's active center. Endocrinology. 2000;141:1127–1135 [DOI] [PubMed] [Google Scholar]
- 33. Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162:71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fiebiger E, Hirsch C, Vyas JM, Gordon E, Ploegh HL, Tortorella D. Dissection of the dislocation pathway for type I membrane proteins with a new small molecule inhibitor, eeyarestatin. Mol Biol Cell. 2004;15:1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cross BC, McKibbin C, Callan AC, et al. Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J Cell Sci. 2009;122:4393–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem. 2008;283:7445–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall JA, Ribich S, Christoffolete MA, et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151:4573–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castillo M, Hall JA, Correa-Medina M, et al. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60:1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carvalho SD, Kimura ET, Bianco AC, Silva JE. Central role of brown adipose tissue thyroxine 5′-deiodinase on thyroid hormone-dependent thermogenic response to cold. Endocrinology. 1991;128:2149–2159 [DOI] [PubMed] [Google Scholar]
- 42. Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 1987;79:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christoffolete MA, Ribeiro R, Singru P, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743 [DOI] [PubMed] [Google Scholar]
- 44. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148 [DOI] [PubMed] [Google Scholar]