Abstract
Gastrointestinal (GI) bleeding from the colon is a common reason for hospitalization and is becoming more common in the elderly. While most cases will cease spontaneously, patients with ongoing bleeding or major stigmata of hemorrhage require urgent diagnosis and intervention to achieve definitive hemostasis. Colonoscopy is the primary modality for establishing a diagnosis, risk stratification, and treating some of the most common causes of colonic bleeding, including diverticular hemorrhage which is the etiology in 30 % of cases. Other interventions, including angiography and surgery, are usually reserved for instances of bleeding that cannot be stabilized or allow for adequate bowel preparation for colonoscopy. We discuss the colonoscopic diagnosis, risk stratification, and definitive treatment of colonic hemorrhage in patients presenting with severe hematochezia.
Keywords: Colonic hemorrhage, Colonoscopy, Hemostasis, Hemoclip, Thermal probe, Diverticulosis, Colitis, Vascular ectasia, Neoplasm, Hemorrhoid, Ulcer, Angiography
Introduction
Acute colonic bleeding (or lower GI bleeding)—defined as that occurring from the colon, rectum, or anus, and presenting as either hematochezia (bright red blood, clots or burgundy stools) or melena—has an annual incidence of hospitalization of approximately 36/100,000 population, about half of that for upper GI bleeding. The rate of hospitalization is even higher in the elderly [1]. Patients usually present with painless hematochezia and a decrease in hematocrit value, but without orthostasis.
Most cases of acute colonic bleeding will stop spontaneously, thereby allowing non-urgent evaluation. However, for patients with severe hematochezia, defined as continued bleeding within the first 24 h of hospitalization with a drop in the hemoglobin of at least 2 g/dL and/or a transfusion requirement of at least 2 units of packed red blood cells, urgent diagnosis and intervention are required to control the bleeding. Clinical factors predictive of severe colonic bleeding include aspirin use, at least two comorbid illnesses, pulse greater than 100/minute, and systolic blood pressure <115 mmHg [2]. The overall mortality rate from colonic bleeding is 2.4–3.9 % [1, 3]. Independent predictors of inhospital mortality are age over 70 years, intestinal ischemia, and two or more comorbidities [3].
This paper reviews the evaluation and management of severe colonic bleeding and hematochezia, including the initial management, differential diagnosis, and diagnostic and therapeutic modalities. Emphasis will be placed on the role of colonoscopy in this setting.
Initial Assessment and Management
Patients presenting with GI bleeding should undergo a directed history and physical examination to look for clues that suggest whether the bleeding source is in the upper tract, colon, or possibly the small bowel, as well as a possible etiology for the hemorrhage. Important historical points to assess include: abdominal pain and weight loss (non-specific, but may suggest inflammatory bowel disease, ischemia, and/or malignancy), medication use (non-steroidal anti-inflammatory drugs—NSAIDS—and other medications that can cause ulcers or intestinal ischemia), recent colonoscopy with polypectomy (post-polypectomy bleed), prior abdominal/pelvic radiation (radiation proctitis/colitis), prior operations (possible anastomotic ulcers), or a history of abdominal aortic aneurysm with or without surgical repair (possible aorto-enteric fistula). A history of alcoholism or chronic liver disease raises the suspicion for bleeding due to portal hypertension, such as varices. The manner in which the patient with bleeding presents can also suggest potential etiologies. Bright red blood is more often seen from ano-rectal and distal colonic sources, but brisk upper GI bleeding can also manifest this way. Painless severe bleeding with clots is more common with diverticular hemorrhage. Bloody diarrhea often occurs with ischemic and inflammatory colitides.
The most important component of the physical exam is the vital signs, including orthostatic measurements. This can help with assessment of vascular volume status, the severity of the hemorrhage, and the need for aggressive volume resuscitation. Other aspects of the examination may be helpful for determining the potential cause of the bleeding, such as stigmata of liver disease that may suggest underlying cirrhosis and portal hypertension. Because about 15 % of hematochezia cases are due to an upper GI source, a nasogastric lavage should be performed in all patients with significant hematochezia, who do not have hematemesis, to assess for blood in the upper GI tract that would warrant an upper endoscopy [4–6]. Similarly, while melena typically indicates bleeding from a foregut location, it may result from bleeding from the small intestine or the right colon, particularly with slow GI bleeding or slow GI transit.
Resuscitation should take place during the course of the initial assessment. The vital signs are essential to determining the degree of volume repletion needed, including assessment of orthostatic changes. Isotonic crystalloid solutions (such as normal saline) should be infused in bolus fashion to maintain a systolic blood pressure of >100 mmHg. This threshold may be lower in some patients with certain comorbidities, such as cirrhosis or those on drugs such as beta-blockers. Blood products should be infused as soon as available. The hemoglobin (Hb) threshold for transfusion of packed red blood cells generally should be 9–10 g/dL, depending on cardiovascular comorbidities. A recent study of blood transfusions for upper GI bleeding found that a more restrictive transfusion threshold of 7 g/dL led to a significantly higher 6-week survival rate and lower rebleeding rate in non-cirrhotic, low-risk patients when compared to a threshold of 9 g/dL. This study, however, excluded high-risk patients and those with colonic bleeding, so it is unclear whether these results would carry over to colonic bleeding scenarios [7•]. The platelet count should be kept above 50,000/mm3 and the international normalized ratio (INR) should be corrected to 1.5 or less.
Most patients have self-limited bleeding and can be evaluated more electively (e.g., after 24 h). Patients with recurrent bleeding, hemodynamic changes, and significant comorbid conditions should be hospitalized and evaluated urgently (e.g., in 12–18 h). Although most hospitalized patients can be admitted to a floor or non-intensive care unit (ICU)-monitored bed, those with shock, hypotension, significant comorbidities, or severe bleeding should initially go the ICU where closer monitoring and more aggressive resuscitation can occur.
Colonic Bleeding Causes
Table 1 lists the most common causes of colonic bleeding, excluding upper GI sources, according to the UCLA-Center for Ulcer Research and Education (CURE) database. The frequencies of these different etiologies are similar to reports from other large centers [8]. Colonic diverticulosis continues to be the most common cause, accounting for about 30 % of lower GI bleeding cases requiring hospitalization. Internal hemorrhoids are the second-most common cause. Ischemic colitis and post-polypectomy bleeding are increasing in frequency, likely due to an increase in medical comorbidities and anti-platelet/anticoagulant use [9]. Our approach to patients hospitalized with severe hematochezia is summarized in Fig. 1.
Table 1.
Causes of lower GI bleeding
| Diagnosis | Frequency (%) |
|---|---|
| Diverticulosis | 30 |
| Hemorrhoids | 14 |
| Ischemic | 12 |
| IBD | 9 |
| Post-polypectomy | 8 |
| Colon cancer/polyps | 6 |
| Rectal ulcer | 6 |
| Vascular ectasia | 3 |
| Radiation colitis/proctitis | 3 |
| Other | 6 |
Source: UCLA-CURE Hemostasis Research Group database
IBD inflammatory bowel disease
Fig. 1.
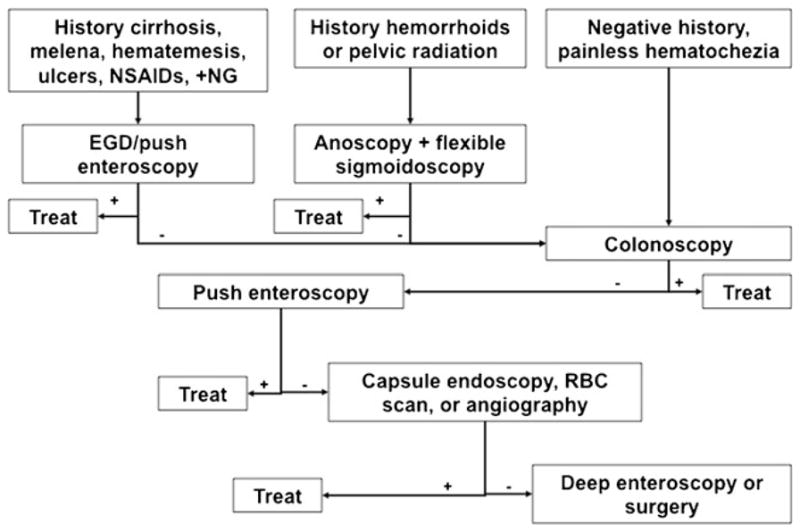
CURE Hemostasis Research Group diagnostic approach to patients hospitalized with severe hematochezia. NSAIDs non-steroidal anti-inflammatory drugs, NG nasogastric lavage, EGD esophagogastroduodenoscopy, RBC red blood cell
Diagnostic and Therapeutic Modalities
Colonoscopy and Hemostasis
Urgent colonoscopy following a rapid bowel purge has been shown to be safe, provide important diagnostic information, and allow therapeutic intervention. Because of these features, colonoscopy generally should be the initial modality used for evaluating acute colonic bleeding or severe hematochezia requiring hospitalization. Because most bleeding stops spontaneously, colonoscopy often is performed semi-electively, 24 h or more after initial hospitalization, to allow the patient to receive blood transfusions and the bowel preparation on the first day of hospitalization. However, the optimal time for performing urgent bowel preparation and colonoscopy is controversial. Early colonoscopy (soon after admission) has been associated with a shorter length of hospitalization, principally because of improved diagnostic yield [10]. Our group has reported that urgent colonoscopy after adequate cleansing of the colon with a bowel purge is more cost effective and associated with higher diagnostic yield than other strategies [11].
Colonoscopic intervention for diverticular hemorrhage appears to be effective for definitive, local therapy. In a landmark study by the UCLA-CURE Hemostasis research group, no patient who underwent colonoscopic treatment rebled (with 3 years of follow-up), compared to 53 % for historical controls who had stigmata of recent hemorrhage, but did not undergo hemostasis [6]. Similar results have been reported elsewhere, although surgery was required in some patients after endoscopic hemostasis [12].
Stratifying the risk of recurrent diverticular bleeding, by applying the same endoscopic stigmata used in high-risk peptic ulcer bleeding (active bleeding, visible vessel, and clot), has been attempted, but the natural history associated with each of these stigmata is underreported. Recently, our group reported that patients with major stigmata of hemorrhage have a worse prognosis, when managed medically, than those with similar stigmata due to peptic ulcers. Among 37 patients with stigmata of recent diverticular hemorrhage (active bleeding in 18, visible vessel in 5, and adherent clot in 14) who did not undergo endoscopic therapy, the rates of rebleeding and emergency surgery were 65 and 43 %, respectively [13••].
Compared to the clinical outcomes of patients managed medically with major stigmata of diverticular hemorrhage, 63 similar patients had very good results after colonoscopic hemostasis with the techniques described by the CURE Hemostasis Research Group [6, 11, 14, 15]. Medically treated patients with these major stigmata of diverticular bleeding had a 30-day rebleeding rate of 65 % (vs. 4.8 % for the colonoscopic hemostasis group). This represents a clinically significant reduction in the rebleeding rate and forms the basis for recommending a major change in the definitive diagnosis and treatment of diverticular hemorrhage. This moves away from the previous practice of angiography or colonic surgical resection to urgent colonoscopy [16]. It is also reported to be more cost effective [10, 11, 13••].
Treatment options for bleeding diverticula are similar to those for peptic ulcer bleeding. What is used depends on the specific stigmata and the location of the bleeding in the diverticulum. If there is active bleeding or a pigmented protuberance at the edge of the diverticulum, we recommend injecting epinephrine (diluted 1:20,000 in saline) in 1-mL aliquots submucosally into four quadrants around the bleeding site. Subsequently, a thermal probe can be applied at a low power setting (10–15 W) for 1–2 s to cauterize the diverticular edge and stop bleeding or flatten the vessel. Alternatively, endoscopic clips (hemoclips) may be applied over the bleeding site or vessel after epinephrine has been injected. The choice between using a thermal probe and hemoclips may be influenced by the degree of difficulty deploying the hemoclips based on position of the bleeding site, as well as comorbid factors or medications such as anticoagulants or antiplatelet agents that may increase the risk of bleeding after cautery. A non-bleeding adherent clot should be injected with epinephrine in a similar manner and the clot removed piecemeal by cold guillotining with a rotatable polyp snare until a ≤3- to 4-mm pedicle is left above the diverticulum. Afterward, the underlying stigma should be treated with a thermal probe or hemoclips as described above.
If the stigma of diverticular hemorrhage is in the base of the diverticulum, hemoclips (with or without epinephrine injection) are be more appropriate due to the greater risk for perforation secondary to thermal injury with a probe. Once hemostasis has been achieved, a submucosal tattoo should be placed around the lesion to allow identification of the site in case repeat colonoscopy or surgery is required for signs or symptoms of recurrent bleeding.
There have been recent studies from Japan suggesting that endoscopic band ligation may be a safe and effective therapy for diverticular bleeding. This technique involves initial localization, marking the site with a hemoclip, withdrawal and placement of a band ligation device onto the colonoscope, relocating the target diverticulum, inverting it with suction into the cap, and subsequent placement of the band. These studies report similar or lower rebleeding rates than with hemoclip placement and their benefit may be most pronounced in the right colon [17, 18]. However, this technique requires two colonoscopies and is not deemed to be as convenient or effective, particularly in Western patients, compared to hemostasis with thermal probes or hemoclips [16].
Flexible Sigmoidoscopy and Hemostasis
Flexible sigmoidoscopy can evaluate the rectum and left side of the colon and can be done with enemas or even in an unprepped colon. This may be an appropriate initial diagnostic (and possibly therapeutic) modality in younger patients whose bleeding is highly suggestive of a distal colon and/or rectal source. However, patients who are of an appropriate age for colorectal cancer and polyp screening, and those who have never had a prior colonoscopy, should have a full colonoscopic evaluation even if the bleeding source is likely to be more distal. Flexible sigmoidoscopy can be useful in patients suspected of having a solitary rectal ulcer, ulcerative colitis, radiation proctitis, infectious colitis, ischemic colitis, post-polypectomy bleeding in cases of polyp(s) removed only from the rectum and/or left colon, or internal hemorrhoids.
Therapeutic hemostasis can be performed depending on the etiology for bleeding. Rectal ulcers and post-polypectomy sites with high-risk stigmata of recent hemorrhage can be treated with epinephrine pre-injection (for active bleeding or adherent clots) and either hemoclips or thermal probe, similar to treating peptic ulcers. Monopolar electrocautery (i.e., snare polypectomy, argon plasma coagulation, hot biopsy forceps) should not be used in a poorly prepped colon to avoid the risk of explosion related to flammable colonic gas [19]. Rubber band ligation is commonly used to treat bleeding due to internal hemorrhoids, although thermal probe and sclerotherapy have also been used [20].
Anoscopy
Anoscopy with a slotted instrument can be useful in patients in whom actively bleeding internal hemorrhoids or other ano-rectal disorders, such as fissures, are suspected. This will facilitate either immediate treatment with rubber band ligation or medical treatment. Most hospitalized patients, however, will also require colonoscopy to evaluate the remainder of the colon.
Radionuclide Imaging
Radionuclide imaging in GI bleeding involves labeling erythrocytes with technetium-99 m and then performing serial scintigraphy (also known as a tagged red blood cell scan) to detect focal collections of radiolabeled material. It can be performed relatively quickly and may help localize the general area of bleeding to guide subsequent endoscopy, angiography, or surgery. Radionuclide scintigraphy has been reported to detect bleeding at a rate as low as 0.04 mL/min. The overall rate of a positive scan is approximately 50 %, with a 66 % accuracy rate in the localization of the true bleeding site, but up to 25 % of bleeding scans suggest a site of bleeding that proves to be incorrect [21, 22]. The most common reason for a false-positive result is rapid transit of luminal blood, so that labeled blood is detected in the colon even though it originated from a more proximal site in the GI tract. Caution is recommended in utilizing the results of delayed scans to localize and target lesions for resective surgery without confirmation from other tests.
Some of the major limitations of scintigraphy are its lack of therapeutic capabilities, that only active bleeding can be detected (rather than other non-bleeding stigmata), and that an etiologic diagnosis is not possible. Therefore, this modality should be viewed as having a complementary role in the evaluation of colonic or small bowel bleeding. In cases of severe colonic bleeding, it may be helpful when recurrent bleeding is presumed to be from colonic diverticula, yet repeated colonoscopic evaluations have failed to identify the bleeding diverticulum. Because it cannot provide for treatment, a positive study should be followed shortly thereafter by mesenteric angiography [22].
Angiography
The main advantage of mesenteric angiography, compared to radionuclide imaging, is its interventional capability. However, the bleeding rate must be at least 0.5 mL/min to detect extravasation into the gut, which is significantly higher than radionuclide imaging. The other disadvantages of radionuclide imaging, the inability to detect non-active bleeding stigmata or to make an etiologic diagnosis, also occur with angiography. Additionally, certain patient factors (such as contrast dye allergy and acute/chronic kidney disease) are potential contraindications to angiography. The diagnostic yield of angiography depends on patient selection, the timing of the procedure, and the skill of the angiographer, with positive results in 10–70 % of cases. Previously, angiographic intervention involved injection of vasoconstrictors to control bleeding in preparation for surgery. However, thanks to technological advances, super-selective embolization of distal arterial branches is now possible. A review of several studies using angiographic embolization for colonic bleeding found that immediate hemostasis was achieved in 96 %, with 22 % experiencing early rebleeding, 26 % experiencing minor complications that did not require surgery, and 17 % experiencing major complications that required surgery or resulted in death [22]. Major complications include bowel ischemia, hematoma formation, femoral artery thrombosis, contrast dye reactions, acute kidney injury, and transient ischemic attacks [23].
Angiography may be bested suited for patients with massive hematochezia that cannot be stabilized to permit adequate bowel preparation and performance of colonoscopy or deep enteroscopy. Depending on the severity of the hemorrhage, it may be preceded by a tagged red blood cell scan or capsule endoscopy for localization of the bleeding site and to help direct the interventional radiologist where to focus contrast injection [24].
Surgery
Surgical management for lower GI bleeding is infrequently necessary since most bleeding is either self-limited or readily managed with medical or colonoscopic therapy. The main indications include malignancy, diffuse bleeding that fails to cease with medical therapy (as in ischemic or ulcerative colitis), and recurrent bleeding from a diverticulum. Every effort should be made to localize the bleeding in order to minimize excessive bowel resection or prevent resection of the incorrect bowel segment.
Conclusions
Because of the broad differential diagnosis for hematochezia, taking a careful medical and surgical history is mandatory to guide the subsequent evaluation. Based on its favorable safety profile, as well as diagnostic and therapeutic capabilities, colonoscopy is the preferred modality for managing patients with severe hematochezia and suspected colonic hemorrhage. Urgent colonoscopy has been reported to increase the diagnostic yield and treatment of bleeding stigmata, as well as reduce the rebleeding rate. While most cases of colonic bleeding can be diagnosed endoscopically and treated appropriately, physicians should be able to recognize the situations when alternatives such as radionuclide imaging, angiographic, or surgical management are indicated.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Kevin A. Ghassemi and Dennis M. Jensen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Kevin A. Ghassemi, Email: kghassemi@mednet.ucla.edu, Division of Digestive Diseases, Center for Esophageal Disorders, David Geffen School of Medicine at UCLA, 100 UCLA Medical Plaza, Suite 700, Los Angeles, CA 90095, USA
Dennis M. Jensen, Email: djensen@mednet.ucla.edu, Departments of Medicine at UCLA and West Los Angeles VA Medical Centers and CURE Digestive Diseases Research Center, David Geffen School of Medicine at UCLA, CURE Hemostasis Research Unit- Bldg 115, Rm 318 11301 Wilshire Blvd, Los Angeles, CA 90073-1003, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190–5. doi: 10.1038/ajg.2012.168. [DOI] [PubMed] [Google Scholar]
- 2.Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838–43. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 3.Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6:1004–10. doi: 10.1016/j.cgh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuckerman GR, Trellis DR, Sherman TM, Clouse RE. An objective measure of stool color for differentiating upper from lower gastrointestinal bleeding. Dig Dis Sci. 1995;40:1614–21. doi: 10.1007/BF02212679. [DOI] [PubMed] [Google Scholar]
- 5.Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia - the role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569–74. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 6.Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 7•.Villanueva C, Colomo A, Bosch A. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;361:11–21. doi: 10.1056/NEJMoa1211801. Study suggesting that lower-risk patients may not need to be transfused with red blood cells as aggressively as previously thought. This study only evaluated upper GI bleeding patients, however. [DOI] [PubMed] [Google Scholar]
- 8.Gayer C, Chino A, Lucas C, et al. Acute lower gastrointestinal bleeding in 1,112 patients admitted to an urban emergency medical center. Surgery. 2009;146:600–7. doi: 10.1016/j.surg.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Chavalitdhamrong D, Jensen DM, Kovacs TOG, et al. Ischemic colitis is a common cause of severe hematochezia and patient outcomes are worse than with other colonic diagnoses. Gastrointest Endosc. 2011;74:852–7. doi: 10.1016/j.gie.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strate LL, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003;98:317–22. doi: 10.1111/j.1572-0241.2003.07232.x. [DOI] [PubMed] [Google Scholar]
- 11.Jensen DM, Machicado GA. Colonoscopy for diagnosis and treatment of severe lower gastrointestinal bleeding: routine outcomes and cost analysis. Gastrointest Endosc Clin N Am. 1997;7:477–98. [PubMed] [Google Scholar]
- 12.Bloomfeld RS, Rockey DC, Shetzline MA. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol. 2001;96:2367–72. doi: 10.1111/j.1572-0241.2001.04048.x. [DOI] [PubMed] [Google Scholar]
- 13••.Jensen DM, Ohning GV, Kovacs TOG, et al. Natural history of definitive hemorrhage based upon stigmata of recent hemorrhage and Doppler blood flow monitoring. Gastroenterology. 2012;142:S-577–8. Large case series report showing that stigmata of hemorrhage associated with diverticula have a poorer prognosis compared to those of peptic ulcer hemorrhage. [Google Scholar]
- 14.Jensen DM, Ohning GV, Kovacs, et al. How to find, diagnose and treat definitive diverticular hemorrhage during urgent colonoscopy in patients with severe hematochezia: results & outcomes of a large prospective study. Gastrointest Endosc. 2012;75:AB179. [Google Scholar]
- 15.Jensen DM, Ohning GV, Kovacs TO, et al. Natural history of definitive diverticular hemorrhage based upon stigmata of recent hemorrhage & Doppler blood flow monitoring. Gastrointest Endosc. doi: 10.1016/j.gie.2015.07.033. In press for DDW 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen DM. The ins and outs of diverticular bleeding. Gastrointest Endosc. 2012;75:388–91. doi: 10.1016/j.gie.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Setoyama T, Ishii N, Fujita Y. Endoscopic band ligation (EBL) is superior to endoscopic clipping for the treatment of colonic diverticular hemorrhage. Surg Endosc. 2011;25:3574–8. doi: 10.1007/s00464-011-1760-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishii N, Setoyama T, Deshpande GA, et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. 2012;75:382–7. doi: 10.1016/j.gie.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Manner H, Plum N, Pech O, et al. Colon explosion during argon plasma coagulation. Gastrointest Endosc. 2008;67:1123–7. doi: 10.1016/j.gie.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Jutabha R, Jensen DM, Chavalitdhamrong D. Randomized prospective study of endoscopic rubber band ligation compared with bipolar coagulation for chronically bleeding internal hemorrhoids. Am J Gastroenterol. 2009;104:2057–64. doi: 10.1038/ajg.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olds GD, Cooper GS, Chak A, et al. The yield of bleeding scans in acute lower gastrointestinal hemorrhage. J Clin Gastroenterol. 2005;39:273–7. doi: 10.1097/01.mcg.0000155131.04821.f3. [DOI] [PubMed] [Google Scholar]
- 22.Strate LL, Naumann CR. The role of colonoscopy and radiologic procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010;8:333–43. doi: 10.1016/j.cgh.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Silver A, Bendick P, Wasvary H. Safety and efficacy of superselective angioembolization in control of lower gastrointestinal hemorrhage. Am J Surg. 2005;189:361–3. doi: 10.1016/j.amjsurg.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Camus M, Jensen DM, Ohning GV, et al. Urgent capsule endoscopy for bleeding site localization & lesion diagnosis of patients with severe hematochezia. Gastrointest Endosc. In press for DDW 2013. [Google Scholar]


