Abstract
Taurine (2-aminoethanesulfonic acid) is a β-amino acid found in many tissues particularly brain, myocardium, and kidney. It plays several physiological roles including cardiac contraction, antioxidation, and blunting of hypertension. Though several lines of evidence indicate that dietary taurine can reduce hypertension in humans and in animal models, evidence that taurine supplementation reduces hypertension in humans has not been conclusive. One reason for the inconclusive nature of past studies may be that taurine having both positive and negative effects on cardiovascular system depending on when it is assessed, some effects may occur early, while others only appear later. Further, other consideration may play a role, e.g., taurine supplementation improves hypertension in spontaneously hypertensive rats on a low salt diet but fails to attenuate hypertension on a high salt diet. In humans, some epidemiologic studies indicate that people with high taurine and low salt diets display lower arterial pressure than those with low taurine and high salt diets. Differences in techniques for measuring arterial pressure, duration of treatment, and animal models likely affect the response in different studies. This review considers both the positive and negative effects of taurine on blood pressure in animal models and their applications for human interventions.
Keywords: Arterial pressure, Circadian rhythm, Hypertension, Spontaneously hypertensive rat, NaCl, Taurine
Core tip: Many reports indicate that dietary taurine can reduce hypertension in humans and in animal models; however, the hypotensive effect of taurine supplementation depends on many factors. Taurine supplementation improves hypertension in spontaneously hypertensive rats on a low salt diet but fails to attenuate hypertension on a high salt diet. In humans, some epidemiologic studies suggest that people with high taurine and low salt diets display lower arterial pressure than those with low taurine and high salt diets. This review considers both positive and negative effects of taurine on blood pressure in animal models of hypertension to apply for human interventions.
INTRODUCTION
Hypertension is a risk factor for both acute and chronic adverse diseases, including stroke and cardiovascular disease[1,2]. Arterial blood pressure displays a diurnal variation, i.e., it is elevated during active behavior periods and decreased during quiescent periods (e.g., sleep)[3]. Thus, in humans, arterial pressure typically increases during the daytime and decreases during the nighttime[4]. In contrast, rats are generally nocturnal animals, and thus their diurnal rhythm is reversed, i.e., their arterial pressures are elevated at night during active behavior, and decreased during the daytime[5]. Established hypertension is associated with decreased amplitude of diurnal arterial pressure variation, in that arterial pressure fails to decrease in the non-active period (e.g., sleep), especially in older adults. In contrast, during the development of hypertension, the arterial pressure amplitude is typically greater than normal. Spontaneously hypertensive rats (SHR) display a circadian rhythm that is directly correlated with activity. However, in adult SHR compared to other strains, arterial pressure declines slowly in the morning (as the animals begin to sleep) and remains in the hypertensive range throughout the sleep period (mean arterial pressure > 110 mmHg)[5,6]. Further, high NaCl diets initially increase arterial pressure in the nighttime and more slowly increase daytime arterial pressure in both SHR and Wistar Kyoto rats (WKY; a normotensive control for SHR). This is especially evident after four nights of high NaCl treatment[5]. In addition, in SHR, high NaCl diets significantly increase daytime arterial pressure after a week of feeding, but they have little effect at that time point on daytime arterial pressures of normotensive WKY[5,7]. In the SHR on either a high or basal NaCl diet, sympathetic blockade greatly decreases arterial pressure rhythm, suggesting that the sympathetic nervous system contributes significantly to SHR hypertension, especially during its development[8,9].
Taurine (2-aminoethanesulfonic acid) is a non-protein, free amino acid found in many tissues particularly brain, myocardium, liver, muscle, and kidney[10-12]. Several lines of evidence indicate that dietary taurine can reduce hypertension in humans and in animal models[13]. For examples, dietary taurine attenuates hypertension in adult SHR[14] and deoxycorticosterone acetate and high NaCl (DOCA-NaCl) rats[15]. Sugar-induced hypertension can also be greatly blunted by dietary taurine and exacerbated by taurine deficiency[16]. Epidemiological studies indicate that people consuming high taurine diets display a low incidence of hypertension and other cardiovascular diseases[17]. Taurine supplementation was also reported to decrease systolic and diastolic blood pressure in young patients with borderline hypertension[18], but not in healthy men[19]. In addition, perinatal taurine exposure affects adult susceptibility to sugar-induced hypertension in rats[20-25].
De novo taurine synthesis is limited in rats and humans; therefore, dietary taurine is needed to maintain taurine in the body, which is especially important during developmental periods[11,12]. Intestinal taurine absorption is via high affinity sodium chloride-dependent active transport[26]. Thus, a high luminal sodium concentration accelerates intestinal taurine absorption, and high taurine transport increases sodium absorption into the blood. This complex relation has been suggested as the reason that in previous studies, taurine supplementation did not prevent NaCl-induced hypertension in SHR[27]. There are no experiments examining the effect of taurine on 24-h arterial pressure in animal models, and such information could elucidate the mechanisms underlying the failure of taurine to reduce arterial pressure in these models. This article reviews the advantage and limitation of taurine’s antihypertensive action based on 24-h arterial pressure monitoring data in SHR.
24-H ARTERIAL PRESSURE DATA
In 1978, Nara et al[14] demonstrated that dietary taurine decreases hypertension in SHR. This finding is later supported by both experimental and epidemiological studies[13,17]. The effective dose of taurine has been between 1%-5% in drinking water for most of animal models of hypertension, and the duration of treatment has usually been more than 2 wk. For examples, Trachtman et al[28] demonstrate that 1% taurine in drinking water significantly decreases arterial pressure by 4 wk of treatment and reaches a maximum antihypertensive effect by 16 wk of treatment. Although taurine supplementation prevents hypertension in DOCA-NaCl sensitive rats, it does not blunt NaCl-sensitive aspects of hypertension in stroke-prone SHR[27]. However, this finding is based on acute arterial pressure measurements that were done during the normal sleep period (daytime), when the rat’s arterial pressure is typically low.
To clarify the diurnal effect of taurine on arterial pressure, we did experiments in young SHR. At 7 wk of age, rats were anesthetized with isoflurane, and the abdominal aorta was exposed via a midline abdominal incision. After a segment of aorta below the renal artery was cleared, the flexible tip of the pressure sensing telemetry transmitter probe was inserted and secured to the vessel with tissue adhesive. The transmitter signal was then tested and the transmitter was surgically sutured to the abdominal wall. After surgically closing the wound, all rats were caged individually in clear cages and recovered on a basal NaCl diet (0.6%) for one week. Thereafter, the rats were fed a high NaCl diet (4.0%; w/w) and given 3% taurine in the drinking water (Taurine; n = 12) or water alone (Control, n = 7) for three weeks. This high NaCl diet has been commonly used to increase arterial pressure in SHR[27], and the 3% taurine in the drinking water has been used previously to attenuate hypertension in several animal models including SHR on a basal NaCl diet[13].
On a basal (0.6%) NaCl diet, both control and taurine-fed groups displayed a similar diurnal variation of mean arterial pressure, i.e., mean arterial pressures were high at night but low in daytime, and they were not significantly different between groups (Figure 1). The high NaCl intake increased daytime and nighttime arterial pressures in both groups, but with different time courses. Throughout the study, taurine had no effect on daytime arterial pressures (Table 1). In contrast, nighttime arterial pressures were significantly higher in the taurine (compared to control) group from the first to ninth night of treatment, but thereafter, taurine did not result in any significant difference in the high NaCl fed SHR for the remainder of the study. The arterial pressure analysis indicated that after starting a high NaCl diet, the taurine group displayed a rapid increase in mean nighttime arterial pressure within the first night, with arterial pressure approaching its maximum in the taurine-treated rats by night 2 (Figures 2 and 3). Thereafter, the nighttime mean arterial pressure of taurine group remained at nearly the same high level throughout the remainder of the study (3 wk).
Figure 1.
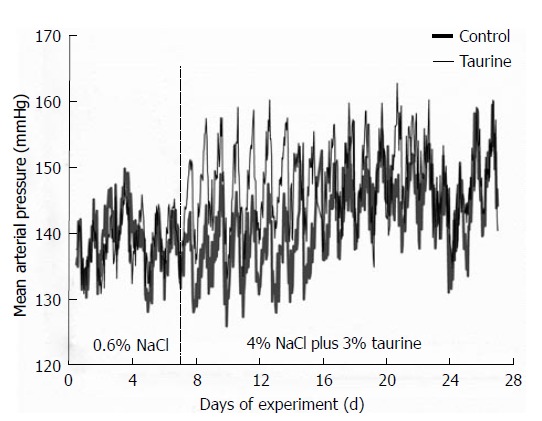
Group average, mean arterial pressures in control (thick line) and taurine (thin line) groups. The values were averaged from seven (control) and twelve (taurine) rats and the standard errors of means were not included to avoid confusion. Significant differences between groups (P < 0.05 by one-way analysis of variance and post hoc Duncan’s multiple range test) were consistently observed in nighttime but not daytime mean arterial pressures from day 1 to day 9 of high salt treatment. The vertical dashed line at day 7 indicates the day that the high NaCl diet and taurine supplementation began.
Table 1.
Noon and midnight mean arterial pressures in control (high salt alone, n = 7) and taurine (high salt plus taurine, n = 12) rats before and after treatment
| Days of treatment |
Control (mmHg) |
Taurine (mmHg) |
||
| Noon | Midnight | Noon | Midnight | |
| Before | 128 ± 5 | 136 ± 4 | 133 ± 2 | 140 ± 2 |
| 1 | 129 ± 3 | 139 ± 4 | 136 ± 3 | 147 ± 2a |
| 3 | 130 ± 3 | 140 ± 3 | 133 ± 2 | 150 ± 4a |
| 7 | 138 ± 6 | 136 ± 4 | 147 ± 5 | 151 ± 3a |
| 14 | 137 ± 6 | 143 ± 8 | 145 ± 2 | 152 ± 5 |
| 21 | 147 ± 5 | 149 ± 8 | 142 ± 3 | 148 ± 3 |
Data are mean ± SE;
P < 0.05 compared to midnight control by one-way analysis of variance and post hoc Duncan’s multiple range test; No significant difference in noon mean arterial pressures between groups.
Figure 2.
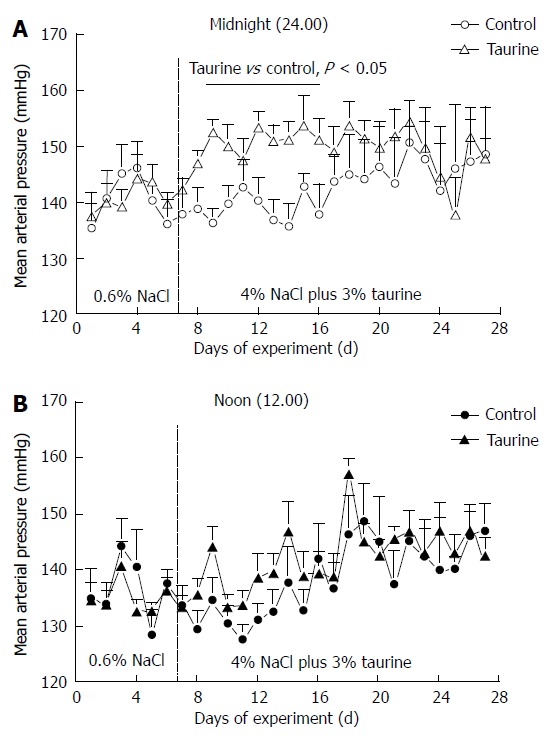
Group averages of mean arterial pressure in control (n = 7) and taurine (n = 12) treated groups at midnight (A) and at noon (B). The daytime mean arterial pressures were not significantly different between groups throughout the study. The vertical dashed line at day 7 indicates the day that the high NaCl diet and taurine supplementation began. Statistical comparisons were performed by one-way analysis of variance and post hoc Duncan’s multiple range test.
Figure 3.
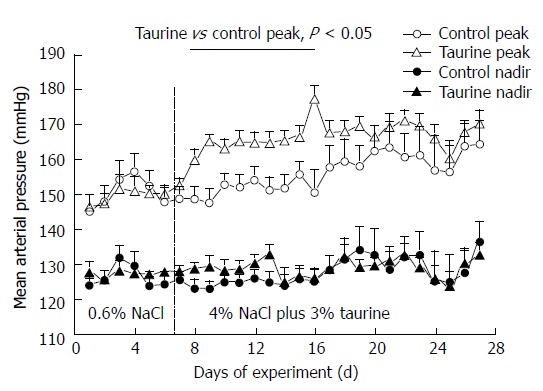
Peak (open symbols) and nadir (closed symbols) mean arterial pressures in control (n = 7) and taurine treated (n = 12) groups. The vertical dashed line at day 7 indicates the day that the high NaCl diet and taurine supplementation began. Statistical comparisons were performed by one-way analysis of variance and post hoc Duncan’s multiple range test.
These data confirm that taurine supplementation does not affect NaCl-induced daytime hypertension in the SHR, and thus, has little effect on mean average precision or on the eventual maximum level of arterial pressure in this model. Unexpectedly, the data also indicate that rather than being hypotensive or having no effect on arterial pressure in the SHR on a high NaCl diet, taurine supplementation accelerates the development of NaCl-sensitive hypertension during the nighttime.
ADVANTAGES AND LIMITATIONS OF TAURINE SUPPLEMENTATION
In most hypertensive rat and mouse models, a high (compared to basal or low) NaCl diet slowly increases nighttime arterial pressure after about 4 d of feeding, but the high dietary NaCl does not increase daytime arterial pressures until much later in these models[5,29,30]. This effect appears to be a consequence of the high dietary NaCl intake leading to Na+ and fluid retention, especially during the active period. This may be exacerbated by taurine supplementation if the taurine increases intestinal sodium absorption, which in SHR on a high NaCl diet may lead to increased NaCl-sensitive hypertension that offsets the normal hypotensive action of taurine.
The underlining mechanism for this effect likely relates to dietary NaCl and fluid retention that leads to increased sympathetic nerve activity, resulting in increased arterial vasoconstriction and cardiac output and ultimately an increase in hypertension (Figure 4). Increased cardiac output significantly contributes to initial phase of essential hypertension, while increased total peripheral resistance sustains it[31,32]. In rats, 24-h measurements of plasma sodium concentration indicate that plasma Na+ has a circadian rhythm that is opposite in phase to diurnal variation of mean arterial pressure[6]. Further, in SHR and WKY, a high NaCl diet increases daytime and nighttime plasma sodium levels, but in SHR compared to WKY on the high NaCl diet, the normal nighttime decrease in plasma sodium is greatly blunted, i.e., plasma sodium remains high during the active phase[6]. This early failure of plasma Na+ to decrease during the active period parallels the rise in nighttime arterial pressure in SHR.
Figure 4.
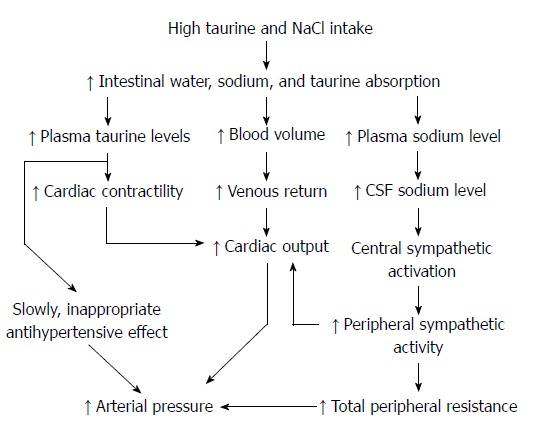
Possible pathways explaining the nighttime increase in arterial pressure after a combination of taurine supplementation and high salt diet in spontaneously hypertensive rats. CSF: Cerebrospinal fluid.
Since intestinal taurine is absorbed via a high affinity sodium chloride-dependent active transport[26], high luminal sodium concentration can accelerate intestinal taurine absorption, and high taurine transport increases sodium absorption into the blood. This potentially causes an early increase in plasma Na+. Further, in Dahl-NaCl sensitive rats, taurine supplementation alone increases sodium and fluid retention after a month on high NaCl diets[33]. It is likely that in the high taurine and NaCl fed SHR, taurine-facilitated Na+ absorption and associated water absorption rapidly increase nighttime arterial pressure, which is already significantly elevated on the first night of high NaCl diet.
The increase in blood pressure in SHR on taurine and a high NaCl diet also may relate to altered brain control of sympathetic nervous system activity and a resulting increase in vasoconstriction. Huang et al[34] demonstrates that in SHR and Dahl-NaCl-sensitive rats, a high NaCl diet (8% NaCl) increases cerebrospinal fluid Na+ concentration within a few days of treatment and 1-2 d before a rise in arterial pressure. This effect is not observed in WKY and Dahl-NaCl-resistance rats. Further, disruption of the hepatorenal natriuresis/diuresis pathway by hepatic denervation heightens nighttime hypertension in WKY rats[30], indicating that the nervous system normally activates the hepatorenal reflex to reduce plasma Na+ concentration by activating renal Na+ excretion. This is particularly effective, since the receptors in the liver very quickly monitor the concentration of Na+ that enters through the gut. Further, an abnormality in this feedback underlies NaCl and fluid retention observed in nephrotic syndrome[35]. These studies suggest that in SHR, high NaCl intake may lead to both peripheral and/or central increases in Na+ concentration, leading to increased sympathetic nerve activity and increased arterial pressure. At least in its developmental phase, NaCl sensitivity in SHR appears to primarily result from sympathetic nervous system overactivity and not alterations in the renin-angiotensin system[7,36].
The hypertensive interactions between dietary NaCl and taurine may be mediated, in part, by taurine effects on Na+ transport across the blood-brain barrier. As in the gut, taurine is transported across the blood-brain barrier by a Na+-dependent, carrier-mediated mechanism[37]. SHR display low taurine content in brain[38,39] and heart[40]. In the SHR brain, taurine content is especially low in the hypothalamus and rostral ventrolateral medulla, both key areas that regulate cardiovascular function[41,42]. In SHR on a basal NaCl diet, long-term (but not short-term) taurine supplementation increases brain taurine levels to those of the WKY and decreases hypertension and related disorders, e.g., cardiac hypertrophy and renal dysfunction. In SHR, the long-term taurine treatment is probably necessary because SHR display slow taurine transport across blood-brain barrier[37], thus decreasing taurine’s ability to rapidly accumulate in the brain after acute treatment. In the short-term study, taurine supplementation may have increased cerebrospinal fluid Na+ concentration in the high NaCl fed SHR before it is able to increase taurine concentration in the brain, leading to the early activation of the sympathetic nervous system[9,43].
In SHR on a basal NaCl diet, overactivity of both the sympathetic nervous system and the renin-angiotensin system contribute importantly to the development of hypertension[7,36], and taurine supplementation reduces both mechanisms in SHR on a basal NaCl diet[13]. Sugar-induced hypertension is also maintained by overactivity of both the sympathetic nervous system and the renin-angiotensin system and is associated with mild insulin resistance[44]. Chronic treatment with taurine improves insulin sensitivity and reduces hypertension in these models of hypertension[16]. The hypotensive action of taurine in DOCA-NaCl rats is also related to inhibition of sympathetic nervous system activity[45]. In humans, taurine supplementation decreases plasma epinephrine levels in borderline hypertension, suggesting a sympathetic nervous system mechanism[18]. Further, epidemiological studies indicate an inverse relationship between taurine-rich diets and sympathetic nervous system activity in hypertension[17]. However, our 24-h arterial pressure study suggests that, while dietary taurine supplementation is antihypertensive in most hypertensive models, at least in SHR, the combination of high dietary NaCl and taurine supplementation causes an early acceleration in the development of NaCl-sensitive hypertension and does not lead to any reduction in arterial pressure at later time points. This indicates that further studies in animals and humans are needed to explore the interactions between dietary supplements and NaCl intake.
Taurine possess positive inotropic effects on cardiac muscle particularly in in vitro experiments (i.e., an acute effect) and in taurine deficient animals[46-48]. These actions are related to taurine-increased calcium inward current and calcium release from sarcoplasmic reticulum. More taurine intake during the nighttime may increase plasma taurine (and likely cardiac taurine concentration), leading to increased cardiac contractility in the nighttime compared to the daytime. In subjects on high taurine and NaCl diets, this positive inotropic effect may increase the Starling’s effect of increased venous return due to increased blood volume, leading to increased cardiac output and eventually increased arterial pressure.
CONCLUSION
Diets high in taurine prevent or decrease hypertension in many animal models of hypertension and in humans[13,17]. In SHR fed a basal NaCl diet, taurine supplementation significantly blunts the development of hypertension; however, taurine supplementation fails to decrease NaCl-induced hypertension in SHR. In contrast to our hypothesis that taurine supplementation lowers arterial pressure, taurine accelerates the hypertensive response to a high NaCl diet in this animal model. The taurine supplementation initially accelerates arterial pressure in SHR fed a high NaCl diet during the nighttime but not the daytime. After the initial 9 d of the high NaCl diet, taurine no longer increases nighttime arterial pressure above that displayed by non-treated SHR on the high NaCl diet. These data suggest that while taurine is generally beneficial to arterial pressure in hypertensive situations, dietary taurine supplementation may have early adverse effects when paired with a high NaCl diet.
Footnotes
Supported by Grants from the National Center for Complementary and Alternative Medicine and the National Institutes of Health (NIH) Office of Dietary Supplements, No.5P50 AT-00477; and the NIH Neuroscience Blueprint Mouse Phenotyping Core at University of Alabama at Birmingham, No.P30 NS-057098; J Michael Wyss, and by a grant from the Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
P- Reviewers: Chawla M, Durandy Y, Miyasaka Y, Sakabe K, Vermeersch P S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.White WB. Relating cardiovascular risk to out-of-office blood pressure and the importance of controlling blood pressure 24 hours a day. Am J Med. 2008;121:S2–S7. doi: 10.1016/j.amjmed.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- 3.Lemmer B, Mattes A, Böhm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension. 1993;22:97–101. doi: 10.1161/01.hyp.22.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34:281–285. doi: 10.1038/hr.2010.241. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun DA, Zhu S, Wyss JM, Oparil S. Diurnal blood pressure variation and dietary salt in spontaneously hypertensive rats. Hypertension. 1994;24:1–7. doi: 10.1161/01.hyp.24.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Fang Z, Carlson SH, Peng N, Wyss JM. Circadian rhythm of plasma sodium is disrupted in spontaneously hypertensive rats fed a high-NaCl diet. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1490–R1495. doi: 10.1152/ajpregu.2000.278.6.R1490. [DOI] [PubMed] [Google Scholar]
- 7.Wyss JM, Roysommuti S, King K, Kadisha I, Regan CP, Berecek KH. Salt-induced hypertension in normotensive spontaneously hypertensive rats. Hypertension. 1994;23:791–796. doi: 10.1161/01.hyp.23.6.791. [DOI] [PubMed] [Google Scholar]
- 8.Wyss JM, Carlson SH. The role of the nervous system in hypertension. Curr Hypertens Rep. 2001;3:255–262. doi: 10.1007/s11906-001-0048-0. [DOI] [PubMed] [Google Scholar]
- 9.Carlson SH, Roysomutti S, Peng N, Wyss JM. The role of the central nervous system in NaCl-sensitive hypertension in spontaneously hypertensive rats. Am J Hypertens. 2001;14:155S–162S. doi: 10.1016/s0895-7061(01)02083-0. [DOI] [PubMed] [Google Scholar]
- 10.Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient. Curr Opin Clin Nutr Metab Care. 2006;9:728–733. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 11.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- 13.Roysommuti S, Wyss JM. Perinatal taurine exposure affects adult arterial pressure control. Amino Acids. doi: 10.1007/s00726-012-1417-5. 2012; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nara Y, Yamori Y, Lovenberg W. Effect of dietary taurine on blood pressure in spontaneously hypertensive rats. Biochem Pharmacol. 1978;27:2689–2692. doi: 10.1016/0006-2952(78)90043-6. [DOI] [PubMed] [Google Scholar]
- 15.Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl. 1984;2:S563–S565. [PubMed] [Google Scholar]
- 16.Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- 17.Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17 Suppl 1:S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–532. doi: 10.1161/01.cir.75.3.525. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–622. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 20.Lerdweeraphon W, Wyss JM, Boonmars T, Roysommuti S. Perinatal taurine exposure affects adult oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2013;305:R95–R97. doi: 10.1152/ajpregu.00142.2013. [DOI] [PubMed] [Google Scholar]
- 21.Roysommuti S, Lerdweeraphon W, Malila P, Jirakulsomchok D, Wyss JM. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv Exp Med Biol. 2009;643:145–156. doi: 10.1007/978-0-387-75681-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roysommuti S, Suwanich A, Jirakulsomchok D, Wyss JM. Perinatal taurine depletion increases susceptibility to adult sugar-induced hypertension in rats. Adv Exp Med Biol. 2009;643:123–133. doi: 10.1007/978-0-387-75681-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roysommuti S, Thaeomor A, Khimsuksri S, Lerdweeraphon W, Wyss JM. Perinatal taurine imbalance alters the interplay of renin-angiotensin system and estrogen on glucose-insulin regulation in adult female rats. Adv Exp Med Biol. 2013;776:67–80. doi: 10.1007/978-1-4614-6093-0_8. [DOI] [PubMed] [Google Scholar]
- 24.Thaeomor A, Wyss JM, Jirakulsomchok D, Roysommuti S. High sugar intake via the renin-angiotensin system blunts the baroreceptor reflex in adult rats that were perinatally depleted of taurine. J Biomed Sci. 2010;17 Suppl 1:S30. doi: 10.1186/1423-0127-17-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaeomor A, Wyss JM, Schaffer SW, Punjaruk W, Vijitjaroen K, Roysommuti S. High sugar intake blunts arterial baroreflex via estrogen receptors in perinatal taurine supplemented rats. Adv Exp Med Biol. 2013;775:437–448. doi: 10.1007/978-1-4614-6130-2_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson CM, Howard A, Walters JR, Ganapathy V, Thwaites DT. Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl(-)-dependent TauT (SLC6A6) J Physiol. 2009;587:731–744. doi: 10.1113/jphysiol.2008.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson R, Liu S, Jung B, Messina S, Eppler B. Effects of high salt diets and taurine on the development of hypertension in the stroke-prone spontaneously hypertensive rat. Amino Acids. 2000;19:643–665. doi: 10.1007/s007260070014. [DOI] [PubMed] [Google Scholar]
- 28.Trachtman H, Lu P, Sturman JA. Immunohistochemical localization of taurine in rat renal tissue: studies in experimental disease states. J Histochem Cytochem. 1993;41:1209–1216. doi: 10.1177/41.8.8331284. [DOI] [PubMed] [Google Scholar]
- 29.Cho TM, Peng N, Clark JT, Novak L, Roysommuti S, Prasain J, Wyss JM. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology. 2007;148:5396–5402. doi: 10.1210/en.2007-0245. [DOI] [PubMed] [Google Scholar]
- 30.Carlson SH, Osborn JW, Wyss JM. Hepatic denervation chronically elevates arterial pressure in Wistar-Kyoto rats. Hypertension. 1998;32:46–51. doi: 10.1161/01.hyp.32.1.46. [DOI] [PubMed] [Google Scholar]
- 31.Beevers G, Lip GY, O’Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001;322:912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkow B. The pathophysiology of hypertension. Differences between young and elderly patients. Drugs. 1993;46 Suppl 2:3–7. doi: 10.2165/00003495-199300462-00003. [DOI] [PubMed] [Google Scholar]
- 33.Fujita T, Sato Y. Changes in blood pressure and extracellular fluid with taurine in DOCA-salt rats. Am J Physiol. 1986;250:R1014–R1020. doi: 10.1152/ajpregu.1986.250.6.R1014. [DOI] [PubMed] [Google Scholar]
- 34.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 35.Jirakulsomchok D, Napawachirahat S, Kunbootsri N, Suttitum T, Wannanon P, Wyss JM, Roysommuti S. Impaired renal response to portal infusion of hypertonic saline in adriamycin-treated rats. Clin Exp Pharmacol Physiol. 2012;39:636–641. doi: 10.1111/j.1440-1681.2012.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyss JM, Mozaffari MS, Roysommuti S. Contribution of the sympathetic nervous system to salt-sensitivity in lifetime captopril-treated spontaneously hypertensive rats. J Hypertens. 1995;13:1037–1042. doi: 10.1097/00004872-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Kang YS. Taurine transport mechanism through the blood-brain barrier in spontaneously hypertensive rats. Adv Exp Med Biol. 2000;483:321–324. doi: 10.1007/0-306-46838-7_36. [DOI] [PubMed] [Google Scholar]
- 38.Kubo T, Kihara M, Misu Y. Altered amino acid levels in brainstem regions of spontaneously hypertensive rats. Clin Exp Hypertens A. 1989;11:233–241. doi: 10.3109/10641968909035339. [DOI] [PubMed] [Google Scholar]
- 39.Kuriyama K, Ida S, Ohkuma S. Alteration of cerebral taurine biosynthesis in spontaneously hypertensive rats. J Neurochem. 1984;42:1600–1606. doi: 10.1111/j.1471-4159.1984.tb12748.x. [DOI] [PubMed] [Google Scholar]
- 40.Chau C, Heu P, Chou SC, Miyahara JT, Ramanathan S. Taurine content of cardiac tissue in spontaneously hypertensive rats. Zhongguo Yao Li Xue Bao. 1983;4:21–23. [PubMed] [Google Scholar]
- 41.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 42.Spyer KM, Lambert JH, Thomas T. Central nervous system control of cardiovascular function: neural mechanisms and novel modulators. Clin Exp Pharmacol Physiol. 1997;24:743–747. doi: 10.1111/j.1440-1681.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- 43.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 45.Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension. 1987;9:81–87. doi: 10.1161/01.hyp.9.1.81. [DOI] [PubMed] [Google Scholar]
- 46.Lake N. Alterations of ventricular contractility and myofibril loss in taurine-deficient hearts. Adv Exp Med Biol. 1994;359:335–342. doi: 10.1007/978-1-4899-1471-2_34. [DOI] [PubMed] [Google Scholar]
- 47.Pisarenko OI. Mechanisms of myocardial protection by amino acids: facts and hypotheses. Clin Exp Pharmacol Physiol. 1996;23:627–633. doi: 10.1111/j.1440-1681.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 48.Schaffer SW, Punna S, Duan J, Harada H, Hamaguchi T, Azuma J. Mechanism underlying physiological modulation of myocardial contraction by taurine. Adv Exp Med Biol. 1992;315:193–198. doi: 10.1007/978-1-4615-3436-5_22. [DOI] [PubMed] [Google Scholar]


