Abstract
Background: Healthcare workers (HCW) are a risk group for tuberculosis (TB). That is why interferon-gamma release assay (IGRA) serial testing is performed on HCWs repeatedly exposed to infectious patients or materials. However, the variability of IGRA in serial testing is not yet well understood. We therefore analysed the prevalence of positive IGRA results as well as conversion and reversion rates in the serial testing of healthcare trainees in a low-incidence country.
Methods: In a prospective cohort study, all trainees (n=194) who began training as a nurse or healthcare worker at the Vivantes Healthcare Training Institute in Berlin on 1 October 2008 or 1 April 2009 were IGRA-tested at three different times during the three years of training. Socio-demographic data and possible risk factors (e.g., TB contacts, time spent abroad, area of work) were recorded by means of a standardised questionnaire. The QuantiFERON Gold In-Tube (QFT) was used as an IGRA.
Results: At the beginning of the training the cohort comprised 194 trainees. 70% were female. Their average age was 23. The prevalence of positive QFT was 2.1% (4/194). In the first follow-up test, 2 out of 154 (1.3%) tested IGRA-positive, 151 (98%) had constantly negative results. One IGRA was constantly positive (0.6%) and there was one conversion and one reversion (0.6% respectively). In the second follow-up (n=142) there was again one conversion (0.7%), one reversion and the one constantly positive test result in all three QFT. This trainee had active TB in 2002. All other test results were constantly negative (n=139; 98%). No case of active tuberculosis was diagnosed over the three-year observation period. Contact with TB patients was reported by 42 (29.6%) trainees during the follow-up. The two trainees with a conversion in QFT had no known contact with TB patients. Discordant results in the three consecutive QFT were observed in three trainees (2.1%). Using a borderline zone from 0.2–0.7 IU/mL reduced the number of trainees with discordant results from three to one – a reversion.
Conclusion: The prevalence rate of latent TB infection is low in healthcare trainees without known risk factors for TB infection in their history. The infection risk seems to be low in this population even though contacts with TB patients during the training were reported. Introducing a borderline zone for the interpretation of reversions and conversions in this cohort appears to be safe and reduces the number of discordant results and helps to avoid unnecessary chest X-rays and preventive treatment.
Keywords: tuberculosis, serial testing, interferon-gamma release assay, healthcare workers, students, occupational disease, latent tuberculosis infection
Zusammenfassung
Hintergrund: Beschäftigte im Gesundheitswesen haben ein erhöhtes Tuberkulose (TB)-Risiko. Deshalb werden sie regelmäßig mit einem Interferon-Gamma Release Assay (IGRA) untersucht, wenn sie Kontakt zu infektiösen Patienten oder Materialien haben. Die Variabilität der IGRA beim seriellen Testen ist bisher nicht gut untersucht. Deshalb haben wir sowohl die Häufigkeit positiver IGRA als auch die Konversions- und Reversionsraten bei Auszubildenden in Gesundheitsberufen in einem Land mit niedriger TB-Inzidenz untersucht.
Methoden: In einer prospektiven Kohorten-Studie wurden alle Auszubildenden (n=194) der Vivantes Pflegeschule in Berlin, die ihre Ausbildung am 1. Oktober 2008 oder 1. April 2009 begannen, zu drei verschiedenen Zeitpunkten während der dreijährigen Ausbildung mit einem IGRA untersucht. Sozio-demografische Daten und mögliche Risikofaktoren (z.B. TB-Kontakte, Reisen in Länder mit hoher TB-Inzidenz, berufliche Risiken) wurden mit einem standardisierten Fragebogen erfasst. Als IGRA wurde der QuantiFERON Gold in Tube (QFT) verwendet.
Ergebnisse: Die Ausbildung wurde von 194 Schülern (Durchschnittsalter 23 Jahre) begonnen, 70% der Berufsanfänger waren weiblich. Einen positiven QFT hatten 2,1% (4/194). Im zweiten QFT waren 2 von 154 (1,3%) Schülern positiv und 151 (98%) hatten konstant negative QFT-Ergebnisse. Ein IGRA war auch bei der ersten Verlaufsuntersuchung positiv (0,6%). Außerdem gab es eine Konversion und eine Reversion (jeweils 0,6%). Im dritten QFT (n=142) gab es wieder eine Konversion (0,7%), eine Reversion und ein konstant positives Ergebnis bei einer Schülerin, die 2002 wegen einer aktiven TB behandelt worden war. Alle anderen QFT waren konstant negativ (n=139; 98%). Während des dreijährigen Follow-up wurde keine aktive TB diagnostiziert. Während der Ausbildung hatten 42 (29,6%) Schüler Kontakt zu TB-Patienten. Die beiden Schüler mit einer Konversion im QFT hatten keinen bekannten Kontakt zu einem TB-Patienten. Drei Schüler (2,1%) hatten abweichende Ergebnisse in den drei aufeinander folgenden QFT. Unter Anwendung einer Borderline-Zone von 0,2–0,7 IU/ml reduzierte sich die Anzahl der instabilen Ergebnisse von drei auf eines – eine Reversion.
Schlussfolgerung: Die latente TB-Infektion ist bei Pflegeschülern selten, wenn sie keine Risikofaktoren für eine TB in der Anamnese haben. Trotz der überraschend häufig angegebenen Kontakte zu TB-Patienten scheint das Infektionsrisiko bei Pflegeschülern gering zu sein. Die Anzahl der Reversionen und Konversionen wird durch die Anwendung einer Borderline-Zone reduziert. Unnötige Röntgenuntersuchungen der Lunge, des Thorax und präventive Chemotherapien können dadurch vermieden werden.
Background
Tuberculosis (TB) incidence in Germany is low – 5.3 cases per 100,000 inhabitants in 2011 [1]. In spite of the low incidence among the general public, healthcare workers (HCW) continue to have an increased risk of TB [2], [3], [4]. In Germany between 60 and 80 cases of TB in HCW are recognized as an occupational disease each year [5].
In countries with a low TB incidence, the relative risk of TB among HCWs might not exceed the risk for the general population because HCW do not share other TB risk factors such as homelessness or drug addiction and not all HCWs have an increased risk of exposure to Mycobacterium tuberculosis [6]. However, the risk of exposure to TB is higher for a wide range of tasks or facilities in healthcare, such as laboratory work, pneumology departments, infection wards, emergency rooms, pathology and geriatric care [7], [8], [9]. For this reason, TB in HCWs is on the list of occupational diseases compiled by the International Labour Organization (ILO) [10]. Different infection control measures have been proposed [11], repeated screening of exposed HCW being one of these infection control measures. Occupational health examinations for HCW raise HCWs’ awareness of possible latent TB infection (LTBI) and suitable preventive treatment. They also serve to reduce the risk of nosocomial infection by an infectious colleague for HCW and patients.
TB screening of HCWs has been performed using the interferon-gamma release assay (IGRA) for several years [9], [12]. However, IGRA variability gives rise to concerns about the usefulness of IGRA in serial testing of HCW [12], [13], [14], [15], [16]. The introduction of a borderline zone for the interpretation of the IGRA might be an option to circumvent the variability problem of IGRA in the serial testing of HCW [13], [17], [18], [19], [20]. Instead of a simple negative-positive interpretation “true” conversions or reversions of the IGRA would be defined as trespassing of the borderline zone. Two IGRA are commercially available, the ELISA-based QuantiFERON®-TB Gold In-Tube (QFT) and the ELISPOT-based T-SPOT.TB®. For the interpretation of the T-SPOT.TB a borderline zone of 5–7 spots has been proposed by CDC and ECDC [21], [22]. However, this recommendation is based on little data [13]. For the QFT different borderline zones were proposed recently [17]. However, one paper cautioned against the use of a borderline zone in HCW with known unprotected exposure to M. tuberculosis [15]. For QFT interpretation in serial TB screenings of German HCW a borderline zone of ≥0.2 to ≤0.7 IU/mL is proposed [23]. Using this borderline zone reduced the number of conversions and reversions considerably without inflating the number of QFT results that fall into the borderline zone and can thus only be interpreted with uncertainty.
In order to gain further insights into the usefulness of a borderline zone for QFT interpretation in serial testing of HCW a prospective follow-up study was conducted in the course of which healthcare trainees were TB-screened three times during their three years of training.
Material and methods
Study population
In the prospective cohort study all trainees who began their training as a healthcare worker or a nurse at the Vivantes Institute for Occupational Education in Health Care in Berlin on 1 October 2008 or 1 April 2009 were QFT-tested on three different occasions. The first test was performed at the start of the training before the first practical deployment. The second was carried out at the midterm of the three-year training period. The third test took place at the end of the training. The results of the first and the second test have been published previously [24]. During the training, the trainees were deployed in different wards of the hospital group.
Data was collected by the supervising doctor and nurse responsible for occupational medicine and was passed on in anonymous form. The repeated tests were arranged independently by the doctor and nurse responsible for occupational medicine, ensuring that the forms filled in at the three examinations could be linked by an anonymous code. The occupational health physician ensured that care of affected persons (those with a positive QFT) was in accordance with the recommendations of the German Central Committee against Tuberculosis (DZK) on contact tracing and consisted of two X-rays to rule out active TB within a year and, if necessary, the prescription of chemoprevention with Isoniazid for at least six months [25].
Ethics
Participation in the study was voluntary. To ensure anonymity, no names or other identifiers were used. All participants gave their written and informed consent for participation in the study, which had been approved by the Hamburg Medical Council’s Ethics Commission.
Diagnostics
The initial occupational health check was conducted when the trainees started training. This included a physical examination to assess suitability for a career in healthcare and a general series of routine laboratory tests. In addition, for study purposes a QFT was performed. The QFT was carried out in accordance with the manufacturer’s instructions. BCG vaccination status was verified either by an entry in the trainee’s vaccination certificate or by means of anamnesis and vaccination scars.
Questionnaire
Standardised questionnaires were used to collect socio-demographic data on age, gender, country of birth and nationality. The first questionnaire also covered details of private infection risk, personal history of TB (exposure), BCG vaccination and previous employment in the social sector. The main focus of questions in the second and third questionnaires was on assessing a possible occupational infection risk. Variables included previous areas of practical work and contact with TB patients as well as the nature and duration of (protected and unprotected) contact with these patients.
Analysis and statistical evaluation
Descriptive data evaluation was undertaken using SPSS, Version 18 (SPSS Inc., Chicago, Illinois). The Friedman test was used to check whether the median differed for the three successive interferon-γ (IFN) concentrations measured.
Results
Description of the study population (baseline)
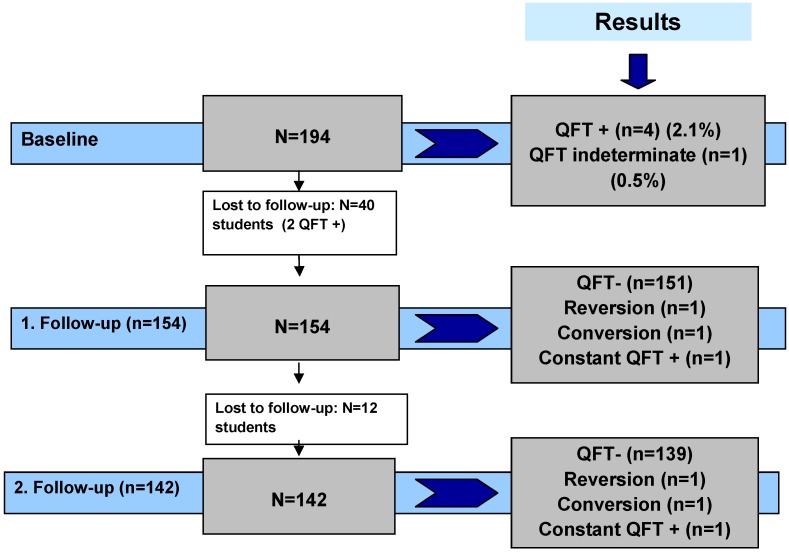
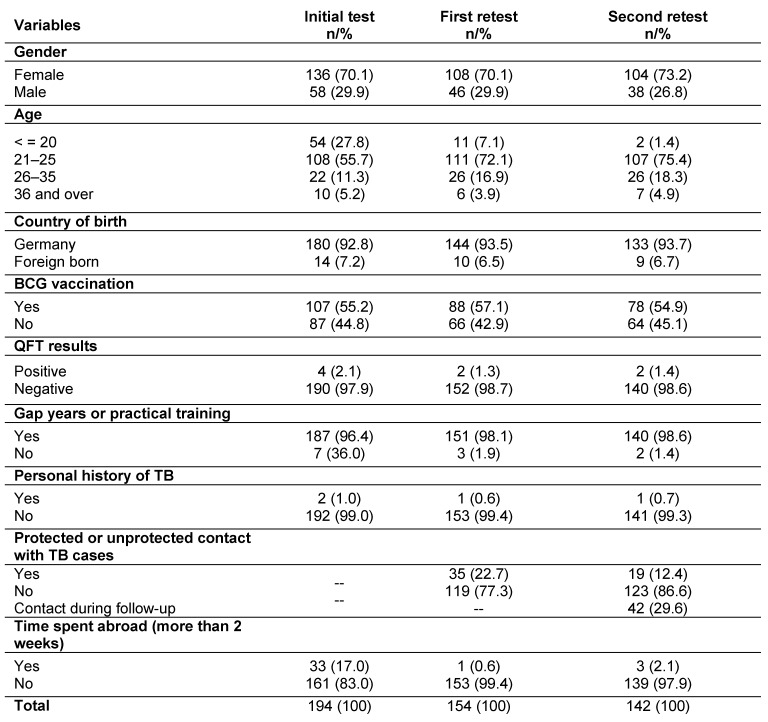
At baseline the study comprised 194 trainees. The three-year follow-up was completed by 142 (73%) participants (Figure 1 (Fig. 1)). All participants reported immune competence. 70% of the trainees were women. The average age was 23 with a standard deviation of 5.5 years. The youngest study participant was 17 and the oldest 53. The majority of trainees (92.8%) were born in Germany, with 14 subjects (7.2%) born abroad (Table 1). The latter all came from countries with an intermediate or a high incidence of TB (annual incidence of TB >20 per 100,000 population). Two were from Africa (Ethiopia and Cameroon), two from Asia (Nepal and Pakistan), one from Chile and nine from Eastern Europe (Poland, Romania, Turkey, Russia and Kosovo, no table).
Figure 1. Flow chart of the study population and the QFT results.
One hundred and seven trainees (55.2%) had undergone BCG vaccination. Most of the trainees (96.4%) had gained previous healthcare experience by means of internships, a voluntary social year, civilian (as an alternative to military) service, work for a rescue service or as helpers in nursing homes. Only seven test subjects stated that they had no previous experience of social work prior to the beginning of training. Anamnesis revealed that one study participant who completed the follow-up had been diagnosed with active TB and had been treated correspondingly in 2002 (Table 1 (Tab. 1)).
Table 1. Description of the study population in three years and three follow-up tests.
During the first year of training 40 trainees (20.6%) dropped out, so the study population for the first follow-up was 154 trainees. A further 12 trainees dropped out of the training after midterm, leaving 142 subjects (73.2%) for testing at the end of the training course (Figure 1). All eligible trainees agreed to be QFT-tested (response rate 100%).
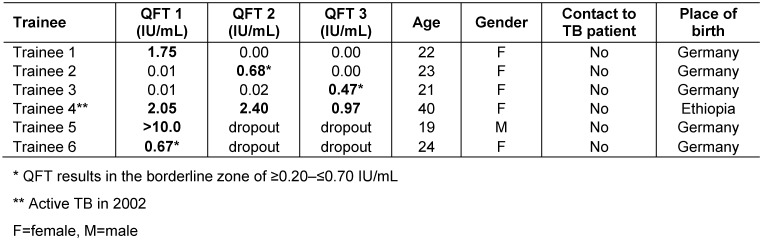
In total, 490 QFT were performed and 8 (2%) were positive. Fourty participants had one QFT, 154 had two QFT and 142 had three consecutive QFT. One student out of 142 (0.6%) was positive in all three QFT. This student had been treated for TB in 2002 and originated from Ethiopia (Table 2 (Tab. 2)). One reversion, one conversion and one fluctuating QFT (neg, pos, neg) were observed. One indeterminate QFT was observed in the first test series (0.5%). The second and third QFT were negative. In the second and third test series no indeterminate QFT was observed.
Table 2. Results of the three QFT for trainees with at least one QFT ≥0.35 IU/mL.
In those six students in whom the QFT was positive at least once, active TB was ruled out by chest X-ray. One participant reported having spent a longer period abroad in a high-incidence country after the first QFT and remained negative in the two following QFT. Three more students spent time abroad after the second QFT and remained negative in the third QFT. Contact with TB patients was reported by 42 trainees (29.6%) during the whole follow-up. In 24 cases these were contacts with both culturally and microscopically confirmed index cases and in three with microscopically negative but culturally confirmed TB index cases. The other 15 participants did not specify the contacts. The trainees with the conversion or the fluctuating QFT had no known TB contact. Trainees were deployed to different healthcare units, from internal medicine, surgery, psychiatry, gynaecology and maternity wards to outpatient work at the welfare centre.
Two participants with a positive QFT at baseline with IFN concentrations of 12.0 IU/mL and 0.67 UI/mL quit training within the first year. The IFN concentrations of the inconsistent positive QFT were between 0.47 and 1.75 IU/mL. If a borderline zone from 0.2 to 0.7 IU/mL was used, the conversion (0.02 to 0.47 IU/mL) and the fluctuating QFT (0.01 to 0.68 to 0.00 IU/mL) pertained to this borderline zone. The number of unexplained positive QFT therefore decreased from three (2.1%) to one (0.7%) by applying this borderline zone for trainees with no known TB contact.
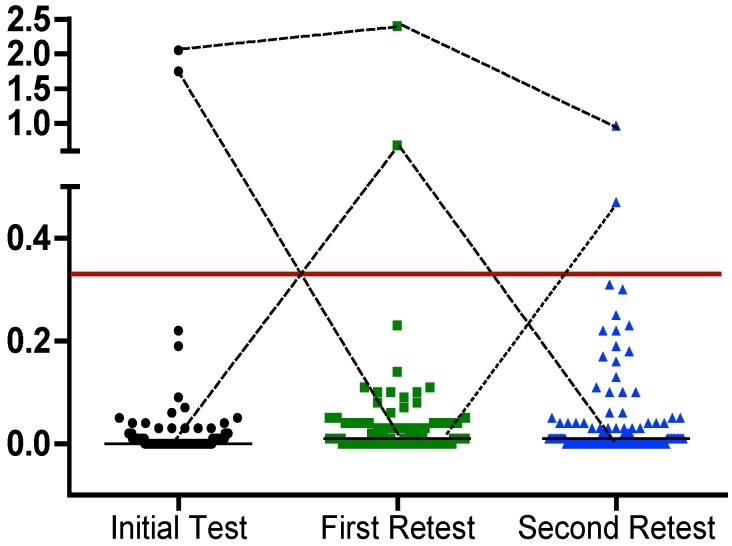
One hundred and thirty-nine participants tested consistently QFT-negative with IFN concentrations of between zero and 0.31 IU/ml. The median of the IFN concentration was changed from 0.00 IU/mL in the initial QFT to 0.01 IU/mL in the second QFT and remained unchanged in the third QFT (Figure 2 (Fig. 2)). No statistically significant difference was observed when comparing the median concentrations of the three consecutive QFT.
Figure 2. Dot plot of individual responses to QFT for 142 healthcare workers available for a third QFT three years after initial testing. The line represents the cut-off of 0.35 IU/mL for IFN (TB antigen minus nil control). The short black horizontal lines represent the respective group’s median IFN values.
No case of active tuberculosis was diagnosed during follow-up over a three-year observational period.
Discussion
This is the first study to investigate the performance of the QFT in serial testing of nursing trainees with a low risk of unprotected exposure to M. tuberculosis over a period of three years with three consecutive QFT. The probability of a positive QFT in this young cohort with limited TB exposure was low. The only person with a continuously positive QFT had a history of active TB. Three further participants showed at least one positive QFT. However, when applying a borderline zone from 0.2 to 0.7 IU/mL, two out of these three participants had a QFT within this zone. As no active TB was diagnosed at baseline and during follow-up and as the few trainees with a QFT conversion had no known TB contact, the use of the proposed borderline zone in this group with a low TB risk seems to be safe.
IGRA are dynamic assays and IFN values tend to fluctuate around the cut-off, leading to apparent conversions and reversions [26]. Reasons for the test variability of IGRA may be based on variations of handling and reading of the test, variability of the immune system and differences in the activity of the M. tuberculosis infection, that is, transient infection, infection with low replication of M. tuberculosis with no stimulation of the immune system or high replication of M. tuberculosis with stimulation of the immune system, and uncontrolled replication causing active TB infection [26]. In addition, reversions may be partly explained by the statistical mechanism of regression toward the mean [13], which is a ubiquitous and inevitable statistical phenomenon that occurs whenever repeated measurements on the same subject are observed with random error, i.e., nonsystematic variation around a true mean [27]. In our study the two reversions are most likely explained by regression toward the mean as no TB risk was known for the respective trainees and the QFT concentration of one positive test was below 0.7 IU/mL. The two conversions we observed are most likely due to unspecific variations as well, because again the respective trainees had no known exposure and the positive QFT results were <0.7 IU/mL. As no active TB was diagnosed in the course of the study, the application of a borderline zone stretching from 0.2 to 0.7 IU/mL for the QFT in this group of HCW with a low TB risk helps to avoid unnecessary chest X-rays and preventive treatment.
In the study by Fong et al. 71% of reversions had IFN values ≤1 IU/mL [18]. The authors therefor argue to extend a putative borderline zone to 0.1 to 1.0 IU/mL. Joshi and colleagues suggested to extend the borderline to 2.0 IU/mL as all reverts in their study had concentrations between 0.35 and 2.0 IU/mL in the baseline QFT [19]. In an attempt to balance sensitivity and specificity of the QFT and to minimize conversions that might lead to unnecessary preventive treatment, a threshold of 1.11 IU/mL was proposed for serial QFT testing of HCW in the US [17]. Following these recommendations, low risk individuals with an initial QFT ≥0.35 IU/mL and ≤0.7, 1.0, 1.11 or 2.0 IU/mL should be retested by clinicians. As in none of these studies active TB at baseline or progression from LTBI to active TB was observed, it is impossible to tell which of the upper limits of a borderline zone should be applied. Our study does not help either to decide which borderline zone is most appropriate as using either of these borderline zones with an upper limit of 0.7, 1.0, 1.1 or 2.0 IU/mL would only affect the interpretation of one positive QFT (1.75 IU/mL, Table 2). As there is no biological but only a statistical reason for introducing a borderline zone for serial testing of HCW, intuitively, the smaller the borderline zone the greater the confidence in the QFT results. Therefore, the tighter borderline zone seems to be appropriate for our study group. However, it should be borne in mind that even this tight borderline zone of 0.2–0.7 IU/mL increases the risk of false negative QFT results in HCW with prevalent active TB or the risk of progression from LTBI to active TB, as it was shown in the only HCW study that reported diagnosis of active TB when performing routine serial testing of HCW [15].
Risk of LTBI infection
LTBI prevalence at the start of training was low (n=4/194, 2.1%). In another German study, no trainees tested positive [28]. There are few comparable studies of TB screening among those beginning careers in the healthcare sector in other countries. Chee and colleagues tested 270 medical students from Singapore in their final semester using the T-SPOT.TB. Here, 4.3% of the students tested positive [29]. Singapore is a country with an intermediate TB incidence. This might account for the prevalence rate of positive IGRA being twice as high as in our study. In a US study, students at the University of Tennessee Health Science Center in Memphis were tested. Retrospectively, all students who had been routinely QFT-tested between June 2005 and August 2006 were included in the study. QFT was positive in 14.8% (8/54) of the students [30]. As only students with positive indicators for a risk of LTBI in their personal history were screened, the high LTBI prevalence rate is not surprising. Hence, the study does not allow for an estimate of the prevalence of LTBI in students in general.
In our study 29.6% of the trainees stated that they had been in contact with TB patients during their practical work but the two participants with the conversions had had no known contact with TB patients. As hygiene standards in Germany are high, contact with TB patients does not necessarily result in exposure to M. tuberculosis. Therefore, it seems plausible that none of the trainees who reported contact with TB patients was positive in the QFT.
The sample of our study is rather small and the follow-up period is limited. Thus, conclusions from our study results should be drawn with caution. Cum grano salis, our data does not support the need for regular TB screenings in healthcare trainees in a country with a low TB incidence in the general population and high in-hospital hygiene standards. We assume that our study population was TB unexposed during the study period. Therefore, our recommendations regarding the use of a borderline zone in the serial testing of HCW should only be applied to unexposed groups with a low a priori risk of LTBI.
Conclusions
We observed a low variability of IGRA results in this young and healthy group of trainees with a low risk of TB exposure in the healthcare sector. In countries with a low TB incidence and high hygiene standards, the infection risk during training in healthcare seems to be low and the application of a borderline zone of 0.2–0.7 IU/mL may help to avoid unnecessary chest X-rays and preventive chemotherapy.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Robert Koch-Institut. Report of the tuberculosis epidemiology in Germany in 2011. [Bericht zur Epidemiologie der Tuberkulose in Deutschland für 2011]. Berlin; 2013. [Google Scholar]
- 2.Diel R, Seidler A, Nienhaus A, Rüsch-Gerdes S, Niemann S. Occupational risk of tuberculosis transmission in a low incidence area. Respir Res. 2005;6:35. doi: 10.1186/1465-9921-6-35. Available from: http://dx.doi.org/10.1186/1465-9921-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries G, Sebek MM, Lambregts-van Weezenbeek CS. Healthcare workers with tuberculosis infected during work. Eur Respir J. 2006 Dec;28(6):1216–1221. doi: 10.1183/09031936.06.00039906. Available from: http://dx.doi.org/10.1183/09031936.06.00039906. [DOI] [PubMed] [Google Scholar]
- 4.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerging Infect Dis. 2011 Mar;17(3):488–494. doi: 10.3201/eid1703.100947. Available from: http://dx.doi.org/10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nienhaus A, Kesavachandran C, Wendeler D, Haamann F, Dulon M. Infectious diseases in healthcare workers - an analysis of the standardised data set of a German compensation board. J Occup Med Toxicol. 2012;7(1):8. doi: 10.1186/1745-6673-7-8. Available from: http://dx.doi.org/10.1186/1745-6673-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007 Jun;11(6):593–605. [PubMed] [Google Scholar]
- 7.Seidler A, Nienhaus A, Diel R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration. 2005 Jul-Aug;72(4):431–446. doi: 10.1159/000086261. Available from: http://dx.doi.org/10.1159/000086261. [DOI] [PubMed] [Google Scholar]
- 8.Nienhaus A, Loddenkemper R, Hauer B, Wolf N, Diel R. Latente Tuberkulose-Infektionen im Gesundheitswesen--Evaluation des Interferon-gamma Release Assay. [Latent tuberculosis infection in healthcare workers--evaluation of an Interferon-gamma release assay]. Pneumologie. 2007 Apr;61(4):219–223. doi: 10.1055/s-2007-959161. (Ger). Available from: http://dx.doi.org/10.1055/s-2007-959161. [DOI] [PubMed] [Google Scholar]
- 9.Nienhaus A, Ringshausen FC, Costa JT, Schablon A, Tripodi D. IFN-γ release assay versus tuberculin skin test for monitoring TB infection in healthcare workers. Expert Rev Anti Infect Ther. 2013 Jan;11(1):37–48. doi: 10.1586/eri.12.150. Available from: http://dx.doi.org/10.1586/eri.12.150. [DOI] [PubMed] [Google Scholar]
- 10.International Labour Organization (ILO) List of occupational diseases (revised 2010) Geneva: International Labour Office; 2010. (Occupational Saftey and Health Series; 74). [Google Scholar]
- 11.Jensen PA, Lambert LA, Iademarco MF, Ridzon R CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005 Dec 30;54(RR-17):1–141. [PubMed] [Google Scholar]
- 12.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012 Jan;67(1):62–70. doi: 10.1136/thx.2010.143180. Available from: http://dx.doi.org/10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 13.Ringshausen FC, Schablon A, Nienhaus A. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol. 2012;7(1):6. doi: 10.1186/1745-6673-7-6. Available from: http://dx.doi.org/10.1186/1745-6673-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai M, Elwood K. Interferon-gamma release assays for screening of health care workers in low tuberculosis incidence settings: dynamic patterns and interpretational challenges. Can Respir J. 2012 Mar-Apr;19(2):81–83. doi: 10.1155/2012/420392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nienhaus A, Costa JT. Screening for tuberculosis and the use of a borderline zone for the interpretation of the interferon-γ release assay (IGRA) in Portuguese healthcare workers. J Occup Med Toxicol. 2013;8(1):1. doi: 10.1186/1745-6673-8-1. Available from: http://dx.doi.org/10.1186/1745-6673-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS) Can Commun Dis Rep. 2008 Oct;34(ACS-6):1–13. [PubMed] [Google Scholar]
- 17.Thanassi W, Noda A, Hernandez B, Newell J, Terpeluk P, Marder D, Yesavage JA. Delineating a Retesting Zone Using Receiver Operating Characteristic Analysis on Serial QuantiFERON Tuberculosis Test Results in US Healthcare Workers. Pulm Med. 2012;2012:291294. doi: 10.1155/2012/291294. Available from: http://dx.doi.org/10.1155/2012/291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong KS, Tomford JW, Teixeira L, Fraser TG, van Duin D, Yen-Lieberman B, Gordon SM, Miranda C. Challenges of interferon-gamma release assay conversions in serial testing of health-care workers in a TB control program. Chest. 2012 Jul;142(1):55–62. doi: 10.1378/chest.11-0992. Available from: http://dx.doi.org/10.1378/chest.11-0992. [DOI] [PubMed] [Google Scholar]
- 19.Joshi M, Monson TP, Woods GL. Use of interferon-gamma release assays in a health care worker screening program: experience from a tertiary care centre in the United States. Can Respir J. 2012 Mar-Apr;19(2):84–88. doi: 10.1155/2012/576324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwerling A, Benedetti A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, Menzies D, Pai M. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PLoS ONE. 2013;8(1):e54748. doi: 10.1371/journal.pone.0054748. Available from: http://dx.doi.org/10.1371/journal.pone.0054748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ECDC - European Centre for Disease Prevention and Control. Use of interferon-gamma release assays in support of TB diagnosis. Stockholm: ECDC; 2011. [Google Scholar]
- 22.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010 Jun 25;59(RR-5):1–25. [PubMed] [Google Scholar]
- 23.Schablon A, Harling M, Diel R, Ringshausen FC, Torres Costa J, Nienhaus A. Serial testing with an interferon-γ release assay in German healthcare workers. GMS Krankenhhyg Interdiszip. 2010;5(2):Doc05. doi: 10.3205/dgkh000148. Available from: http://dx.doi.org/10.3205/dgkh000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schablon A, Diel R, Diner G, Anske U, Pankow W, Ringshausen FC, Nienhaus A. Specificity of a whole blood IGRA in German nursing students. BMC Infect Dis. 2011;11:245. doi: 10.1186/1471-2334-11-245. Available from: http://dx.doi.org/10.1186/1471-2334-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diel R, Loytved G, Nienhaus A, Castell S, Detjen A, Geerdes-Fenge H, Haas W, Hauer B, Königstein B, Maffei D, Magdorf K, Priwitzer M, Zellweger JP, Loddenkemper R. Neue Empfehlungen für die Umgebungsuntersuchungen bei Tuberkulose. Deutsches Zentralkomitee zur Bekämpfung der Tuberkulose. [New recommendations for contact tracing in tuberculosis. German Central Committee against Tuberculosis]. Pneumologie. 2011 Jun;65(6):359–378. doi: 10.1055/s-0030-1256439. (Ger). Available from: http://dx.doi.org/10.1055/s-0030-1256439. [DOI] [PubMed] [Google Scholar]
- 26.Andersen P, Doherty TM, Pai M, Weldingh K. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol Med. 2007 May;13(5):175–182. doi: 10.1016/j.molmed.2007.03.004. Available from: http://dx.doi.org/10.1016/j.molmed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Ringshausen FC, Nienhaus A, Torres Costa J, Knoop H, Schlösser S, Schultze-Werninghaus G, Rohde G. Within-subject variability of Mycobacterium tuberculosis-specific gamma interferon responses in German health care workers. Clin Vaccine Immunol. 2011 Jul;18(7):1176–1182. doi: 10.1128/CVI.05058-11. Available from: http://dx.doi.org/10.1128/CVI.05058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schablon A, Harling M, Diel R, Nienhaus A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: a multicentre prevalence study. BMC Infect Dis. 2010;10:107. doi: 10.1186/1471-2334-10-107. Available from: http://dx.doi.org/10.1186/1471-2334-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chee CB, Lim LK, Barkham TM, Koh DR, Lam SO, Shen L, Wang YT. Use of a T cell interferon-gamma release assay to evaluate tuberculosis risk in newly qualified physicians in Singapore healthcare institutions. Infect Control Hosp Epidemiol. 2009 Sep;30(9):870–875. doi: 10.1086/599284. Available from: http://dx.doi.org/10.1086/599284. [DOI] [PubMed] [Google Scholar]
- 30.Veeser PI, Smith PK, Handy B, Martin SR. Tuberculosis screening on a health science campus: use of QuantiFERON-TB Gold Test for students and employees. J Am Coll Health. 2007 Sep-Oct;56(2):175–180. doi: 10.3200/JACH.56.2.175-180. Available from: http://dx.doi.org/10.3200/JACH.56.2.175-180. [DOI] [PubMed] [Google Scholar]